Abstract
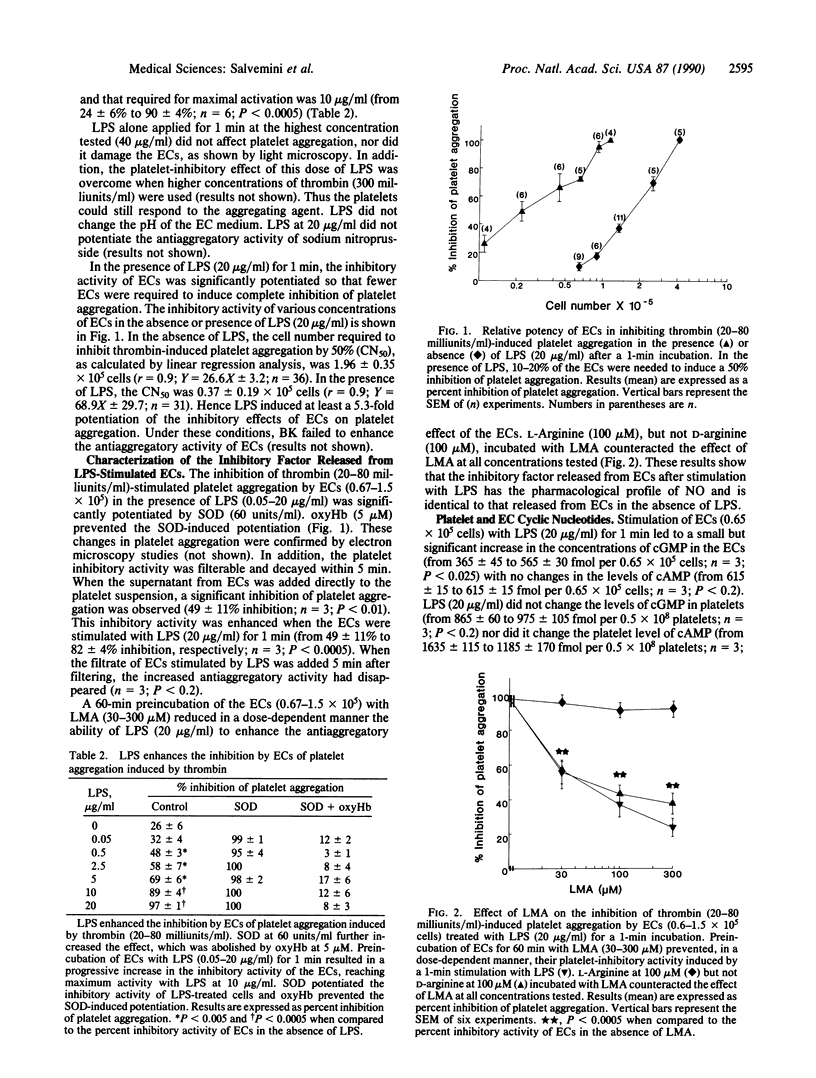
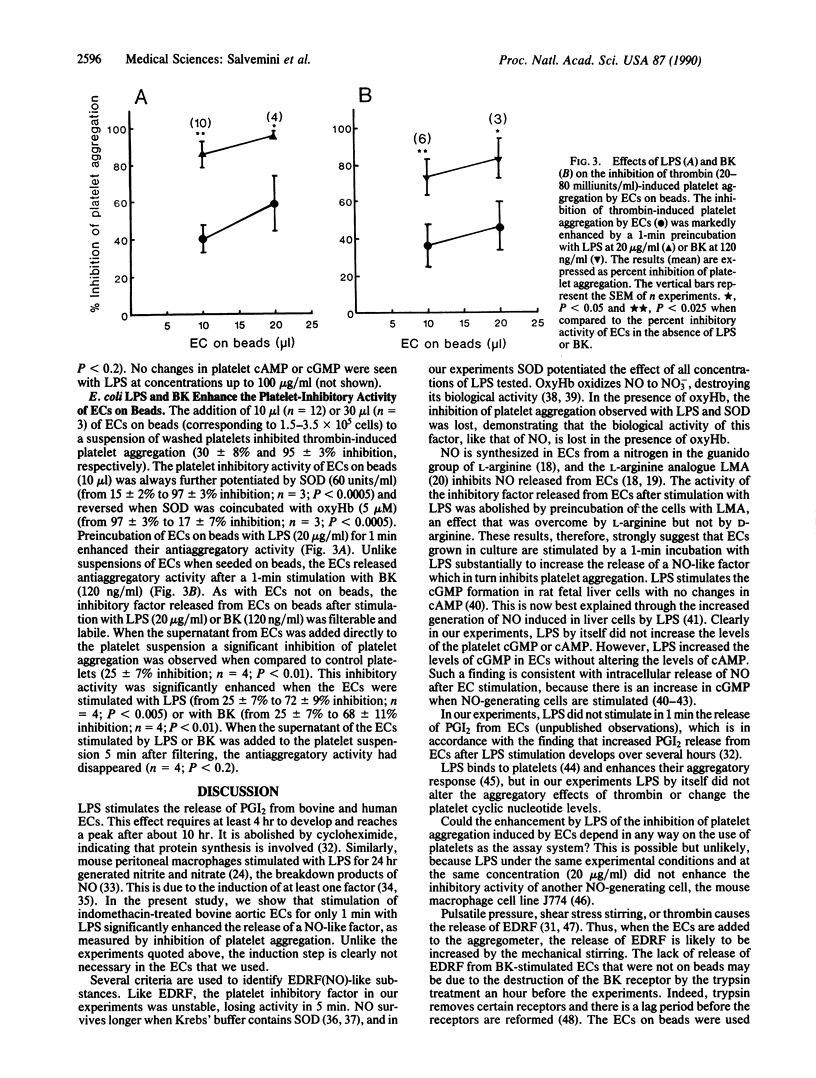
Incubation of human washed platelets with bovine aortic endothelial cells (ECs) treated with indomethacin resulted in an inhibition of thrombin-induced platelet aggregation that was dependent on the number of ECs added. Preincubation of ECs with Escherichia coli lipopolysaccharide (LPS; 0.5-2.0 micrograms/ml) for 1 min significantly enhanced their inhibitory activity. This effect was potentiated by superoxide dismutase (60 units/ml) and reversed by oxyhemoglobin (5-10 microM), indicating that the inhibition was due to the release of endothelium-derived relaxing factor (nitric oxide). When the ECs were pretreated with NG-monomethyl-L-arginine (30-300 microM) before LPS, the antiaggregatory activity was strongly reduced. The reduction of activity by NG-monomethyl-L-arginine was reversed by L-arginine (100 microM) but not by D-arginine (100 microM). Under similar conditions, LPS also enhanced the antiaggregatory activity of ECs grown on beads. The immediate enhancement by LPS of the release of endothelium-derived relaxing factor from endothelial cells may contribute to the rapid fall in blood pressure associated with endotoxin shock in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisaka K., Gross S. S., Griffith O. W., Levi R. NG-methylarginine, an inhibitor of endothelium-derived nitric oxide synthesis, is a potent pressor agent in the guinea pig: does nitric oxide regulate blood pressure in vivo? Biochem Biophys Res Commun. 1989 Apr 28;160(2):881–886. doi: 10.1016/0006-291x(89)92517-5. [DOI] [PubMed] [Google Scholar]
- Alheid U., Frölich J. C., Förstermann U. Endothelium-derived relaxing factor from cultured human endothelial cells inhibits aggregation of human platelets. Thromb Res. 1987 Sep 1;47(5):561–571. doi: 10.1016/0049-3848(87)90361-6. [DOI] [PubMed] [Google Scholar]
- Azuma H., Ishikawa M., Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986 Jun;88(2):411–415. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORN G. V., CROSS M. J. THE AGGREGATION OF BLOOD PLATELETS. J Physiol. 1963 Aug;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Stuehr D. J., Ferrari F. K., Simmons R. L. Evidence that activation of Kupffer cells results in production of L-arginine metabolites that release cell-associated iron and inhibit hepatocyte protein synthesis. Surgery. 1989 Aug;106(2):364–372. [PubMed] [Google Scholar]
