Abstract
An improved analytical method compared with conventional ones was developed for simultaneous determination of 13 mycotoxins (deoxynivalenol, nivalenol, 3-acetylnivalenol, aflatoxin B1, aflatoxin B2, aflatoxin G1, aflatoxin G2, fumonisin B1, fumonisin B2, T-2, HT-2, zearalenone, and ochratoxin A) in cereal grains by liquid chromatography-tandem mass spectrometry (LC/MS/MS) after a single immunoaffinity column clean-up. The method showed a good linearity, sensitivity, specificity, and accuracy in mycotoxin determination by LC/MS/MS. The levels of 13 mycotoxins in 5 types of commercial grains (brown rice, maize, millet, sorghum, and mixed cereal) from South Korea were determined in a total of 507 cereal grains. Mycotoxins produced from Fusarium sp. (fumonisins, deoxynivalenol, nivalenol, and zearalenone) were more frequently (more than 5%) and concurrently detected in all cereal grains along with higher mean levels (4.3–161.0 ng/g) in positive samples than other toxins such as aflatoxins and ochratoxin A (less than 9% and below 5.2 ng/g in positive samples) from other fungal species.
Keywords: aflatoxins, deoxynivalenol, fumonisins, HT-2, nivalenol, ochratoxin A, T-2, zearalenone, grains, LC/MS/MS
1. Introduction
Mycotoxins are biologically active secondary metabolites produced by a variety of fungi such as Aspergillus, Penicillium, and Fusarium sp. To date, approximately 400 compounds have been identified as mycotoxins such as aflatoxins (AFs), ochratoxin A (OTA), fumonisins (Fs), nivalenol (NIV), deoxynivalenol (DON), zearalenone (ZEN), T-2 toxin (T-2), and HT-2 toxin (HT-2) [1]. The International Agency for Research on Cancer [2] assigned major mycotoxins into one of 5 groups based on their carcinogenicity. Aflatoxin B1 (AFB1) is categorized as a Group 1 human carcinogen due to its potent carcinogenic properties in liver [2]. OTA, fumonisin B1 (FB1), and fumonisin B2 (FB2) are classified as possible carcinogens (Group 2B) to human since OTA causes nephrotoxicity, immune suppression, carcinogenicity, and teratogenicity in laboratory animals, and it has been associated with a fatal human kidney disease known as Balkan Endemic Nephropathy, and FB1 and FB2 cause equine leukoencephalomalacia (ELEM) in horses and porcine pulmonary edema (PPE) in pigs [3]. However, DON, ZEN, and T-2 are categorized into Group 3 (not classifiable as to its carcinogenicity to humans) because there is no evidence in their mutagenicity and carcinogenicity [4].
These mycotoxins occur in agricultural crops during pre-harvest and storage. As the mycotoxins are chemically very stable, they are not degraded during food processing, causing a variety of adverse and toxic health effects in target organs such as liver, kidney, and nerve systems in human. Thus, most of the countries started to reinforce management of mycotoxins in foods and feeds, and the European Union and the Codex have made efforts to set common regulatory limits for mycotoxins. South Korea has also set and regulated the maximum allowable limits for important mycotoxins in foods and feeds based on Food Sanitation Act and Control of Livestock and Fish Feed Act. Currently, risks of mycotoxins found in agricultural crops are assessed by analyses of only one toxin among several mycotoxins, which can contaminate the same agricultural crops, and the maximum allowable limits and analytical methods for major mycotoxins are established only for individual toxin in South Korea as well as other countries. Moreover, these mycotoxins found in a contaminated agricultural commodity can cause serious synergistic effects in human and animals when consumed simultaneously by them. Boeira and co-workers reported a synergistic toxicity between Fusarium mycotoxins (DON and ZEA) on the growth of brewing yeasts [5]. Other researchers have demonstrated that a combined toxicity of either DON and Fs or AFs and FB1 in liver in piglets or barrows caused higher histopathological lesion and immune suppression [6,7]. Severe reductions in growth and immune response were found in broilers by dietary combinations of AFs and OTA [8]. Also, synergistic cytotoxic effects of mycotoxin combinations were shown in mammalian cell [9,10,11,12]. Because co-occurrence of mycotoxins is very common in agricultural commodities, a reliable and sensitive analytical method is needed for simultaneous determination of multi-mycotoxins. In addition, a prerequisite for the analyses of multi-toxins is good recoveries of the toxins at toxin extraction and clean-up steps. Most of recently developed multi-mycotoxin analytical methods employ acetonitrile-water mixtures for co-extraction of mycotoxins at toxin extraction steps [13]. In order to purify toxin extracts at toxin clean-up steps, commercial immunoaffinity columns (IAC) have been successfully applied for simultaneous determination of mycotoxins by liquid chromatography-tandem mass spectrometry (LC/MS/MS). There are two toxin elution methods at IAC steps; single elution [13] and double elution [14]. In the current study, in order to develop analytical methods for rapid and efficient determination of major mycotoxins in food, we developed a new rapid method to co-elute all 13 major mycotoxins by one step using 5 mL of 80% methanol (MeOH) containing 0.5% acetic acid at the same time and established an analytical method for simultaneous determination of the 13 mycotoxins by LC/MS/MS. The method was successfully applied for rapid and simultaneous determination of the 13 mycotoxins in grains collected from retail markets in South Korea. To the best of our knowledge, this is the first report on improved co-extraction of 13 mycotoxins in a variety of grains and simultaneous analyses of the multi-mycotoxins in the cereal grains collected from markets in South Korea by LC/MS/MS.
2. Results
2.1. Linearity of Calibration Curves for 13 Mycotoxins
The linearity of 13 mycotoxins in the analytical method was assessed by each standard curve using 5 levels of standard solutions for each toxin. An extract ion chromatogram (EIC) of 13 mycotoxins analyzed by the LC/MS/MS is shown in Figure 1. The linearity of the calibration curves was determined by linear regression analysis. The curves for all 13 mycotoxins showed r2 values of 0.9932–1.0000 (Figure S1). Therefore, we concluded that the standard curves for all 13 mycotoxins were linear in the range of 1.3–53 ng/mL.
