Abstract
BACKGROUND
When using area under the concentration-time curve-based strategies for dosing carboplatin, accurate estimation of glomerular filtration rate is required for determining dose. Commonly, the Cockcroft–Gault equation is used, which is dependent on measurement of serum creatinine (SCr). Because analysis of SCr changed to an isotope dilution mass spectrometry (IDMS) standard, we sought to determine the impact of this assay change on carboplatin dosing and related toxicity.
METHODS
This was a single-center, retrospective chart review of adults treated with carboplatin between April 2008 and April 2010 divided into cohorts that initiated carboplatin before or after IDMS standardization. End points included grade 3 thrombocytopenia, decrease in platelet count, and hospitalization and were evaluated in cohorts based on concomitant chemotherapy.
RESULTS
The chart review identified 158 patients, with 63 patients in the pre-IDMS group and 95 patients in the post-IDMS group. Average SCr (pre 1.01 mg/dl vs post 0.86 mg/dl, p<0.001) and average carboplatin dose (pre 580 mg vs post 703 mg, p<0.001) were significantly different between the groups. The frequency of grade 3 thrombocytopenia was not statistically significant across three partner chemotherapy cohorts before and after IDMS implementation.
CONCLUSION
IDMS standardization led to an overall decrease in SCr with subsequent increase in carboplatin doses. However, no increase in recorded adverse events was observed, suggesting that the clinical relevance in toxicity from higher doses was minimal.
Keywords: carboplatin, glomerular filtration rate, serum creatinine, creatinine clearance
Carboplatin is a platinum-containing alkylating agent with activity in a wide array of hematologic malignancies and solid tumors.1 Renal elimination via filtration accounts for approximately 65% of clearance after dosing.2, 3 The initial approach to carboplatin dosing on the basis of body surface area resulted in wide variability in exposure and toxicity, suggesting improved approaches using pharmacokinetic and pharmacodynamic end points were needed.4 Due to predominant renal elimination, an estimated area under the concentration-time curve (AUC) may be determined by using glomerular filtration rate (GFR) and a simple, elegant linear equation first published in 1989.5 Dose calculation determined by desired AUC now provides a more predictable risk of thrombocytopenia.
The Calvert equation was derived using chromium-51–labeled ethylenediaminetetraacetic acid (51Cr-EDTA) to accurately measure GFR. The use of radioisotopes to determine kidney function is not standard practice at many institutions; therefore, most use the Cockcroft–Gault equation, an estimation of creatinine clearance, as an estimate of GFR.6 The Cockcroft–Gault equation is dependent on patient age, weight, sex, and measured serum creatinine (SCr).
Before 2006, a number of assays were used to determine SCr, leading to significant variability among institutions and a general overestimation of SCr with historical methods.7, 8 In 2006, the National Kidney Disease Education Program (NKDEP) published a recommendation to standardize SCr assays to an isotope dilution mass spectrometry (IDMS) standard in an effort to reduce variability among reported SCr values used to make a diagnosis of chronic kidney disease.9 While the IDMS-traceable assay standard provides accurate values for SCr, the use of IDMS was not commonly used before the NKDEP recommendations. As institutions converted to the IDMS method, differences in carboplatin doses received before and after standardization have been observed.10 Clinically, the dose-limiting toxicity of carboplatin is thrombocytopenia. The incidence and severity of thrombocytopenia increase as doses approach and exceed an AUC of 7.11 No study to date has attempted an analysis of the incidence of carboplatin toxicities as a result of IDMS standardization. Therefore, the purpose of this study was to determine if standardization to the IDMS method of reporting SCr has led to an increase in the incidence of grade 3 thrombocytopenia in patients receiving carboplatin-based chemotherapy.
