Supplemental Digital Content is available in the text
Keywords: cardiopulmonary bypass, meta-analysis, respiratory insufficiency, ventilation
Abstract
Background:
Cardiopulmonary bypass (CPB) is necessary for most cardiac surgery, which may lead to postoperative lung injury. The objective of this paper is to systematically evaluate whether ventilation during CPB would benefit patients undergoing cardiac surgery.
Methods:
We searched randomized controlled trials (RCTs) through PubMed, Embase, and Cochrane Library from inception to October 2016. Eligible studies compared clinical outcomes of ventilation versus nonventilation during CPB in patients undergoing cardiac surgery. The primary outcome includes oxygenation index (PaO2/FiO2 ratio) or alveolar to arterial oxygen tension difference (AaDO2) immediately after weaning from bypass. The secondary outcomes include postoperative pulmonary complications (PPCs), shunt fraction (Qs/Qt), hospital stay, and AaDO2 4 hours after CPB.
Results:
Seventeen trials with 1162 patients were included in this meta-analysis. Ventilation during CPB significantly increased post-CPB PaO2/FiO2 ratio (mean difference [MD] = 21.84; 95% confidence interval [CI] = 1.30 to 42.37; P = 0.04; I2 = 75%) and reduced post-CPB AaDO2 (MD = –50.17; 95% CI = –71.36 to –28.99; P <0.00001; I2 = 74%). Qs/Qt immediately after weaning from CPB showed a significant difference between groups (MD = –3.24; 95% CI = –4.48 to –2.01; P <0.00001; I2 = 0%). Incidence of PPCs (odds ratio [OR] = 0.79; 95% CI = 0.42 to 1.48; P = 0.46; I2 = 37%) and hospital stay (MD = 0.09; 95% CI = –23 to 0.41; P = 0.58; I2 = 37%) did not differ significantly between groups.
Conclusion:
Ventilation during CPB might improve post-CPB oxygenation and gas exchange in patients who underwent cardiac surgery. However, there is no sufficient evidence to show that ventilation during CPB could influence long-term prognosis of these patients. The beneficial effects of ventilation during CPB are requisite to be evaluated in powerful and well-designed RCTs.
1. Introduction
Despite the improvement in perioperative management, the postoperative respiratory dysfunction is still a widely reported complication of cardiopulmonary bypass (CPB), leading to increased mortality and morbidity in cardiac surgery.[1,2] Various strategies including perioperative management of mechanical ventilation (MV), restrictive transfusion, technical modifications of CPB, and medication administration such as steroids and aprotinin have been developed to reduce impairment of pulmonary function.[3–5] Ventilation during CPB is an important element of MV management strategies and determined by anesthesiologists in the operation room. Continuous positive airway pressure (CPAP), low-volume ventilation, positive end-expiratory pressure (PEEP), and vital capacity maneuvers (VCMs) are adjustable parameters composing ventilation techniques.
So far, available researches regarding whether ventilation during CPB could improve respiratory outcomes are still controversial. Some studies found that the application of CPAP during CPB was an effective adjunct.[6,7] Gaudriot et al[8] suggested that maintaining MV during CPB could diminish immune dysfunction after surgery. However, others reported that the utilization of CPAP did not show a significant difference compared with the controls when it came to attenuating the post-CPB impairment of lung function.[9,10]
Additionally, it has been reported that the application of low tidal volume (TV)–low frequency ventilation could decrease the occurrence of CPB-related lung injury because it could decrease inflammatory reactions and some negative immune markers such as interleukin 10 and tumor necrosis factor-α (TNF-α).[11–14] But the protective function of continuous ventilation during CPB is still debatable because many studies found it is not a necessary technique for an improved respiratory outcome.[9,15] To provide the latest and more convincing evidence, we systematically reviewed the present available literature to evaluate the efficacy and safety of ventilation during CPB in patients who were scheduled to undergo cardiac surgery.