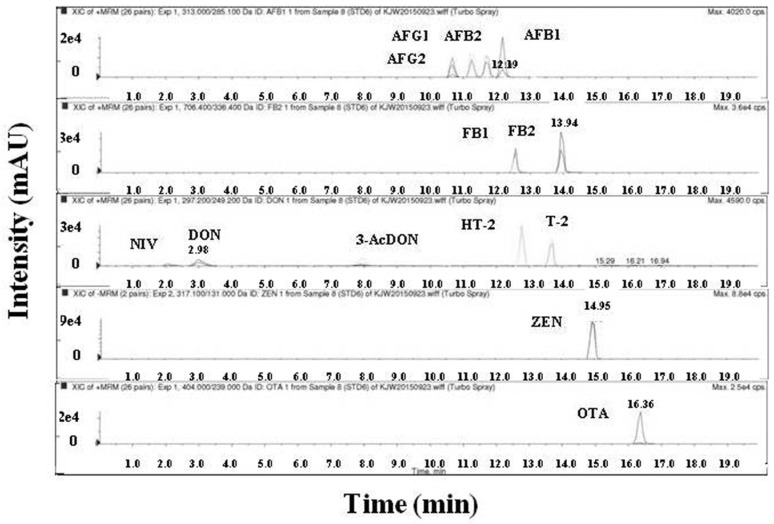
Figure 1.
Chromatograms of 13 mycotoxins using LC/MS/MS. Extract ion chromatogram of 13 mycotoxins. Ten microliters of the toxin mixture standard was injected into the LC/MS/MS system. The retention times of peaks corresponding to each toxin are as follow: NIV, 2.08 min; DON, 2.98 min; 3-AcDON, 7.95 min; AFG2, 10.67 min; AFG1, 11.25 min; AFB2, 11.71 min; AFB1, 12.19 min; FB1, 12.54 min; HT-2, 12.77 min; T-2, 13.68 min; FB2, 13.94 min; ZEN, 14.95 min; OTA, 16.36 min.
2.2. Extraction of 13 Mycotoxins from 5 Different Matrices and the Effects of the Matrices on Toxin Extraction
Previously, toxins have been extracted by two steps using water and MeOH, which require time-consuming and laborious shaking and clean-up [14,15]. In this study our method describes a rapid and efficient co-extraction and co-elution of 13 mycotoxins in 5 different matrices including mixed cereal with organic solvents containing acetic acid. Our one step method for extraction of multi-toxins shortened the toxin extraction procedure.
Extraction of toxins from grain samples with organic solvents entails the possibility of analytical problems (matrix effects) due to the co-extraction of matrix components in the samples. The matrix effects can affect the ionization efficiency of toxins, leading to suppression or enhancement of the signal in LC/MS/MS depending on combinations of types of toxins and matrices. Thus, the effects of matrices on the determination of 13 mycotoxins in 5 types of grains were evaluated.
The signal suppression/enhancement was calculated by the following equation:
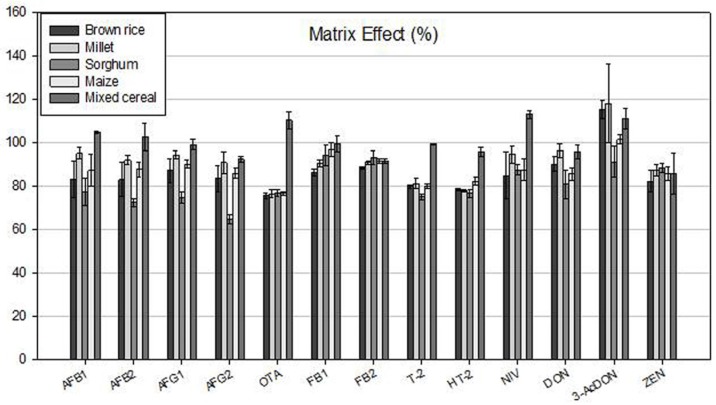
Five types of grain samples showed minor matrix effects on the determination of all 13 toxins in the samples (74.5%–112.2%) (Figure 2). Of these toxins, signals for levels of 3-AcDON in all 5 types of samples were slightly enhanced by the matrices (102.9%–112.2%), whereas those for all other toxins in the samples were a little suppressed by the matrices (74.5%–90.0%).
Figure 2.
Signal suppression/ enhancement (SSE) effect of 13 mycotoxins in 5 types of matrices. Five levels (1.325, 2.65, 13.25, 26.5, and 53 ng/mL) of standard solutions for each toxin were prepared by mixing the extract from each type of grain sample. The standard solutions for each toxin were injected into LC/MS/MS in triplicate.
2.3. Recovery of 13 Mycotoxins from 5 Different Matrices
Recoveries for 13 mycotoxins extracted from each matrix spiked with each toxin standard solution were evaluated by using our newly developed co-extraction and co-elution method.
The recoveries were calculated by the following equation:
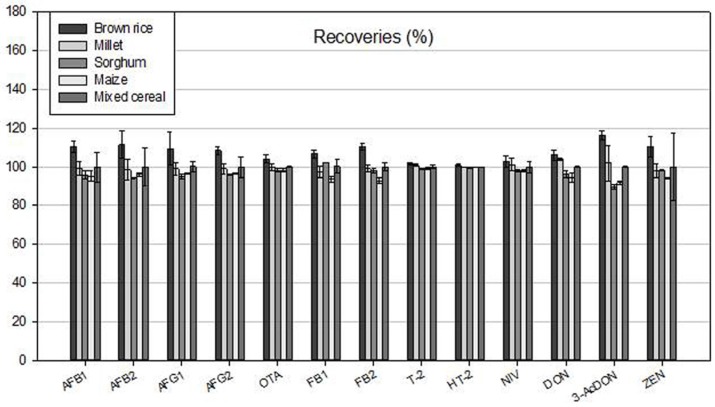
The recoveries were measured by injecting toxins extracted from each matrix, which was naturally uncontaminated with 13 mycotoxins and spiked with 1.2–326.1 ng/mL of each toxin standard solution as described in materials and methods, into LC/MS/MS. The recovery rates for 13 mycotoxins in 5 types of matrices were 73.9%–133.0% along with relative standard deviation (RSDr) of 0.1%–14.3% (Figure 3). These recovery rates of 13 mycotoxins satisfied allowable limits of the recovery and RSD recommended by Codex or Association of Official Analytical Chemists (AOAC) [16,17]. The Codex recommends 60%–120% of recovery rates in food samples contaminated with 1–10 μg/kg of mycotoxins and the guideline for the recoveries by AOAC is 70%–125% in food samples contaminated with 10 μg/kg of mycotoxins. In addition, RSDr values of the 13 mycotoxins (0.1%–14.3%) were below 15%, which is recommended for food samples contaminated with 10 μg/kg of mycotoxins by AOAC. Thus, we concluded that the analytical method by co-extraction and co-elution using MeOH containing acetic acid had good recoveries from 5 types of matrices.
