Abstract
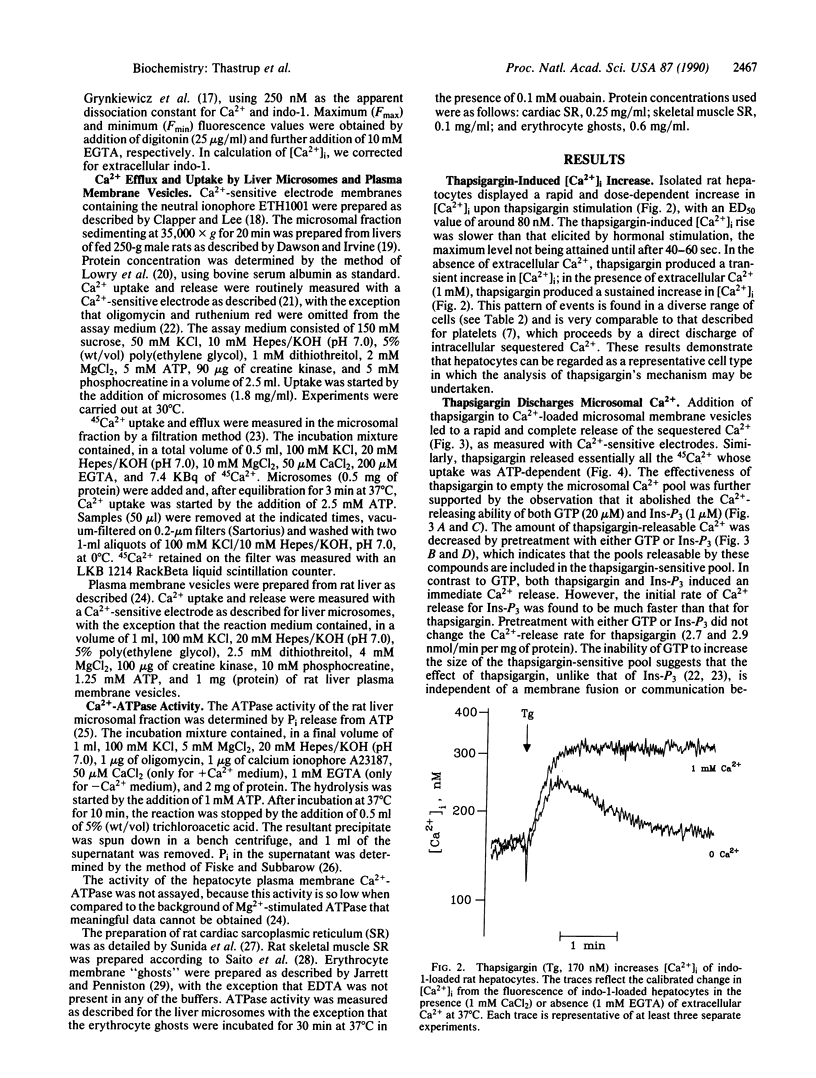
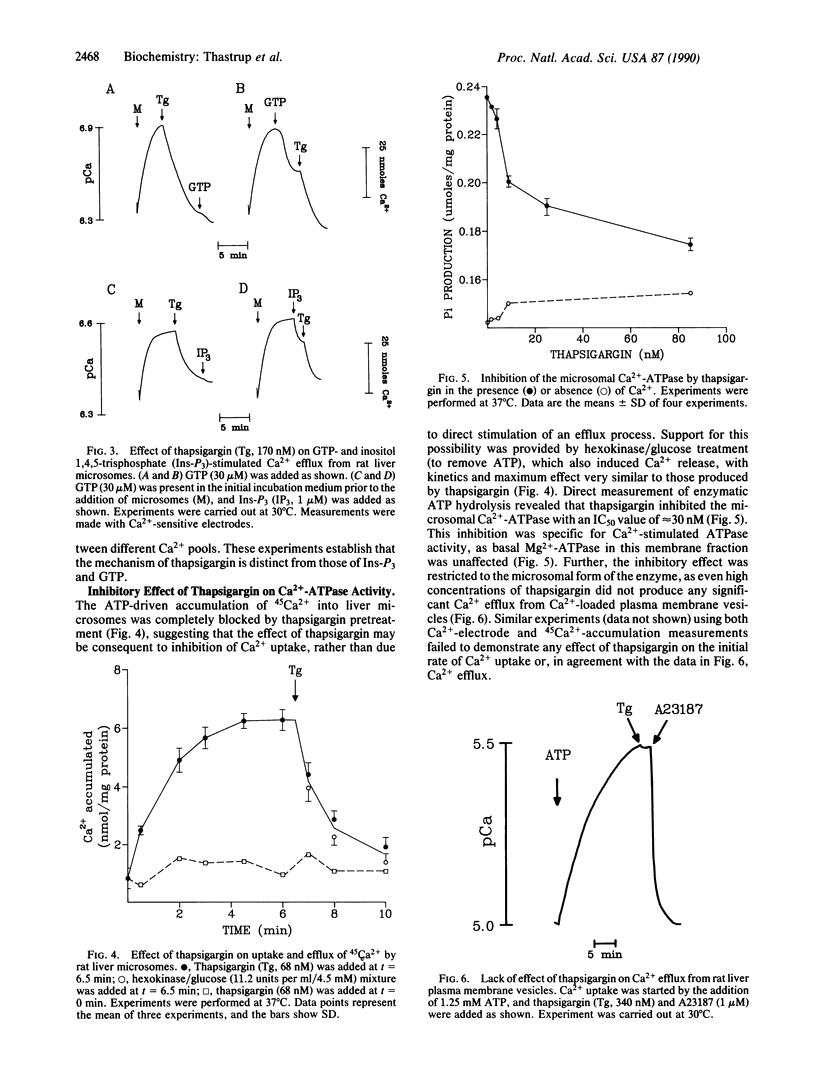
Thapsigargin, a tumor-promoting sesquiterpene lactone, discharges intracellular Ca2+ in rat hepatocytes, as it does in many vertebrate cell types. It appears to act intracellularly, as incubation of isolated rat liver microsomes with thapsigargin induces a rapid, dose-dependent release of stored Ca2+. The thapsigargin-releasable pool of microsomal Ca2+ includes the pools sensitive to inositol 1,4,5-trisphosphate and GTP. Thapsigargin pretreatment of microsomes blocks subsequent loading with 45Ca2+, suggesting that its target is the ATP-dependent Ca2+ pump of endoplasmic reticulum. This hypothesis is strongly supported by the demonstration that thapsigargin causes a rapid inhibition of the Ca2(+)-activated ATPase activity of rat liver microsomes, with an identical dose dependence to that seen in whole cell or isolated microsome Ca2+ discharge. The inhibition of the endoplasmic reticulum isoform of the Ca2(+)-ATPase is highly selective, as thapsigargin has little or no effect on the Ca2(+)-ATPases of hepatocyte or erythrocyte plasma membrane or of cardiac or skeletal muscle sarcoplasmic reticulum. These results suggest that thapsigargin increases the concentration of cytosolic free Ca2+ in sensitive cells by an acute and highly specific arrest of the endoplasmic reticulum Ca2+ pump, followed by a rapid Ca2+ leak from at least two pharmacologically distinct Ca2+ stores. The implications of this mechanism of action for the application of thapsigargin in the analysis of Ca2+ homeostasis and possible forms of Ca2+ control are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali H., Christensen S. B., Foreman J. C., Pearce F. L., Piotrowski W., Thastrup O. The ability of thapsigargin and thapsigargicin to activate cells involved in the inflammatory response. Br J Pharmacol. 1985 Jul;85(3):705–712. doi: 10.1111/j.1476-5381.1985.tb10567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch-Machin M. A., Dawson A. P. Effects of chelating agents on the Ca2+-stimulated ATPase of rat liver plasma membranes. Biochim Biophys Acta. 1986 Feb 27;855(2):277–285. doi: 10.1016/0005-2736(86)90175-6. [DOI] [PubMed] [Google Scholar]
