Abstract
Objective:
To identify cell-surface antibodies in patients with neuromyotonia and to describe the main clinical implications.
Methods:
Sera of 3 patients with thymoma-associated neuromyotonia and myasthenia gravis were used to immunoprecipitate and characterize neuronal cell-surface antigens using reported techniques. The clinical significance of antibodies against precipitated proteins was assessed with sera of 98 patients (neuromyotonia 46, myasthenia gravis 52, thymoma 42; 33 of them with overlapping syndromes) and 219 controls (other neurologic diseases, cancer, and healthy volunteers).
Results:
Immunoprecipitation studies identified 3 targets, including the Netrin-1 receptors DCC (deleted in colorectal carcinoma) and UNC5A (uncoordinated-5A) as well as Caspr2 (contactin-associated protein-like 2). Cell-based assays with these antigens showed that among the indicated patients, 9 had antibodies against Netrin-1 receptors (7 with additional Caspr2 antibodies) and 5 had isolated Caspr2 antibodies. Only one of the 219 controls had isolated Caspr2 antibodies with relapsing myelitis episodes. Among patients with neuromyotonia and/or myasthenia gravis, the presence of Netrin-1 receptor or Caspr2 antibodies predicted thymoma (p < 0.05). Coexisting Caspr2 and Netrin-1 receptor antibodies were associated with concurrent thymoma, myasthenia gravis, and neuromyotonia, often with Morvan syndrome (p = 0.009). Expression of DCC, UNC5A, and Caspr2 proteins was demonstrated in paraffin-embedded thymoma samples (3) and normal thymus.
Conclusions:
Antibodies against Netrin-1 receptors (DCC and UNC5a) and Caspr2 often coexist and associate with thymoma in patients with neuromyotonia and myasthenia gravis.
Classification of evidence:
This study provides Class III evidence that antibodies against Netrin-1 receptors can identify patients with thymoma (sensitivity 21.4%, specificity 100%).
Neuromyotonia is a clinical and electrophysiologic syndrome that results from a variety of diseases affecting peripheral motor nerve axons.1, 2 Acquired autoimmune neuromyotonia may arise as a paraneoplastic phenomenon associated mainly with thymoma or, less frequently, with lung cancer.1 Frequently, patients with thymoma also develop myasthenia gravis with antibodies against the acetylcholine receptor, dysautonomia, and CNS symptoms such as delirium or severe insomnia (Morvan syndrome).3 Clinical and experimental data suggest that circulating antibodies against cell-surface antigens might cause axonal hyperexcitability in a subgroup of these patients. Although electrophysiologic studies have suggested a dysfunction of voltage-gated potassium channels,4, 5 cell-based assays did not reveal that antibodies target these channels.6 It was later demonstrated that a percentage of patients had antibodies against contactin-associated protein-like 2 (Caspr2) or, less frequently, against leucine-rich glioma-inactivated 1.7–10 However, the frequency and diagnostic utility of these antibodies in patients with or without thymoma and whether other antibodies are implicated in the pathogenesis of motor hyperexcitability symptoms are unclear.
In this study, we used a systematic immunoproteomic approach aiming to identify new membrane antigens with the sera of patients with paraneoplastic neuromyotonia associated with myasthenia gravis and thymoma. We also determined the clinical significance of the antibodies of interest and the expression of the corresponding antigens in thymoma samples and normal thymus.
METHODS
Patients and controls.