- Bottoms G. D., Templeton C. B., Fessler J. F., Johnson M. A., Roesel O. F., Ewert K. M., Adams S. B. Thromboxane, prostaglandin I2 (epoprostenol), and the hemodynamic changes in equine endotoxin shock. Am J Vet Res. 1982 Jun;43(6):999–1002. [PubMed] [Google Scholar]
- Cybulsky M. I., Chan M. K., Movat H. Z. Acute inflammation and microthrombosis induced by endotoxin, interleukin-1, and tumor necrosis factor and their implication in gram-negative infection. Lab Invest. 1988 Apr;58(4):365–378. [PubMed] [Google Scholar]
- Delvos U., Janssen B., Müller-Berghaus G. Effect of lipopolysaccharides on cultured human endothelial cells. Relationship between tissue factor activity and prostacyclin release. Blut. 1987 Aug;55(2):101–108. doi: 10.1007/BF00631779. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Ogilvie M. L. Peptides and monoclonal antibodies which bind to platelet glycoproteins IIb and/or IIIa inhibit clot retraction. Thromb Res. 1988 Jan 1;49(1):43–53. doi: 10.1016/0049-3848(88)90358-1. [DOI] [PubMed] [Google Scholar]
- Goligorsky M. S. Role of endothelium in endotoxin blockade of voltage-sensitive Ca2+ channels in smooth muscle cells. Am J Physiol. 1989 Nov;257(5 Pt 1):C875–C881. doi: 10.1152/ajpcell.1989.257.5.C875. [DOI] [PubMed] [Google Scholar]
- Graber S. E., Clancey M. A. Further characterization of the effect of bacterial lipopolysaccharide preparations on cyclic GMP levels: the importance of macromolecular synthesis. Proc Soc Exp Biol Med. 1985 Oct;180(1):163–169. doi: 10.3181/00379727-180-42159. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Harris R. H., Zmudka M., Maddox Y., Ramwell P. W., Fletcher J. R. Relationship of TXB2 and 6-keto PGF1 alpha to the hemodynamic changes during baboon endotoxic shock. Adv Prostaglandin Thromboxane Res. 1980;7:843–849. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J. Heme-dependent activation of soluble guanylate cyclase by nitric oxide: regulation of enzyme activity by porphyrins and metalloporphyrins. Semin Hematol. 1989 Jan;26(1):63–76. [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaf C. D., Wishnok J. S., Tannenbaum S. R. L-arginine is a precursor for nitrate biosynthesis in humans. Biochem Biophys Res Commun. 1989 Sep 15;163(2):1032–1037. doi: 10.1016/0006-291x(89)92325-5. [DOI] [PubMed] [Google Scholar]