2. Methods
2.1. Search strategy
This meta-analysis was performed in accordance with PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines.[16] We searched PubMed, Embase, and Cochrane Library from inception to October 2016, without a language restriction. Two reviewers conducted the searching work independently and the other 2 helped to resolve the conflicts in the process and find out the missed studies identified through other sources manually. A list of the search terms used for each electronic database is presented in Fig. S1 (Supplemental content). There is no requirement for ethical approval and patient consent as this is a meta-analysis of published studies.
2.2. Selection criteria
Study inclusion criteria were as follows: patients: adult patients (≥18 years) scheduled to undergo cardiac surgery with CPB procedure; interventions: different ventilation strategies during CPB period including either CPAP or low TV ventilation (PEEP and VCMs were not necessary factors); control: patients did not receive any type of ventilation during CPB; outcomes: the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2 ratio), alveolar to arterial oxygen tension difference (AaDO2), shunt fraction (Qs/Qt), postoperative pulmonary complications (PPCs), and duration of hospital stay. At least 1 primary outcome, that is, either PaO2/FiO2 or AaDO2, must be reported in the eligible cites. Design: randomized controlled trials (RCTs).
Study exclusion criteria were as follows: the ventilation interventions carried out before or after the CPB period, or in the intensive care unit after surgery, or carried discontinuously (e.g., only intermittent mandatory ventilation at weaning from CPB); (the CPB procedure was not used in cardiac surgery; publication type: conference abstracts, corresponding to other trial reports, or reviews; the interventions of control group was not nonventilation during CPB; other reasons including sub-studies and small sample-sized studies (<10 patients).
2.3. Data abstraction and quality assessment
Two independent reviewers conducted data abstraction and quality assessment. Conflicts were solved by discussion. Data extracted from articles included the following: trial characteristics: author, year of publication, study design, surgery type, sample size, inclusion criteria, experimental, and control arms; overall average baseline patient characteristics: age, male percent, body mass index (BMI), length of CPB, and length of surgery; endpoints: relevant primary endpoints (PaO2/FiO2 and AaDO2) and recording time of primary endpoints; and relevant secondary endpoints: Qs/Qt, PPCs, length of hospital stay and others. The secondary outcomes were not analyzed unless the data were available in at least 3 trials.
We assessed the risk of bias of all eligible studies according to the standard of Cochrane Collaboration. The studies were assessed from the following aspects: randomized sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. In this way, the studies were assessed as low risk, high risk, and unclear risk.[17]
2.4. Quality of evidence
GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system was used for assessment of evidence quality. The quality of evidence classified by GRADE system is in 1 of 4 levels: high, moderate, low, and very low. Meta-analysis based on RCTs starts as high-quality evidence, and the confidence may be decreased because of the following reasons: study limitation, inconsistency of results, indirectness of evidence, imprecision, and reporting bias.[18]
2.5. Statistical analysis
Outcomes of continuous variables were expressed as the mean with standard difference. Mean difference (MD) and 95% confidence interval (CI) were calculated. Outcomes of discontinuous variables presented with events and total patients. Odds ratio (OR) and 95% CI were figured up. The primary outcome referred to PaO2/FiO2 or AaDO2 immediately after weaning from CPB. The secondary outcome referred to the PPCs, hospital stay, Qs/Qt immediately after weaning from CPB, and AaDO2 4 hours after weaning from CPB.
Homogeneity assumption was tested with I2 statistics. It is calculated as I2 = 100% × (Q – df)/Q, where Q is Cochran's heterogeneity statistic. Heterogeneity was suggested if P ≤0.10. An I2 value of 0%–24.9% indicated no heterogeneity, 25%–49.9% mild heterogeneity, 50%–74.9% moderate heterogeneity, and 75%–100% considerable heterogeneity.
