Abstract
Organophosphorus insecticide (OP) resistance in several Culex species is associated with increased esterase activity resulting from amplification of the corresponding structural gene. In Culex pipiens quinquefasciatus, high levels of OP resistance (approximately 800 times) are due to the esterase B1 gene, which is amplified at least 250-fold. This gene has now been sequenced, and the structure of the amplification unit (amplicon) encompassing the structural gene has been partially characterized. The inferred amino acid sequence of the enzyme revealed regions of strong homology with other eukaryotic serine-esterases, such as cholinesterases, which are the target of OPs. The amplicon covers at least 30 kilobases and contains a constant and highly conserved "core" of 25 kilobases. This core carries a single copy of the esterase gene (2.8 kilobases) as well as other sequences that are present as single or low number copies in the genomes of mosquitoes lacking overproduction of the esterase B1 protein. In the amplicon, the esterase gene is framed by two DNA sequences that are repeated in other parts of the genome of resistant mosquitoes and found in the genome of susceptible mosquitoes but not near the esterase B1 gene. It is suggested that these repetitive sequences may have a role in the amplification process.
Full text
PDF
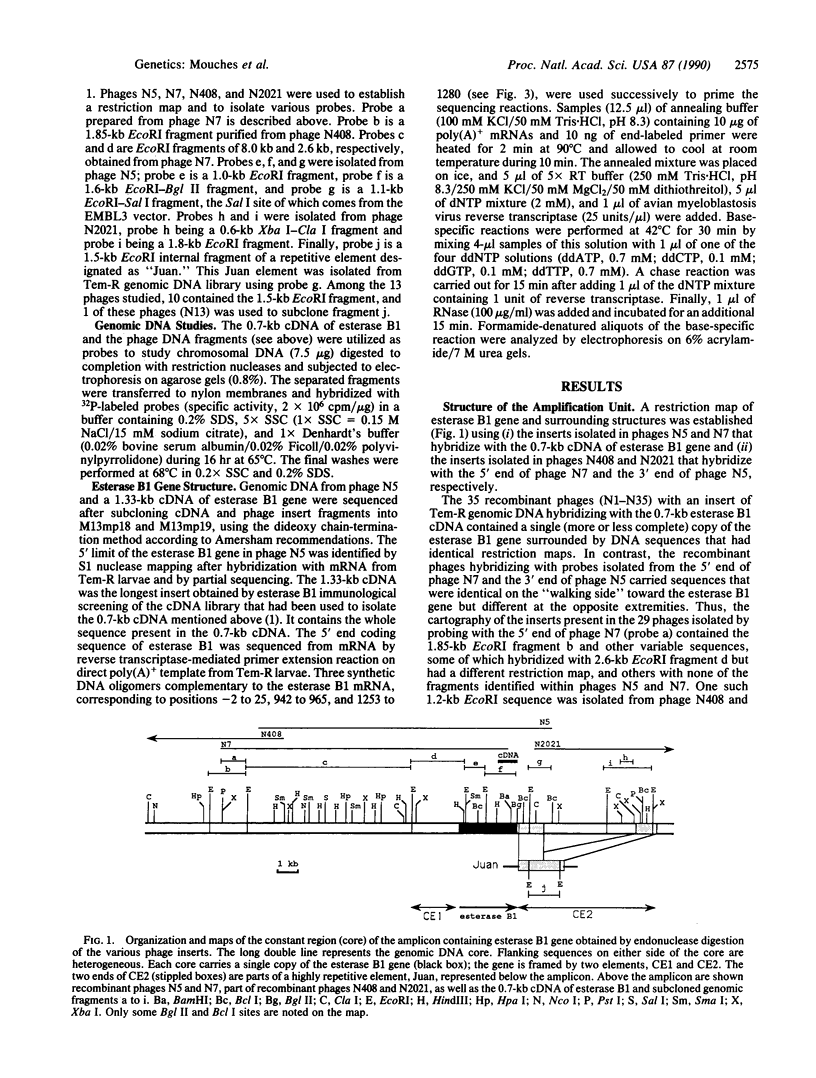

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Donn G., Tischer E., Smith J. A., Goodman H. M. Herbicide-resistant alfalfa cells: an example of gene amplification in plants. J Mol Appl Genet. 1984;2(6):621–635. [PubMed] [Google Scholar]
- Field L. M., Devonshire A. L., Forde B. G. Molecular evidence that insecticide resistance in peach-potato aphids (Myzus persicae Sulz.) results from amplification of an esterase gene. Biochem J. 1988 Apr 1;251(1):309–312. doi: 10.1042/bj2510309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto M., Shevrin D. H., Roninson I. B. Analysis of gene amplification in human tumor cell lines. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6846–6850. doi: 10.1073/pnas.85.18.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey E. P., Santi D. V. Stable amplified DNA in drug-resistant Leishmania exists as extrachromosomal circles. Science. 1986 Aug 1;233(4763):535–540. doi: 10.1126/science.3726545. [DOI] [PubMed] [Google Scholar]
