Abstract
An interface coordinating lipid metabolism with proteins that regulate membrane trafficking is necessary to regulate Golgi morphology and dynamics. Such an interface facilitates the membrane deformations required for vesicularization, forms platforms for protein recruitment and assembly on appropriate sites on a membrane surface and provides lipid co-factors for optimal protein activity in the proper spatio-temporally regulated manner. Importantly, Sec14 and Sec14-like proteins are a unique superfamily of proteins that sense specific aspects of lipid metabolism, employing this information to potentiate phosphoinositide production. Therefore, Sec14 and Sec14 like proteins form central conduits to integrate multiple aspects of lipid metabolism with productive phosphoinositide signaling.
Keywords: Phosphatidylinositol, Pi-transfer protein, Sec14 domain, Golgi, Phosphatidylinositol 4-kinase
9.1 Lipid Metabolism and the Golgi System
The functional integrity of the Golgi network requires cooperation between protein function and lipid metabolism. Lipid metabolism interfaces with the proteinaceous membrane trafficking machinery in three primary ways. First, it facilitates the membrane deformations that accompany vesicle budding, fusion and tubulation. Second, lipid metabolism creates platforms for protein recruitment to appropriate sites on a membrane surface. Third, it produces lipids which serve as co-factors for optimal protein activity in a spatio-temporally regulated manner. Thus, the interface of lipid metabolism with mechanisms of membrane trafficking is complex, and this interface is a major factor in controlling Golgi morphology and dynamics.
9.2 PtdIns-4-Phosphate and Golgi Function
Phosphoinositides (PIPs) are phosphorylated in all combinations on either the D-3, D-4 or D-5 hydroxyl moieties of the inositol headgroup of phosphatidylinositol (Ptdlns). The observation that Ptdlns and phosphoinositides regulate membrane trafficking describes the first established case for lipids playing an active role in regulating membrane trafficking reactions. Herein, we focus on the functions of PtdIns-4-phosphate within the Golgi system.
Two distinct classes of Ptdlns 4-OH kinases, Pik1 and Lsb6 in yeast or PI4KIIIβ and PI4KIIα in mammals respectively, associate with the Golgi network (Balla and Balla 2006; Strahl and Thorner 2007). The PI4KIIIβ enzymes have been more extensively studied and provide the focus herein. PI4KIIIβ activity is stimulated by heterodimerization with NCS1 (frequenin in yeast), a small myristoylated Ca2+ -binding protein (Hendricks et al. 1999; Zhao et al. 2001). Upon association with the Golgi membrane PI4KIIIβ enzymes have been found to directly interact with components of the vesicular trafficking machinery, PI4KIIIβ interacts with the GTP-bound form of Arf1 on the Golgi in mammalian cells (Godi et al. 1999; Haynes et al. 2005). In contrast to mammalian PI4KIIβ, yeast Pik1 does not directly interact with Arf1, instead it interacts with the ARF1 specific GTP exchange factor, Sec7 (Gloor et al. 2010).
Membrane associated Ptdlns 4-OH kinases employs dual activities to regulate Golgi secretory function. Firstly, PI4KIIIβ directly binds to and regulates the activity of the small Rab GTPase, Rabll (the mammalian orthologue of yeast Ypt31). Therefore, one critical activity of PI4KIIIβ kinases is to function as a scaffold for the recruitment of key components of the membrane trafficking machinery (Polevoy et al. 2009). Secondly, the catalytic activity of Ptdlns 4-OH kinase is necessary for Golgi function. Acute inactivation of yeast Pik1 kinase activity (Hama et al. 1999; Walch-Solimena and Novick 1999), or evoked recruitment of the Sac1 phosphoinositide phosphatase catalytic domain which degrades PtdIns-4-phosphate to Ptdlns (Szentpetery et al. 2010) to the Golgi induces membrane trafficking defects through this organelle. In addition, inactivation of Ptdlns-binding proteins (e.g. Sec14; see below), which potentiate the Ptdlns 4-OH kinase of these enzymes, also compromises protein trafficking through the Golgi complex (Bankaitis et al. 1990; Schaaf et al. 2008).
PtdIns-4-phosphate regulates Golgi secretory functions by several mechanisms. PtdIns-4-phosphate serves as an adaptor required to recruit peripheral membrane proteins required for vesicle biogenesis. These include adaptor proteins for clathrin binding (i.e. AP-1; Carlton and Cullen 2005; Wang et al. 2003), GGA proteins which potentiate Arf1-GTP activity (Wang et al. 2007; Demmel et al. 2008), Rab GTPases and their concomitant guanidine nucleotide exchange factors (de Graaf et al. 2004; Sciorra et al. 2005; Mizuno-Yamasaki et al. 2010a), oxysterol binding proteins which interface with phosphoinositide-4-phosphate signaling (Li et al. 2002a; Litvak et al. 2005; Stefan et al. 2011), and other lipid binding/transfer proteins which further remodel Golgi membrane lipid composition (see below). Second, PtdIns-4-phosphate directly regulates the activities of other Golgi resident proteins. One example is the amino-phospholipid flippase Drs2, a Type-IV integral membrane ATPase (Natarajan et al. 2004; Muthusamy et al. 2009), which transfers phosphatidylserine (PtdSer) and phosphatidylethanolamine (PtdEtn) from the luminal leaflet to the cytosolic face of Golgi/endosomal membranes. Drs2 executes significant pro-secretory functions in these compartments, and its flippase activity is stimulated by binding to both Ptdlns-4-phosphate and to a GTPase exchange factor for Arf1 (Chantalat et al. 2004). How Drs2 dependent aminophospholipid flippase activity regulates membrane trafficking pathways remains unknown.
Another requirement for PtdIns-4-phosphate is to maintain the integrity of the Golgi apparatus. Sec2, the major Sec4 Rab GTPase exchange factor, binds to Ptdlns-4-phosphate, which inhibits Sec2 binding to the Sec15 component of the exocyst complex. Therefore, PtdIns-4-phosphate inhibits the inappropriate assembly of the exocyst complex on Golgi membranes (Mizuno-Yamasaki et al. 2010b). The exocyst marks secretory vesicles for fusion to the plasma membrane, therefore PtdIns-4-phosphate inhibits the premature recruitment of the exocyst to Golgi membranes (i.e. membranes from which secretory vesicles form)—an event which could potentially confuse the distinction between secretory vesicles and the Golgi system.
In addition to PtdIns-4-phosphate promoting anterograde membrane trafficking pathways from the Golgi, recent studies show that PtdIns-4-phosphate also regulates retrograde membrane trafficking from endosomes back to the Golgi (Mousley et al. 2008; Wood et al. 2009). Specifically, the yeast Vps74 protein, which facilitates the sorting of escaped Golgi resident glycosyltransferases to retrograde carriers for retrieval back to the Golgi system, is a PtdIns-4-phosphate binding protein. Vps74 binds directly to the cytosolic tails of the cargo glycosyltransferases as well as to the COP1 subunit of the coatomer complex whose assembly is regulated by Arf1-GTP (Wood et al. 2009). Vps74 is the yeast functional ortholog of the mammalian GOLPH3 protein that has been implicated in MYO18A-dependent control of Golgi morphology (Wood et al. 2009). GOLPH3 is reported to interact with at least one subunit of the retromer complex which functions in retrograde trafficking in the endosomal pathway (Vergés et al. 2006) therefore GOLPH3 may execute Vps74-like functions in cargo sorting and retrieval in mammals.