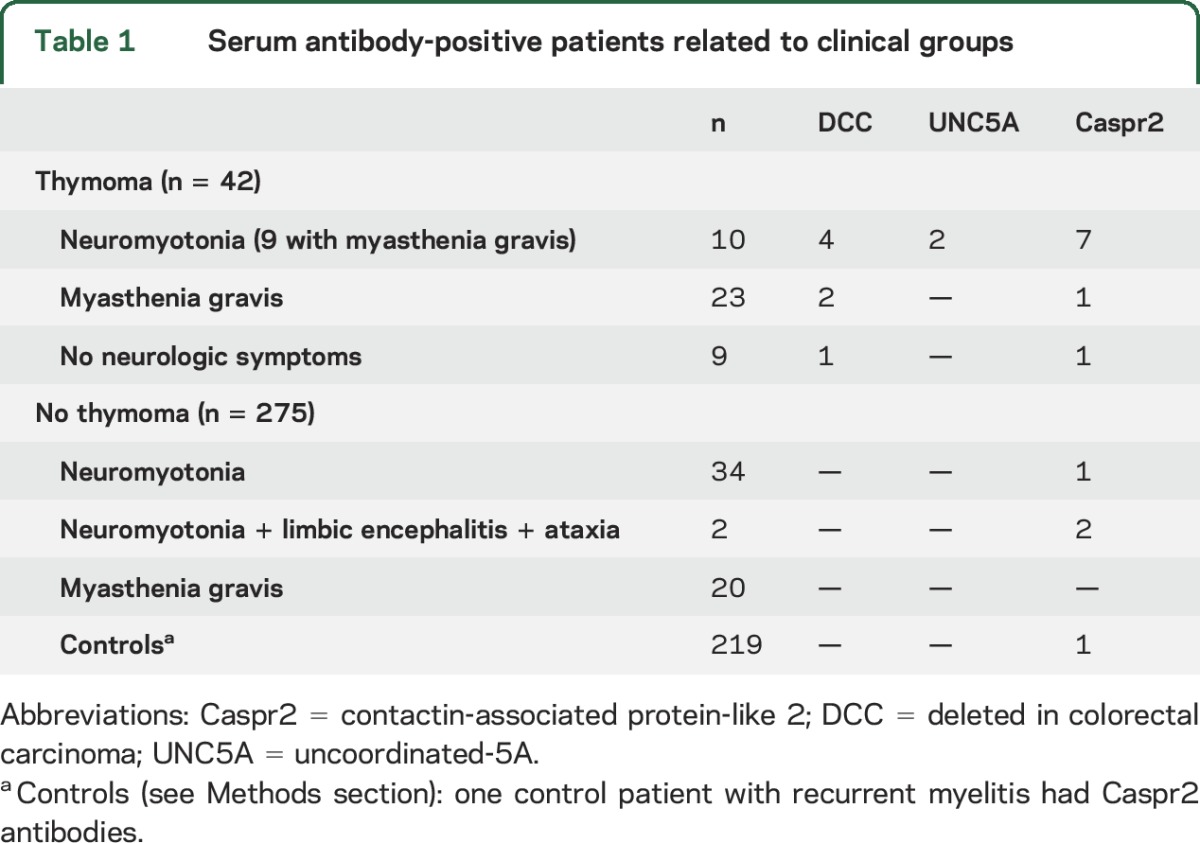
The sera from 3 patients with neuromyotonia, myasthenia gravis, and thymoma were used for immunoprecipitation studies, as previously reported.11 Control samples for immunoprecipitation experiments included sera from 3 patients with anti-IgLON5 syndrome,12 3 children with opsoclonus-myoclonus associated with neuroblastoma,11 and 3 healthy volunteers. Presence of antibodies against immunoprecipitated proteins of interest was investigated with cell-based assays (see below) with the serum of 317 individuals. Participants included a cohort of 46 patients with neuromyotonia (40 from the Neuromuscular Diseases Unit of Hospital Universitario y Politécnico La Fe, Valencia, Spain, and 6 from the French Reference Center on Paraneoplastic Neurological Syndromes, Lyon, France).13 The inclusion criteria for the patients with neuromyotonia were defined as follows: muscle twitching or cramps involving at least 2 skeletal muscle regions (cranial, upper limbs, trunk, lower limbs) and EMG studies showing myokymic (40–150 Hz) or neuromyotonic (150–300 Hz) discharges that affect at least 2 skeletal muscle regions.2 Two patients had additional symptoms of limbic encephalitis (short-term memory deficits, temporal seizures, and an increased T2 MRI signal in the medial temporal lobes). Participants also included 43 patients who had myasthenia gravis with anti–acetylcholine receptor antibodies, 9 patients with thymoma but no neurologic symptoms, and 219 controls, including those with other neurologic disorders (10 subacute and 39 chronic sporadic cerebellar ataxia of unknown origin, 20 CNS demyelinating syndromes, 20 Guillain-Barré syndrome, and 50 Alzheimer disease), cancer without neurologic symptoms (10 breast cancer, 10 ovarian cancer, 10 skin cancer, 4 lung cancer, 10 brain primary tumors, 5 lymphoma, 5 leukemia), and 50 healthy volunteers (table 1). All patients were seen by the authors. The thymoma tumor stage was classified according to the Masaoka system.14
Table 1.
Serum antibody-positive patients related to clinical groups
Immunoprecipitation experiments.
CNS synaptosomes were isolated and used for the immunoprecipitation experiments. Precipitated proteins were identified by mass spectrometry (e-Methods at Neurology.org).
Cell-based assay using human embryonic kidney cells.
