Significance
Light-emitting materials have been widely studied for their promising applications in chemical and biological science. Here we report three phenanthrene-21-crown-7 functionalized fluorescent Pt(II) rhomboidal metallacycles which were then converted into fluorescent supramolecular oligomers by reacting with a bis-ammonium linker. One of these constructs shows concentration-dependent fluorescence in a wide color range, where orange emission at high concentration and blue emission at low concentration was observed. Moreover, at a concentration of 29 µM the same ensemble emits white light that has emerged from the integration of the complementary orange and blue color from the metallacycles and linker, respectively. This study shows how light-emitting materials can be obtained by the proper implementation of multiple orthogonal interactions in a single process.
Keywords: fluorescence, supramolecular metallacycles, host–guest interactions, white-light emission, orthogonal interactions
Abstract
Control over the fluorescence of supramolecular assemblies is crucial for the development of chemosensors and light-emitting materials. Consequently, the postsynthetic modification of supramolecular structures via host–guest interactions has emerged as an efficient strategy in recent years that allows the facile tuning of the photophysical properties without requiring a tedious chemical synthesis. Herein, we used a phenanthrene-21-crown-7 (P21C7)-based 60° diplatinum(II) acceptor 8 in the construction of three exohedral P21C7 functionalized rhomboidal metallacycles 1–3 which display orange, cyan, and green emission colors, respectively. Although these colors originate from the dipyridyl precursors 10–12, containing triphenylamine-, tetraphenylethene-, and pyrene-based fluorophores, respectively, the metal–ligand coordination strongly influences their emission properties. The metallacycles were further linked into emissive supramolecular oligomers by the addition of a fluorescent bis-ammonium linker 4 that forms complementary host–guest interactions with the pendant P21C7 units. Notably, the final ensemble derived from a 1:1 mixture of 1 and 4 displays a concentration-dependent emission. At low concentration, i.e., <25 µM, it emits a blue color, whereas an orange emission was observed when the concentration exceeds >5 mM. Moreover, white-light emission was observed from the same sample at a concentration of 29 µM, representing a pathway to construct supramolecular assemblies with tunable fluorescence properties.
Fluorescent supramolecular assemblies have received much attention due to their broad applications as biological and chemical sensors (1–5), bioimaging agents (6–10), light-emitting materials (11–15), etc. One method used to prepare such assemblies is coordination-driven self-assembly (16–29), because the incorporation of metal coordination not only endows the resultant assemblies with good stabilities but also at times provides unique emissions that are different from the fluorescent ligands and/or metal ions. By the incorporation of tetraphenylene derivatives into supramolecular coordination complexes (SCC) platforms, our group has prepared a series of highly emissive metal–organic assemblies that are capable of sensing nitroaromatics, cell imaging, etc. (30–34), although the precursors are weakly- or nonemissive. Similarly, Yoshizawa and coworkers prepared a series of M2L4 (M = Zn, Cu, Pt, Ni, Co, and Mn) capsules among which only the Zn(II) capsule emits blue fluorescence with a quantum yield of 80%, whereas the other capsules are nonemissive (35).
Fluorophores with aggregation-induced emission (AIE) (36–39) properties are good candidates for the construction of fluorescent supramolecular polymers because both the formation of supramolecular polymers and the accomplishment of a reasonable emission from the AIE cores need high concentrations. However, supramolecular oligomers/polymers with tunable emission have been rarely reported, although some progress has recently been made on the construction of discrete fluorescent supramolecular assemblies (29). The introduction of additional fluorophores via noncovalent interactions provides an alternative approach to further tune the emission of a given SCC (40, 41), leading to the formation of fluorescent supramolecular polymers. Herein we report three emissive metallacycles with pendant crown ether units by the incorporation of three different fluorescent dipyridyl ligands. Upon the addition of a fluorescent bis-ammonium linker, supramolecular oligomers are formed and the emission properties of these metallacycles were further tuned. Moreover, using the orange-emissive metallacycle 1 with AIE properties and the blue-emissive linker 4 with aggregation-caused quenching (ACQ) properties (39), the emission of the resultant supramolecular oligomers can be tuned from orange to blue as the concentration decreases. Interestingly, the same sample emits white-light emission at a concentration of 29 µM. This is an example where white-light emission was achieved by the integration of complementary emission properties (AIE and ACQ), complementary colors (orange and blue), and complementary host–guest interactions (crown ether and ammonium salts).
Results and Discussion
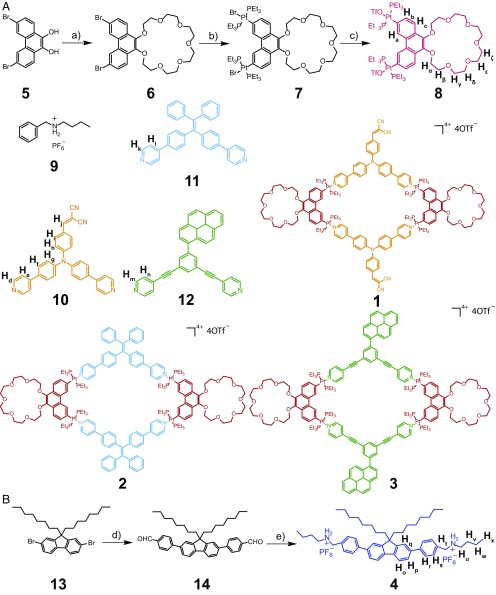
The synthetic procedures for rhomboidal metallacycles 1, 2, and 3 and a bis-ammonium linker 4 are shown in Fig. 1. A phenanthrene-21-crown-7 (P21C7)-based 60° diplatinum(II) acceptor 8 was synthesized in a three-step pathway starting from 3,6-dibromophenanthrene-9,10-diol 5 (Fig. 1A). The key intermediate 3,6-dibromophenanthrene-21-crown-7 6 was prepared by a potassium-templated cyclization reaction of 5 and hexethylene glycol ditosylate. The oxidative addition of 6 to Pt(PEt3)4 at each bromide site furnished 7, whose bromide anions were then exchanged by treating with AgOTf, leading to the formation of 8 in a good yield. The rhomboidal Pt(II) metallacycles 1, 2, and 3 were prepared by stirring the 60° platinum acceptor 8 and the corresponding 120° dipyridyl donor 10, 11, or 12, respectively, in dichloromethane at room temperature for 24 h. The bis-ammonium linker 4 was prepared from commercially available 2,7-dibromo-9,9-dioctyl-9H-fluorene 13 (Fig. 1B). Reaction of 13 with (4-formylphenyl)boronic acid via a Suzuki coupling provided compound 14 with two aldehyde groups, which were further reacted to afford bis-ammonium salt 4.