9.3 Diacylglycerol and Golgi Function
Diacylglycerol (DAG) is a neutral lipid which regulates vesicle budding at multiple steps in the exocytic pathway including transport from the TGN (Litvak et al. 2005; Antonny et al. 1997; Kearns et al. 1997; Yanagisawa et al. 2002; Baron and Malhotra 2002) and the formation of COP1-vesicles for retrograde trafficking from early Golgi cisternae back to the ER. It regulates vesicular budding in two general ways. First, DAG is known to directly regulate protein components of the membrane trafficking machinery. It stimulates the activities of Arf-GTPase activating proteins in both yeast and mammalian systems (Antonny et al. 1997; Yanagisawa et al. 2002). Also, in mammalian cells, DAG is necessary and sufficient to recruit protein kinase D (PKD) isoforms to Golgi membranes. Upon activation, PKD coordinates DAG metabolism and signaling to downstream lipid metabolic events that optimize membrane trafficking from the TGN (Liljedahl et al. 2001; Bard and Malhotra 2006; Bossard et al. 2007). A prominent feature of this circuit is that PI4KIIIβ is recruited to the Golgi, the consequence of which is described above. DAG recruits a number of other signaling proteins to Golgi membranes as well including various protein kinases C isoforms and Ras guanosine nucleotide release proteins (Lehel et al. 1995; Maissel et al. 2006; Wang et al. 1999; Caloca et al. 2003). Included in this cohort is PKCη′, which phosphorylates and activates PKD (Díaz Ael and Malhotra 2005).
The second regulatory mechanism utilizes the unique topological properties of DAG to orchestrate the nucleation and/or propagation of the membrane deformations necessary for vesicle budding and scission (Chernomordik et al. 1995; Burger 2000). The extreme inverted-cone shapes assumed by DAG species (due to their small headgroup to acyl chain axial area ratios) are compatible with non-bilayer lipid arrangements. The biophysical properties and signaling capacity of DAG is likely coupled. DAG regulates at least two steps in COP1-dependent vesicle biogenesis. At an early point in vesicle formation, it enables membrane deformation required to generate buds/tubules. Later, DAG regulates the scission event required to release the newly formed vesicle from its donor membrane (Asp et al. 2009). Budding/tubulation does not require the involvement of the DAG-activated ArfGAP1 and is posited to act primarily as a topological regulator of membrane curvature. The DAG involvement at the scission step does require ArfGAP1 activity—suggesting that DAG potentiates scission both by activating ArfGAP1 and by promoting formation of the non-bilayer membrane structures which characterize terminal fission intermediates (Asp et al. 2009). The DAG-activated PKD involvements in membrane trafficking from the TGN also display scission-step execution points (Liljedahl et al. 2001; Bard and Malhotra 2006).
9.4 Lipid Transfer Proteins
The extensive involvement of lipids in regulating the functional integrity of the Golgi complex requires coordination between both lipid metabolism and lipid signaling. Lipid transfer proteins have been found to perform such a task and Ptdlns/PtdCho transfer proteins (PITPs) represents a suitable example of this coordination. Sec14, the major yeast PITP, regulates an essential interface between lipid metabolism and membrane trafficking from the Golgi network. Historically, it has been proposed that Sec14 utilizes its intrinsic ability to transfer Ptdlns or PtdCho monomers in-between bilayers to deliver lipids from the endoplasmic reticulum to the Golgi. Genetic studies in yeast have proven invaluable in determining what biological activities are regulated by Sec14p and other Sec14-like PITPs; the findings of which are not consistent with classical transfer mechanisms for Sec14p function. Intracellular Sec14p levels are approximately 100-fold in excess of the levels needed for cell viability (Salama et al. 1990; Cleves et al. 1991), thus, for a transfer model to fit biologically, cells must demonstrate an extremely low threshold for Ptdlns transfer. Second, disruption of the CDP-choline pathway for PtdCho biosynthesis or Sac1 dependent PtdIns-4-phosphate catabolism bypasses the essential Sec14p requirement for yeast viability (Rivas et al. 1999; Xie et al. 1998; Phillips et al. 1999). Thus, Sec14p regulates lipid metabolism rather than Ptdlns supply/transport. Third, vectoral phospholipid transfer models predict that increased affinity of a PITP for Ptdlns versus PtdCho is an important functional property; yet, Sec14p activity is surprisingly insensitive to specific reductions in Ptdlns binding affinity (Salama et al. 1990; Sha et al. 1998). Finally, transfer models predict that alternative supply of Ptdlns to membranes bypass essential Sec14p requirement. Contrary to this observation, manipulating the yeast lipidome by increasing Ptdlns to 40 mol% of total glycerophospholipid mass (which should solve all Ptdlns supply demands) fails to relieve cells of the essential Sec14p requirement (Rivas et al. 1999).
A description for Sec14 activity is better served where it functions primarily as a lipid sensor that instructs specific enzymes when and where to execute biochemical reactions. Such an activity provides a mechanism of coincidence detection that integrates multiple aspects of lipid metabolism with PIP signaling. Ultimately, this provides additional layers by which the membrane trafficking machinery is regulated. These alternative possibilities suggest concepts that might translate into functional mechanisms for other LTPs. Utilization of this class of proteins for linking specific channels of metabolic information with the action of interfacial lipid-modifying enzymes represents a novel theme in cell signaling.
9.5 The Sec14 Superfamily of PITPs
The Sec14 domain (SMART entry: smart00516), for which the yeast Sec14p is the prototype (Sha et al. 1998), represents an ancient and versatile structural unit restricted to eukaryotes. To date 1551 Sec14 domains, representing 1550 proteins, are annotated in the NCBI database (http://www.ncbi.nlm.nih.gov). Even simple eukaryotes express multiple Sec14 family members. Saccharomyces cerevisiae expresses five Sec14-like proteins in addition to Sec14p (Li et al. 2002b), and Homo sapiens, Mus musculus, Drosophila melanogaster, Caenorhabditis elegans and Aradidopsis thaliana each possess > 20 individual genes that encode distinct Sec14 superfamily members.
As expected from the diversity of the superfamily, proteins containing a Sec14 domain interface with a multitude of cellular activities. Studies in yeast and plants demonstrate multiple roles for Sec14-like PITPs in regulating: housekeeping membrane-trafficking pathways (Phillips et al. 1999; Wu et al. 2000; Carmen-Lopez et al. 1994), developmental membrane-trafficking circuits for dimorphic growth and sporulation in yeast (Carmen-Lopez et al. 1994; Nakase et al. 2001; Rudge et al. 2004), and root hair biogenesis in plants (Vincent et al. 2005). In addition, Sec14 domains are frequently observed in proteins that regulate activities of small GTPases of the Ras, Rho and Rac families. Examples include the Ras-GAP neurofibromins NF1 and NF2 (Aravind et al. 1999), Rho-GAPs and Cdc42-GAPs of the BCH and BNIP families (Shang et al. 2003; Tcherkezian and Lamarche-Vane 2007; Sirokmany et al. 2005), and the Rho-GEFs Trio, Dbl and Duo (Ueda et al. 2004; Debant et al. 1996). The PTP-MEG2 protein tyrosine phosphatase also harbors a Sec14 domain (Gu et al. 1992).
The Sec14 domain is also associated with uncharacterized modules that include nodulin domains (in higher plants) (Vincent et al. 2005; Kapranov et al. 2001), Golgi dynamics (GOLD) domains in metazoans and higher plants (Anantharaman and Aravind 2002), metazoan-specific PRELI domains (thought to function as mitochondrial targeting motifs) (Dee and Moffat 2005), and a GTPase motif of uncertain function (Habermehl et al. 2004). Stand-alone Sec14 domain proteins are more prevalent in simpler eukaryotes. Higher eukaryotes often couple Sec14 domains with more complex arrangements in addition to expressing stand-alone Sec14 domains. Examples of mammalian stand-alone Sec14 domain proteins include cellular retinaldehyde binding protein (CRALBP) (Liu et al. 2005), caytaxin (Bomar et al. 2003), and α-tocopherol and retinaldehyde transfer proteins (Gotoda et al. 1995; Hentati et al. 1996; Golovleva et al. 2003). The biological importance of Sec14 superfamily members in mammals is demonstrated by the linkage of human diseases to dysfunction of Sec14-like proteins and of Sec14 domains in multidomain proteins. Such diseases include autosomal-dominant cancers attributed to neurofibromin insufficiencies (Cichowski and Jacks 2001), ataxia with vitamin E deficiency arising from diminished α-TTP function (Ouachi et al. 1995), ataxia caused by loss of caytaxin function (Hentati et al. 1996), and retinal degeneration syndromes elicited by CRALBP dysfunction (Maw et al. 1997; Fishman et al. 2004).