- Brandt P., Zurini M., Neve R. L., Rhoads R. E., Vanaman T. C. A C-terminal, calmodulin-like regulatory domain from the plasma membrane Ca2+-pumping ATPase. Proc Natl Acad Sci U S A. 1988 May;85(9):2914–2918. doi: 10.1073/pnas.85.9.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden D. J., Hanley M. R., Thastrup O., Cuthbert A. W. Thapsigargin, a new calcium-dependent epithelial anion secretagogue. Br J Pharmacol. 1989 Nov;98(3):809–816. doi: 10.1111/j.1476-5381.1989.tb14609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek T. R., Thastrup O. Internal Ca2+ mobilization and secretion in bovine adrenal chromaffin cells. Cell Calcium. 1989 May-Jun;10(4):213–221. doi: 10.1016/0143-4160(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Clapper D. L., Lee H. C. Inositol trisphosphate induces calcium release from nonmitochondrial stores i sea urchin egg homogenates. J Biol Chem. 1985 Nov 15;260(26):13947–13954. [PubMed] [Google Scholar]
- Comerford J. G., Dawson A. P. The mechanism of action of GTP on Ca2+ efflux from rat liver microsomal vesicles. Measurement of vesicle fusion by fluorescence energy transfer. Biochem J. 1988 Jan 1;249(1):89–93. doi: 10.1042/bj2490089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Fulton D. V. Some properties of the Ca2+-stimulated ATPase of a rat liver microsomal fraction. Biochem J. 1983 Feb 15;210(2):405–410. doi: 10.1042/bj2100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P. GTP enhances inositol trisphosphate-stimulated Ca2+ release from rat liver microsomes. FEBS Lett. 1985 Jun 3;185(1):147–150. doi: 10.1016/0014-5793(85)80759-6. [DOI] [PubMed] [Google Scholar]
- Dawson A. P., Hills G., Comerford J. G. The mechanism of action of GTP on Ca2+ efflux from rat liver microsomal vesicles. Biochem J. 1987 May 15;244(1):87–92. doi: 10.1042/bj2440087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Irvine R. F. Inositol (1,4,5)trisphosphate-promoted Ca2+ release from microsomal fractions of rat liver. Biochem Biophys Res Commun. 1984 May 16;120(3):858–864. doi: 10.1016/s0006-291x(84)80186-2. [DOI] [PubMed] [Google Scholar]
- Dich J., Bro B., Grunnet N., Jensen F., Kondrup J. Accumulation of triacylglycerol in cultured rat hepatocytes is increased by ethanol and by insulin and dexamethasone. Biochem J. 1983 Jun 15;212(3):617–623. doi: 10.1042/bj2120617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foder B., Scharff O., Thastrup O. Ca2+ transients and Mn2+ entry in human neutrophils induced by thapsigargin. Cell Calcium. 1989 Oct;10(7):477–490. doi: 10.1016/0143-4160(89)90025-0. [DOI] [PubMed] [Google Scholar]