Materials and Methods
The design was a single-center retrospective review of patients who were treated with carboplatin-containing chemotherapy between April 6, 2008, and April 6, 2010. These dates were selected as 1-year periods before and after IDMS implementation on April 6, 2009. Adult patients who received two or more doses of the same carboplatin-based regimen in groups before and after IDMS implementation were evaluated for inclusion in the study. Patients were included in the study if they were 18 years of age or greater, were naïve to chemotherapy, received a 21-day cycle, and had carboplatin doses calculated using a current body weight and SCr value. Exclusion criteria were those receiving carboplatin in preparation for an autologous hematopoietic stem cell transplant or who were enrolled in a clinical trial because of differences in dosing practices compared with routine clinical use. Patients who received their first two doses of carboplatin-based therapy overlapping the date of IDMS implementation were also excluded from analysis. The primary objective was to compare the incidence of grade 3 or higher thrombocytopenia at any time during the first 2 cycles of chemotherapy within cohorts receiving the same chemotherapy regimen (e.g., carboplatin and paclitaxel, carboplatin and pemetrexed). Secondary end points included comparison of SCr values and carboplatin doses received pre-and post-IDMS standardization. Secondary toxicity objectives included incidence of grade 2 thrombocytopenia, grade 3 neutropenia, hospitalization, and total change in platelet count during the first two cycles of chemotherapy. Objectives were compared among patients who received AUC 6-based carboplatin dosing, as too few patients receiving AUC 5 were identified for meaningful analysis.
Statistical Analysis
Baseline demographic values were estimated using standard descriptive statistics. Clinical laboratory values (platelet count, absolute neutrophil count, SCr, and albumin) and carboplatin doses were compared between pre-and post-IDMS groups by using an analysis of variance approach. Incidence of severe thrombocytopenia and neutropenia (grade ≥ 3) and incidence of hospitalization were compared between groups using either χ2 tests or Fisher’s exact test, where appropriate. For the comparison of carboplatin doses, patients were divided into subgroups based on AUC. Additional subgroup analysis of patients aged ≥ 65 years and patients with body mass index (BMI) ≤ 25 kg/m2 were also performed. For all comparisons, a p-value of <0.05 was considered statistically significant. All statistical analysis was performed with SAS v 9.3 (SAS Institute Inc., Cary, NC). A multivariable logistic regression model was fit, where the odds of developing grade 3 thrombocytopenia was modeled as a function of IDMS group, AUC, chemotherapy regimen, age, and sex. In addition, a multivariable linear regression model was fit, where percent platelet reduction was modeled as a function of the same variables listed here, and multicollinearity was checked.
Results
A total of 520 patients were evaluated for inclusion. Of those evaluated, 158 met all entry criteria, with 63 assigned to the pre-IDMS group and 95 to the post-IDMS group (Figure 1).
Figure 1.

Patient identification. Process of chart review and patient selection, including those not meeting study criteria.
Basic demographics of the study population are shown in Table 1. Overall, patients were similar among the comparators of race, sex, age, and chemotherapy regimen. Mean weight (71.6 vs 78.7 kg, p=0.018), body surface area (1.8 vs 1.89 m2, p=0.03), and BMI (25.5 vs 27.4 kg/m2, p=0.034) were statistically different between the two groups, with higher values in the post-IDMS standardization group.
Table 1.
Demographics
| Pre-IDMS n=63 |
Post-IDMS n=95 |
p-value | |
|---|---|---|---|
| Age, median yrs (range) | 65 (30–90) | 62 (19–84) | 0.09 |
| Sex, female | 41 (65%) | 55 (57%) | 0.365 |
| Race | |||
| White | 43 (68%) | 62 (65%) | |
| Black | 15 (24%) | 26 (27%) | |
| Hispanic | 0 (0%) | 3 (3%) | |
| Asian | 4 (6%) | 3 (3%) | |
| Other | 1 (%) | 1 (1%) | |
| Height, median, cm (range) | 168 (145–188) | 168 (152–196) | 0.168 |
| Weight, median, kg (range) | 71 (42–124) | 75 (46–153) | 0.018 |
| Albumin, median g/dl (range) | 3.7 (1.7–4.7) | 3.7 (2.2–4.8) | 0.24 |
| Malignancy | |||
| Breast | 12 (19%) | 12 (13%) | |
| Lung | 26 (41%) | 50 (52%) | |
| Ovarian | 9 (14%) | 10 (11%) | |
| Other | 16 (26%) | 23 (24%) | |
| Other chemotherapy | |||
| Paclitaxel | 27 (43%) | 41 (43%) | |
| Pemetrexed | 11 (17%) | 17 (18%) | |
| Docetaxel | 15 (24%) | 21 (22%) | |
| Etoposide | 10 (16%) | 10 (11%) | |
| Other | 0 (0%) | 2 (2%) | |
| None | 0 (0%) | 1 (1%) | |
IDMS = isotope dilution mass spectrometry.