How is the diversity of the Sec14 superfamily employed in the context of the lipid signaling circuitry of eukaryotic cells? Together it is proposed that the Sec14-protein superfamily has been engineered for sensing specific aspects of lipid metabolism and for transduction of sensing information to an activity that employs PIP signaling for action. Below, we describe aphysical picture of how the Sec14 superfamily generates a productive ‘crosstalk’ between the larger lipidome, PIP signaling, and membrane trafficking control.
9.6 Ligand Binding by Sec14-like Proteins
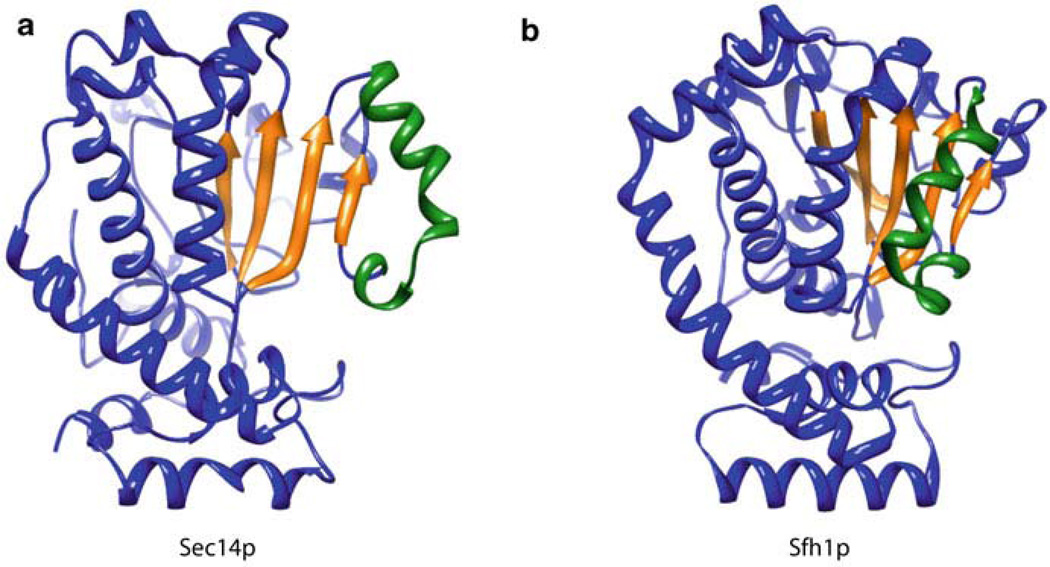
Available crystal structures of Sec14-like proteins include detergent-bound forms of Sec14p, several phospholipid-bound forms of yeast Sfh1p, ligand-bound and unbound versions of α-tocopherol transfer protein (α-TTP), the mammalian Sec14-GOLD protein Sec14L2, and detergent-bound and phospholipid-bound forms of the neurofibromin Sec14-like domain (Schaaf et al. 2008; Sha et al. 1998; Min et al. 2003; Meier et al. 2003; Stacker and Baumann 2003; D’Angelo et al. 2006; Welti et al. 2007). These structures show that the Sec14 fold is structurally conserved comprising approximately 280-residue two-lobed globular structure. In the case of apo-Sec14p, the amino-terminal region consists of four anti-parallel α-helices whereas the carboxy-terminal lobe (also referred to as the CRAL_TRIO domain) forms the phospholipid binding pocket (Fig. 9.1). The Sec14p lipid binding cavity consists of five parallel β-strands comprising a β-sheet that is sandwiched by two long α-helices on one side and two short α-helices plus one 310-helix on the other. Molecular dynamics simulations (Ryan et al. 2007) and comparisons of apo-Sec14p and holo-Sfh1p structures (Schaaf et al. 2008; Sha et al. 1998) demonstrate that access to the hydrophobic lipid-binding cavity is regulated by a helical gate (Fig. 9.1). This gate is closed when Sec14 is bound to ligand. Transitions from open to closed conformations bring about rigid body motions which displaces the helical gate. These conformational dynamics are coupled to interfacial phospholipid exchange reactions on the surface of biological membranes. Other members of the Sec14 superfamily probably undergo similar conformational dynamics during the course of ligand loading/unloading reactions. A regulatory substructure, the approximately 20-residue gating module, regulates an H-bond network that transduces conformational information to the helical gate (Ryan et al. 2007), probably in response to conformational changes initiated by membrane docking. Examples of human disease missense mutations that map to the gating modules of Sec14 superfamily proteins confirm the functional importance of this substructure (Ryan et al. 2007).
Fig. 9.1.
A helical substructure of the Sec14-like proteins regulates entrance of the ligand into the hydrophobic binding pocket. Ribbon diagrams of (a) open Sec14p (1AUA, open conformer bound to the detergent β-octylglucoside) and (b) closed Sfh1p (3B7N, closed conformer bound to Ptdlns) are shown. Comparisons of open versus closed conformers provide insight into how the helical substructure (green) regulates entry into the binding pocket, α-helices and turns are depicted in blue and the binding cavity floor β-sheets are depicted in yellow
A notable property of Sec14 is that the hydrophobic gradient within the lipid binding domain closely matches that found in the cytosolic leaflet of a membrane. This similarity implies that lipid binding/release by Sec14p, and presumably other Sec14-like proteins, is driven by simple substrate partitioning from one aprotic environment to another (Smirnova et al. 2007). How Sec14 selects lipid ligands on the membrane surface, and how these are subsequently configured to enable abstraction from a membrane bilayer evoke questions for future analysis. The local membrane deformations that must proceed to enable these selection/configuration processes also remain unknown.