Inverse variance statistical method was used in continuous variables data analysis, and the Mantel–Haenszel statistical method was used in discontinuous variables data analysis. Moreover, random-effects model was used for synthesis of the data. The publication biases were assessed by Egger's test for the asymmetry of funnel plots by regression methods. The presence of publication bias was indicated by Skewed and asymmetrical funnel plots. Sensitivity analyses were carried out for different subgroups according to a variety of differences in study design. All statistical analyses were executed by using Review Manager V.5.3 (RevMan, The Cochrane Collaboration, Oxford, UK). Significant differences were set at a 2-sided P value <0.05.
3. Results
3.1. Literature identification and study characteristics
A total of 1469 potentially eligible records were yielded from the initial database search (400 from PubMed, 878 from Embase, 191 from CENTRAL and 0 from other sources). After removing 488 duplicates, 4 independent authors screened 981 citations according to the inclusion criteria. Based on title and abstract, 954 records were excluded for various reasons (conference abstracts, letters, case reports, animal studies, pediatric patients, not RCTs in cardiac surgery, or irrelevant to the study). The remaining 27 full texts were screened, of which 10 citations were excluded for the following reasons: 3 studies had been published twice; 1 was an observational study; the full-text of 1 study was not available; and 5 studies assessed the effects of VCMs at the weaning from CPB. Finally, 17 citations were included in the meta-analysis (Fig. 1).[7,9,11,19–32] The main characteristics of the included trials are described in appendix (Table S1). The assessment of risk of bias regarding included studies is shown in appendix (Fig. S2, Supplemental content).
Figure 1.
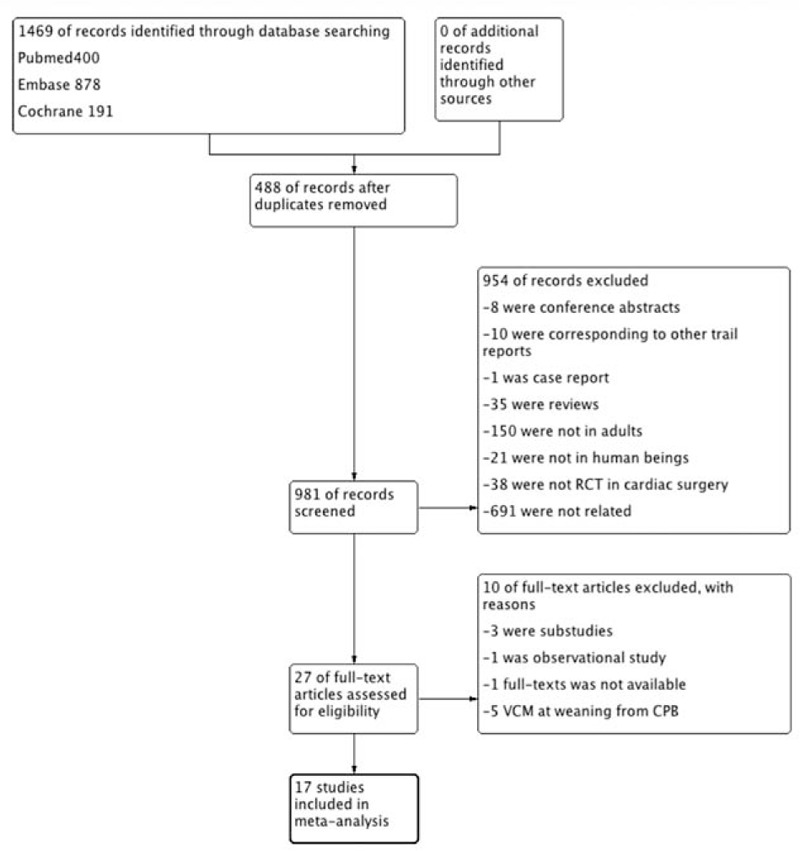
Flowchart of selecting process for meta-analysis.
3.2. Primary outcomes
There were 10 studies with 588 patients who reported PaO2/FiO2 ratio immediately after CPB. Our meta-analysis showed that there was a significant difference of PaO2/FiO2 between the ventilation and nonventilation groups in these studies (MD = 21.84; 95% CI = 1.30 to 42.37; P = 0.04; P for heterogeneity <0.01; I2 = 75% (Fig. 2).