Figure 3.
Recovery of 13 mycotoxins from 5 types of matrices. Each matrix, which was naturally uncontaminated with 13 mycotoxins, was spiked with 1.2–326.1 ng/mL of each toxin standard solution as described in materials and methods. Toxins extracted from each matrix were injected into LC/MS/MS.
2.4. LOD and LOQ of an Analytical Method for Determination of Levels of 13 Mycotoxins
The sensitivity of the analytical method using LC/MS/MS was determined by a limit of detection (LOD) and limit of quantification (LOQ). They were calculated as signal-to-noise (S/N) ratios of 3 and 10, respectively, which were measured by using Analyst 1.6 software. The LODs of the analytical method for 13 mycotoxins in 5 types of cereal grains were between 0.1 and 18.1 ng/g, whereas the LOQs of the method for the mycotoxins in cereal grains were between 0.4 and 54.8 ng/g (Table S1). They were as low as those for detection of trace amounts of the toxins. It indicates that the method using LC/MS/MS is highly sensitive for determination of all 13 mycotoxins in cereal grains.
2.5. Monitoring Levels of 13 Mycotoxins in Commercial Cereal Grains
The analytical method validated above was used for the determination of levels of 13 mycotoxins in 5 types of 507 cereal grains (brown rice, maize, millet, sorghum, and mixed cereal) collected from local markets in South Korea. The occurrence and levels of all 13 toxins in the commercial products are summarized in Table 1. Mycotoxins produced from Fusarium sp. (FB1, FB2, DON, NIV, and ZEN) were more frequently detected in all grain samples than other toxins such as AFs and OTA from other fungal species.
Table 1.
Mean and range of levels and incidence of 13 mycotoxins in a total of 507 brown rice, millet, sorghum, maize, and mixed cereal collected from retail market.
| Mycotoxin1 | Grain Sample | |||||
|---|---|---|---|---|---|---|
| Item | Brown Rice | Millet | Sorghum | Maize | Mixed Cereal | |
| AFB1 | Incidence (%) | 1 | 9 | 4 | 1 | 4 |
| Mean2 (ng/g) | 1.1 | 1.3 | 1.0 | 5.2 | 4.3 | |
| Range (ng/g) | 1.1 | 0.4–5.6 | 0.7–1.7 | 5.2 | 0.7–12.4 | |
| AFB2 | Incidence (%) | 0 | 1 | 0 | 0 | 0 |
| Mean2 (ng/g) | 0 | 0.5 | 0 | 0 | 0 | |
| Range (ng/g) | - | 0.5 | - | - | - | |
| FB1 | Incidence (%) | 42 | 52 | 95 | 47 | 74 |
| Mean2 (ng/g) | 13.6 | 12.4 | 160.8 | 136.5 | 17.3 | |
| Range (ng/g) | 2.1–22.8 | 2.0–32.6 | 5.8–890.0 | 3.8–2990.0 | 3.1–80.1 | |
| FB2 | Incidence (%) | 44 | 50 | 89 | 59 | 58 |
| Mean2 (ng/g) | 9.6 | 12.0 | 42.8 | 45.2 | 14.6 | |
| Range (ng/g) | 1.6–18.8 | 1.6–31.1 | 4.0–223.5 | 1.9–620.0 | 1.8–22.1 | |
| T-2 | Incidence (%) | 0 | 0 | 0 | 2 | 0 |
| Mean2 (ng/g) | 0 | 0 | 0 | 10.0 | 0 | |
| Range (ng/g) | - | - | - | 6.4–13.7 | - | |
| HT-2 | Incidence (%) | 0 | 0 | 0 | 0 | 1 |
| Mean2 (ng/g) | 0 | 0 | 0 | 0 | 4.3 | |
| Range (ng/g) | - | - | - | - | 4.3 | |
| OTA | Incidence (%) | 0 | 0 | 0 | 0 | 1 |
| Mean2 (ng/g) | 0 | 0 | 0 | 0 | 0.5 | |
| Range (ng/g) | - | - | - | - | 0.5 | |
| DON | Incidence (%) | 7 | 25 | 70 | 13 | 19 |
| Mean2 (ng/g) | 5.6 | 46.5 | 64.0 | 180.4 | 41.5 | |
| Range (ng/g) | 6.0–12.3 | 12.1–212.0 | 18.1–257.0 | 17.0–1405.0 | 14.5–162.0 | |
| NIV | Incidence (%) | 5 | 16 | 53 | 18 | 40 |
| Mean2 (ng/g) | 26.3 | 45.3 | 48.2 | 116.1 | 50.2 | |
| Range (ng/g) | 16.3–36.8 | 15.7–102.0 | 18.1–211.5 | 12.7–570.0 | 13.8–175.0 | |
| 3-AcDON | Incidence (%) | 0 | 0 | 0 | 2 | 0 |
| Mean2 (ng/g) | 0 | 0 | 0 | 17.8 | 0 | |
| Range (ng/g) | - | - | - | 7.9–27.7 | - | |
| ZEN | Incidence (%) | 32 | 14 | 62 | 7 | 47 |
| Mean2 (ng/g) | 5.2 | 7.4 | 37.5 | 4.3 | 6.1 | |
| Range (ng/g) | 0.4–37.6 | 0.7–61.5 | 0.9–313.0 | 0.9–14.7 | 0.2–36.0 | |
1 AFG1 and AFG2 were not detected in all grain samples; 2 Mean indicates an average in positive samples.
The AFB1 showed 1%–9% of incidence in all cereal grains (Table 1). The highest levels of AFB1 in maize and millet were 5.2 and 5.6 ng/g, respectively, which were below the maximum allowable limit (10 ng/g) set by the Korean Food and Drug Administration (KFDA) [18]. However, levels of AFB1 in mixed cereal (12.4 ng/g) exceeded the allowable limit. The occurrence of AFB2 was much lower than that of AFB1; the occurrence of AFB2 was below 1% in millet. AFG1 and AFG2 were not detected in any types of grain samples.