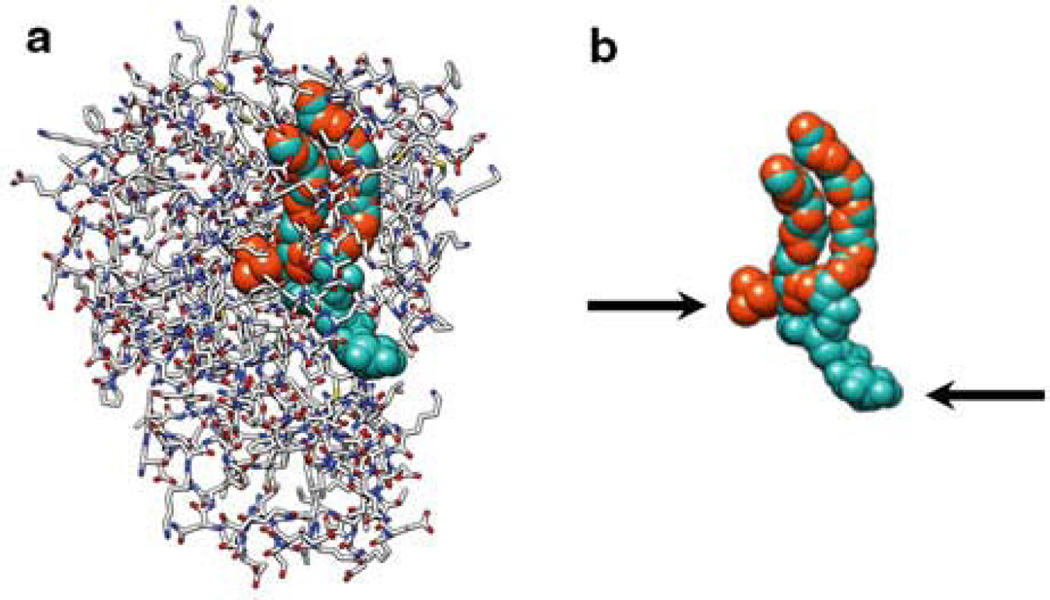
The most surprising feature of both Sfh1p and Sec14p is that the headgroups of PtdCho and Ptdlns are bound at distinct sites within the lipid binding pocket (Schaaf et al. 2008, Fig. 9.2). The inositol binding site is situated near the protein surface (Schaaf et al. 2008, Fig. 9.2). By contrast, the PtdCho headgroup is buried within the interior of the hydrophobic cleft, an interaction stabilized by tyrosine-mediated cation–π interactions (Schaaf et al. 2008). Another unusual feature of Sfh1p and Sec14p is their ability to accommodate phospholipid molecules with different volumes (cavity volumes for Sfh1p–Ptdlns and Sfh1p–PtdCho are 4050.6 Å3 and 3068.7 Å3, respectively) without significantly effecting the shape of the protein surface. This is accomplished in part by loading the unoccupied PtdCho headgroup binding site with ordered water molecules in the Sec14–Ptdlns complex and, reciprocally, loading the unoccupied Ptdlns headgroup binding site with ordered water in the Sec14–PtdCho complex (Schaaf et al. 2008). The flux of water into and from the hydrophobic pocket during heterotypic exchange reactions is a major factor in overcoming the differences in Sec14p relative binding affinities for Ptdlns and PtdCho so that heterotypic exchange reactions (Ptdlns for PtdCho or PtdCho for Ptdlns) can actually take place. That Sfh1, a protein that shares 64% sequence identity with Sec14, is functionally distinct to Sec14 is proposed to be attributed to specific reconfigurations in atomic interactions between amino acid side chains and ordered water molecules within the lipid binding cavity (Schaaf et al. 2011). Such altered dynamics reconstitutes a functional gating module that communicates conformational energy from within the hydrophobic pocket to the helical structure that gates access to the pocket (Schaaf et al. 2011). It is predicted that a consequence of this is that the rates of phospholipid cycling into and out of the Sfh1 and Sec14 hydrophobic pocket differs such that Sfh1 cannot substitute for Sec14 activity. This remains open for future investigation.
Fig. 9.2.
Sec 14-like PITPs bind phospholipid head groups at two different sites, (a) 3B7Z Bound Ptdlns (Aqua spheres) and PtdCho (Orange spheres) is shown in a complex with the Sec14-like PlTP, Sfh1p (Grey, red and blue wire). (b) The Sfh1 bound phospholipid configurations are depicted without the PHP. The arrows indicate the lipid headgroups. Extraordinarily, the acyl chains of these two different lipid species occupy the same hydrophobic cavity of the transfer protein, but their headgroups bind at distinct sites with a clear physical barrier between binding regions. We propose that this physical barrier between the headgroup binding sites in Sec14p molecule forms the basis for how heterotypic exchange reactions present a Ptdlns headgroup to the lipid kinase
9.7 Coincidence Sensors that Couple Lipid Metabolic Inputs to PIP Synthesis
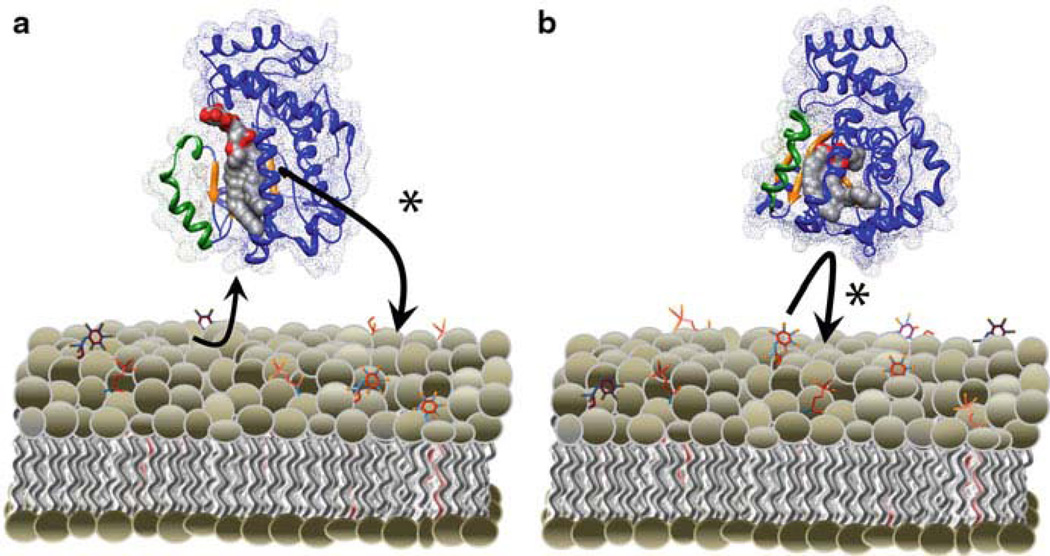
Both Ptdlns- and PtdCho-binding activities must reside on the same Sec14p molecule to generate a biologically functional protein able to stimulate Ptdlns 4–OH kinase activity (Schaaf et al. 2008). Thus, heterotypic exchange reactions are required for Sec14p-mediated stimulation of Ptdlns kinases (and PIP synthesis) in vivo. This indicates that Sec14p cannot stimulate Ptdlns 4-OH kinases in cells unless sufficient amounts of PtdCho are present to facilitate heterotypic exchange reactions necessary to activate Ptdlns kinases (Fig. 9.3). Together this connects Sec14p as a PtdCho sensor which transmits PtdCho metabolic information to PIP synthesis. This activity is consistent with the apparent coupling between the cytidine diphosphate (CDP)–choline pathway for PtdCho biosynthesis and membrane trafficking control (Cleves et al. 1991; Skinner et al. 1993).
Fig. 9.3.
Heterotypic exchange promotes Ptdlns presentation. Heterotypic exchange reactions can support Ptdlns presentation by two different models. a PtdCho vectorial displaces a Sec14p bound Ptdlns in a head-first manner. The displaced Ptdlns exits the binding pocket through a portal distinct from the portal through which PtdCho invades. Ptdlns4-OH Kinase (not shown) modifies the exiting Ptdlns during this exchange. b A second mode by which heterotypic exchange promotes Ptdlns presentation is by frustration of an invading Ptdlns. In this mode, Ptdlns attempts to invade into the hydrophobic pocket of a PtdCho bound Sec14p. The bound PtdCho frustrates the Ptdlns, allowing Ptdlns 4-OH kinase to modify its substrate. Both models satisfy the requirement that Ptd-4-phosphate cannot collapse back into the hydrophobic pocket, as this complex results in locked Sec14p-PIP species that cannot be reversed by phospholipid exchange.
The Sec14p requirement for coordinating the PtdCho biosynthesis/membrane trafficking interface ensures that the DAG pools necessary for TGN/endosomal trafficking are not exhausted by the CDP–choline pathway for PtdCho biosynthesis. Thus Sec14p senses PtdCho as a readout for DAG consumption (Skinner et al. 1995). As PtdCho levels increase from synthesis through the CDP–choline pathway, Sec14p is activated for heterotypic Ptdlns/PtdCho exchange, stimulating PIP production by Ptdlns 4-OH kinases (Fig. 9.3). As a consequence, PtdIns-4-phosphate synthesis would serve to activate downstream effectors that promote vesicle budding from TGN/endosomes. In addition, PtdIns-4-phosphate might also inhibit the cholinephosphate cytidylyltransferase, the rate-determining enzyme of the CDP–choline pathway (Fig. 9.3).