Figure 2.
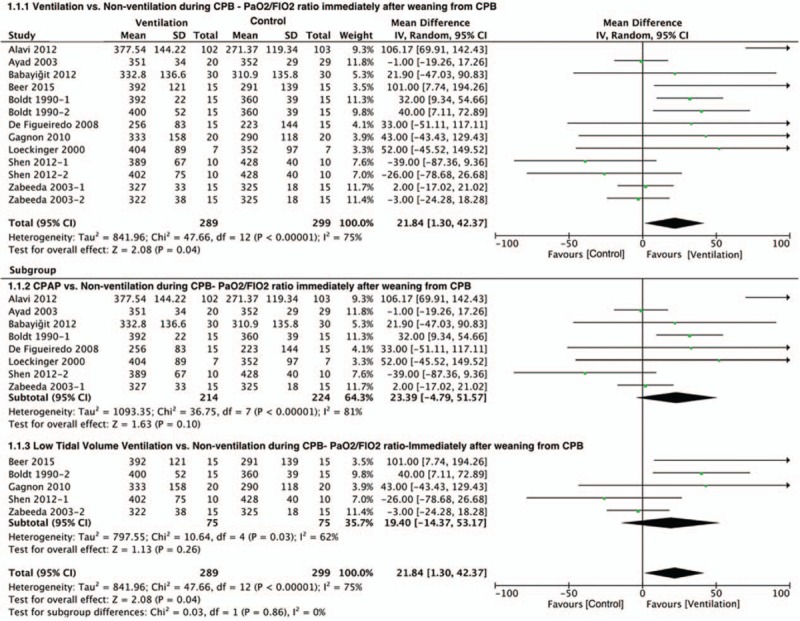
Forest plot showing the effect of ventilation during CPB on PaO2/FiO2 ratio immediately after CPB. CPB = cardiopulmonary bypass, PaO2/FiO2 ratio = the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen.
Data for the AaDO2 of ventilation during CPB were available from 10 RCTs in this analysis. Compared with the control group, ventilation during CPB was associated with significant improvement in AaDO2 immediately after weaning from CPB (MD = –50.17; 95% CI = –71.36 to –28.99; P <0.00001; P for heterogeneity <0.0001, I2 = 74%) (Fig. 3).
Figure 3.

Forest plot showing the effect of ventilation during CPB on AaDO2 immediately after CPB. AaDO2 = alveolar to arterial oxygen tension difference, CPB = cardiopulmonary bypass.
To determine the source of heterogeneity, we made a sensitivity analysis and subgroup analysis according to ventilation strategies (Figs. 2 and 3). The sensitivity analysis showed that Alavi et al[20] and Altmay et al[19] separately had the greatest influence of heterogeneity on PaO2/FiO2 ratio and AaDO2. The subgroups were divided into the CPAP group and the low TV ventilation group based on the ventilation type. In the oxygenation index analysis, using CPAP during CPB showed a greater heterogeneity (I2 = 81%) than low TV ventilation (I2 = 62%). But there was no difference between 2 groups by using either CPAP (P = 0.1) or low TV ventilation strategy (P = 0.26). When it came to the AaDO2 in the subgroup analysis, there was a significant difference between 2 groups by using either CPAP (P = 0.004) or TV ventilation (P = 0.03).
3.3. Secondary outcomes
In secondary outcomes, the association between ventilation strategies during CPB and Qs/Qt immediately after weaning from CPB, the incidence of PPCs, hospital stay, and AaDO2 after 4 hours weaning from CPB were analyzed. The incidence of PPCs (OR = 0.79; 95% CI = 0.42 to 1.48; P = 0.46; P for heterogeneity = 0.18, I2 = 25%) and hospital stay (MD = 0.09; 95% CI = –23 to 0.41; P = 0.58; P for heterogeneity –0.16, I2 = 37%) did not differ significantly between 2 groups. However, AaDO2 4 hours after weaning from CPB (MD = –26.62; 95% CI = –48.18 to –5.06; P = 0.02; P for heterogeneity = 0.0010, I2 = 73%) and Qs/Qt immediately after weaning from CPB showed a significant difference between 2 groups (MD = –3.24; 95% CI = –4.48 to –2.01; P <0.00001; P for heterogeneity <0.75, I2 = 0%) (Fig. 4).