- Martin W., White D. G., Henderson A. H. Endothelium-derived relaxing factor and atriopeptin II elevate cyclic GMP levels in pig aortic endothelial cells. Br J Pharmacol. 1988 Jan;93(1):229–239. doi: 10.1111/j.1476-5381.1988.tb11426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrick B. O., Ryan U. S., Brigham K. L. Direct effects of E coli endotoxin on structure and permeability of pulmonary endothelial monolayers and the endothelial layer of intimal explants. Am J Pathol. 1986 Jan;122(1):140–151. [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M., Kaplan K. L., Nossel H. L. Prostacyclin production by perturbed bovine aortic endothelial cells in culture. Blood. 1984 Oct;64(4):801–806. [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987 Nov 7;2(8567):1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983 Nov 10;306(5939):174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Romero J. C., Vanhoutte P. M. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986 Jun;250(6 Pt 2):H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Salvemini D., de Nucci G., Gryglewski R. J., Vane J. R. Human neutrophils and mononuclear cells inhibit platelet aggregation by releasing a nitric oxide-like factor. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6328–6332. doi: 10.1073/pnas.86.16.6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D., de Nucci G., Sneddon J. M., Vane J. R. Superoxide anions enhance platelet adhesion and aggregation. Br J Pharmacol. 1989 Aug;97(4):1145–1150. doi: 10.1111/j.1476-5381.1989.tb12572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schini V., Schoeffter P., Miller R. C. Effect of endothelium on basal and on stimulated accumulation and efflux of cyclic GMP in rat isolated aorta. Br J Pharmacol. 1989 Jul;97(3):853–865. doi: 10.1111/j.1476-5381.1989.tb12025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorer A. E., Kaplan M. E., Rao G. H., Moldow C. F. Interleukin 1 stimulates endothelial cell tissue factor production and expression by a prostaglandin-independent mechanism. Thromb Haemost. 1986 Dec 15;56(3):256–259. [PubMed] [Google Scholar]
- Schröder H., Schrör K. Cyclic GMP stimulation by vasopressin in LLC-PK1 kidney epithelial cells is L-arginine-dependent. Naunyn Schmiedebergs Arch Pharmacol. 1989 Oct;340(4):475–477. doi: 10.1007/BF00167052. [DOI] [PubMed] [Google Scholar]