To test for the presence of antibodies, human embryonic kidney (HEK293T) cells (CRL-1573; American Type Culture Collection, Manassas, VA) were transfected with the plasmids that contained Caspr2 (SC115167), Netrin-1 uncoordinated-5A (UNC5A) receptor (RG212389), deleted in colorectal carcinoma (DCC, as in reference 10; kindly provided by Dr. Masaki Fukata), Neurexin-1 (RG215381,) or metabotropic glutamate receptor 3 (RG207379). Twenty-four hours after transfection with Lipofectamine 2000 (Invitrogen, Carlsbad, CA), cells were washed with phosphate-buffered saline, pH 7.4, fixed with 4% paraformaldehyde, permeabilized with 0.3% triton X-100, and incubated overnight at 4°C with patients' serum (1:40) or commercial antibodies (1:100) specific for Caspr2 (rabbit polyclonal; Ab33994, Abcam, Cambridge, UK), UNC5A (goat polyclonal; sc-67903, Santa Cruz Biotechnologies, Dallas, TX), DCC (rabbit polyclonal; sc-11437, Santa Cruz Biotechnologies), Neurexin-1 (goat polyclonal; Ab77596, Abcam), or metabotropic glutamate receptor 3 (mouse monoclonal; sc-271899, Santa Cruz Biotechnologies) and incubated with the corresponding Alexa Fluor 594/488/594 (1:1,000) secondary antibodies (Molecular Probes, Eugene, OR). Results were visualized and photographed under a confocal laser-scanning microscope (Fluoview FV1000; Olympus, Tokyo, Japan) using the FV viewer software. Samples were considered positive if there was concordance between 2 independent investigators (E.T.-V., L.B.) blinded to clinical information.
Statistical methods.
An association between the categorical variables (presence of antibodies or specific clinical diagnostic categories: neuromyotonia, myasthenia gravis, thymoma) was assessed by the χ2 and Fisher exact tests, and odds ratio (OR) estimations were obtained. Univariate and multivariate logistic regressions were used to test for the significance of the predictor variables on a specific diagnosis and to provide adjusted ORs. The significance level was set at 5% (α = 0.05).
Immunohistochemical analysis of the expression of Caspr2, DCC, and UNC5A in thymomas and normal thymus.
Formalin-fixed paraffin-embedded tissue samples of 3 thymomas (patients 1, 2, and 8 in table 2) and 7 normal thymic tissues (from fetal or perinatal autopsies) were studied. After heat-induced epitope retrieval in citrate buffer, pH 6, for 20 minutes (Dako, Glostrup, Denmark), 4-μm-thick sections were incubated with primary antibodies specific for Caspr2 (mouse monoclonal 1:50 for 20 minutes; NBP1-49575, Novus Biologicals, Littleton, CO), DCC (mouse monoclonal 1:50 for 30 minutes; MAB5884, Novus Biologicals), UNC5A (rabbit polyclonal 1:50 for 45 minutes; LS-B11622, Lifespan