The incidence (42%–95%) and levels of FB1 and FB2 were similar to each other in the same grain group, and the levels of Fs were in the range of 1.6–2990 ng/g in positive samples (Table 1). In particular, Fs were detected in relatively high concentrations (1.9–2990 ng/g of the range and 42.8–160.8 ng/g of the mean levels in positive samples) in maize and sorghum compared to those (1.6–80.1 ng/g of the range and 9.6–17.3 ng/g of the mean levels in positive samples) in other types of cereal grains. Nevertheless, the levels of FB1 and FB2 detected in all types of grains were below the maximum allowable limit (4 µg/g for FB1 and FB2 in maize) set by the KFDA.
Of trichothecenes (TCs), DON and NIV were more frequently detected in all types of cereal grains than T-2, HT-2, and 3-AcDON; the incidence of DON and NIV was between 16%–70% in all grains except 7% for DON and 5% for NIV in brown rice, and the mean levels of DON and NIV were in the range of 6.0-1405 ng/g in positive samples (Table 1). Except the levels of DON in maize, the levels of DON and NIV detected in all types of cereal grains were below the maximum allowable limit (1 µg/g for DON in grains and their products) set by the KFDA when the limit of DON is used for levels of NIV since the legal limits of NIV are not set yet in South Korea [18]. However, the occurrence of T-2, HT-2, and 3-AcDON was less than 2% in the cereal grains, and the ranges of those in positive samples were lower than 27.7 ng/g. The ranges of T-2, HT-2, and 3-AcDON were far below than 1 µg/g, which was set for DON as the legal limit by KFDA [18]. Thus, the levels of DON and NIV as well as those of 3AcDON, T-2, and HT-2 in all types of cereal grains except for DON in maize could not pose a health risk in South Korea.
The incidence of ZEN, one of mycotoxins from Fusarium sp., was between 7%-62% in all types of cereal grains, and its range was between 0.2–313.0 ng/g (Table 1). In particular, the levels of ZEN detected in sorghum were above the maximum allowable limit (200 ng/g) set by the KFDA [18]. Therefore, the level of ZEN in sorghum could pose a risk to public health in South Korea.
Finally, OTA was detected rarely (0%–1% of incidence) in the cereal grains, and its range was 0–0.5 ng/g. The levels of OTA detected in all grains were below the maximum allowable limit (5 ng/g) set by the KFDA [18].
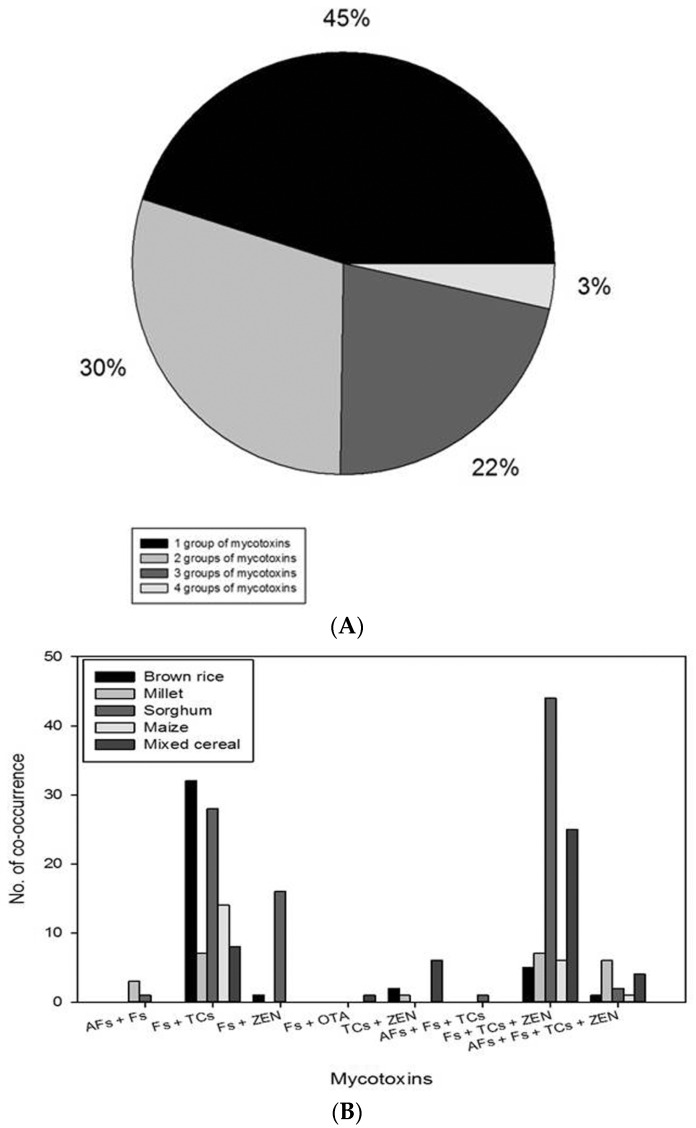
The co-occurrence of the mycotoxins in 507 cereal grains was as follows; 30% of 2 groups of micotoxins, 22% of 3 groups of mycotoxins, and 3% of 4 groups of mycotoxins as shown in Figure 4A. In samples which showed co-occurrence of 2 groups of mycotoxins, Fs and TCs were frequently detected. Fs, TCs, and ZEN were major mycotoxins in the category of 3 groups of mycotoxins. These data on the co-occurrence of the mycotoxins in the same grain sample indicate that mycotoxins produced from Fusarium sp. (FB1, FB2, HT-2, T-2, DON, NIV, 3-AcDON, and ZEN) were concurrently detected in the same samples. In addition, they were detected more frequently along with AFB1 or AFB2 in the same samples than along with OTA, and any samples were not co-contaminated with AFs and OTA; AFs with Fusarium mycotoxins in 17 samples and OTA with Fusarium mycotoxins in only 1 mixed cereal sample (Figure 4B).
Figure 4.
Co-occurrence of mycotoxins in the same sample of 5 types of cereal grains collected from retail market. (A) Percentage of co-occurrence of mycotoxins in a total of 507 cereal grains; (B) Number of co-occurrence of mycotoxins in 5 types of cereal grains.
Overall, although the levels of AFB1 (except for mixed cereal), total AFs, FB1, FB2, and OTA detected in all 5 types of grain samples were below the maximum allowable limit set by the KFDA, they should have been much lower than observed levels because even low exposure to dietary toxins could pose a carcinogenic risk to human. In addition, the levels of DON and NIV in all 5 types of grains (except the levels of DON in maize) as well as those of 3AcDON, T-2, and HT-2 in the cereal grains were below the maximum allowable limit set for DON by KFDA. Also, the level of ZEN detected in sorghum was above the maximum allowable limit set by KFDA. Therefore, monitoring the levels of 13 mycotoxins in cereal grains marketed in South Korea should be continued.