- Semeraro N., Fumarola D., Telesforo P., Vermylen J. Platelet-endotoxin interaction: a review. Boll Ist Sieroter Milan. 1976;55(6):577–586. [PubMed] [Google Scholar]
- Sneddon J. M., Vane J. R. Endothelium-derived relaxing factor reduces platelet adhesion to bovine endothelial cells. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2800–2804. doi: 10.1073/pnas.85.8.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J., Mendelsohn M. E., Amarante P., Smick D., Andon N., Davies P. F., Cooke J. P., Loscalzo J. N-acetylcysteine potentiates platelet inhibition by endothelium-derived relaxing factor. Circ Res. 1989 Sep;65(3):789–795. doi: 10.1161/01.res.65.3.789. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Gross S. S., Sakuma I., Levi R., Nathan C. F. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989 Mar 1;169(3):1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Gross S. S., Thiel B. A., Levi R., Nathan C. F. Synthesis of nitrogen oxides from L-arginine by macrophage cytosol: requirement for inducible and constitutive components. Biochem Biophys Res Commun. 1989 Jun 15;161(2):420–426. doi: 10.1016/0006-291x(89)92615-6. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Synthesis of nitrite and nitrate in murine macrophage cell lines. Cancer Res. 1987 Nov 1;47(21):5590–5594. [PubMed] [Google Scholar]
- Sung C. P., Arleth A. J., Shikano K., Zabko-Potapovich B., Berkowitz B. A. Effect of trypsinization in cell culture on bradykinin receptors in vascular endothelial cells. Biochem Pharmacol. 1989 Feb 15;38(4):696–699. doi: 10.1016/0006-2952(89)90219-0. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Oliver R. C., MacIntyre D. E., Gordon J. L. Endotoxin-induced platelet aggregation and secretion. II. Changes in plasma membrane proteins. J Cell Sci. 1977 Dec;28:225–236. doi: 10.1242/jcs.28.1.225. [DOI] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989 Oct 28;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Lopez-Belmonte J., Rees D. D. Modulation of the vasodepressor actions of acetylcholine, bradykinin, substance P and endothelin in the rat by a specific inhibitor of nitric oxide formation. Br J Pharmacol. 1989 Oct;98(2):646–652. doi: 10.1111/j.1476-5381.1989.tb12639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