Do these concepts hold true for other members of the Sec14 superfamily? Bioinformatic analyses have identified primary sequence ‘bar codes’ for Ptdlns and PtdCho binding (Schaaf et al. 2008). It is apparent that PtdCho binding is not a conserved feature of Sec14-like proteins. However, the holo-Sec14L2 and α-TTP structures, and the biochemical properties of CRALBP and related proteins, show that members of the Sec14 superfamily lacking key PtdCho-binding residues are able to bind to alternative hydrophobic ligands (Schaaf et al. 2008; Min et al. 2003; Meier et al. 2003; Stacker and Baumann 2003; D’Angelo et al. 2006; Welti et al. 2007). In contrast to the PtdCho binding motif, bioinformatics identifies the Ptdlns-binding ‘bar code’ to be ubiquitous to the superfamily. It is attractive to propose that a two-ligand PITP-mediated mechanism for Ptdlns kinase activation (analogous to that described for Sec14p and Sfh1p) might be broadly utilized by the Sec14 superfamily members. Together, the Sec14 superfamily of proteins link diverse aspects of the lipid metabolome with PIP signaling.
9.8 Instructive Mechanisms for Regulating Ptdlns-kinase Activity
That Sec14p and Sec14-like PITPs are required physiologically to potentiate yeast Ptdlns 4-OH kinase activity suggests that these enzymes, alone, inadequately engage membrane-incorporated Ptdlns. Infact, to circumvent the poor interfacial utilization of Ptdlns as a substrate by Ptdlns 4-OH kinase in an in vitro Ptdlns kinase assay Ptdlns is presented to the kinase in the context of mixed phospholipid-detergent micelles. Thus, it is attractive to speculate that Ptdlns 4-OH kinases have evolved to be inefficient interfacial enzymes to enable opportunities for regulating lipid signaling. That is, Sec14-like PITPs solve the inadequacies of Ptdlns 4-OH kinases in engaging lipid metabolism with a plethora of intracellular signaling pathways.
Insights into Sec14 functionality predict that the priming of Sec14-like PITPs through association with their cognate sensory ligand (PtdCho in the case of Sec14) triggers, through heterotypic exchange, the ability to present Ptdlns headgroups to Ptdlns 4-OH kinases in a conformation readily susceptible to phosphorylation. In this model, the efficiency with which Ptdlns 4-OH kinase modifies Ptdlns is low, a consequence attributed to the short window of opportunity that a Ptdlns kinase has to phosphorylate its substrate. However, these exchange reactions occur rapidly and independent of ATP consumption, therefore, the high frequency with which a PITP presents Ptdlns to a kinase compensates for this inefficient system.
The term ‘heterotypic exchange’ implies complete transition, in one cycle, of a holo-Sec14p–PtdCho complex to a holo-Sec14p–PtdIns complex or vice versa. This definition might be too strict. The effect of heterotypic exchange reactions on interfacial presentation of Ptdlns to Ptdlns 4-OH kinases could reflect consequences of abortive heterotypic exchange reactions (Schaaf et al. 2008). Assuming that the Sec14p–PtdCho state represents the ‘primed’ Sec14p, sequestration of PtdCho deep within the hydrophobic pocket might result in a ‘slow’ exit of this phospholipid from the pocket. Thus, a deep ligand obstructs the pocket from an invading Ptdlns molecule. During abortive heterotypic exchange, where PtdCho binding is a prerequisite to Ptdlns presentation, the invading lipid is neither embedded in the membrane nor sequestered by the engaged Sec14p enabling the lipid headgroup to become highly susceptible to by Ptdlns 4-OH kinase mediated phosphorylation. Multiple abortive Ptdlns exchange events could theoretically accompany a complete heterotypic exchange event, particularly if the rate of Ptdlns invasion exceeds the rate of PtdCho egress. In support of this idea the rates of Ptdlns transfer are approximately 20-fold faster than that of PtdCho. In an alternative scenario, however, an invading PtdCho molecule would drive the ejection of a pre-bound Ptdlns ligand from the PITP lipid binding pocket. Thus, during its egress, the Ptdlns headgroup would become susceptible to Ptdlns 4-OH dependent modification. These alternative mechanisms might not be mutually exclusive and make distinguishing experimental predictions of lipid trajectories during heterotypic exchange. Molecular dynamic approaches will be required to investigate these mechanisms further.
9.9 Definition of Sensing Territories
Spatial and temporal restriction of PITP activity can be achieved by multiple paths. Protein–protein or protein–lipid interactions that involve the Sec14-like PITP surface (as opposed to the hydrophobic binding pocket) can restrict localization to membrane subdomains. Indeed, several yeast Sec14-like PITPs stably target specific intracellular locations. The identities of proteins (or lipids) that impart specific localization to PITPs have yet to be described. PITP receptors are of interest, as such proteins (or lipid platforms) define ‘sensing’ territories. In that regard, the Ptdlns kinases themselves represent obvious candidates for PITP receptors. Is there a requirement for dedicated physical interactions between PITPs and the kinases? This requirement is unlikely to be the case as Sec14p defects are rescued by the expression in yeast of mammalian class 1 PITPs (i.e. proteins with no primary sequence homology or structural similarity to Sec14p).
Sec14 membrane association is transient yet it is still sufficient to productively activate Ptdlns kinases. Presentation mechanisms remain plausible given that the rate with which Sec14 dissociates from membranes (or any given PITP) is much slower than the rate of heterotypic exchange. The residence time of a single Sec14 molecule is unknown. However, reasonable estimates suggest that a Sec14 molecule could execute up to 10 exchange cycles on a membrane surface in a 2 s residency. Direct measurements of the time scales for lipid exchange by individual PITP molecules, especially when considered relative to PITP membrane dwell times, define important future directions essential for assessing nanoreactor versus lipid transfer models.
In this regard, Sec14 domains in modular proteins (e.g. Ras/Rho GAPs and GEFs and the MEG2 PTP) (Aravind et al. 1999; Shang et al. 2003; Tcherkezian and Lamarche-Vane 2007; Sirokmany et al. 2005; Ueda et al. 2004; Debant et al. 2004; Gu et al. 1992) are well-engineered to act as intrinsic lipid sensing units for instructing local changes in PIP environment in response to metabolic cues. Both GEF and GAP activities are responsive to PIPs, therefore it is attractive to postulate that the Sec14 domain ‘senses’ the lipid environment, then directs Ptdlns kinases for ‘on-demand’ PIP synthesis, and finally, recruits GEF/GAP/PTP catalytic domains to the newly synthesized pool of PIPs (e.g. as in Dbl and Dbs). Some authors suggesting that lipid binding by the Sec14 domain recruits Ras/Rho-GEFs to membrane surfaces (Sirokmany et al. 2005; Debant et al. 1996; Kostenko et al. 2004). However, it is more likely that such membrane-targeting activities probably involve protein-lipid interactions on the Sec14 domain surface rather than the hydrophobic pocket.
Similar designs might also apply to Sec14-nodulin proteins of higher plants (Aravind et al. 1999; Anantharaman and Aravind 2002). The AtSfh1 Sec14-nodulin protein expressed in Arabidopsis root hair cells both stimulates PtdIns-4,5-P2 synthesis and distribution in growing root hairs. In this manner, an AtSfh1-Ptdlns-kinase-PIP-kinase axis enables polarized programs for membrane trafficking, actin organization and calcium signaling in growing root hairs (Vincent et al. 2005). AtSfh1 might help develop the PIP landscape via a sensing role for the Sec14 domain and consequently signals and potentiates PIP synthesis by neighboring Ptdlns 4-OH and PtdIns-4-phosphate 5-OH kinases (Preuss et al. 2006; Stenzel et al. 2008).