Biosciences, Seattle, WA), HLA-DR (mouse monoclonal 1:30 for 20 minutes; M0775, Dako), CD3 (mouse monoclonal; M7254, Dako), CD20 (mouse monoclonal; M0755, Dako), and cytokeratin AE1/AE3 (mouse monoclonal; M3515, Dako), ready to use for 20 minutes. For antibody detection, Envision detection system peroxidase/DAB (Dako) was used. The procedure was performed on a Dako Autostainer Link 48. Image acquisition and analyses were performed with a Leica DMD108 digital microscope.
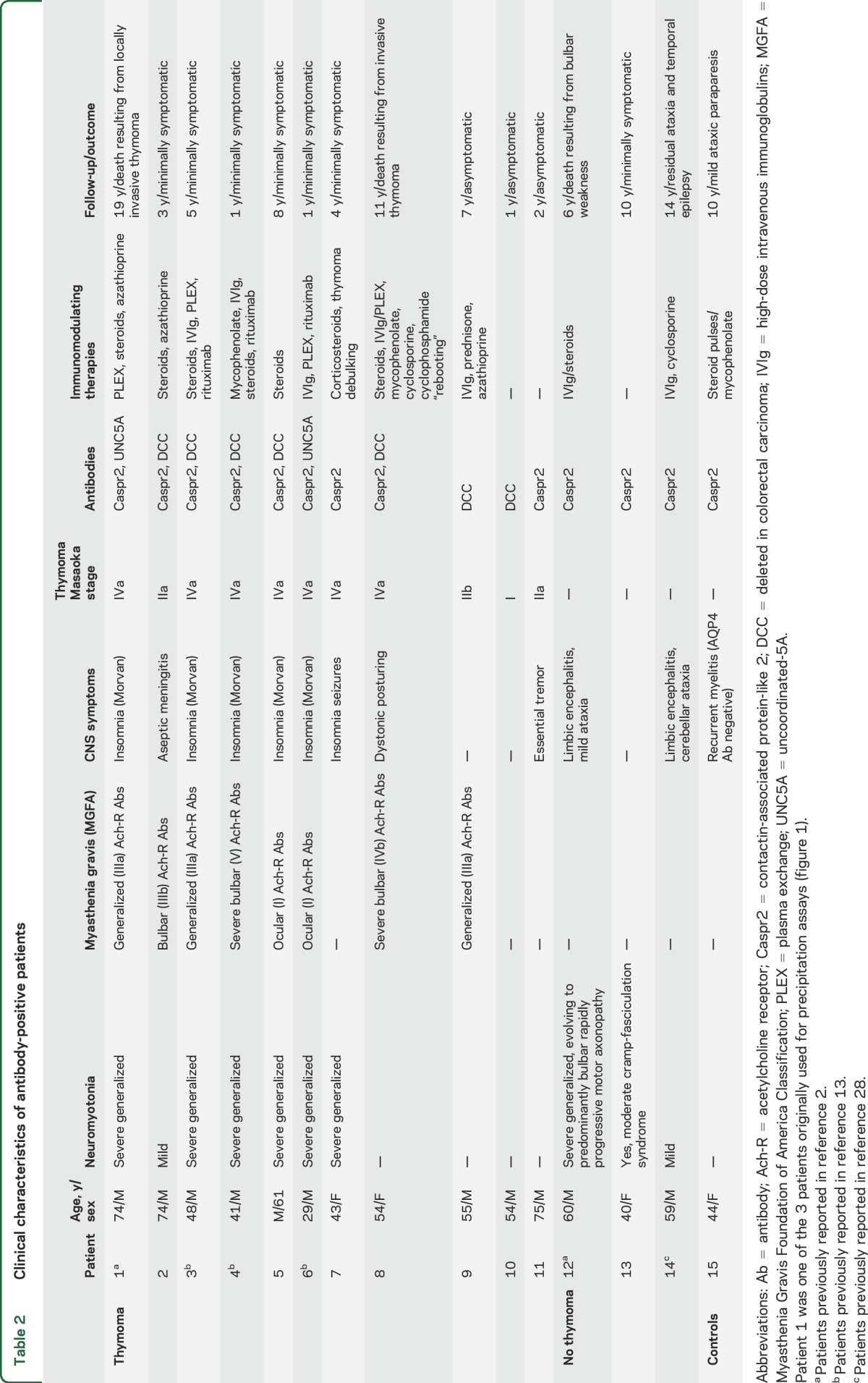
Table 2.
Clinical characteristics of antibody-positive patients
Standard protocol approvals, registrations, and patient consents.
Written informed consent for storing and using samples for research purposes was obtained from all the patients and controls. Serum samples were stored at −80°C and treated in accordance with current legislation (Spanish RD 1716/2011, November 18) in the Biobank of the Hospital Universitario y Politécnico La Fe and the Hospital Central de Asturias. The study was approved by the ethics committee of the Hospital Universitario y Politécnico La Fe, Valencia, Spain (CEIB 2012/0145).
Primary research questions.
We determined the presence of antibodies against novel cell-membrane antigens in the serum of patients with neuromyotonia and myasthenia gravis and examined whether the identification of those antibodies associated with clinical neurologic manifestations or the presence of thymoma. Our study is rated Class III because of the case-control design and the risk of spectrum bias in the interpretation of the cell-based assays.
RESULTS
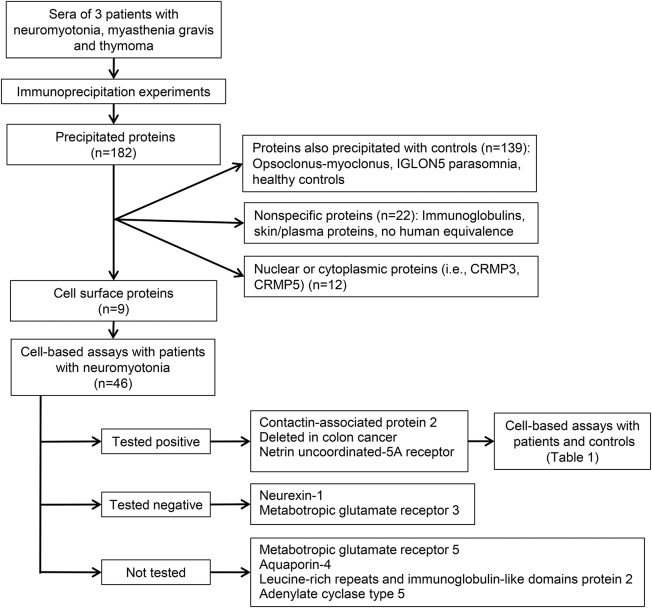
The immunoprecipitation studies (figure 1) led to the isolation of Caspr2 and 2 Netrin-1 receptors, DCC and UNC5A, as candidate antigens (tables e-1 and e-2). The cell-based assays with 98 patients and 219 controls are summarized in table 1. Fifteen patients were positive for any of these antibodies: 9 had antibodies against Netrin-1 receptors (7 DCC, 2 UNC5A) (7 with additional Caspr2 antibodies) and 6 had isolated Caspr2 antibodies (figure 2, figure e-1). The clinical characteristics of these patients are summarized in table 2. All 9 patients with Netrin-1 receptor antibodies had thymoma. Six of them had neuromyotonia, myasthenia gravis, and severe insomnia, all with the Netrin-1 receptor plus Caspr2 antibodies. In 4 patients in this group, myasthenia gravis with thymoma preceded by 1 to 10 years the development of peripheral nerve hyperexcitability symptoms, which were manifested simultaneously in 2 patients. Of the 3 remaining patients with thymoma and Netrin-1 receptor antibodies, 2 had isolated myasthenia and one had no neurologic symptoms.