3. Discussion
In this study we developed an improved rapid one-step method to co-elute all 13 major mycotoxins from Myco6in1 with IAC using 5 mL of 80% MeOH containing 0.5% acetic acid and established an analytical method for simultaneous determination of the mycotoxins by LC/MS/MS. The method was then used for rapid and simultaneous determination of levels of the 13 mycotoxins in grains collected from retail markets in South Korea. For 100% elution of 13 mycotoxins from IACs, all bonds between analytes and antibodies should be broken. In our one-step elution, 5 mL of 80% MeOH can provide enough time to break the bonds, and the acidic condition produced by 0.5% acetic acid makes the bond breakage occur easily, which improves the recoveries of the mycotoxins in this study. The recovery rates for 13 mycotoxins in 5 types of matrices were 73.9%–133.0% using the improved toxin extraction method (Figure 3). In contrast, a previous study described by Lattanzio and collaborators showed that recovery rates were relatively low (63%–93%) in simultaneous determination of multi-mycotoxins in corn and wheat samples [14].
Our data showed that the incidence of AFB1 in all cereal grains was from 1% to 9% (Table 1). Of these grains, the occurrence of AFB1 in maize was 1% in our study, while AFB1 was not detected in corn collected in South Korea in one study described by Park and co-workers [19]. One of the reasons for this discrepancy may have come from the small sample size (n = 18) in the previous study.
Also, the occurrences of FB1 and FB2 in brown rice and millet were about 50% (42% in brown rice and 52% in millet for FB1, and 44% in brown rice and 50% in millet for FB2) in the current study (Table 1), while both mycotoxins were not detected in brown rice and millet collected in South Korea in one study described by Seo and collaborators [20]. Again, the small sample size (n = 12) in the previous study may have been one of the reasons for this discrepancy.
Table 1 showed that the incidence and the mean levels of DON and NIV in maize were similar to each other (13% and 180 ng/g for DON, and 18% and 116 ng/g for NIV). These results show lower occurrences and levels of DON and NIV compared to those detected in corn collected in South Korea in 1993, in which authors reported occurrences of 65.2% of DON (310 ng/g of the mean level) and 34.8% of NIV (77 ng/g of the mean level) [21]. One of possible reasons for this discrepancy may have been due to different climate when the maize was harvested in the field or differences between the regions where the maize was harvested.
The incidence and the mean level of ZEN, another mycotoxin from Fusarium sp., in maize were 7% and 4.3 ng/g in the present study (Table 1). The level of ZEN in maize was lower than that (151 ng/g of the mean level) detected in corn collected in South Korea in 1993 as described by Kim and co-workers, while the occurrence of ZEN in maize in our study was similar to that (8%) detected in the previous study [21]. The same reason (different climate when maize was harvested or different regions where maize was harvested) as that for DON, and NIV may be able to explain this discrepancy.
Finally, OTA was detected rarely (1% of incidence) in all cereal grains, and its mean level was 0–0.5 ng/g in our study (Table 1). Of these grains, OTA was not detected in maize in this study, which was the same as that (0%) in corn collected in South Korea in 2002 [19].
On the other hand, Figure 4B showed that 17 samples were co-contaminated with AFs and mycotoxins from Fusarium sp. (FB1, FB2, HT-2, T-2, DON, NIV, 3-AcDON, and ZEN), whereas only one sample (mixed cereal) was concurrently contaminated with OTA and Fusarium mycotoxins. These data are in agreement with Ok and co-workers’ study in which they detected DON and ZEN concurrently in 12 out of 70 corn and barley samples collected from South Korea in 2005 and 2006 [22]. In addition, another study from South Korea reported the co-occurrence of AFB1, FB1, and ZEN in corn and barley [19]. Moreover, studies from other countries also showed the co-occurrence of the mycotoxins from Fusarium sp. One study from Finland reported that the same samples of cereal grains such as barley, oats, and wheat were contaminated simultaneously with DON, 3-AcDON, HT-2, T-2, and ZEN [23]. Another study by Ali and collaborators also reported that Fs, DON, NIV, and ZEN were co-contaminated with AFs in corn from Indonesia, and they isolated Aspergillus flavus and Aspergillus parasiticus along with Fusarium moniliforme in the same samples [24]. These data suggest that our cereal samples may also have been co-infected with the Aspergillus sp. and Fusarium sp.
Because the co-occurrence of these mycotoxins is common in cereal grains and they can cause synergistic effects to human and animals, it is necessary that efficient control methods are developed to prevent and monitor contamination of multi-mycotoxins and fungi in grains.
4. Conclusions
In our current study we developed a highly sensitive and reliable analytical method for simultaneous determination of levels of 13 mycotoxins (AFB1, AFB2, AFG1, AFG2, DON, NIV, 3-AcDON, FB1, FB2, T-2, HT-2, ZEN, and OTA) in cereal grains by LC/MS/MS after IAC clean-up. We were able to minimize any interfering materials against determination of levels of the mycotoxins in grains using the Myco6in1 with IAC columns and were able to elute all of the mycotoxins simultaneously from the IAC using 5 mL of 80% MeOH containing 0.5% acetic acid. The analytical method established in this study showed a good linearity, sensitivity, specificity, and accuracy in determination of levels of the mycotoxins by LC/MS/MS. The recovery rates of the mycotoxins in rice were 73.9%–133.0% along with RSDr of 0.1%–14.3%, which satisfied the legal limits of the recovery and RSD recommended by Codex or AOAC. The LODs of the analytical method for all of the mycotoxins were in the range of 0.1–18.5 µg/kg at a signal-to-noise (S/N) ratio of 3, and the LOQs of the method for the mycotoxins were in the range of 0.4–56.1 µg/kg at an S/N ratio of 10.