There was a significant difference in mean SCr values among all patients pre-versus post-IDMS standardization (1.01 vs 0.86 mg/dl, p<0.001). Carboplatin doses were evaluated among patients who were to receive doses to achieve an AUC of 6. The mean doses received pre- versus post-IDMS were also found to differ significantly among patients receiving an AUC of 6 (580 vs 703 mg, p<0.001), which represented a 21% average dose increase (Figure 2).
Figure 2.
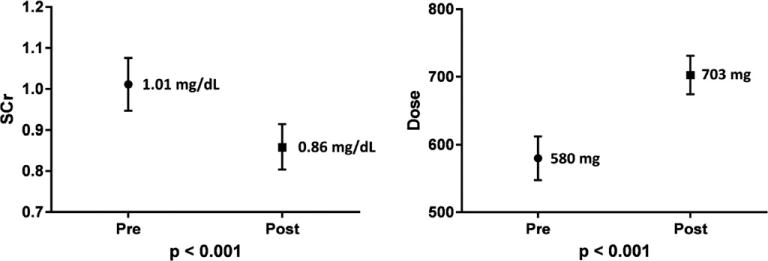
Serum creatinine measures and carboplatin dosing. Numbers displayed are mean values with lines displaying 95% confidence intervals.
Chemotherapy Cohorts
Statistical analysis was completed on three cohorts of patients receiving the most commonly observed partner chemotherapy agents:paclitaxel, pemetrexed, and docetaxel (Table 2). The primary end point of incidence of grade ≥ 3 thrombocytopenia was not significantly different in the paclitaxel and pemetrexed cohorts. Within the docetaxel cohort, there were no grade ≥ 3 thrombocytopenia events.
Table 2.
Primary and Secondary Toxicity Outcomes (Carboplatin AUC = 6)
| Pre-IDMS n=24 |
Post-IDMS n=39 |
p | |
|---|---|---|---|
| Paclitaxel | |||
| Grade 3+ thrombocytopenia | 0 (0%) | 4 (10%) | 0.288 |
| Grade 2+ thrombocytopenia | 1 (4%) | 7 (18%) | 0.141 |
| Median platelet reduction | 87 | 88 | 0.853 |
| Grade 3+ neutropenia | 9 (38%) | 12 (31%) | 0.582 |
| Hospitalization rate | 7 (29%) | 10 (26%) | 0.759 |
| Pemetrexed | n=7 | n=17 | |
| Grade 3+ thrombocytopenia | 1 (14%) | 0 (0%) | 0.292 |
| Grade 2+ thrombocytopenia | 2 (29%) | 0 (0%) | 0.076 |
| Median platelet reduction | 51 | 17 | 0.021 |
| Grade 3+ neutropenia | 0 (0%) | 1 (6%) | 1 |
| Hospitalization rate | 3 (43%) | 5 (29%) | 0.647 |
| Docetaxel | n=14 | n=21 | |
| Grade 3+ thrombocytopenia | 0 (0%) | 0 (0%) | |
| Grade 2+ thrombocytopenia | 0 (0%) | 3 (14%) | 0.259 |
| Median platelet reduction | 39 | 79 | 0.054 |
| Grade 3+ neutropenia | 3 (21%) | 6 (29%) | 0.712 |
| Hospitalization rate | 1 (7%) | 3 (14%) | 0.635 |
AUC = area under the concentration-time curve; IDMS = isotope dilution mass spectrometry.
There were no significant differences in secondary end points (grade ≥ 2 thrombocytopenia, median platelet reduction, grade ≥ 3 neutropenia, hospitalizations) within the three cohorts pre-IDMS vs. post-IDMS except regarding mean platelet reduction in the pemetrexed group. Mean absolute reduction in platelet count (51 vs 17, p=0.021) was statistically significant in the pemetrexed cohort, with a greater reduction occurring in the pre-IDMS group.