Figure 1. Flowchart of the study.
CRMP3 and CRMP5 = collapsin response mediator proteins 3 and 5.
Figure 2. Venn diagram of Netrin-1 receptor antibody–positive patients in clinical groups.
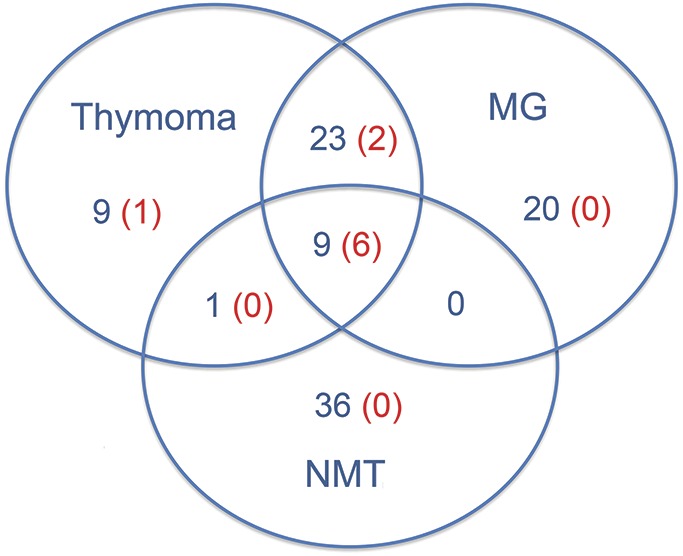
Number of patients in blue; number of patients with Netrin 1 receptor antibodies in red. MG = myasthenia gravis; NMT = neuromyotonia.
Four patients without thymoma had isolated Caspr2 antibodies: 2 had mild to severe neuromyotonia with associated limbic encephalitis and cerebellar ataxia, one had isolated mild neuromyotonia, and one woman had relapsing episodes of myelitis associated with coeliac disease.
To assess for the clinical significance of the antibodies, a statistical analysis of their association with neuromyotonia, myasthenia gravis, and thymoma was performed. In the patients with neuromyotonia, presence of Netrin-1 receptor antibodies (or Caspr2 antibodies) predicted thymoma (p < 0.05; positive predictive value [PPV] of 70% for Caspr2 antibodies and 100% for Netrin-1 receptor antibodies in our study population). Similarly, in the patients with myasthenia gravis, presence of any of these antibodies predicted thymoma (p < 0.05; PPV of 100% for Caspr2 and Netrin-1 receptor antibodies in our study population). Conversely, in the patients with thymoma, presence of any of the antibodies predicted neuromyotonia (p < 0.05; PPV of 78% and 66% for Caspr2 and Netrin-1 receptor antibodies, respectively) but was not associated with myasthenia gravis. In addition, when patients with Caspr2 antibodies were analyzed, the association of a second (anti–Netrin-1 receptor) antibody was strongly associated with the combination of neuromyotonia, myasthenia gravis, and thymoma (often with severe insomnia, suggesting Morvan syndrome) (p = 0.009). Caspr2 antibodies alone (p = 0.035) or in combination with Netrin-1 receptor antibodies (p = 0.041) were associated with a higher Masaoka stage (≥III) of thymoma.