Fig. 1.
Synthetic routes and chemical structures of compounds. Synthetic routes of 60° diplatinum (II) acceptor 8 (A) and bis-ammonium linker 4 (B). Conditions: a) hexethylene glycol ditosylate, K2CO3, CH3CN, reflux, 72 h; 64%; b) Pt(PEt3)4, toluene, 95 °C, 72 h; 68%; c) AgOTf, dry CH2Cl2, room temperature, 12 h; 95%; d) (4-formylphenyl)boronic acid, K2CO3, Pd(PPh3)4, dioxane/water (2:1), 90 °C, 48 h; 63%; e) (i) n-butylamine, CH3OH, reflux, 12h; (ii) NaBH4, room temperature, 24 h; (iii) HCl (aq), and (iv) NH4PF6 (aq); 27% in four steps.
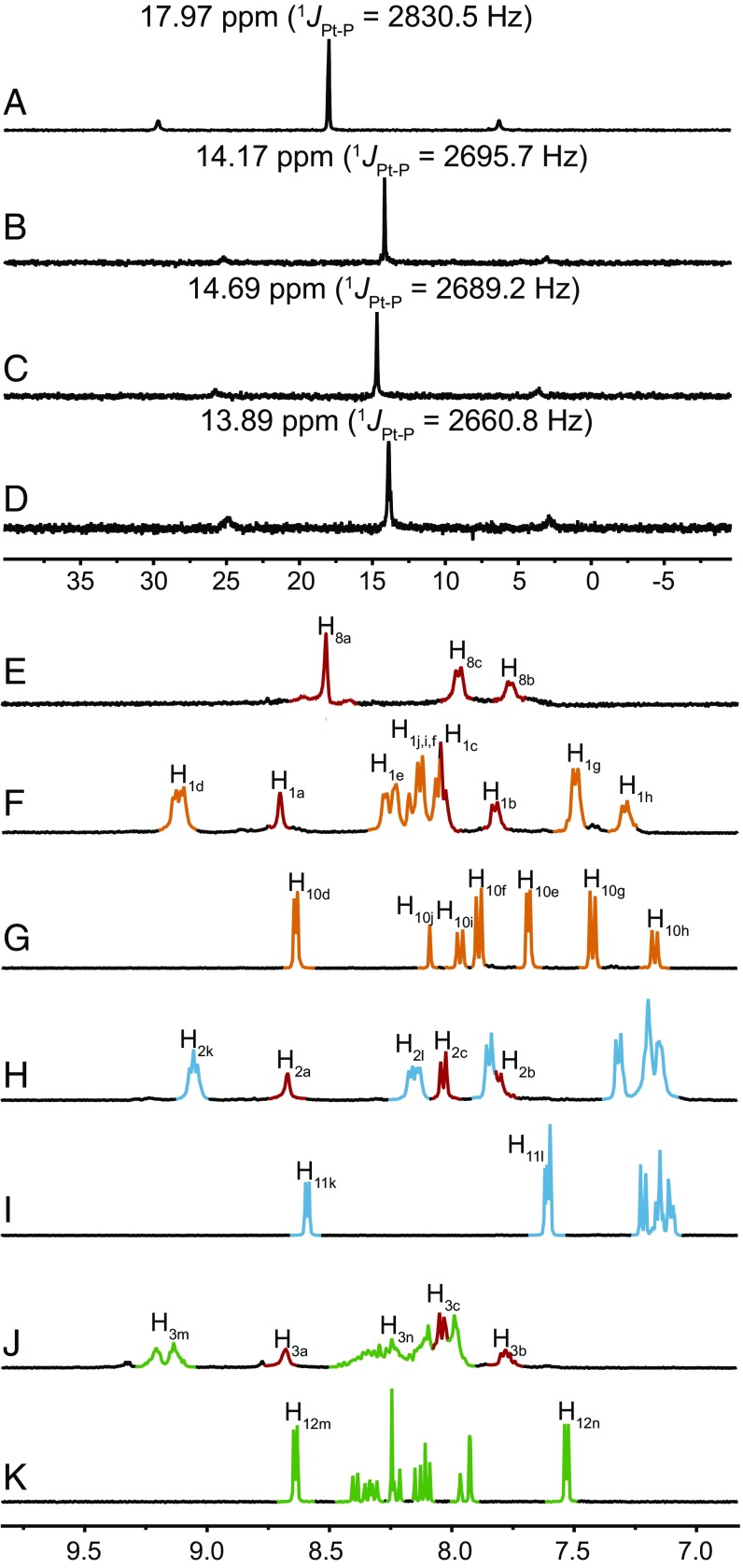
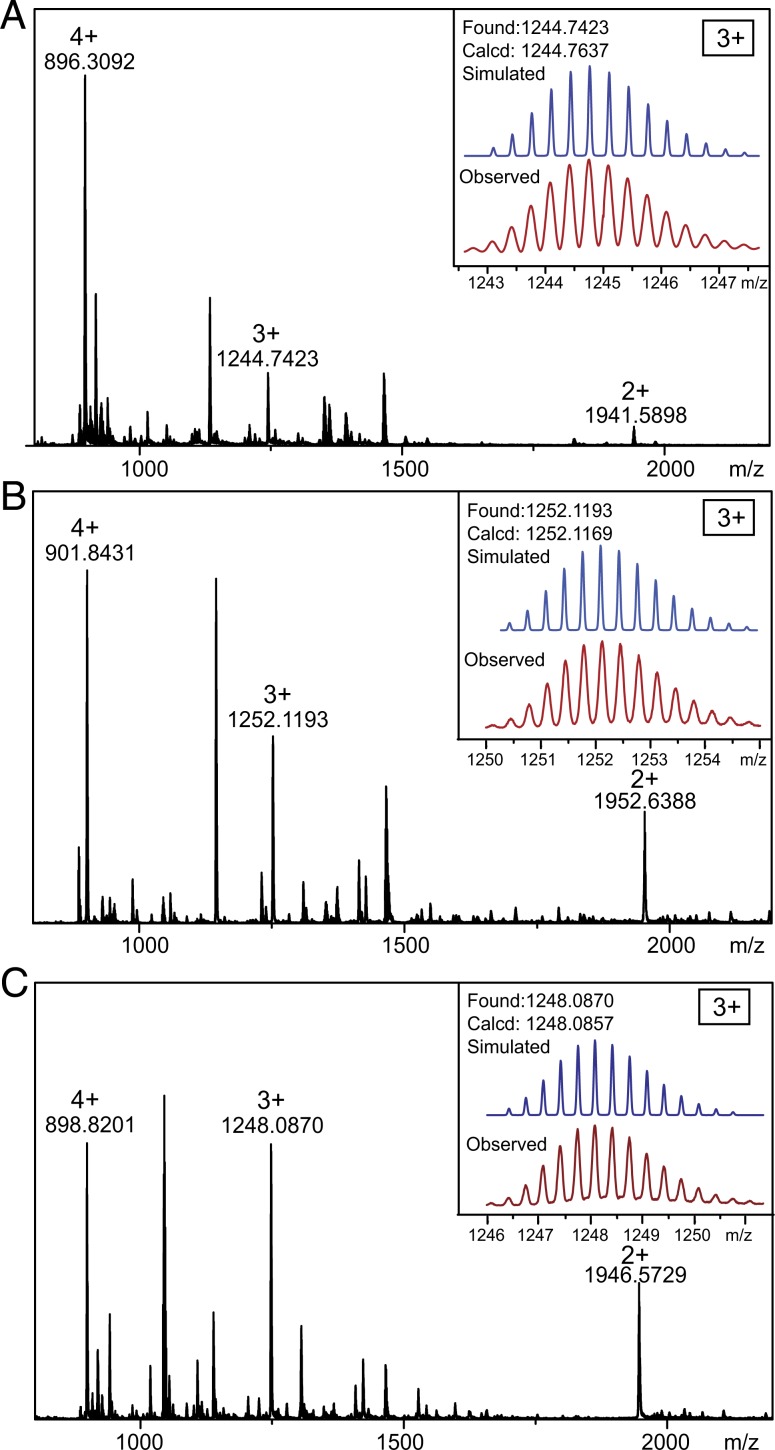
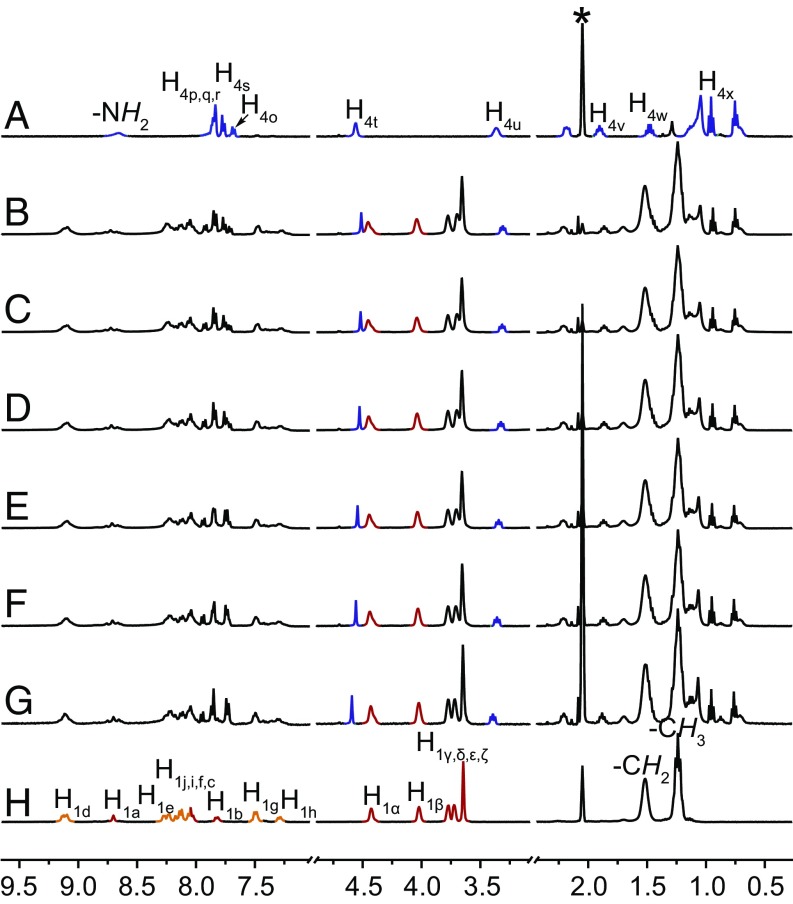
The formation of metallacycles 1, 2, and 3 was confirmed by multinuclear NMR (31P and 1H) analysis and electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS). The 31P{1H} NMR spectra of 1, 2, and 3 exhibit sharp singlets with concomitant 195Pt satellites at 14.17 ppm for 1, 14.69 ppm for 2, and 13.89 ppm for 3 (Fig. 2, spectra B–D), corresponding to a single phosphorous environment, indicating the formation of discrete, highly symmetric metallacycles (31–34). In the 1H NMR spectra of these metallacycles, downfield chemical shifts were observed for the α-pyridyl protons Hd (from 8.64 to 9.13 and 9.10 ppm), Hk (from 8.59 to 9.07 and 9.05 ppm), and Hm (from 8.64 to 9.22 and 9.15 ppm) and β-pyridyl protons He (from 7.69 to 8.24 and 8.15 ppm), Hl (from 7.61 to 8.17 and 8.15 ppm), and Hn (from 7.53 to 8.31 and 8.26 ppm) and both split into two set of signals (Fig. 2, spectra F–K). The aromatic protons of Ha, Hb, and Hc of platinum(II) acceptor also shifted downfield. All these chemical shift changes are similar to their analogous reaction systems (31–34), providing evidence for the formation of rhomboidal metallacycles. ESI-TOF-MS provided evidence for the stoichiometry of formation of discrete rhomboidal Pt(II) metallacycles. Prominent sets of peaks with charge states (from 2+ to 4+) were observed for all of the metallacycles due to the loss of counterions (OTf−), and each peak closely matched the corresponding simulated isotope pattern (Fig. 3), supporting the composition of 1–3.