- Fujiki H., Suganuma M., Nakayasu M., Hakii H., Horiuchi T., Takayama S., Sugimura T. Palytoxin is a non-12-O-tetradecanoylphorbol-13-acetate type tumor promoter in two-stage mouse skin carcinogenesis. Carcinogenesis. 1986 May;7(5):707–710. doi: 10.1093/carcin/7.5.707. [DOI] [PubMed] [Google Scholar]
- Gould G. W., McWhirter J. M., East J. M., Lee A. G. A model for the uptake and release of Ca2+ by sarcoplasmic reticulum. Biochem J. 1987 Aug 1;245(3):739–749. doi: 10.1042/bj2450739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hakii H., Fujiki H., Suganuma M., Nakayasu M., Tahira T., Sugimura T., Scheuer P. J., Christensen S. B. Thapsigargin, a histamine secretagogue, is a non-12-O-tetradecanoylphorbol-13-acetate (TPA) type tumor promoter in two-stage mouse skin carcinogenesis. J Cancer Res Clin Oncol. 1986;111(3):177–181. doi: 10.1007/BF00389230. [DOI] [PubMed] [Google Scholar]
- Hanley M. R., Jackson T. R., Vallejo M., Patterson S. I., Thastrup O., Lightman S., Rogers J., Henderson G., Pini A. Neural function: metabolism and actions of inositol metabolites in mammalian brain. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):381–398. doi: 10.1098/rstb.1988.0083. [DOI] [PubMed] [Google Scholar]
- Jackson T. R., Patterson S. I., Thastrup O., Hanley M. R. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J. 1988 Jul 1;253(1):81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett H. W., Penniston J. T. Purification of the Ca2+-stimulated ATPase activator from human erythrocytes. Its membership in the class of Ca2+-binding modulator proteins. J Biol Chem. 1978 Jul 10;253(13):4676–4682. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lytton J., MacLennan D. H. Molecular cloning of cDNAs from human kidney coding for two alternatively spliced products of the cardiac Ca2+-ATPase gene. J Biol Chem. 1988 Oct 15;263(29):15024–15031. [PubMed] [Google Scholar]
- Mallat A., Pavoine C., Dufour M., Lotersztajn S., Bataille D., Pecker F. A glucagon fragment is responsible for the inhibition of the liver Ca2+ pump by glucagon. Nature. 1987 Feb 12;325(6105):620–622. doi: 10.1038/325620a0. [DOI] [PubMed] [Google Scholar]