Age and BMI subgroup analysis
Serum creatinine values and carboplatin doses for AUC 6 were further evaluated within 2 separate subgroups of patients with age ≥ 65 years and BMI ≤ 25 kg/m2 (Figure 3).
Figure 3.
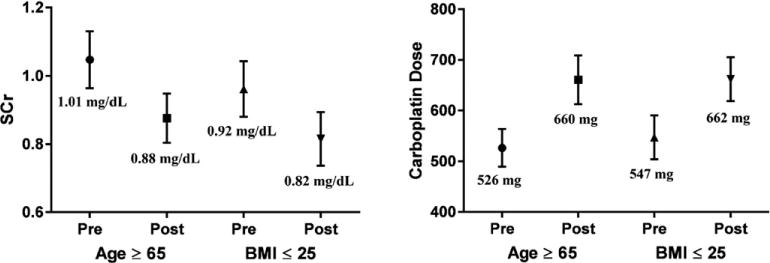
Subgroup analysis of creatinine measures and carboplatin dosing by age and body mass index. Numbers displayed are mean values with lines displaying 95% confidence intervals.
Mean SCr was significantly lower within the post-IDMS group of patients age ≥ 65 years (pre 1.01 vs post 0.88 mg/dl, p<0.015). Mean SCr within the BMI ≤ 25 kg/m2 subgroup, however, was not statistically significant. Carboplatin doses for both subgroups were significantly higher in the post-IDMS group (age ≥ 65 years: 526 vs 661 mg, p<0.001; BMI ≤ 25 kg/m2: 547 vs 662 mg, p<0.001). Patients in the age ≥ 65 years subgroup received a 25% higher dose after IDMS standardizations and those within the BMI ≤ 25 kg/m2 subgroup received a 21% higher dose post-IDMS.
Multivariable Analysis
Multivariable analysis was conducted that accounted for the covariates of pre-versus post-IDMS, carboplatin AUC, secondary chemotherapy drug, age, and sex. For the outcome of grade ≥ 3 thrombocytopenia, there was no significant relationship among the selected variables. However, secondary chemotherapy drug (p=0.014) and AUC (p=0.04) were significantly associated with percent reduction in platelets from baseline when adjusting for the other covariates (Table 3).
Table 3.
Multivariable Analysis of Hematologic Toxicity
| Covariate | Logistic Regression – Grade 3 Thrombocytopenia
|
Linear Regression – Percent Platelet Reduction
|
||||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio p-value | Type 3 p-value | β (95% CI) | β p-value | Type 3 p-value | |
| Pre-IDMS | 0.76 (0.18–3.21) | 0.71 | 0.71 | −2.44 (−10.71 to 5.83) | 0.56 | 0.56 |
| Post-IDMS | ||||||
| AUC = 5 | 0.08 (0.00–1.79) | 0.11 | 0.11 | −14.29 (−27.95 to −0.63) | 0.04 | 0.04 |
| AUC = 6 | ||||||
| Pemetrexed | 0.14 (0.01–1.34) | 0.087 | 0.091 | −20.77 (−35.8 to −5.78) | 0.007 | 0.014 |
| Paclitaxel | 0.13 (0.02–0.96) | 0.045 | −11.24 (−25.44 to 2.96) | 0.12 | ||
| Docetaxel | 0.02 (0.00–0.57) | 0.022 | −21.22 (−36.7 to −5.74) | 0.008 | ||
| Other | – | – | – | |||
| Age | 0.95 (0.9–1.01) | 0.113 | 0.113 | −0.32 (−0.68 to 0.05) | 0.088 | 0.088 |
| Female | 1.62 (0.39–6.79) | 0.507 | 0.507 | −0.16 (−8.48 to 8.16) | 0.969 | 0.969 |
| Male | – | |||||
AUC = area under the concentration-time curve; CI = confidence interval; IDMS = isotope dilution mass spectrometry. Number of observations used = 155.
No variables were removed from the model.