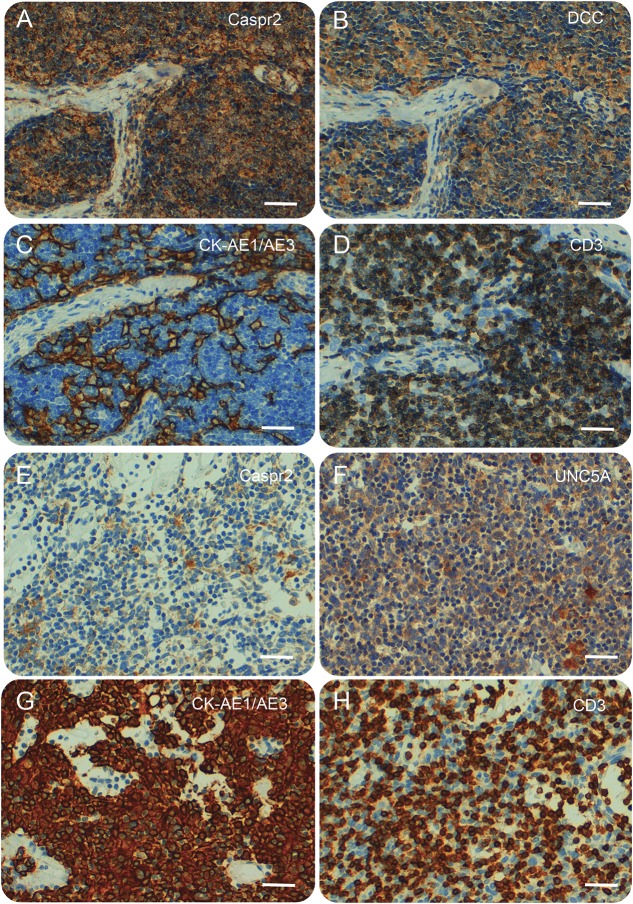
Thymoma tissue samples from 3 patients were available for immunohistochemical analysis (patients 1, 2, and 8 in table 2, all with Caspr2 and Netrin-1 receptor antibodies) (table e-3). Neoplastic epithelial thymoma cells stained on the cell membrane, cytoplasm, or both for Caspr2, DCC, and UNC5a in all 3 evaluated samples (figure 3). In addition, Caspr2 and UNC5A were expressed by epithelial stromal cells contained in the cortex and medulla of all 7 evaluated normal thymus; DCC was expressed in 4 of the 7 normal thymus samples in a similar pattern. Staining was especially intense in areas surrounding the Hassall corpuscles (figure e-2).
Figure 3. Immunohistochemistry studies of thymomas.
Immunohistochemical staining for contactin-associated protein-like 2 (Caspr2), deleted in colorectal carcinoma (DCC), uncoordinated-5A (UNC5A), cytokeratin (CK)-AE1/AE3, and CD3 on the thymoma samples of patients 2 (A–D) and 8 (E–H). CK-AE1/AE3 staining reveals a neoplastic epithelial cell network around CD3-positive T cells (thymocytes). Neoplastic epithelial thymoma cells were immunoreactive on the cell membrane, cytoplasm, or both for Caspr2, DCC, and UNC5A. Scale bars: 50 μm.
DISCUSSION
This study shows that Netrin-1 receptor antibodies associate with thymoma in patients with neuromyotonia and myasthenia gravis. These antibodies might add to other cancer-predicting antibodies such as SOX1 antibodies in the Lambert-Eaton myasthenic syndrome15 or titin antibodies in early-onset myasthenia gravis.16 Currently, screening for an occult neoplasm is warranted in most patients with these autoimmune neuromuscular syndromes regardless of their antibody status.1,17 However, if future studies confirm our findings, patients with Netrin-1 receptor antibodies may need more frequent or prolonged tumor surveillance.
In a previous report, DCC antibodies were found in 7 patients with Morvan syndrome (most with thymoma and coexisting Caspr2 or leucine-rich glioma-inactivated 1 antibodies).10 Consistently in our study, Netrin-1 receptor antibodies frequently coexisted with Caspr2 antibodies, and this combination strongly associated with overlapping presynaptic and postsynaptic neuromuscular syndromes triggered by thymoma.18 Netrin-1 receptor antibodies were also occasionally observed in patients with isolated myasthenia or thymoma. Because there was a 37-fold increased risk of thymoma in the patients with myasthenia gravis in our study population (OR = 37.1; p < 0.001), presence of Netrin-1 receptor antibodies in these patients was likely related to thymoma-associated autoimmunity rather than myasthenic symptoms. It should be noted that manifestations of muscular hyperexcitability might be less apparent in myasthenia gravis because of the coexisting defect of neuromuscular transmission and thus could be underdiagnosed at early stages.18
Netrin-1 is a protein involved in axon guidance during nervous system development through its interaction with its 2 main receptors, DCC and UNC5A, which lead to axonal attraction or repulsion, respectively. Netrin-1 receptors are also expressed in the adult nervous system with functions that go beyond axon guidance such as CNS synaptic plasticity19 or Schwann cell-axon interactions in the paranodal regions of myelinated peripheral nerves.20 Future studies should explore whether antibodies against Netrin-1 receptors have direct pathogenic effects on their targets.