Nodulin domains represent membrane association elements, and the chemical properties of the AtSfh1 nodulin domain and of other nodulin domains suggest additional layers of regulation. These units present basic carboxy-terminal tails (Vincent et al. 2005; Kapranov et al. 2001), thereby resembling known PIP-binding motifs that operate via membrane surface electrostatics (e.g. the MARCKS peptide (McLaughlin and Murray 2005). It is a testable proposition that AtSfh1 functionally specifies the production and organization of dedicated PIP pools. Regulated electrostatic interactions suggest mechanisms for imprinting ‘caged’ PIP patterns for subsequent and regulated PIP release in a spatially organized program of downstream signaling (McLaughlin and Murray 2005). The multiplicity of plant Sec14-nodulin proteins forecasts a large diversity in such a strategy for coupling distinct Sec14-like modules of multidomain proteins to developmental pathways for membrane morphogenesis (Vincent et al. 2005). Sec14-GOLD domain proteins that bind PIPs (e.g. PATELLIN 1) might also operate similarly (Peterman et al. 2004).
9.10 Concluding Remarks
New lines of research are providing novel understandings into the mechanisms of how lipid metabolism plays a central role in regulating membrane trafficking and signaling. Recognition of Sec14-like PITPs’ integral function in lipid metabolism and PIP signaling, and the recent advances that demonstrate a physical appreciation of how such integration could work, present the trafficking and signaling fields with new questions to address. Although we propose that PITPs primarily function as nanoreactores for regulating lipid metabolism, as opposed to the classic “lipid carrier” model, the mechanism will vary from case to case. For instance, the metazoan-specific PITPs (e.g. Drosophila RdgBα and mammalian NIR2) are structurally unrelated to the Sec14-like PITPs, yet bind Ptdlns and PtdCho in the same binding siter. Do these PITPs function as signaling nanoreactors by potentiating PIP synthesis or do they simply carry lipids from one membrane to another? Furthermore, the nanoreactor/lipid-carrier dichotomy may be applied to other putative transfer proteins that operate in cells (e.g. oxysterol binding proteins and ceramide transfer protein. These competing conceptual frameworks can now be experimentally examined, thereby providing an escape from the circular arguments that have plagued the lipid transfer protein field since its inception.
A unique way of regulating signal transduction pathways maybe the use of non-enzymatic protein biosensors, such as Sec14-like PITPs, to couple metabolic cues with the action of interfacial lipid modifying enzymes. If Sec14p can bind other inositol-phospholipids in addition to Ptdlns, then the versatility of Sec14p as a biosensor might be more than previously anticipated. There is no structural rationale to exclude the possibility that Sec14p may also bind inositol-phosphoceramides in vivo, a property that could additionally allow Sec14p the ability to transmit PtdCho metabolic information to inositol sphingolipid metabolism.
Currently, our understanding of spatial and temporal regulation of lipid metabolism is inadequate for a complete biological evaluation. Technical complications will be a major challenge in the future and impede scientific growth and information. Understanding when and where Sec14-like PITPs are active is a central component to understanding lipid metabolism. Thus, engineering reliable conformational biosensors will be required to elucidate the roles of Sec14-like PITPs at the interface between membrane trafficking and signaling in living cells.
References
- Anantharaman V, Aravind L. The GOLD domain, a novel protein module involved in Golgi function and secretion. Genome Biol. 2002;3:0023. doi: 10.1186/gb-2002-3-5-research0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonny B, et al. Activation of ADP-ribosylation factor 1 GTPase-activating protein by phosphatidylcholine-derived diacylglycerols. J Biol Chem. 1997;272:30848–30851. doi: 10.1074/jbc.272.49.30848. [DOI] [PubMed] [Google Scholar]
- Aravind L, et al. Sec14p-like domains in NF1 and Db1-like proteins indicate lipid regulation of Ras and Rho signaling. Curr Biol. 1999;9:R195–R197. doi: 10.1016/s0960-9822(99)80127-4. [DOI] [PubMed] [Google Scholar]
- Asp L, et al. Early stages of Golgi vesicle and tubule formation require diacylglycerol. Mol Biol Cell. 2009;20:780–790. doi: 10.1091/mbc.E08-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla A, Balla T. Phosphatidylinositol 4-kinases: old enzymes with emerging functions. Trends Cell Biol. 2006;16:351–361. doi: 10.1016/j.tcb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Bankaitis VA, et al. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347:561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- Bard F, Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol. 2006;22:439–455. doi: 10.1146/annurev.cellbio.21.012704.133126. [DOI] [PubMed] [Google Scholar]
- Baron CL, Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 2002;295:325–328. doi: 10.1126/science.1066759. [DOI] [PubMed] [Google Scholar]
- Bomar JM, et al. Mutations in a novel gene encoding a CRAL-TRIO domain cause human Cayman ataxia and ataxia/dystonia in the jittery mouse. Nat Genet. 2003;35:264–269. doi: 10.1038/ng1255. [DOI] [PubMed] [Google Scholar]
- Bossard C, et al. Dimeric PKD regulates membrane fission to form transport carriers at the TGN. J Cell Biol. 2007;179:1123–1131. doi: 10.1083/jcb.200703166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger KNJ. Greasing membrane fusion and fission machineries. Traffic. 2000;1:605–613. doi: 10.1034/j.1600-0854.2000.010804.x. [DOI] [PubMed] [Google Scholar]
- Caloca MJ, et al. Exchange factors of the RasGRP family mediate Ras activation in the Golgi. J Biol Chem. 2003;278:33465–33473. doi: 10.1074/jbc.M302807200. [DOI] [PubMed] [Google Scholar]
- Carlton JG, Cullen PJ. Coincidence detection in phosphoinositide signaling. Trends Cell Biol. 2005;15:540–547. doi: 10.1016/j.tcb.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmen-Lopez M, et al. A phosphatidylinositol/phosphatidylcholine transfer protein is required for differentiation of the dimorphic yeast Yarrowia lipolytica from the yeast to the mycelial form. J Cell Biol. 1994;124:113–127. doi: 10.1083/jcb.125.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantalat S, et al. The Arf activator Gea2p and the P-type ATPase Drs2p interact at the Golgi in Saccharomyces cerevisiae. J Cell Sci. 2004;117:711–722. doi: 10.1242/jcs.00896. [DOI] [PubMed] [Google Scholar]
- Chernomordik L, et al. Lipids in biological membrane fusion. J Membr Biol. 1995;146:1–14. doi: 10.1007/BF00232676. [DOI] [PubMed] [Google Scholar]
- Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001;104:593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Cleves AE, et al. Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell. 1991;64:789–800. doi: 10.1016/0092-8674(91)90508-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo I, et al. A novel bipartite phospholipid-binding module in the neurofibromatosis type 1 protein. EMBO Rep. 2006;7:174–179. doi: 10.1038/sj.embor.7400602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debant A, et al. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci U S A. 1996;93:5466–5471. doi: 10.1073/pnas.93.11.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dee CT, Moffat KG. A novel family of mitochondrial proteins is represented by the Drosophila genes slmo, preli-like and real-time. Dev Genes Evol. 2005;215:248–254. doi: 10.1007/s00427-005-0470-4. [DOI] [PubMed] [Google Scholar]