- Hall L. M., Spierer P. The Ace locus of Drosophila melanogaster: structural gene for acetylcholinesterase with an unusual 5' leader. EMBO J. 1986 Nov;5(11):2949–2954. doi: 10.1002/j.1460-2075.1986.tb04591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockridge O., Adkins S., La Du B. N. Location of disulfide bonds within the sequence of human serum cholinesterase. J Biol Chem. 1987 Sep 25;262(27):12945–12952. [PubMed] [Google Scholar]
- Mercken L., Simons M. J., Swillens S., Massaer M., Vassart G. Primary structure of bovine thyroglobulin deduced from the sequence of its 8,431-base complementary DNA. Nature. 1985 Aug 15;316(6029):647–651. doi: 10.1038/316647a0. [DOI] [PubMed] [Google Scholar]
- Mouchès C., Magnin M., Bergé J. B., de Silvestri M., Beyssat V., Pasteur N., Georghiou G. P. Overproduction of detoxifying esterases in organophosphate-resistant Culex mosquitoes and their presence in other insects. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2113–2116. doi: 10.1073/pnas.84.8.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchès C., Pasteur N., Bergé J. B., Hyrien O., Raymond M., de Saint Vincent B. R., de Silvestri M., Georghiou G. P. Amplification of an esterase gene is responsible for insecticide resistance in a California Culex mosquito. Science. 1986 Aug 15;233(4765):778–780. doi: 10.1126/science.3755546. [DOI] [PubMed] [Google Scholar]
- Myers M., Richmond R. C., Oakeshott J. G. On the origins of esterases. Mol Biol Evol. 1988 Mar;5(2):113–119. doi: 10.1093/oxfordjournals.molbev.a040485. [DOI] [PubMed] [Google Scholar]
- Oakeshott J. G., Collet C., Phillis R. W., Nielsen K. M., Russell R. J., Chambers G. K., Ross V., Richmond R. C. Molecular cloning and characterization of esterase-6, a serine hydrolase of Drosophila. Proc Natl Acad Sci U S A. 1987 May;84(10):3359–3363. doi: 10.1073/pnas.84.10.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M., Beyssat-Arnaouty V., Sivasubramanian N., Mouchès C., Georghiou G. P., Pasteur N. Amplification of various esterase B's responsible for organophosphate resistance in Culex mosquitoes. Biochem Genet. 1989 Aug;27(7-8):417–423. doi: 10.1007/BF02399670. [DOI] [PubMed] [Google Scholar]
- Risler J. L., Delorme M. O., Delacroix H., Henaut A. Amino acid substitutions in structurally related proteins. A pattern recognition approach. Determination of a new and efficient scoring matrix. J Mol Biol. 1988 Dec 20;204(4):1019–1029. doi: 10.1016/0022-2836(88)90058-7. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Camp S., Maulet Y., Newton M., MacPhee-Quigley K., Taylor S. S., Friedmann T., Taylor P. Primary structure of Torpedo californica acetylcholinesterase deduced from its cDNA sequence. 1986 Jan 30-Feb 5Nature. 319(6052):407–409. doi: 10.1038/319407a0. [DOI] [PubMed] [Google Scholar]