Fig. 2.
NMR characterization ligands 8, 10, 11, and 12 and metallacycles 1, 2, and 3. Partial (A−D) 31P and (E−K) 1H NMR spectra (CD3COCD3, 295 K) of platinum acceptor 8 (A and E), ligands 10 (G), 11 (I), and 12 (K), and metallacycles 1 (B and F), 2 (C and H), and 3 (D and J).
Fig. 3.
Mass spectra of metallacycles 1 (A), 2 (B), and 3 (C).
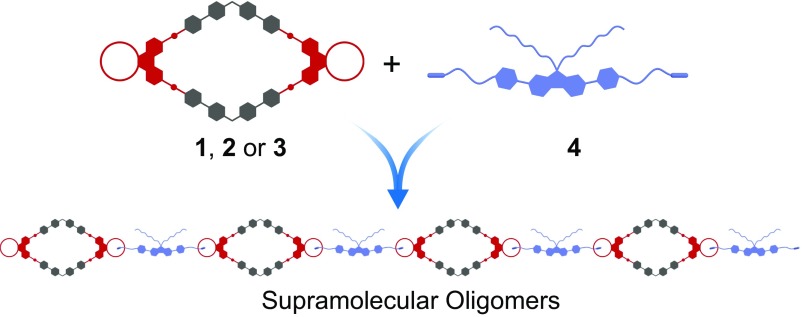
The complexation between crown ether 8 and secondary ammonium salt 9 was first investigated as a model system (SI Appendix, Figs. S34–S36). The association constant (Ka) of 8·9 in acetone was determined by a 1H NMR titration method, with a value of 9.4 (±1.0) × 102 M−1, which is comparable to that of benzo-21-crown-7·9 (6.2 (±0.4) × 102 M−1) (42) and suitable for the construction of supramolecular assemblies (Fig. 4).
Fig. 4.
Cartoon representation of the formation of supramolecular oligomers.
Concentration-dependent 1H NMR measurements were then carried out to study the formation of the supramolecular oligomers. Therefore, a 1:1 mixture of 1 and 4 was chosen as the onset. The concentration of the sample was increased from 1.0 mM to 40.0 mM, leading to noticeable chemical shift changes for both precursors. Upfield chemical shifts were observed for the benzyl protons Ht and methylene protons Hu of the bis-ammonium linker 4, whereas the ethyl protons Hα and Hβ of the metallacycle 1 shifted downfield (Fig. 5). Moreover, the chemical shifts of the linker 4 in the latter ensemble appeared in a similar region to that of 9 in the complex 8·9, indicating the formation of supramolecular oligomers via host–guest complexation. Two-dimensional diffusion-ordered NMR experiments were also carried out to test the size of the supramolecular oligomers in solution. As the concentration of the sample increased from 5.00 to 40.0 mM, the measured weight average diffusion coefficient (D) decreased from 3.19 × 10−10 to 1.16 × 10−10 m2⋅s−1 (SI Appendix, Fig. S37), indicating the formation of high molecular weight supramolecular assemblies (43–46). To investigate the effect of the counterions in the assembly process, the counterions of 4 were also changed from hexafluorophosphate (PF6) to trifluoromethanesulfonate (OTf) anions and the 1H NMR spectrum of a mixture of 1 and 4•OTf at 5 mM was measured (SI Appendix, Fig. S38). However, this shows negligible effects compared with the 1H NMR spectrum of a mixture containing 1 and 4•PF6 salt, suggesting that the counterions do not influence these assembly processes.
Fig. 5.
Partial 1H NMR spectra (CD3COCD3, 295 K, 400 MHz) of bis-ammonium linker 4 (A), and equal molar 4 and 1 at the concentration of 40 mM (B), 30 mM (C), 20 mM (D), 10 mM (E), 5.0 mM (F), 1.0 mM (G), and metallacycle 1 (H). The acetone peaks are marked with asterisks.