- Demmel L, et al. The clathrin adaptor Gga2p is a phosphatidylinositol-4-phosphate effector at the Golgi exit. Mol Biol Cell. 2008;19:1991–2002. doi: 10.1091/mbc.E06-10-0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz Ael AM, Malhotra V. PKCeta is required for β1γ2/β3γ2 and PKD mediated transport to the cell surface and the organization of the Golgi apparatus. J Cell Biol. 2005;169:83–91. doi: 10.1083/jcb.200412089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman GA, et al. Vovel mutations in the cellular retinaldehyde-binding protein gene (RLBP1) associated with retinitis punctata albescens: evidence of interfamilial genetic heterogeneity and fundus changes in heterozygotes. Arch Opthamol. 2004;122:70–75. doi: 10.1001/archopht.122.1.70. [DOI] [PubMed] [Google Scholar]
- Gloor Y, et al. Interaction between Sec7p and Pik1p: the first clue for the regulation of a coincidence detection signal. Eur J Cell Biol. 2010;89:575–583. doi: 10.1016/j.ejcb.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Godi A, et al. ARF mediates recruitment of PtdIns 4-OH kinase beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- Golovleva I, et al. Disease causing mutations in the cellular retinaldehyde binding protein tighten and abolish ligand interactions. J Biol Chem. 2003;278:12397–12402. doi: 10.1074/jbc.M207300200. [DOI] [PubMed] [Google Scholar]
- Gotoda T, et al. Adult-onset spinocerebellar dysfunction caused by a mutation in the gene for alpha-tocopherol transfer protein. N Engl J Med. 1995;333:1313–1318. doi: 10.1056/NEJM199511163332003. [DOI] [PubMed] [Google Scholar]
- de Graaf P, et al. Phosphatidylinositol 4-kinase beta is critical for functional association of rab 11 with the Golgi complex. Mol Biol Cell. 2004;15:2038–2047. doi: 10.1091/mbc.E03-12-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M, et al. Cloning and expression of a cytosolic megakaryocyte protein-tyrosine-phosphatase with sequence homology to retinaldehyde-binding protein and yeast SEC14p. Proc Natl Acad Sci U S A. 1992;89:2980–2984. doi: 10.1073/pnas.89.7.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermehl D, et al. Recombinant SEC14-like proteins (TAP) possess GTPase activity. Biochem Biophys Res Commun. 2004;326:254–259. doi: 10.1016/j.bbrc.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Hama H, et al. Direct involvement of phosphatidylinositol-4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:34294–34301. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- Haynes LP, et al. Interaction of neuronal calcium sensor-1 (NCS-1) and ADP-ribosylation factor 1 allows bidirectional control of phosphatidylinositol-4-kinase beta and trans-Golgi network-plasma membrane traffic. J Biol Chem. 2005;280:6047–6054. doi: 10.1074/jbc.M413090200. [DOI] [PubMed] [Google Scholar]
- Hendricks KB, et al. Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nat Cell Biol. 1999;1:234–241. doi: 10.1038/12058. [DOI] [PubMed] [Google Scholar]
- Hentati A, et al. Human alpha-tocopherol transfer protein: gene structure and mutations in familial vitamin E deficiency. Ann Neurol. 1996;39:295–300. doi: 10.1002/ana.410390305. [DOI] [PubMed] [Google Scholar]
- Kapranov P, et al. Nodule-specific regulation of phosphatidylinositol transfer protein expression in Lotus japonicus. Plant Cell. 2001;13:1369–1382. doi: 10.1105/tpc.13.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns BG, et al. Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature. 1997;387:101–105. doi: 10.1038/387101a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostenko EV, et al. The Sec14 homology domain regulates the cellular distribution and transforming activity of the Rho-specific guanine nucleotide exchange factor Dbs. J Biol Chem. 2004;280:2807–2817. doi: 10.1074/jbc.M411139200. [DOI] [PubMed] [Google Scholar]
- Lehel C, et al. Protein kinase C epsilon subcellular localization domains and proteolytic degradation sites. A model for protein kinase C conformational changes. J Biol Chem. 1995;270:19651–19658. doi: 10.1074/jbc.270.33.19651. [DOI] [PubMed] [Google Scholar]
- Li X, et al. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J Cell Biol. 2002a;157:63–77. doi: 10.1083/jcb.200201037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. Identification of a novel family of nonclassic yeast phosphatidylinositol transfer proteins whose function modulates phospholipase D activity and Sec14p-independent cell growth. Mol Biol Cell. 2002b;11:1989–2005. doi: 10.1091/mbc.11.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljedahl M, et al. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. doi: 10.1016/s0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- Litvak V, et al. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat Cell Biol. 2005;7:225–234. doi: 10.1038/ncb1221. [DOI] [PubMed] [Google Scholar]
- Liu T, et al. Structural insights into the cellular retinaldehyde-binding protein (CRALBP) Proteins Struct Funct Bioinform. 2005;61:412–422. doi: 10.1002/prot.20621. [DOI] [PubMed] [Google Scholar]
- Maissel A, et al. PKCeta is localized in the Golgi, ER and nuclear envelope and translocates to the nuclear envelope upon PMA activation and serum-starvation: C1b domain and the pseudo-substrate containing fragmenttarget PKCeta to the Golgi and the nuclear envelope. Cell Signal. 2006;18:1127–1139. doi: 10.1016/j.cellsig.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Maw MA, et al. Mutation of the gene encoding cellular retinaldehyde-binding protein in autosomal recessive retinitis pigmentosa. Nat Genet. 1997;17:198–200. doi: 10.1038/ng1097-198. [DOI] [PubMed] [Google Scholar]
- McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- Meier R, et al. The molecular basis of vitamin E retention: structure of human alpha-tocopherol transfer protein. J Mol Biol. 2003;331:725–734. doi: 10.1016/s0022-2836(03)00724-1. [DOI] [PubMed] [Google Scholar]
- Min KC, et al. Crystal structure of α-tocopherol transfer protein bound to its ligand: Implications for ataxia with vitamin E deficiency. Proc Natl Acad Sci U S A. 2003;100:14713–14718. doi: 10.1073/pnas.2136684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno-Yamasaki E, et al. Phosphatidylinositol-4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2. Dev Cell. 2010a;18:828–840. doi: 10.1016/j.devcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno-Yamasaki E, et al. Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev Cell. 2010b;18(5):828–840. doi: 10.1016/j.devcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousley CJ, et al. Trans-Golgi network and endosome dynamics connect ceramide homeostasis with regulation of the unfolded protein response and TOR signaling in yeast. Mol Biol Cell. 2008;19:4785–4803. doi: 10.1091/mbc.E08-04-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusamy B-P, et al. Linking phospholipid flippases to vesicle-mediated transport. Biochim Biophys Acta. 2009;179:612–619. doi: 10.1016/j.bbalip.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase Y, et al. The Schizosaccharomyces pombe spo20(+) gene encoding a homologue of Saccharomyces cerevisiae Sec14 plays an important role in forespore membrane formation. Mol Biol Cell. 2001;4:901–917. doi: 10.1091/mbc.12.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan P, et al. Regulation of a golgi flippase by phosphoinositides and an Arf-GEF. Proc Natl Acad Sci U S A. 2004;101:10614–10619. doi: 10.1073/pnas.0404146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouachi K, et al. Ataxia with vitamin E deficiency is caused by mutations in the α-tocopherol transfer protein. Nat Genet. 1995;9:141–145. doi: 10.1038/ng0295-141. [DOI] [PubMed] [Google Scholar]
- Peterman TK, et al. Patellin1, a novel Sec14-like protein, localizes to the cell plate and binds phosphoinositides. Plant Physiol. 2004;136:3080–3094. doi: 10.1104/pp.104.045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SE, et al. Yeast Sec14p deficient in phosphatidylinositol transfer activity is functional in vivo. Mol Cell. 1999;4:187–197. doi: 10.1016/s1097-2765(00)80366-4. [DOI] [PubMed] [Google Scholar]