Discussion
This analysis in 520 patients supports prior reports that the switch to an IDMS-corrected standard for creatinine measurement leads to an increase in carboplatin dose. This increase in dose was found across all treatment cohorts as well as the age and BMI subgroups. While there was a statistically significant increase in dose, we did not detect a change in the incidence of grade ≥ 3 thrombocytopenia. Given the small number of events (n=7) and the small sample size in each of the treatment cohorts, the study was not adequately powered to show a 7% difference in an end point with an estimated incidence of 5%. Other plausible explanations for the lack of a statistically significant findings regarding toxicity include the fact that paclitaxel and pemetrexed were the two most common partner chemotherapy agents. Together, these two cohorts account for 60% of the total population studied. Paclitaxel has been shown to have a platelet sparing effect when administered with carboplatin.12 The mechanism of this protection is not well understood beyond knowing that coadministration does not reduce antitumor effects.13 Given this effect, the incidence of grade 2+ thrombocytopenia is likely to be lower within this cohort, requiring a higher sample size to show a significant difference between the prestandardization and poststandardization groups. In addition, pemetrexed is less myelosuppressive than many other chemotherapy agents, due to the coadministration of cyanocobalamin and folate, which all of our patients received.14 As a result, the incidence of hematologic toxicity in this group is less likely to be statistically different in small sample sizes.
In addition to the pharmacodynamic effects of partner chemotherapy, the method by which GFR is estimated could also contribute to the low incidence of carboplatin toxicity. The initial study that determined the Calvert equation used 51Cr-EDTA to measure GFR. In our center, creatinine clearance (CrCl) is estimated using SCr in the Cockcroft–Gault equation, and the result is substituted for GFR in the Calvert equation. The Cockcroft–Gault equation has been shown to underestimate true GFR compared with standard of 51Cr-EDTA–measured GFR.15 As a result, carboplatin dosing determined using a Cockcroft–Gault equation–estimated CrCl is significantly lower compared with dosing determined using radioisotope-measured GFR.16, 17 As corroborated in our study findings, doses may differ by as much as 20%. This suggests that the conversion to IDMS may, in reality, provide a more similar dose derivation to that using gold standard GFR measures. However, other studies have documented that IDMS Cockcroft–Gault estimation of GFR has less predictable variability compared with 51Cr-EDTA GFR, with 18% of patients being under dosed by > 100 mg and 23% of patients being overdosed by the same amount.18 Regardless of the low incidence of toxicity endpoints within the study, comparison of SCr values and related carboplatin doses shows that IDMS standardization has led to a significant increase in carboplatin doses received by patients.
Demographically, the subjects of the study were similar between groups with the exception of weight. However, even though weights were statistically different, median weights between patients differed only by 4 kg and, therefore, are not clinically significant when estimating GFR with the Cockcroft–Gault equation. As this difference in weight would not clinically affect reporting of estimated GFR, the difference in weights would unlikely be responsible for affecting dosing and incidence of toxicity between pre-IDMS and post-IDMS treatment groups.
Although our study was not adequately powered to show a difference in carboplatin toxicity between the cohorts, the difference found in carboplatin doses received by patients after transition to the IDMS correction provides confirmation of previous studies. In addition, our subgroup analyses show that among patients of advanced age and lower body weight, this significant increase in carboplatin dose is preserved. As the expense of radiologically measured GFR can be prohibitive, many centers are unlikely to transition from SCr-estimated GFR to determine carboplatin doses. Therefore, additional research into the toxic effects of using estimated GFR for carboplatin dosing with larger sample sizes would be beneficial in determining if this difference in dose translates into an increase in carboplatin-related toxicity. Another potential avenue of study might include following patients over more cycles of chemotherapy or including more myelosuppressive carboplatin-containing regimens (e.g., gemcitabine). With these changes, the incidence of toxicity may be higher and may become statistically significant.
Acknowledgments
This project received no funding.
Footnotes
No author has any conflicts of interest or disclosures.