Two recent reports have contributed to a better definition of the clinical profile of patients with Caspr2 autoimmunity.21,22 The 2 most frequent syndromes of these patients include limbic encephalitis (rarely associated with cancer) and Morvan syndrome (frequently associated with malignant thymoma). Because our article focused on neuromyotonia, Caspr2 antibodies were found to be predictive of thymoma. Notably, we identified only 2 patients with Caspr2 antibodies and limbic encephalitis, both without thymoma, which emphasizes that patients with Caspr2 antibody–associated encephalitis do not usually have an underlying tumor.7
We found that the Caspr2 antibody–positive patients with neuromyotonia were largely confined to the group of patients with thymoma. This contrasts with previous reports in which antibody positivity was not segregated to cases with thymoma.23 In the present study, only one of 34 patients with nonthymomatous isolated neuromyotonia had Caspr2 antibodies. Other as-yet unknown antibodies may account for the axonal hyperexcitability of these patients. Because our study used rat CNS synaptosomes for the immunoprecipitation experiments, it is conceivable that other implicated peripheral nerve antigens might have been missed.
Most paraneoplastic neurologic syndromes associated with classic onconeuronal antigens develop at early oncological disease stages; tumors express the onconeuronal antigen; and the prognosis of these patients improves after successful treatment of the underlying cancer. Thymoma-associated immunity differs in many aspects from this classic model. In our study, Caspr2 and Netrin-1 receptor immunity developed at advanced metastatic Masaoka stages of thymoma and frequently in patients previously diagnosed with thymoma-associated myasthenia gravis. Thymectomy, chemotherapy, and thymoma recurrence have all been previously reported as triggers of Caspr2-related immunity.24,25 Expression of Netrin-1 receptors and Caspr2 was confirmed in 3 available thymoma samples and, as expected, in thymic epithelial cells of normal thymus. Previous reports have shown thymoma expression of epitopes of antigens involved in paraneoplastic immunity (collapsin response mediator protein 5, titin, acetylcholine receptors), whereas the corresponding whole functional protein is usually absent.26,27 Intratumoral T-cell selection and activation in an abnormal microenvironment that expresses the antigens are probably important for the development of Netrin-1 receptor and Caspr2 autoimmunity. The task for the future is to determine the pathogenicity of these antibodies and to confirm with larger series of patients their potential utility as biomarkers of thymoma in patients with autoimmune neuromuscular or CNS diseases.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Drs. Miguel Baquero, Bonaventura Casanova, Yania Yáñez, and Victoria Castel (Hospital Universitario y Politécnico La Fe, Valencia, Spain) and Dr. Aurora Astudillo (Hospital Universitario Central de Asturias, Oviedo, Spain) for providing control sera samples; Dr. Masaki Fukata (National Institute for Physiological Sciences, Okazaki, Japan) for providing the DCC plasmid; Lola Bataller for image editing; and Juan Luis Gómez-Martínez, stHalley Statistics, for the statistical analysis.
GLOSSARY
- Caspr2
contactin-associated protein-like 2
- DCC
deleted in colorectal carcinoma
- OR
odds ratio
- PPV
positive predictive value
- UNC5A
uncoordinated-5A
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Estefania Torres-Vega: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. Nuria Mancheño: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data. Arantxa Cebrián-Silla, Vicente Herranz-Pérez, María José Chumillas, and Germán Moris: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. Bastien Joubert: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval. Jerome Honnorat: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data. Teresa Sevilla: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. Juan Vílchez: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval. Josep Dalmau: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval, contribution of vital reagents/tools/patients, obtaining funding. Francesc Graus: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data. Jose Manuel García-Verdugo: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. Luis Bataller: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, study supervision, obtaining funding.
STUDY FUNDING
Supported by Fondo de Investigaciones Sanitarias (Instituto de Salud Carlos III) (PS12/01031) and Asociación Miastenia de España.