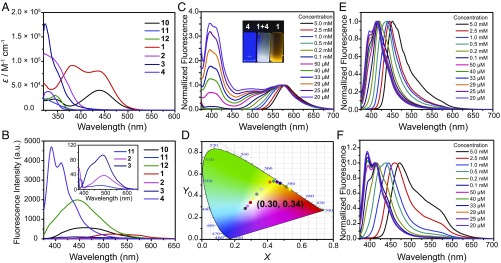
The UV-vis absorption and emission spectra of ligands 10, 11, and 12, rhomboidal metallacycles 1, 2, and 3 and bis-ammonium linker 4 in acetone are shown in Fig. 6. Ligand 10 displays two broad absorption bands centered at 346 and 440 nm with molar absorption coefficients (ε) of 2.28 × 104 M−1⋅cm−1 and 4.09 × 104 M−1⋅cm−1, respectively (Fig. 6, spectrum A). Ligand 11 shows one broad absorption band in the range of 320–370 nm, whereas ligand 12 exhibits one broad absorption band centered at 344 nm with ε = 3.03 × 104 M−1⋅cm−1. Metallacycle 1 exhibits two absorption bands centered at 380 and 436 nm with ε = 9.13 × 104 M−1⋅cm−1 and 8.04 × 104 M−1⋅cm−1, respectively (Fig. 6, spectrum A). There is one absorption band centered at 322 nm for 2, 323 nm for 3, and 331 nm for 4 with ε = 1.16 × 105 M−1⋅cm−1, 1.77 × 105 M−1⋅cm−1, and 3.85 × 104 M−1⋅cm−1, respectively. It is worth noting that the absorption of metallacycles 1, 2, and 3 is greatly increased compared with their precursors 10, 11, and 12 due to the inclusion of multiple ligands in one metallacycle structure.
Fig. 6.
Spectral characterization of ligands 10, 11, 12, metallacycles 1, 2, 3, and bis-ammonium linker 4. (A) UV-vis absorption spectra of ligands 10, 11, 12, metallacycles 1, 2, 3, and bis-ammonium linker 4 in acetone (c = 10 µM). (B) Emission spectra of ligands 10, 11, 12, metallacycles 1, 2, 3, and bis-ammonium linker 4 in acetone (λex = 365 nm, c = 10 µM). (C) Emission spectra of equal molar 1 and 4 at different concentrations; (Inset) photograph of 1, 4, and mixture of equal molar 1 and 4 in acetone upon excitation at 365 nm using a UV lamp at 298 K (c = 29 µM). (D) CIE chromaticity coordinates of equal molar 1 and 4 at different concentrations, according to the fluorescence spectra recorded in C. (E) Emission spectra of equal molar 2 and 4 at different concentrations. (F) Emission spectra of equal molar 3 and 4 at different concentrations.
Ligands 10, 11, and 12 display moderate emission bands centered at 465, 472, and 445 nm in acetone, respectively (Fig. 6, spectrum B). Upon the formation of rhomboidal metallacycles, red-shifts were observed for 1 and 2. Specifically, rhomboids 1, 2, and 3 exhibit one emission band centered at 563, 490, and 445 nm, respectively, corresponding to the orange, cyan, and green emission according to the 1931 Commission Internationale de L’Eclairage (CIE) chromaticity diagram. Bis-ammonium linker 4 shows two emission bands centered at 391 and 411 nm, corresponding to the blue emission. Compared with their dipyridyl precursors 10, 11, and 12, the emissions of metallacycles 1 and 3 decrease whereas that of metallacycle 2 increases. The decrease of the emission is due to heavy atom effect which was also observed in other SCC systems (29). Although this effect also influences the emission of 2, the metal coordination inhibits the free rotation of the aromatic rings of the tetraphenylene derivatives, which enhances the emission of 2 relative to 11 (31–34).
The emission properties of the supramolecular assemblies formed by metallacycles (1, 2, or 3) and bis-ammonium 4 were investigated (Fig. 6, spectrum C). Interestingly, the supramolecular assemblies formed by 1 and 4 (1:1 molar ratio) show concentration-dependent fluorescence in a wide color range, where an orange emission at high concentration (>0.5 mM) and a blue emission at low concentration (<25 µM) were observed. This is likely because in the ensemble the metallacycle 1 acts as an AIE fluorophore, whereas the bis-ammonium linker 4 is an ACQ fluorophore (SI Appendix, Figs. S39 and S40). At high concentration, the AIE fluorophore plays a more important role so the sample mainly shows orange emission derived from 1. However, at low concentration, the ACQ fluorophore dominates the fluorescence, thus the ensemble exhibits a blue emission derived from 4. Moreover, the same sample displays white-light emission (CIE chromaticity coordinate: 0.30, 0.34) at a concentration of 29 µM (Fig. 6, spectrum D), which has only been rarely found in such supramolecular assemblies. At 29 µM, both the orange emission and the blue emission are observed and the emission covers the entire visible spectral region (400∼700 nm), endowing the solution with an overall white-light emission. Tunable emission was also observed in the assemblies formed by 2 and 4 or 3 and 4 (Fig. 6, spectra E and F). However, in these two assemblies, the color of the emission cannot be tuned in a wide range because of the strong overlap between the emission of 2 (or 3) and 4. This study provides a simple yet efficient approach for finely tuning the emission of supramolecular assemblies via concentration by the incorporation of both AIE and ACQ fluorophores as their building blocks.
Conclusion
In summary, three Pt(II) rhomboidal metallacycles with orange (1), cyan (2), and green (3) emissions were prepared by the metal-coordination-driven self-assembly of the 60° diplatinum(II) acceptor 8 and 120° dipyridyl donors 10–12. Supramolecular oligomers were further obtained via the host–guest interactions between the P21C7 units of the metallacyles and ammonium salts. The emissions of these supramolecular assemblies as well as their precursors were studied. These supramolecular oligomers show concentration-dependent fluorescence. Notably, the assemblies formed by orange-emissive metallacycle 1 and blue-emissive bis-ammonium linker 4 emit from orange to blue as the concentration decreases, whereas white-light emission was obtained at the concentration of 29 µM. This study provides a strategy to prepare light-emitting metal–organic assemblies by the precise manipulation of the AIE and ACQ properties (39) of the fluorophores.