References
- 1.Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol. 1999;17:409–22. doi: 10.1200/JCO.1999.17.1.409. [DOI] [PubMed] [Google Scholar]
- 2.Calvert AH, Harland SJ, Newell DR, et al. Early clinical studies with cis-diammine-1,1-cyclobutane dicarboxylate platinum II. Cancer Chemother Pharmacol. 1982;9:140–7. doi: 10.1007/BF00257742. [DOI] [PubMed] [Google Scholar]
- 3.Harland SJ, Newell DR, Siddik ZH, et al. Pharmacokinetics of cis-diammine-1,1-cyclobutane dicarboxylate platinum(II) in patients with normal and impaired renal function. Cancer Res. 1984;44:1693–7. [PubMed] [Google Scholar]
- 4.Foster BJ, Clagett-Carr K, Leyland-Jones B, Hoth D. Results of NCI-sponsored phase I trials with carboplatin. Cancer Treat Rev. 1985;12(Suppl A):43–9. doi: 10.1016/0305-7372(85)90017-9. [DOI] [PubMed] [Google Scholar]
- 5.Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7:1748–56. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 6.Smith J. The controversy remains, a consensus is needed: how to assess renal function for dosing carboplatin. Hematol Pharm Assoc News. 2010;3:2–4. [Google Scholar]
- 7.Ando M, Minami H, Ando Y, et al. Multi-institutional validation study of carboplatin dosing formula using adjusted serum creatinine level. Clin Cancer Res. 2000;6:4733–8. [PubMed] [Google Scholar]
- 8.Séronie-Vivien S, Galteau M-M, Carlier M-C, et al. Impact of standardized calibration on the inter-assay variation of 14 automated assays for the measurement of creatinine in human serum. Clin Chem. 2005;43:1227–33. doi: 10.1515/CCLM.2005.213. [DOI] [PubMed] [Google Scholar]
- 9.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 10.Murray B, Bates J, Buie L. Impact of a new assay for measuring serum creatinine levels on carboplatin dosing. Am Health-Syst Pharm. 2012;69:1136–41. doi: 10.2146/ajhp110560. [DOI] [PubMed] [Google Scholar]
- 11.Jodrell DI, Egorin MJ, Canetta RM, et al. Relationships between carboplatin exposure and tumor response and toxicity in patients with ovarian cancer. J Clin Oncol. 1992;10:520–8. doi: 10.1200/JCO.1992.10.4.520. [DOI] [PubMed] [Google Scholar]
- 12.Belani CP, Kearns CM, Zuhowski EG, et al. Phase I trial, including pharmacokinetic and pharmacodynamic correlations, of combination paclitaxel and carboplatin in patients with metastatic non-small-cell lung cancer. J Clin Oncol. 1999;17:676–84. doi: 10.1200/JCO.1999.17.2.676. [DOI] [PubMed] [Google Scholar]
- 13.Daga H, Isobe T, Miyazaki M, Fujitaka K, Kondo K, Kohno N. Investigating the relationship between serum thrombopoietin kinetics and the platelet-sparing effect: a clinical pharmacological evaluation of combined paclitaxel and carboplatin in patients with non-small cell lung cancer. Oncol Rep. 2004;11:1225–31. [PubMed] [Google Scholar]
- 14.Zukin M, Barrios CH, Pereira JR, et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol. 2013;31:2849–53. doi: 10.1200/JCO.2012.48.1911. [DOI] [PubMed] [Google Scholar]
- 15.de Lemos ML, Hsieh T, Hamata L, et al. Evaluation of predictive formulae for glomerular filtration rate for carboplatin dosing in gynecological malignancies. Gynecol Oncol. 2006;103:1063–9. doi: 10.1016/j.ygyno.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Dooley MJ, Poole SG, Rischin D. Dosing of cytotoxic chemotherapy: impact of renal function estimates on dose. Ann Oncol. 2013;24:2746–52. doi: 10.1093/annonc/mdt300. [DOI] [PubMed] [Google Scholar]
- 17.Cathomas R, Klingbiel D, Geldart TR, et al. Relevant risk of carboplatin underdosing in cancer patients with normal renal function using estimated GFR: lessons from a stage I seminoma cohort. Ann Oncol. 2014;25:1591–7. doi: 10.1093/annonc/mdu129. [DOI] [PubMed] [Google Scholar]
- 18.Whittle J, Graham J, Ismail H, et al. Carboplatin dosing based on estimated Glomerular Filtration Rate (GFR) using IDMS creatinine: a comparison of estimated GFR based on IDMS creatinine in the Cockroft-Gault (CG) formula (IDMS-GFR), with measured GFR using 51Cr-EDTA (51Cr-GFR) J Clin Oncol. 2015;33:2576. [Google Scholar]