DISCLOSURE
E. Torres-Vega, N. Mancheño, A. Cebrián-Silla, V. Herranz-Pérez, M. Chumillas, G. Moris, B. Joubert, J. Honnorat, T. Sevilla, and J. Vílchez report no disclosures relevant to the manuscript. J. Dalmau receives royalties for patents from Athena Diagnostics for the use of Ma2 and NMDA as autoantibody tests and licensing fees from Euroimmun for a patent for the use of NMDAR as an autoantibody test. F. Graus, J. Manuel García-Verdugo, and L. Bataller report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Hart IK, Maddison P, Newsom-Davis J, Vincent A, Mills KR. Phenotypic variants of autoimmune peripheral nerve hyperexcitability. Brain 2002;125:1887–1895. [DOI] [PubMed] [Google Scholar]
- 2.Rubio-Agusti I, Perez-Miralles F, Sevilla T, et al. Peripheral nerve hyperexcitability: a clinical and immunologic study of 38 patients. Neurology 2011;76:172–178. [DOI] [PubMed] [Google Scholar]
- 3.Evoli A, Lancaster E. Paraneoplastic disorders in thymoma patients. J Thorac Oncol 2014;9:S143–S147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newsom-Davis J, Mills KR. Immunological associations of acquired neuromyotonia (Isaacs' syndrome): report of five cases and literature review. Brain 1993;116:453–469. [DOI] [PubMed] [Google Scholar]
- 5.Sinha S, Newsom-Davis J, Mills K, Byrne N, Lang B, Vincent A. Autoimmune aetiology for acquired neuromyotonia (Isaacs' syndrome). Lancet 1991;338:75–77. [DOI] [PubMed] [Google Scholar]
- 6.Vincent A, Irani SR. Caspr2 antibodies in patients with thymomas. J Thorac Oncol 2010;5:S277–S280. [DOI] [PubMed] [Google Scholar]
- 7.Lancaster E, Huijbers MG, Bar V, et al. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann Neurol 2011;69:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain 2010;133:2734–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 2010;9:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohkawa T, Fukata Y, Yamasaki M, et al. Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. J Neurosci 2013;33:18161–18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres-Vega E, Durán-Moreno M, Sánchez del Pino M, et al. Immunoproteomic studies on paediatric opsoclonus-myoclonus associated with neuroblastoma. J Neuroimmunol 2016;297:98–102. [DOI] [PubMed] [Google Scholar]
- 12.Sabater L, Gaig C, Gelpi E, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol 2014;13:575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laurencin C, Andre-Obadia N, Camdessanche JP, et al. Peripheral small fiber dysfunction and neuropathic pain in patients with Morvan syndrome. Neurology 2015;85:2076–2078. [DOI] [PubMed] [Google Scholar]
- 14.Masaoka A. Staging system of thymoma. J Thorac Oncol 2010;5:S304–S312. [DOI] [PubMed] [Google Scholar]
- 15.Sabater L, Titulaer M, Saiz A, Verschuuren J, Gure AO, Graus F. SOX1 antibodies are markers of paraneoplastic Lambert-Eaton myasthenic syndrome. Neurology 2008;70:924–928. [DOI] [PubMed] [Google Scholar]
- 16.Voltz RD, Albrich WC, Nagele A, et al. Paraneoplastic myasthenia gravis: detection of anti-MGT30 (titin) antibodies predicts thymic epithelial tumor. Neurology 1997;49:1454–1457. [DOI] [PubMed] [Google Scholar]
- 17.Titulaer MJ, Lang B, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurol 2011;10:1098–1107. [DOI] [PubMed] [Google Scholar]
- 18.Vernino S, Auger RG, Emslie-Smith AM, Harper CM, Lennon VA. Myasthenia, thymoma, presynaptic antibodies, and a continuum of neuromuscular hyperexcitability. Neurology 1999;53:1233–1239. [DOI] [PubMed] [Google Scholar]
- 19.Horn KE, Glasgow SD, Gobert D, et al. DCC expression by neurons regulates synaptic plasticity in the adult brain. Cell Rep 2013;3:173–185. [DOI] [PubMed] [Google Scholar]
- 20.Webber CA, Christie KJ, Cheng C, et al. Schwann cells direct peripheral nerve regeneration through the Netrin-1 receptors, DCC and Unc5H2. Glia 2011;59:1503–1517. [DOI] [PubMed] [Google Scholar]
- 21.Joubert B, Saint-Martin M, Noraz N, et al. Characterization of a subtype of autoimmune encephalitis with anti-contactin-associated protein-like 2 antibodies in the cerebrospinal fluid, prominent limbic symptoms, and seizures. JAMA Neurol 2016;73:1115–1124. [DOI] [PubMed] [Google Scholar]
- 22.van Sonderen A, Ariño H, Petit-Pedrol M, et al. The clinical spectrum of Caspr2 antibody-associated disease. Neurology 2016;87:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein CJ, Lennon VA, Aston PA, et al. Insights from LGI1 and CASPR2 potassium channel complex autoantibody subtyping. JAMA Neurol 2013;70:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irani SR, Pettingill P, Kleopa KA, et al. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol 2012;72:241–255. [DOI] [PubMed] [Google Scholar]
- 25.Fleisher J, Richie M, Price R, Scherer S, Dalmau J, Lancaster E. Acquired neuromyotonia heralding recurrent thymoma in myasthenia gravis. JAMA Neurol 2013;70:1311–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camdessanche JP, Lassabliere F, Meyronnet D, et al. Expression of the onconeural CV2/CRMP5 antigen in thymus and thymoma. J Neuroimmunol 2006;174:168–173. [DOI] [PubMed] [Google Scholar]
- 27.Marx A, Porubsky S, Belharazem D, et al. Thymoma related myasthenia gravis in humans and potential animal models. Exp Neurol 2015;270:55–65. [DOI] [PubMed] [Google Scholar]
- 28.Becker EB, Zuliani L, Pettingill R, et al. Contactin-associated protein-2 antibodies in non-paraneoplastic cerebellar ataxia. J Neurol Neurosurg Psychiatry 2012;83:437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.