Figure 4.

Forest plot showing the secondary outcomes of ventilation during CPB. CPB = cardiopulmonary bypass.
3.4. Quality of evidence
GRADE system grades of evidence were very low for PaO2/FiO2, AaDO2 immediately after weaning from CPB, and all the secondary outcomes (Fig. S3, Supplemental content).
4. Discussion
The main findings of this meta-analysis were ventilation during CPB indicated an increase in oxygenation index level and a decrease in AaDO2 level immediately after weaning from CPB in patients who underwent elective cardiac surgery. In addition, shunt fraction and AaDO2 4 hours after CPB were significantly different between groups. However, the PPCs and hospital stay did not differ significantly between ventilation and nonventilation group during CPB.
CPB-related pulmonary dysfunction is a multifactorial postoperative problem with high morbidity and mortality. Bignami et al[2] reviewed the literature concerning CPB-related respiratory insufficiency and lung damage, concluding that correct ventilation during CPB, as a paramount part of multidisciplinary approach, might diminish the occurrence of postoperative lung injury. A meta-analysis reported by Schreiber et al[33] showed that CPAP or VCMs administrated during CPB had a potential trend for lung protection based on some surrogate endpoints but no sustained effect postoperatively was found. Previous studies showed that the potential mechanisms of CPB-related lung dysfunction involved pulmonary atelectasis, intrapulmonary shunt, and change of systemic immune and inflammatory status.[2] This meta-analysis examined the efficacy and safety of ventilation during CPB in patients who underwent elective cardiac surgery. It is not only an update of Schreiber et al's study but also provides different comparison direction and endpoints. On one hand, our study analyzed the combined effect of ventilation during CPB compared with the nonventilation group. On the other hand, we performed subgroup analysis to compare the impact of separate ventilation strategies (CPAP or low TV ventilation alone). But the results from the above-mentioned comparison need to be interpreted with caution. The results from the combined effect of ventilation showed that ventilation during CPB could improve PaO2/FiO2 ratio immediately after weaning from CPB (P = 0.04) but the effect from CPAP or low TV ventilation alone got an opposite conclusion (P = 0.10; 0.26). The difference might be explained by the following: sample size: all included studies were small sample size trials and the subgroup analysis made the total sample size smaller, which thus could affect the results; the influence of PEEP: the use of PEEP was not uniform in each study, so the use of PEEP might be a potential confounding factor. In order to figure out the influence of PEEP, we performed subgroup analysis based on whether PEEP was used (Fig. S4, Supplemental content). Compared with the nonventilation group, the influence of low TV ventilation with or without PEEP on the PaO2/FiO2 ratio immediately after weaning from CPB was different from each other (low TV ventilation with PEEP: MD = 54.25, 95% CI = 3.66 to 104.84, P = 0.04; low TV ventilation without PEEP: MD = –3.79, 95% CI = –23.02 to 15.45, P = 0.70). Based on this result, we could conclude that the application of PEEP might improve oxygenation after CPB when the intervention measure was low TV ventilation. None of papers about CPAP mentioned the use of PEEP so we did not perform subgroup analysis about CPAP with or without PEEP. We also performed a funnel plot to access the publication bias of the literatures. In regard to PaO2/FiO2 immediately after weaning from CPB, the symmetry of the funnel plot was not very good suggesting that there might be publication bias (Fig. S5, Supplemental content). When it came to the AaDO2 immediately after weaning from CPB, the funnel plot was symmetrical in general showing that publication bias for the included studies was controlled passably (Fig. S6, Supplemental content).