- Ohuchi K., Sugawara T., Watanabe M., Hirasawa N., Tsurufuji S., Fujiki H., Christensen S. B., Sugimura T. Analysis of the stimulative effect of thapsigargin, a non-TPA-type tumour promoter, on arachidonic acid metabolism in rat peritoneal macrophages. Br J Pharmacol. 1988 Jul;94(3):917–923. doi: 10.1111/j.1476-5381.1988.tb11604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen U., Brøogger Christensen S., Sandberg F. Thapsigargine and thapsigargicine, two new histamine liberators from Thapsia garganica L. Acta Pharm Suec. 1978;15(2):133–140. [PubMed] [Google Scholar]
- Saito A., Seiler S., Chu A., Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol. 1984 Sep;99(3):875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff O., Foder B., Thastrup O., Hofmann B., Møller J., Ryder L. P., Jacobsen K. D., Langhoff E., Dickmeiss E., Christensen S. B. Effect of thapsigargin on cytoplasmic Ca2+ and proliferation of human lymphocytes in relation to AIDS. Biochim Biophys Acta. 1988 Dec 9;972(3):257–264. doi: 10.1016/0167-4889(88)90200-5. [DOI] [PubMed] [Google Scholar]
- Sumida M., Wang T., Mandel F., Froehlich J. P., Schwartz A. Transient kinetics of Ca2+ transport of sarcoplasmic reticulum. A comparison of cardiac and skeletal muscle. J Biol Chem. 1978 Dec 25;253(24):8772–8777. [PubMed] [Google Scholar]
- Tada M., Kadoma M. Regulation of the Ca2+ pump ATPase by cAMP-dependent phosphorylation of phospholamban. Bioessays. 1989 May;10(5):157–163. doi: 10.1002/bies.950100505. [DOI] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Foder B., Scharff O. The calcium mobilizing tumor promoting agent, thapsigargin elevates the platelet cytoplasmic free calcium concentration to a higher steady state level. A possible mechanism of action for the tumor promotion. Biochem Biophys Res Commun. 1987 Feb 13;142(3):654–660. doi: 10.1016/0006-291x(87)91464-1. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Linnebjerg H., Bjerrum P. J., Knudsen J. B., Christensen S. B. The inflammatory and tumor-promoting sesquiterpene lactone, thapsigargin, activates platelets by selective mobilization of calcium as shown by protein phosphorylations. Biochim Biophys Acta. 1987 Jan 19;927(1):65–73. doi: 10.1016/0167-4889(87)90066-8. [DOI] [PubMed] [Google Scholar]
- Vrolix M., Raeymaekers L., Wuytack F., Hofmann F., Casteels R. Cyclic GMP-dependent protein kinase stimulates the plasmalemmal Ca2+ pump of smooth muscle via phosphorylation of phosphatidylinositol. Biochem J. 1988 Nov 1;255(3):855–863. doi: 10.1042/bj2550855. [DOI] [PMC free article] [PubMed] [Google Scholar]