Finally, we investigated levels of 13 mycotoxins in 5 types of commercial cereal grains (brown rice, maize, millet, sorghum, and mixed cereal) collected from local markets in South Korea using the analytical method established in this study. The levels of DON and NIV in all types of cereal grains (except levels of DON in maize) and those of 3-AcDON, T-2, and HT-2 in cereal grains were below the maximum allowable legal limit (1 µg/g) set for DON or NIV by the KFDA. The levels of AFB1 (except for mixed cereal), total AFs, FB1, FB2, ZEN (except for sorghum) and OTA in the cereal grains were also below the maximum allowable limit set by the KFDA in South Korea. Because levels of DON in maize, those of AFB1 in mixed cereal, and those of ZEN in sorghum were higher than the maximum legal limits set by KFDA, extensive and active research should be continued for monitoring all 13 mycotoxins in cereal grains. Furthermore, establishment of legal limits of trichothecenes including NIV, 3-AcDON, T-2, and HT-2 for grains marketed in South Korea is required for monitoring them.
5. Experimental Sections
5.1. Samples
Brown rice, millet, sorghum, maize, and mixed cereal were used for the development of the analytical method and the determination of levels of 13 mycotoxins in these grains. The 5 types of 507 cereal grains were purchased from retail markets in South Korea. The grain samples were stored at 4 °C until use.
5.2. Standard Solutions and Reagents
The standards of Aflatoxins Mix 1 (2 µg/mL for AFB1 and AFG1, and 0.5 µg/mL for AFB2 and AFG2), OTA (10 µg/mL), Fumonisin Mix 3 (50 µg/mL for FB1 and FB2), DON (100 µg/mL), NIV (100 µg/mL), and 3-AcDON (100 µg/mL) were obtained from Biopure (Cambridge, MA, USA), while those of ZEN (100 µg/mL), T-2 (100 µg/mL), and HT-2 (100 µg/mL) were obtained from Sigma-Aldrich (St. Louis, MO, USA). The standard of 3 TCs mixture (DON, NIV, and 3-AcDON) was prepared by mixing 100 µL of each TC (101.9 µg/mL of DON, 100.9 µg/mL of NIV, and 100.6 µg/mL of 3-AcDON, which were diluted with acetonitrile [ACN]) with 490 µL of ACN, followed by dilution of the mixture with 410 µL of MeOH. The phosphate buffered saline (PBS, pH 7.4) for pH adjustment of toxin extracts was purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC grade ACN and MeOH were obtained from Merck (Darmstart, Germany). Stock solutions for each toxin were prepared by dilution of the standard solutions with 100% ACN (except for FB1 and FB2 for which 50% ACN was used), and working solutions with a series of toxin concentrations were made by dilution with 50% MeOH containing 1% acetic acid.
5.3. Assessment of the Precision, Linearity, and Sensitivity of the Analytical Method forDetermination ofLevels of 13 Mycotoxins
The linearity of a series of concentrations of 13 toxins in the analytical method was assessed by each standard curve using five levels (1.325, 2.65, 13.25, 26.5, and 53 ng/mL) of standard solutions for each toxin. The calibration curve for each toxin was constructed by plotting the peak areas (y axis) versus concentrations of each toxin (x axis) in LC/MS/MS analyses (Figure S1).
Five types of cereal grains that were not naturally contaminated with toxins were spiked with a mixed standard solution including 13 mycotoxins to determine the precision of the analytical method at levels of the toxins as follows: NIV, 321.0 ng/g; DON, 326.1 ng/g; 3-AcDON, 321.9 ng/g; AFB1, 8.5 ng/g; AFB2, 8.5 ng/g; AFG1, 8.2 ng/g; AFG2, 8.0 ng/g; FB1, 163.2 ng/g; FB2, 160.3 ng/g; HT-2, 161.6 ng/g, T-2, 64.6 ng/g; ZEN, 64.3 ng/g; OTA, 16.1 ng/g. Extraction and clean-up of analytes from the spiked samples were performed in triplicate by the procedures described below.
The sensitivity of the analytical method using LC/MS/MS was determined by LOD and LOQ. They were calculated as signal-to-noise (S/N) ratios of 3 and 10, respectively, which were determined by using HPLC software (Analyst 1.6 software program, Sciex, Framingham, MA, USA).
5.4. Toxin Extraction Procedure
Each sample (12.5 g) was weighed and placed in 100 mL of Erlenmeyer flasks after being ground into powders by a food grinder (Hallde, KISTA, Sweden). Fifty milliliters of ACN containing acetic acid (ACN: water: acetic acid = 79.5:20.0:0.5; v:v:v) as a selected solvent was added to it, and the 13 toxins were extracted by shaking at 320 rpm for 1 h with a wrist action shaker (EYELA, Tokyo, Japan). After the extracts were centrifuged at 3000× g for 5 min under 4 °C, supernatants were filtered through Whatman No. 4 filter paper (WhatmanTM, Maidstone, UK). Five milliliters of each filtrate was diluted with 75 mL of PBS (pH 7.4) and then filtered through a GF/A filter paper (WhatmanTM, Maidstone, UK). Sixty-five milliliters of the filtrate was loaded onto an IAC (Myco6in1+ column, VICAM, Milford, MA, USA) and passed through at a flow rate of one—two drops/sec. The column was washed with 10 ml of PBS and distilled water until 2–3 mL of air passed through it, and toxins were finally eluted from the column with 5 mL of 80% MeOH containing 0.5% acetic acid. The eluates were evaporated to dryness under a stream of N2 at 50 °C using a vacuum manifold (Agilent, Santa Clara, CA, USA), and the residues were re-dissolved in 1 mL of 50% MeOH containing 0.5% acetic acid. The solutions were vortexed for 1 min and filtered through a 0.22 μm syringe filter.
5.5. Matrix Effects on Toxin Extraction
After extraction of matrix components from the 5 types of cereal grains by the same procedure as the toxin extraction method described above, 265 ng/mL of each toxin standard solution was mixed with the extract from each type of grain sample at a ratio (v:v) of 4 to 1 to make 53 ng/mL of each toxin standard solution containing the extracted matrix components. Then, a series of five levels (1.325, 2.65, 13.25, 26.5, and 53 ng/mL) of standard solutions for each toxin were prepared by serial dilution of 53 ng/mL of the standard solution with the extract containing matrix components. After LC/MS/MS analyses, each calibration curve for each toxin was constructed as described above. The matrix-matched calibration curves were used for calculation of recovery rates of 13 mycotoxins from 5 types of matrices.