A variety of lung-protective techniques, including CPAP, low TV ventilation, and VCMs, have been reported to be beneficial when applied during CPB.[2,5] As VCM is not a continuous ventilation type and has been recommended as a lung protective strategy in clinical practice at the weaning of CPB, VCM treatment alone was excluded and we mainly discuss CPAP or low TV ventilation during CPB. And owing to the endpoints of interest in the included studies were gathered at various time points, we chose the most frequent overlapped time point (immediately after CPB) to analyze the endpoints of patients. Besides, parameter details in the intervention group were set in a large range that is trials evaluating the effect of CPAP during CPB used CPAP from 5 to 15 cmH2O. Only 1 trial used CPAP of 15 cmH2O in one of the groups but this parameter setting was too high to get a better outcome of pulmonary function.[22] We just excluded these data and used a moderate CPAP from 5 to 10 cmH2O. In addition, the FiO2 used for CPAP or low TV ventilation ranged from 0.21 to 1.0. There were 2 trials that investigated the relationship of different FiO2 levels (FiO2 = 0.21; 1.0) with the PaO2/FiO2[22,32] and they suggested that perioperative hyperoxia was potentially harmful by increasing the expression of reactive oxygen species and decreasing receptors in cells.[34] Considering the lung injury caused by high FiO2, we excluded the group that using 100% oxygen in those 2 trials.
When interpreting the present results, several potential limitations should be taken into consideration. To begin with, the sample sizes of many included studies were limited. Clinical trial with small size and low quality might lead to bias and high heterogeneity (Fig. S2, Supplemental content). Presently, a large-scale RCT comparing the effects of no ventilation during CPB, CPAP with a PEEP of 5 cmH2O during CPB, and low TV ventilation of 2–3 mL/kg with a PEEP of 3–5 cmH2O during CPB in cardiac surgery is being performed (clinicaltrials.gov identifier NCT02090205). Nevertheless, the comparability of eligible cites was influenced by the heterogeneity of study endpoints and protocols. Moreover, owing to long-term outcomes that were rarely reported in the eligible cites and influenced by multiple factors such as the lung protection strategies before and after CPB, the main outcomes were surrogate endpoints that were not directly linked to long-term prognosis. Only 5 trials showed the incidence of PPCs and 7 trials referred to the length of hospital stay. Thus, we could not draw the conclusion that ventilation during CPB influences long-term prognosis of patients who underwent cardiac surgery.
5. Conclusion
Our meta-analysis indicated that ventilation during CPB might improve post-CPB oxygenation and gas exchange in patients who were scheduled to undergo cardiac surgery. However, long-term outcomes need to be further investigated through other large-sized and high-quality clinical trials. Different respiratory parameters setting during CPB, such as low or high FiO2 values and low TV ventilation with or without PEEP, may impact clinical outcomes.
Acknowledgments
The authors sincerely thank the authors of primary clinical studies.
Supplementary Material
Footnotes
Abbreviations: AaDO2 = alveolar to arterial oxygen tension difference, ARDS = acute respiratory distress syndrome, BMI = body mass index, CI = confidence interval, CPAP = continuous positive airway pressure, CPB = cardiopulmonary bypass, FiO2 = the fraction of inspired oxygen, ICU = intensive care unit, IMV = intermittent mandatory ventilation, IL-10 = interleukin 10, MD = mean difference, MV = mechanical ventilation, OR = odds ratio, PaO2 = partial pressure of arterial oxygen, PaO2/FiO2 ratio = the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen, PEEP = positive end-expiratory pressure, PPCs = postoperative pulmonary complications, RCTs = randomized controlled trials, TV = tidal volume, TNF-α = tumor necrosis factor-α, VCMs = vital capacity maneuvers.
Declaration: This document is a unique submission and it has never been published in any other medium, in part or in full.