Materials and Methods
All reagents and deuterated solvents were commercially available and used without further purification. Hexa(ethylene glycol) ditosylate (42), Pt(PEt3)4 (47), 5 (48), 9 (49), 10 (31), and 11 (50) were prepared according to the literature procedures. NMR spectra were recorded on a Varian Unity 300- or 400-MHz spectrometer. 1H and 13C NMR chemical shifts are reported relative to residual solvent signals, and 31P{1H} NMR chemical shifts are referenced to an external unlocked sample of 85% H3PO4 (δ 0.0 ppm). Mass spectra were recorded on a Micromass Quattro II triple-quadrupole mass spectrometer using electrospray ionization with a MassLynx operating system. The melting points were collected on an SHPSIC WRS-2 automatic melting point apparatus. The UV-vis experiments were conducted on a Hitachi U-4100 absorption spectrophotometer. The fluorescent experiments were conducted on a Hitachi F-7000 fluorescence spectrophotometer. Quantum yields were determined using quinine sulfate at 365 nm as reference (ΦF = 56%).
Metallacycles 1, 2, and 3 were synthesized by stirring 8 with 10, 11, or 12 in a 1:1 molar ratio at room temperature for 12 h. Then diethyl ether was added to the homogeneous solution to give the corresponding metallacycles as precipitates. Bis-ammonium salt 4 was synthesized by the reaction of compound 14 with n-butylamine, followed by reduction, protonation, and ion exchange. The supramolecular assemblies were prepared by mixing metallacycles (1, 2, or 3) with 4 in a 1:1 molar ratio in acetone at room temperature.
Metallacycle 1: 1H NMR (400 MHz, CD3COCD3, 295 K): 9.11 (dd, J1 = 12.1 Hz, J2 = 5.4 Hz, 8H), 8.70 (s, 4H), 8.18 (d, J = 5.6 Hz, 8H), 8.01–8.23 (m, 18H), 7.82 (d, J = 8.5 Hz, 4H), 7.49 (d, J = 7.8 Hz, 8H), 7.29 (d, J = 7.8 Hz, 4H), 4.37–4.48 (m, 8H), 3.97–4.07 (m, 8H), 3.74–3.83 (m, 8H), 3.69–3.74 (m, 8H), 3.56–3.68 (m, 16H), 1.41–1.66 (m, 48H), 1.15–1.34 (m, 72H). 31P{1H} NMR (121.4 MHz, CD3COCD3, 295 K): 14.17 ppm (s, 195Pt satellites, 1JPt–P = 2,674.2 Hz). ESI-TOF-MS: m/z 896.3092 [1 – 4OTf]4+, 1244.7423 [1 – 3OTf]3+, 1,941.5898 [1 – 2OTf]2+.
Metallacycle 2: 1H NMR (400 MHz, CD3COCD3, 295 K): 9.06 (t, J = 6.2 Hz, 8H), 8.68 (s, 4H), 8.15 (dd, J1 = 16.7 Hz, J2 = 5.3 Hz, 8H), 8.04 (d, J = 8.4 Hz, 4H), 7.86 (d, J = 8.2 Hz, 8H), 7.82 (d, J = 8.6 Hz, 4H), 7.33 (d, J = 8.2 Hz, 8H), 7.10–7.29 (m, 20H), 4.37–4.52 (m, 8H), 3.98–4.08 (m, 8H), 3.75–3.82 (m, 8H), 3.68–3.75 (m, 8H), 3.61–3.68 (m, 16H), 1.41–1.62 (m, 48H), 1.14–1.30 (m, 72H). 31P{1H} NMR (121.4 MHz, CD3COCD3, 295 K): 14.69 ppm (s, 195Pt satellites, 1JPt–P = 2,784.6 Hz). ESI-TOF-MS: m/z 901.3514 [2 – 4OTf]4+, 1,252.1193 [2 – 3OTf]3+, 1,952.6388 [2 – 2OTf]2+.
Metallacycle 3: 1H NMR (400 MHz, CD3COCD3, 295 K): 9.09–9.30 (m, 8H), 8.69 (s, 4H), 8.21–8.47 (m, 12H), 7.94–8.18 (m, 24H), 7.79 (d, J = 7.0 Hz, 4H), 4.35–4.50 (m, 8H), 3.97–4.07 (m, 8H), 3.74–3.82 (m, 8H), 3.67–3.74 (m, 8H), 3.57–3.67 (m, 16H), 1.36–1.61 (m, 48H), 1.07–1.31 (m, 72H). 31P{1H} NMR (121.4 MHz, CD3COCD3, 295 K): 13.89 ppm (s, 195Pt satellites, 1JPt–P = 2655.5 Hz). ESI-TOF-MS: m/z 898.3780 [3 – 4OTf]4+, 1,247.4213 [3 – 3OTf]3+, 1,945.6079 [3 – 2OTf]2+.
Supplementary Material
Acknowledgments
P.J.S. thanks National Science Foundation (Grant 1212799) for financial support. Y.S. thanks National Natural Science Foundation of China (Grants 21574034 and 21274034) and Zhejiang Provincial Natural Science Foundation of China (Grant LY16B040006) for financial support.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702510114/-/DCSupplemental.