5.6. LC/MS/MS Conditions
HPLC (1260 series, Agilent, Santa Clara, CA, USA) equipped with a AB SCIEX QTRAP mass spectrometer (AB 3200, Applied Biosystems, Foster city, CA, USA) was used to detect the 13 toxins. Separation of the toxins was carried out on a Scherzo SM-C18 column (3 mm × 150 mm, 3 µm particle size; Imtakt, Kyoto, Japan). The two elution solutions used were (A) 0.1% formic acid in water containing 2 mM ammonium acetate and (B) 0.1% formic acid in MeOH containing 2 mM ammonium acetate. The solutions were pumped at a flow rate of 0.5 mL/min and a gradient elution program was applied as shown in Table 2. The injection volume of the samples was 10 μL. Analysis software (version 1.6, Sciex, Framingham, MA, USA) was used to control the LC/MS/MS system and to acquire and process data. The mass spectrometer was operated in the positive ESI (electrospray ionization) mode with MRM (multiple reaction monitoring) at unit resolution. The main MS parameters were optimized and finally set as follows: curtain gas, 20 psi; collision gas (CAD), medium; capillary temperature (TEM), 500 °C; ion spray voltage, ± 4500 V; ion source gas 1 (GS1), 50 psi; ion source gas 2 (GS2), 50 psi; interface heater (ihe), on. Nitrogen was used as the nebulizer, heater, curtain, and collision gas.
Table 2.
A gradient condition of a mobile phase composed of two solutions in analyses of multi-mycotoxins by HPLC.
| Total Time (min) | Flow Rate (mL/min) | A Solution (%) | B Solution (%) |
|---|---|---|---|
| 0 | 0.5 | 70 | 30 |
| 3 | 0.5 | 70 | 30 |
| 13 | 0.5 | 10 | 90 |
| 16 | 0.5 | 10 | 90 |
| 18 | 0.5 | 70 | 30 |
| 20 | 0.5 | 70 | 30 |
MRM parameters for detection of 13 mycotoxins by the mass spectrometer are shown in Table 3.
Table 3.
Multiple reaction monitoring (MRM) parameters for detection of 13 mycotoxins by the mass spectrometer.
| Mycotoxin | Precursor ion | Q1 (m/z) | Q3 (m/z) | Time (msec) | DP (volts) | EP (volts) | CE (volts) | CXP (volts) |
|---|---|---|---|---|---|---|---|---|
| NIV | [M + CH3COO−]− | 313 313 |
175 295 |
100 100 |
41 41 |
5 5 |
19 15 |
6 6 |
| DON | [M + CH3COO−]− | 297.2 297.2 |
249.2 231.2 |
100 100 |
40 40 |
7.5 7.5 |
12 12 |
4 4 |
| 3-AcDON | [M + CH3COO−]− | 339 339 |
231 213 |
100 100 |
15 15 |
3.5 3.5 |
15 15 |
2 2 |
| AFB1 | [M + H]+ | 313 313 |
285.1 115 |
100 100 |
70 70 |
11 11 |
51 89 |
4 4 |
| AFB2 | [M + H]+ | 313 313 |
287.1 259.1 |
100 100 |
70 70 |
10 10 |
50 50 |
3 3 |
| AFG1 | [M + H]+ | 329 329 |
243.0 215.1 |
100 100 |
70 70 |
11 10 |
25 50 |
4 4 |
| AFG2 | [M + H]+ | 331 331 |
189 257 |
100 100 |
70 70 |
10.0 10.5 |
50 47 |
3 4 |
| FB1 | [M + H]+ | 722.4 722.4 |
334.3 352.4 |
100 100 |
91 91 |
8 8 |
53 43 |
6 6 |
| FB2 | [M + H]+ | 706.4 706.4 |
336.4 318.2 |
100 100 |
70 70 |
9 9 |
45 47 |
6 6 |
| HT-2 | [M + NH4]+ | 442 442 |
263 215 |
100 100 |
26 26 |
3 3 |
19 19 |
4 4 |
| T-2 | [M + NH4]+ | 484.1 484.1 |
215.2 185.2 |
100 100 |
21 21 |
6 6 |
29 31 |
4 4 |
| ZEN | [M − H]− | 317.1 317.1 |
131 175 |
100 100 |
−50 −50 |
−4.5 −4.5 |
−40 −34 |
−2 −2 |
| OTA | [M + H]+ | 404 404 |
239 220.9 |
100 100 |
41 41 |
5 5 |
31 19 |
4 4 |
Acknowledgments
This work was supported by the National Agricultural Products Quality Management Service and the Chung-Ang University Research Scholarship Grants in 2015.
Abbreviations
The following abbreviations are used in this manuscript:
| LC/MS/MS | Liquid Chromatography-Tandem Mass Spectrometry |
| AFs | aflatoxins |
| DON | deoxynivalenol |
| 3-AcDON | 3-Acetyldeoxynivalenol |
| OTA | ochratoxin A |
| Fs | fumonisins |
| NIV | nivalenol |
| ZEN | zearalenone |
| T-2 | T-2 toxin |
| HT-2 | HT-2 toxin |
| TCs | Trichothecenes |
| TIC | Total Ion Chromatogram |
| EIC | Extract Ion Chromatogram |
| RSDr | relative standard deviation |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| ACN | acetonitrile |
| MeOH | methanol |
| MRM | multiple reaction monitoring |
Supplementary Materials
The following are available online at www.mdpi.com/2072-6651/9/3/106/s1, Figure S1: Calibration curves of 13 mycotoxins, Table S1: LODs and LOQs of 13 mycotoxins in 5 types of cereal grains.
Author Contributions
D.-H.K. and S.H.C. conceived and designed the experiments; D.-H.K., S.-Y.H., and J.W.K. performed the experiments; D.-H.K., S.-Y.H., J.W.K., S.M.C., K.R.L., T.K.A., and S.H.C. analyzed the data; S.-Y.H., C.L., and S.H.C. wrote the paper.
Conflicts of Interest
The authors declared no conflict of interests.