DC, CC, and HY had the original idea and wrote the initial draft of the manuscript. HY had full access to all the data and took responsibility for the integrity of the data and the accuracy of the data analysis. BL had access to some data analysis and provided some guiding suggestions. YS and WW contributed substantially to the study design, data analysis and revision, and final approval of the manuscript. DC, CC, YS, WW and YM contributed equally to this article.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Apostolakis E, Filos KS, Koletsis E, et al. Lung dysfunction following cardiopulmonary bypass. J Cardiac Surg 2010;25:47–55. [DOI] [PubMed] [Google Scholar]
- [2].Bignami E, Guarnieri M, Saglietti F, et al. Mechanical ventilation during cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2016;30:1668–75. [DOI] [PubMed] [Google Scholar]
- [3].Hill GE, Alonso A, Spurzem JR, et al. Aprotinin and methylprednisolone equally blunt cardiopulmonary bypass-induced inflammation in humans. J Thorac Cardiovasc Surg 1995;110:1658–62. [DOI] [PubMed] [Google Scholar]
- [4].Rahman A, Ustunda B, Burma O, et al. Does aprotinin reduce lung reperfusion damage after cardiopulmonary bypass? Eur J Cardiothorac Surg 2000;18:583–8. [DOI] [PubMed] [Google Scholar]
- [5].Apostolakis EE, Koletsis EN, Baikoussis NG, et al. Strategies to prevent intraoperative lung injury during cardiopulmonary bypass. J Cardiothorac Surg 2010;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Claxton B, Morgan P, McKeague H, et al. Alveolar recruitment strategy improves arterial oxygenation after cardiopulmonary bypass. Anaesthesia 2003;58:111–6. [DOI] [PubMed] [Google Scholar]
- [7].Loeckinger A, Kleinsasser A, Lindner KH, et al. Continuous positive airway pressure at 10 cm H2O during cardiopulmonary bypass improves postoperative gas exchange. Anesth Analg 2000;91:522–7. [DOI] [PubMed] [Google Scholar]
- [8].Gaudriot B, Uhel F, Gregoire M, et al. Immune dysfunction after cardiac surgery with cardiopulmonary bypass: beneficial effects of maintaining mechanical ventilation. Shock (Augusta, Ga) 2015;44:228–33. [DOI] [PubMed] [Google Scholar]
- [9].Berry C, Butler P, Myles P. Lung management during cardiopulmonary bypass: is continuous positive airways pressure beneficial? Brit J Anaesth 1993;71:864–8. [DOI] [PubMed] [Google Scholar]
- [10].Stanley TH, Liu W-S, Gentry S. Effects of ventilatory techniques during cardiopulmonary bypass on post-bypass and postoperative pulmonary compliance and shunt. Anesthesiology 1977;46:391–5. [PubMed] [Google Scholar]
- [11].Beer L, Szerafin T, Mitterbauer A, et al. Continued mechanical ventilation during coronary artery bypass graft operation attenuates the systemic immune response. Eur J Cardiothorac Surg 2012;ezs659. [DOI] [PubMed] [Google Scholar]
- [12].Beer L, Szerafin T, Mitterbauer A, et al. Low tidal volume ventilation during cardiopulmonary bypass reduces postoperative chemokine serum concentrations. Thorac Cardiovasc Surg 2014;62:677–82. [DOI] [PubMed] [Google Scholar]
- [13].Beer L, Szerafin T, Mitterbauer A, et al. Ventilation during cardiopulmonary bypass: impact on heat shock protein release. J Cardiovasc Surg 2014;55:849–56. [PubMed] [Google Scholar]
- [14].Beer L, Warszawska JM, Schenk P, et al. Intraoperative ventilation strategy during cardiopulmonary bypass attenuates the release of matrix metalloproteinases and improves oxygenation. J Surg Res 2015;195:294–302. [DOI] [PubMed] [Google Scholar]
- [15].Ng CS, Wan S, Yim AP, et al. Pulmonary dysfunction after cardiac surgery. CHEST J 2002;121:1269–77. [DOI] [PubMed] [Google Scholar]
