Abstract
Emerging evidence indicates that alcohol intake is associated with human cancers in different organs. However, the molecular mechanism of alcohol-associated human cancers remains to be elucidated. Here, this paper aimed to clarify a novel mechanism of alcohol-promoted cell transformation and tumor development. Alcohol induces JNK1 activation and increases cellular levels of c-Jun to upregulate Brf1 expression and Pol III gene transcription, leading to an enhancement of rates of cell transformation and tumor formation.
Keywords: Alcohol, HCC, Breast cancer, Brf1, Pol III genes
Alcohol has been classified as carcinogenic in human (1–2). Target sites for alcohol-related carcinogenesis include the liver, breast, oral cavity, pharynx, esophagus, larynx, ovary, stomach and pancreas (3–5). Is there a common mechanism which mediates alcohol-associated cancer development in different organs? Cancer cells have a consistent cytological feature of nucleolar hypertrophy, where RNA polymerase I and III-dependent genes are transcribed (6). This feature provides the possibility to explore a common mechanism of alcohol-associated human cancers by elucidating the deregulation of RNA polymerase III-dependent genes (Pol III genes). To investigate the common mechanism and organ specificity, we currently focus our studies on alcohol-associated liver and breast cancers (7–9). Thus, this paper aims to present the progress and significance of the studies on alcohol-induced deregulation of Pol III genes in liver and breast.
Alcohol intake and cancers
Alcohol consumption is widely thought to enhance the risk for liver cancer (10–13). Besides hepatitis viruses (HBV and HCV) and aflatoxin, alcohol presents a major etiological factor in hepatocarcinogenesis. Alcohol abuse is a major cause of liver fibrosis. Liver fibrosis represents a wound-healing process in response to a variety of chronic injurious stimuli. Eventually, alcohol-induced liver fibrosis promotes formation of liver cirrhosis, which increases the risk of HCC (hepatocellular carcinoma) (14). Breast cancer is the most common malignant disease in females. Overall, one woman in every nine will get breast cancer at some time in her life. Breast cancer ranks second among cancer deaths in women (15). Epidemiological studies have indicated that alcohol consumption has most consistently been associated with breast cancer risk in both premenopausal and postmenopausal women, regardless of the type of alcohol beverage consumed (16–19). Animal experiments have shown that ethanol intake can cause mammary tumorigenesis (20–22). Epidemiologic studies have indicated that alcohol is more associated with ER+ (estrogen receptor positive) than ER- (estrogen receptor negative) breast cancer cases (23–24). How alcohol causes human breast cancer remains to be elucidated. It is critically important to clarify whether alcohol causes deregulation of Pol III genes to promote tumor development in liver and breast.
Deregulation of RNA Pol III genes and cancer
The transcription machinery of Pol III tRNA genes consists of RNA Pol III, TFIIIB and TFIIIC. TFIIIB is composed of TBP, Brf1 and Bdp1, which is much more associated with cancers than TFIIIC (7). TBP is the most central eukaryotic transcription component, as it is required by all three cellular Pol I, Pol II and Pol III genes; whereas Brf1 and Bdp1 specifically regulate Pol III gene transcription.
It has been known for over a century that nucleolar hypertrophy is a consistent cytological feature of cancer cells (6). Enlarged nucleoli are used by pathologists as a strong diagnostic indicator of cell transformation and neoplasia (6). This implies that transformation in situ is closely linked to the deregulation of RNA Pol I- and Pol III-dependent transcription, the size of the nucleolus reflects the level of rRNA synthesis. RNA Pol III is responsible for the synthesis of untranslated RNAs, such as tRNAs and 5S rRNAs. 5S rRNA is a component of ribosome, which is a complex of rRNAs and proteins to perform protein synthesis, whereas tRNAs transfers amino acids to ribosome for peptide formation. Previous studies showed that the alteration of RNA Pol III transcription affects transformation of Rat1 cells and tumorigenesis in nude mouse (7–9,25). Increasing expression of Myc or TBP enhanced transformation of Rat1A cells and elevated Pol III gene transcription; in contrast, inhibiting Pol III gene transcription repressed cell transformation and tumorigenesis (8–9,25–28). HBV X, a hepatitis viral oncogenic protein, enhanced the transcription of Pol III genes (29–30). EGF and DEN (diethylnitrosamine), which are a mitogen and carcinogen, respectively, enhance Pol III gene transcription and, increase the rates of transformation of MCF-10A and AML-12 cells (8–9,27). The above evidence demonstrates that deregulation of Pol III genes is closely associated with cell transformation and tumor development.