References
- 1.Dsouza RN, Pischel U, Nau WM. Fluorescent dyes and their supramolecular host/guest complexes with macrocycles in aqueous solution. Chem Rev. 2011;111(12):7941–7980. doi: 10.1021/cr200213s. [DOI] [PubMed] [Google Scholar]
- 2.Lee MH, Kim JS, Sessler JL. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem Soc Rev. 2015;44(13):4185–4191. doi: 10.1039/c4cs00280f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daly B, Ling J, de Silva AP. Current developments in fluorescent PET (photoinduced electron transfer) sensors and switches. Chem Soc Rev. 2015;44(13):4203–4211. doi: 10.1039/c4cs00334a. [DOI] [PubMed] [Google Scholar]
- 4.You L, Zha D, Anslyn EV. Recent advances in supramolecular analytical chemistry using optical sensing. Chem Rev. 2015;115(15):7840–7892. doi: 10.1021/cr5005524. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Yim D, Jang WD, Yoon J. Recent progress in the design and applications of fluorescence probes containing crown ethers. Chem Soc Rev. 2016 doi: 10.1039/C6CS00619A. [DOI] [PubMed] [Google Scholar]
- 6.Schultz-Sikma EA, Meade TJ. Supramolecular chemistry in biological imaging in vivo. In: Gale PA, Steed JW, editors. Supramolecular Chemistry: From Molecules to Nanomaterials. John Wiley & Sons; Chichester, UK: 2012. pp. 1851–1876. [Google Scholar]
- 7.Peng H-Q, et al. Biological applications of supramolecular assemblies designed for excitation energy transfer. Chem Rev. 2015;115(15):7502–7542. doi: 10.1021/cr5007057. [DOI] [PubMed] [Google Scholar]
- 8.Ma X, Zhao Y. Biomedical applications of supramolecular systems based on host-guest interactions. Chem Rev. 2015;115(15):7794–7839. doi: 10.1021/cr500392w. [DOI] [PubMed] [Google Scholar]
- 9.Webber MJ, Appel EA, Meijer EW, Langer R. Supramolecular biomaterials. Nat Mater. 2016;15(1):13–26. doi: 10.1038/nmat4474. [DOI] [PubMed] [Google Scholar]
- 10.Shi B, et al. Nanoparticles with near-infrared emission enhanced by pillararene-based molecular recognition in water. J Am Chem Soc. 2016;138(1):80–83. doi: 10.1021/jacs.5b11676. [DOI] [PubMed] [Google Scholar]
- 11.Ajayaghosh A, Praveen VK, Vijayakumar C, George SJ. Molecular wire encapsulated into pi organogels: Efficient supramolecular light-harvesting antennae with color-tunable emission. Angew Chem Int Ed Engl. 2007;46(33):6260–6265. doi: 10.1002/anie.200701925. [DOI] [PubMed] [Google Scholar]
- 12.Abbel R, et al. White-light emitting hydrogen-bonded supramolecular copolymers based on pi-conjugated oligomers. J Am Chem Soc. 2009;131(2):833–843. doi: 10.1021/ja807996y. [DOI] [PubMed] [Google Scholar]
- 13.Chen P, Li Q, Grindy S, Holten-Andersen N. White-light-emitting lanthanide metallogels with tunable luminescence and reversible stimuli-responsive properties. J Am Chem Soc. 2015;137(36):11590–11593. doi: 10.1021/jacs.5b07394. [DOI] [PubMed] [Google Scholar]
- 14.Ni XL, Chen S, Yang Y, Tao Z. Facile cucurbit[8]uril-based supramolecular approach to fabricate tunable luminescent materials in aqueous solution. J Am Chem Soc. 2016;138(19):6177–6183. doi: 10.1021/jacs.6b01223. [DOI] [PubMed] [Google Scholar]
- 15.Zhang QW, et al. Multicolor photoluminescence including white-light emission by a single host-guest complex. J Am Chem Soc. 2016;138(41):13541–13550. doi: 10.1021/jacs.6b04776. [DOI] [PubMed] [Google Scholar]
- 16.Stang PJ, Olenyuk B. Self-assembly, symmetry, and molecular architecture: Coordination as the motif in the rational design of supramolecular metallacyclic polygons and polyhedra. Acc Chem Res. 1997;30(12):502–518. [Google Scholar]
- 17.Leininger S, Olenyuk B, Stang PJ. Self-assembly of discrete cyclic nanostructures mediated by transition metals. Chem Rev. 2000;100(3):853–908. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]
- 18.Fujita M, Tominaga M, Hori A, Therrien B. Coordination assemblies from a Pd(II)-cornered square complex. Acc Chem Res. 2005;38(4):369–378. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]
- 19.Oliveri CG, Ulmann PA, Wiester MJ, Mirkin CA. Heteroligated supramolecular coordination complexes formed via the halide-induced ligand rearrangement reaction. Acc Chem Res. 2008;41(12):1618–1629. doi: 10.1021/ar800025w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Northrop BH, Zheng Y-R, Chi K-W, Stang PJ. Self-organization in coordination-driven self-assembly. Acc Chem Res. 2009;42(10):1554–1563. doi: 10.1021/ar900077c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De S, Mahata K, Schmittel M. Metal-coordination-driven dynamic heteroleptic architectures. Chem Soc Rev. 2010;39(5):1555–1575. doi: 10.1039/b922293f. [DOI] [PubMed] [Google Scholar]
- 22.Chakrabarty R, Mukherjee PS, Stang PJ. Supramolecular coordination: Self-assembly of finite two- and three-dimensional ensembles. Chem Rev. 2011;111(11):6810–6918. doi: 10.1021/cr200077m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook TR, Zheng Y-R, Stang PJ. Metal-organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis, and functionality of metal-organic materials. Chem Rev. 2013;113(1):734–777. doi: 10.1021/cr3002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown CJ, Toste FD, Bergman RG, Raymond KN. Supramolecular catalysis in metal-ligand cluster hosts. Chem Rev. 2015;115(9):3012–3035. doi: 10.1021/cr4001226. [DOI] [PubMed] [Google Scholar]
- 25.Cook TR, Stang PJ. Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem Rev. 2015;115(15):7001–7045. doi: 10.1021/cr5005666. [DOI] [PubMed] [Google Scholar]