References
- 1.Binder E.M., Tan L.M., Chin L.J., Handl J., Richard J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007;137:265–282. doi: 10.1016/j.anifeedsci.2007.06.005. [DOI] [Google Scholar]
- 2.IARC . Monograph on the Evaluation of Carcinogenic Risk to Humans. World Health Organization; Lyon, France: 2002. pp. 171–175. [Google Scholar]
- 3.Park J.W., Chung S.H., Kim Y.B. Ochratoxin a in Korean food commodities: Occurrence and safety evaluation. J. Agric. Food Chem. 2005;53:4637–4642. doi: 10.1021/jf050227j. [DOI] [PubMed] [Google Scholar]
- 4.Ok H.E., Kim H.J., Cho T.Y., Oh K.S., Chun H.S. Determination of deoxynivalenol in cereal-based foods and estimation of dietary exposure. J. Toxicol. Environ. Health A. 2009;72:1424–1430. doi: 10.1080/15287390903212832. [DOI] [PubMed] [Google Scholar]
- 5.Boeira L.S., Bryce J.H., Stewart G.G., Flannigan B. The effect of combinations of Fusarium mycotoxins (deoxynivalenol, zearalenone and fumonisin b1) on growth of brewing yeasts. J. Appl. Microbiol. 2000;88:388–403. doi: 10.1046/j.1365-2672.2000.00972.x. [DOI] [PubMed] [Google Scholar]
- 6.Grenier B., Loureiro-Bracarense A.P., Lucioli J., Pacheco G.D., Cossalter A.M., Moll W.D., Schatzmayr G., Oswald I.P. Individual and combined effects of subclinical doses of deoxynivalenol and fumonisins in piglets. Mol. Nutr. Food Res. 2011;55:761–771. doi: 10.1002/mnfr.201000402. [DOI] [PubMed] [Google Scholar]
- 7.Harvey R.B., Edrington T.S., Kubena L.F., Elissalde M.H., Rottinghaus G.E. Influence of aflatoxin and fumonisin b1-containing culture material on growing barrows. Am. J. Vet. Res. 1995;56:1668–1672. [PubMed] [Google Scholar]
- 8.Verma J., Johri T.S., Swain B.K., Ameena S. Effect of graded levels of aflatoxin, ochratoxin and their combinations on the performance and immune response of broilers. Br. Poult. Sci. 2004;45:512–518. doi: 10.1080/00071660412331286226. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz M.J., Macakova P., Juan-Garcia A., Font G. Cytotoxic effects of mycotoxin combinations in mammalian kidney cells. Food Chem. Toxicol. 2011;49:2718–2724. doi: 10.1016/j.fct.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Tammer B., Lehmann I., Nieber K., Altenburger R. Combined effects of mycotoxin mixtures on human t cell function. Toxicol. Lett. 2007;170:124–133. doi: 10.1016/j.toxlet.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Creppy E.E., Chiarappa P., Baudrimont I., Borracci P., Moukha S., Carratu M.R. Synergistic effects of fumonisin B1 and ochratoxin A: Are in vitro cytotoxicity data predictive of in vivo acute toxicity? Toxicology. 2004;201:115–123. doi: 10.1016/j.tox.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Klaric M.S., Rumora L., Ljubanovic D., Pepeljnjak S. Cytotoxicity and apoptosis induced by fumonisin B1, beauvericin and ochratoxin a in porcine kidney pk15 cells: Effects of individual and combined treatment. Arch. Toxicol. 2008;82:247–255. doi: 10.1007/s00204-007-0245-y. [DOI] [PubMed] [Google Scholar]
- 13.Vaclavikova M., MacMahon S., Zhang K., Begley T.H. Application of single immunoaffinity clean up for simultaneous determination of regulated mycotoxins in cereals and nuts. Talanta. 2013;117:345–351. doi: 10.1016/j.talanta.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Lattanzio V.M., Ciasca B., Powers S., Visconti A. Improved method for the simultaneous determination of aflatoxins, ochratoxin A and Fusarium toxins in cereals and derived products by liquid chromatography-tandem mass spectrometry after multi-toxin immunoaffinity clean up. J. Chromatogr. A. 2014;1354:139–143. doi: 10.1016/j.chroma.2014.05.069. [DOI] [PubMed] [Google Scholar]
- 15.Lattanzio V.M., Solfrizzo M., Powers S., Visconti A. Simultaneous determination of aflatoxins, ochratoxin A and Fusarium toxins in maize by liquid chromatography/tandem mass spectrometry after multitoxin immunoaffinity cleanup. Rapid Commun. Mass Spectrom. 2007;21:3253–3261. doi: 10.1002/rcm.3210. [DOI] [PubMed] [Google Scholar]
- 16.AOAC . Sub-Committee on Feed Additives and Contaminants. AOAC; Rockville, MD, USA: 2008. pp. 41–42. [Google Scholar]
- 17.Food and Agriculture Organization . Codex General Standard for Contaminants and Toxins in Food And Feed (Codex Stan 193–1995) Food and Agriculture Organization; Geneva, Switzerland: 1995. [Google Scholar]
- 18.KFDA . Food Standards Codex. Korea Foods Industry Association; Seoul, Korea: 2010. pp. 10–16. [Google Scholar]
- 19.Park J.W., Kim E.K., Shon D.H., Kim Y.B. Natural co-occurrence of aflatoxin B1, fumonisin B1 and ochratoxin A in barley and corn foods from Korea. Food Addit. Contam. 2002;19:1073–1080. doi: 10.1080/02652030210151840. [DOI] [PubMed] [Google Scholar]
- 20.Seo E., Yoon Y., Kim K., Shim W.B., Kuzmina N., Oh K.S., Lee J.O., Kim D.S., Suh J., Lee S.H., et al. Fumonisins B1 and B2 in agricultural products consumed in South Korea: An exposure assessment. J. Food Prot. 2009;72:436–440. doi: 10.4315/0362-028X-72.2.436. [DOI] [PubMed] [Google Scholar]
- 21.Kim J.C., Kang H.J., Lee D.H., Lee Y.W., Yoshizawa T. Natural occurrence of Fusarium mycotoxins (trichothecenes and zearalenone) in barley and corn in Korea. Appl. Environ. Microbiol. 1993;59:3798–3802. doi: 10.1128/aem.59.11.3798-3802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ok H.E., Chang H.J., Choi S.W., Lee N., Kim H.J., Koo M.S., Chun H.S. Co-occurrence of deoxynivalenol and zearalenone in cereals and their products. J. Food Hyg. Saf. 2007;22:375–381. [Google Scholar]
- 23.Eskola M., Rizzo A., Soupas L. Occurrence and amounts of some Fusarium toxins in finnish cereal samples in 1998. Acta Agric. Scand. 2001;50:183–186. [Google Scholar]
- 24.Ali N., Sardjono, Yamashita A., Yoshizawa T. Natural co-occurrence of aflatoxins and Fusarium mycotoxins (fumonisins, deoxynivalenol, nivalenol and zearalenone) in corn from Indonesia. Food Addit. Contam. 1998;15:377–384. doi: 10.1080/02652039809374655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.