- Polevoy G, et al. Dual roles for the Drosophila PI 4-kinase four wheel drive in localizing Rab11 during cytokinesis. J Cell Biol. 2009;187:847–858. doi: 10.1083/jcb.200908107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss ML, et al. A role for the RabA4b effector protein PI-4Kbeta1 in polarized expansion of root hairs in Arabidopsis thaliana. J Cell Biol. 2006;172:261–268. doi: 10.1083/jcb.200508116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas MP, et al. Relationship between altered phospholipid metabolism, DAG, ‘bypass Sec14p’, and the inositol auxotrophy of yeast sac1 mutants. Mol Biol Cell. 1999;10:2235–2250. doi: 10.1091/mbc.10.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routt SM, et al. Nonclassical PITPs activate phospholipase D via an Stt4p-dependent pathway and modulate function of late stages of the secretory pathway in vegetative yeast cells. Traffic. 2005;6:1157–1172. doi: 10.1111/j.1600-0854.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- Rudge SA, et al. Roles of phosphoinositides and of Spo14p (phospholipase D)-generated phosphatidic acid during yeast sporulation. Mol Biol Cell. 2004;15:207–218. doi: 10.1091/mbc.E03-04-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MM, et al. Conformational dynamics of the major yeast phosphatidylinositol transfer protein Sec14: Insights into the mechanisms of PL exchange and diseases of Sec14-like protein deficiencies. Mol Biol Cell. 2007;18:1928–1942. doi: 10.1091/mbc.E06-11-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama SR, et al. Cloning and characterization of the Kluyveromyces lactis SEC14: A gene whose product stimulates Golgi secretory function in S. cerevisiae. J Bacteriol. 1990;172:4510–4521. doi: 10.1128/jb.172.8.4510-4521.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf G, et al. The functional anatomy of phospholipid binding and regulation of phosphoinositide homeostasis by proteins of the Sec14-superfamily. Mol Cell. 2008;29:191–206. doi: 10.1016/j.molcel.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf G, et al. Resurrection of a functional phosphatidylinositol transfer protein from a pseudo-Sec14 scaffold by directed evolution. Mol Biol Cell. 2011;22(6):892–905. doi: 10.1091/mbc.E10-11-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciorra V, et al. Synthetic gene array analysis of the PtdIns 4-kinase Pik1p identifies components in a Golgi specific Ypt31/rab-GTPase signaling pathway. Mol Biol Cell. 2005;15:2038–2047. doi: 10.1091/mbc.E04-08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha B, et al. Crystal structure of the Saccharomyces cerevisiae phosphatidylinositol transfer protein Sec14. Nature. 1998;391:506–510. doi: 10.1038/35179. [DOI] [PubMed] [Google Scholar]
- Shang X, et al. Concerted regulation of cell dynamics by BNIP-2 and Cdc42GAP homology/Sec14p-like, proline-rich, and GTPase-activating protein domains of a novel rhoGTPase-activating protein, BPGAP1. J Biol Chem. 2003;278:45903–45914. doi: 10.1074/jbc.M304514200. [DOI] [PubMed] [Google Scholar]
- Sirokmany G, et al. Sec14 homology domain targets p50RhoGAP to endosomes and provides a link between Rab- and Rho GTPases. J Biol Chem. 2005;281:6096–6105. doi: 10.1074/jbc.M510619200. [DOI] [PubMed] [Google Scholar]
- Skinner HB, et al. Phospholipid transfer activity is relevant to but not sufficient for the essential function of the yeast SEC14 gene product. EMBO J. 1993;12:4775–4784. doi: 10.1002/j.1460-2075.1993.tb06166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HB, et al. Phosphatidylinositol transfer protein stimulates yeast Golgi secretory function by inhibiting choline-phosphate cytidylyltransferase activity. Proc Natl Acad Sci U S A. 1995;92:112–116. doi: 10.1073/pnas.92.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova T, et al. Local polarity and hydrogen bonding inside the Sec14 PL-binding cavity: high-field multifrequency studies. Biophys J. 2007;92:3686–3695. doi: 10.1529/biophysj.106.097899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, Emr SD. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell. 2011;144:389–401. doi: 10.1016/j.cell.2010.12.034. [DOI] [PubMed] [Google Scholar]
- Stenzel I, et al. The type B phosphatidylinositol-4-phosphate 5-kinase 3 is essential for root hair formation in Arabidopsis thaliana. Plant Cell. 2008;20:124–141. doi: 10.1105/tpc.107.052852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker A, Baumann U. Supernatant protein factor in complex with RRR-alpha-tocopherylquinone: a link between oxidized vitamin E and cholesterol biosynthesis. J Mol Biol. 2003;332:759–765. doi: 10.1016/s0022-2836(03)00924-0. [DOI] [PubMed] [Google Scholar]
- Strahl T, Thorner J. Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 2007;1771:353–404. doi: 10.1016/j.bbalip.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentpetery Z, et al. Acute manipulation of Golgi phosphoinositides to assess their importance in membrane trafficking and signaling. Proc Natl Acad Sci U S A. 2010;107:8225–8230. doi: 10.1073/pnas.1000157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell. 2007;26:67–86. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- Ueda S, et al. Role of the Sec14-like domain of Db1 family exchange factors in the regulation of Rho family GTPases in different subcellular sites. Cell Signal. 2004;16:826–906. doi: 10.1016/j.cellsig.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Vergés M, et al. The mammalian retromer regulates transcytosis of the polymeric immunoglobulin receptor. Nat Cell Biol. 2006;6:763–769. doi: 10.1038/ncb1153. [DOI] [PubMed] [Google Scholar]
- Vincent P, et al. A Sec14p-nodulin domain phosphatidylinositol transfer protein polarizes membrane growth of Arabidopsis thaliana root hairs. J Cell Biol. 2005;168:801–812. doi: 10.1083/jcb.200412074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Novick P. The yeast phosphatidylinositol-4-OH kinase Pik1 regulates secretion at the Golgi. Nat Cell Biol. 1999;1:523–555. doi: 10.1038/70319. [DOI] [PubMed] [Google Scholar]
- Wang QJ, et al. Differential localization of protein kinase C delta by phorbol esters and related compounds using a fusion protein with green fluorescent protein. J Biol Chem. 1999;274:37233–37239. doi: 10.1074/jbc.274.52.37233. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. Phosphatidylinositol-4-phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114:299–310. doi: 10.1016/s0092-8674(03)00603-2. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. PI4P promotes the recruitment of the GGA adaptor proteins to the trans-Golgi network and regulates their recognition of the ubiquitin sorting signal. Mol Biol Cell. 2007;18:2646–2655. doi: 10.1091/mbc.E06-10-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welti S, et al. The sec14 homology module of neurofibromin binds cellular glycerophospholipids: mass spectrometry and structure of a lipid complex. J Mol Biol. 2007;366:551–562. doi: 10.1016/j.jmb.2006.11.055. [DOI] [PubMed] [Google Scholar]
- Wood CS, et al. PtdIns4P recognition by Vps74/GOLPH3 links PtdIns 4-kinase signaling to retrograde Golgi trafficking. J Cell Biol. 2009;187:967–975. doi: 10.1083/jcb.200909063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WI, et al. A new gene involved in the transport-dependent metabolism of phosphatidylserine, PSTB2/PDR17, shares sequence similarity with the gene encoding the phosphatidylinositol/phosphatidylcholine transfer protein, SEC14. J Biol Chem. 2000;275:14446–14456. doi: 10.1074/jbc.275.19.14446. [DOI] [PubMed] [Google Scholar]
- Xie Z, et al. Phospholipase D activity is required for suppression of yeast phosphatidylinositol transfer protein defects. Proc Natl Acad Sci U S A. 1998;95:12346–12351. doi: 10.1073/pnas.95.21.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa L, et al. Activity of specific lipid-regulated ARFGAPs is required for Sec14p-dependent Golgi secretory function in yeast. Mol Biol Cell. 2002;13:2193–2206. doi: 10.1091/mbc.01-11-0563.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, et al. Interaction of neuronal calcium sensor-1 (NCS-1) with phosphatidylinositol-4-kinase beta stimulates lipid kinase activity and affects membrane trafficking in COS-7 cells. J Biol Chem. 2001;276:40183–40189. doi: 10.1074/jbc.M104048200. [DOI] [PubMed] [Google Scholar]