- [16].Moher D, Liberati A, Tetzlaff J, et al. Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Altmay E, Karaca P, Yurtseven N, et al. Continuous positive airway pressure does not improve lung function after cardiac surgery. Can J Anesth 2006;53:919–25. [DOI] [PubMed] [Google Scholar]
- [20].Alavi M, Pakrooh B, Mirmesdagh Y, et al. The effects of positive airway pressure ventilation during cardiopulmonary bypass on pulmonary function following open heart surgery. Res Cardiovasc Med 2013;2:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ayad AE, Hamed HF. Continuous positive airway pressure (CPAP) during cardiopulmonary bypass attenuates postoperative pulmonary dysfunction and complications. Egypt J Anaesth 2003;19:345–51. [Google Scholar]
- [22].Boldt J, King D, Scheid H, et al. Lung management during cardiopulmonary bypass: influence on extravascular lung water. J Cardiothorac Anesth 1990;4:73–9. [DOI] [PubMed] [Google Scholar]
- [23].Babayiğit M, Özgencil GE, Çatav S, et al. Effect of ventilation during cardiopulmonary bypass in open heart surgery on postoperative pulmonary functions. Anestezi Dergisi 2012;20:92–8. [Google Scholar]
- [24].Cogliati A, Menichetti A, Tritapepe L, et al. Effects of three techniques of lung management on pulmonary function during cardiopulmonary bypass. Acta Anaesthesiol Belgica 1995;47:73–80. [PubMed] [Google Scholar]
- [25].Davoudi M, Farhanchi A, Moradi A, et al. The effect of low tidal volume ventilation during cardio-pulmonary bypass on postoperative pulmonary function. J Tehran Univ Heart Center 2010;5:128–31. [PMC free article] [PubMed] [Google Scholar]
- [26].De Figueiredo LC, Araújo S, Abdala RCS, et al. CPAP at 10 cm H2O during cardiopulmonary bypass does not improve postoperative gas exchange. Braz J Cardiovasc Surg 2008;23:209–15. [DOI] [PubMed] [Google Scholar]
- [27].Durukan AB, Gurbuz HA, Salman N, et al. Ventilation during cardiopulmonary bypass did not attenuate inflammatory response or affect postoperative outcomes. Cardiovasc J Africa 2013;24:224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].John LC, Ervine IM. A study assessing the potential benefit of continued ventilation during cardiopulmonary bypass. Interac Cardiovasc Thorac Surg 2008;7:14–7. [DOI] [PubMed] [Google Scholar]
- [29].Gagnon J, Laporta D, Beique F, et al. Clinical relevance of ventilation during cardiopulmonary bypass in the prevention of postoperative lung dysfunction. Perfusion 2010;25:205–10. [DOI] [PubMed] [Google Scholar]
- [30].Ng CS, Arifi AA, Wan S, et al. Ventilation during cardiopulmonary bypass: impact on cytokine response and cardiopulmonary function. Ann Thorac Surg 2008;85:154–62. [DOI] [PubMed] [Google Scholar]
- [31].Shen SE, Wang YW. Effects of different ventilation modes during cardiopulmonary bypass on pulmonary function after cardiac surgery. J Shanghai Jiaotong Univ (Medical Science) 2010;30:843–7. +864. [Google Scholar]
- [32].Zabeeda D, Gefen R, Medalion B, et al. The effect of high-frequency ventilation of the lungs on postbypass oxygenation: A comparison with other ventilation methods applied during cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2003;17:40–4. [DOI] [PubMed] [Google Scholar]
- [33].Schreiber J-U, Lancé MD, de Korte M, et al. The effect of different lung-protective strategies in patients during cardiopulmonary bypass: a meta-analysis and semiquantitative review of randomized trials. J Cardiothorac Vasc Anesth 2012;26:448–54. [DOI] [PubMed] [Google Scholar]
- [34].Garcia-Delgado M, Navarrete-Sanchez I, Colmenero M. Preventing and managing perioperative pulmonary complications following cardiac surgery. Curr Opin Anaesthesiol 2014;27:146–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