- 26.Newkome GR, Moorefield CN. From 1 → 3 dendritic designs to fractal supramacromolecular constructs: Understanding the pathway to the Sierpiński gasket. Chem Soc Rev. 2015;44(12):3954–3967. doi: 10.1039/c4cs00234b. [DOI] [PubMed] [Google Scholar]
- 27.McConnell AJ, Wood CS, Neelakandan PP, Nitschke JR. Stimuli-responsive metal-ligand assemblies. Chem Rev. 2015;115(15):7729–7793. doi: 10.1021/cr500632f. [DOI] [PubMed] [Google Scholar]
- 28.Lifschitz AM, Rosen MS, McGuirk CM, Mirkin CA. Allosteric supramolecular coordination constructs. J Am Chem Soc. 2015;137(23):7252–7261. doi: 10.1021/jacs.5b01054. [DOI] [PubMed] [Google Scholar]
- 29.Saha ML, Yan X, Stang PJ. Photophysical properties of organoplatinum(II) compounds and derived self-assembled metallacycles and metallacages: Fluorescence and its applications. Acc Chem Res. 2016;49(11):2527–2539. doi: 10.1021/acs.accounts.6b00416. [DOI] [PubMed] [Google Scholar]
- 30.Yan X, Cook TR, Wang P, Huang F, Stang PJ. Highly emissive platinum(II) metallacages. Nat Chem. 2015;7(4):342–348. doi: 10.1038/nchem.2201. [DOI] [PubMed] [Google Scholar]
- 31.Yan X, et al. A suite of tetraphenylethylene-based discrete organoplatinum(II) metallacycles: Controllable structure and stoichiometry, aggregation-induced emission, and nitroaromatics sensing. J Am Chem Soc. 2015;137(48):15276–15286. doi: 10.1021/jacs.5b10130. [DOI] [PubMed] [Google Scholar]
- 32.Yan X, et al. Light-emitting superstructures with anion effect: Coordination-driven self-assembly of pure tetraphenylethylene metallacycles and metallacages. J Am Chem Soc. 2016;138(13):4580–4588. doi: 10.1021/jacs.6b00846. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Z, et al. Immobilizing tetraphenylethylene into fused metallacycles: Shape effects on fluorescence smission. J Am Chem Soc. 2016;138(40):13131–13134. doi: 10.1021/jacs.6b07173. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M, et al. Fluorescent metallacycle-cored polymers via covalent linkage and their use as contrast agents for cell imaging. Proc Natl Acad Sci USA. 2016;113(40):11100–11105. doi: 10.1073/pnas.1612898113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Kishi N, Hasegawa K, Akita M, Yoshizawa M. Highly fluorescent M2L4 molecular capsules with anthracene shells. Chem Commun (Camb) 2011;47(30):8605–8607. doi: 10.1039/c1cc12946e. [DOI] [PubMed] [Google Scholar]
- 36.Hong Y, Lam JWY, Tang BZ. Aggregation-induced emission. Chem Soc Rev. 2011;40(11):5361–5388. doi: 10.1039/c1cs15113d. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Z, Lam JWY, Tang BZ. Tetraphenylethene: A versatile AIE building block for the construction of efficient luminescent materials for organic light-emitting diodes. J Mater Chem. 2012;22(45):23726–23740. [Google Scholar]
- 38.Mei J, et al. Aggregation-induced emission: The whole is more brilliant than the parts. Adv Mater. 2014;26(31):5429–5479. doi: 10.1002/adma.201401356. [DOI] [PubMed] [Google Scholar]
- 39.Mei J, Leung NL, Kwok RT, Lam JW, Tang BZ. Aggregation-induced emission: Together we shine, united we soar! Chem Rev. 2015;115(21):11718–11940. doi: 10.1021/acs.chemrev.5b00263. [DOI] [PubMed] [Google Scholar]
- 40.Yamashina M, et al. Preparation of highly fluorescent host–guest complexes with tunable color upon encapsulation. J Am Chem Soc. 2015;137(29):9266–9269. doi: 10.1021/jacs.5b06195. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Z, Yan X, Cook TR, Saha ML, Stang PJ. Engineering functionalization in a supramolecular polymer: Hierarchical self-organization of triply orthogonal non-covalent interactions on a supramolecular coordination complex platform. J Am Chem Soc. 2016;138(3):806–809. doi: 10.1021/jacs.5b12986. [DOI] [PubMed] [Google Scholar]
- 42.Zhang C, et al. Benzo-21-crown-7/secondary dialkylammonium salt [2]pseudorotaxane- and [2]rotaxane-type threaded structures. Org Lett. 2007;9(26):5553–5556. doi: 10.1021/ol702510c. [DOI] [PubMed] [Google Scholar]
- 43.De Greef TFA, et al. Supramolecular polymerization. Chem Rev. 2009;109(11):5687–5754. doi: 10.1021/cr900181u. [DOI] [PubMed] [Google Scholar]
- 44.Yang L, Tan X, Wang Z, Zhang X. Supramolecular polymers: Historical development, preparation, characterization, and functions. Chem Rev. 2015;115(15):7196–7239. doi: 10.1021/cr500633b. [DOI] [PubMed] [Google Scholar]
- 45.Yan X, et al. Supramolecular polymers with tunable topologies via hierarchical coordination-driven self-assembly and hydrogen bonding interfaces. Proc Natl Acad Sci USA. 2013;110(39):15585–15590. doi: 10.1073/pnas.1307472110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Wang Z, Zhang X. Characterization of supramolecular polymers. Chem Soc Rev. 2012;41(18):5922–5932. doi: 10.1039/c2cs35084j. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida T, Matsuda T, Otsuka S, Parshall GW, Peet WG. Tetrakis(triethylphosphine)platinum(0) Inorg Synth. 1990;28:122–123. [Google Scholar]
- 48.Kim HJ, Lee E, Park HS, Lee M. Dynamic extension-contraction motion in supramolecular springs. J Am Chem Soc. 2007;129(36):10994–10995. doi: 10.1021/ja073554b. [DOI] [PubMed] [Google Scholar]
- 49.Ashton PR, et al. Pseudorotaxanes formed between secondary dialkylammonium salts and crown ethers. Chemistry. 1996;2(6):709–728. [Google Scholar]
- 50.Qu Y, et al. A new triphenylamine fluorescent dye for sensing of cyanide anion in living cell. Tetrahedron Lett. 2013;54(36):4942–4944. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.