Alcohol induces deregulation of Pol III genes
Deregulation of Pol III gene transcription, enhancing cellular tRNAs and 5S rRNAs production, leads to an increase in translational capacity to promote cell proliferation and transformation and tumor formation. However, it remains to be investigated whether alcohol affects Pol III gene transcription. Using cell culture and animal models (7–9,28), the studies indicate that ethanol increased transcription of Pol III genes in engineered HepG2-ADH (alcohol dehydrogenase) cells, compared to HepG2-vector cells (7). Immunoblot analysis reveals that alcohol increased expression of c-Jun (7). Down-regulation of c-Jun expression abrogated the ethanol-mediated increases in TBP and Brf1 expression and Pol III gene transcripts, whereas TFIIIC63 was unaffected by decreased c-Jun expression (7). These results support the idea that ethanol-increased expression of c-Jun drives the enhanced expression of TBP, Brf1, and Pol III genes. An increase in Pol III gene transcription is observed in the livers of wild type C57BL/6 mice that were fed an ethanol liquid diet, compared to the wild type mice fed a control diet. The ethanol-fed mice display steatohepatitis, but do not have obvious inflammation and tumors. Whereas the chronic feeding of HCV NS5A (Non-structure 5A) transgenic mice with an ethanol liquid diet promotes HCC development (7). Pol III gene transcription was dramatically enhanced in livers of ethanol-fed NS5A transgenic mice, compared to the livers in mice given a control diet (7). Hence, induction of Pol III gene transcription occurs in vivo after chronic alcohol administration. Transcription levels of Pol III genes, such as pre-tRNALeu and 5S rRNA, were elevated in HCC tissues of alcohol-fed NS5A mice, compared to the levels of the Pol III genes in the non-tumor liver tissues from non-alcohol-fed and alcohol-fed NS5A mice. More interestingly, the cellular level of TBP and Brf1 mRNA in HCC tissues of alcohol-fed NS5A mice is also significantly higher than in the non-tumor liver tissues of these mice with or without alcohol intake. Recent studies indicate that Brf1 expression is increased in human biopsies of HCC cases (28). The overall survival period is shorter for the patients with high expression of Brf1 (28). Further analysis indicates that levels of Brf1 and Pol III gene transcription in HCC patients with alcohol consumption is higher than the cases of non-HCC with or without alcohol intake (28). Induction of Brf1 and Pol III genes by ethanol in hepatoma cells is higher than in non-tumor liver cells. Ethanol increases the rate of transformation of liver AML-12 cells. Repression of Brf1 inhibits alcohol-promoted AML-12 cell transformation. Brf1 is overexpressed in HCC cases and liver tumor cell lines (28). Alcohol enhances Brf1 expression to promote cell transformation (7,28).
Alcohol slightly increases Pol III gene transcription in ER- non-tumor breast cell lines (MCF-10A, MCF-10F, MCF-12A) and ER- breast cancer cell lines (MDA-MB231, SKBR-3), whereas alcohol dramatically increases Pol III gene transcription in ER+ breast cancer cell lines (MCF-7 and T47D) (8–9). ER ligand, E2 (17β-estradiol) causes a small alteration of these genes in MCF-7 cells. However, ethanol works with E2 to dramatically enhance Pol III gene transcription (8). Alcohol increases cellular levels of ERα mRNA and protein, whereas reduction of ERα by its siRNA decreases Brf1 expression and Pol III gene transcription. Additionally, while control cells and ethanol-treated MCF10A cells do not undergo colony formation, E2 alone induces colony formation of the cells. Ethanol in combination with E2 produces larger and more numerous colonies than E2 alone. Repression of ERα or Brf1 by their siRNAs decreases alcohol-induced cell transformation (8). These studies support the idea that alcohol affects Pol III gene transcription in a ERα-dependent manner.
Tamoxifen (Tam) is an antagonist of ER in breast tissue, which competitively binds to ER, producing a nuclear complex that decreases DNA synthesis and inhibits estrogen effects. Tam is currently used for the treatment of both early and advanced ER+ breast cancer in women (31). Recent studies demonstrate that Tam inhibits the induction of Brf1 and Pol III genes by alcohol in ER+ breast cancer cells (9). Further analysis indicates that alcohol increases c-Jun expression in MCF-7 cells to upregulate the transcription of Brf1 and Pol III genes, whereas Tam reduces c-Jun expression to repress the transcription of Brf1 (9). Repression of cJun decreases cellular levels of ERα and Brf1. Alcohol-dependent increased occupancy of Brf1 in Pol III gene promoters is reduced by Tam (9). The repression of Brf1 and Pol III genes by Tam reduces alcohol-induced cell proliferation and colony formation (9). Recently, we have analyzed the breast cancer biopsies over two hundred cases from the patients before Tam treatment. Results indicate that Brf1 expression was increased in the patients of breast cancer. Very interestingly, the patients, who accepted Tam treatment after surgery operation, with high Brf1 expression display longer survival periods (unpublished). This is because cellular levels of Brf1 and Pol III gene expression are decreased by Tam. These results further support the earlier studies that Tam inhibits Brf1 and Pol III gene expression (9). Together, these results indicate that Tam inhibits alcohol-induced Brf1 expression through c-Jun and ERα to downregulate Pol III gene transcription. The studies uncover a new mechanism of Tam-treated ER+ breast cancer, by which Tam inhibits tumor growth through repressing Brf1 expression and Pol III gene transcription. Together, these studies mentioned above indicate that Brf1 is a new biomarker of diagnosis and prognosis of HCC and breast cancer.
Alcohol-induced JNK1 activation to mediate Pol III gene transcription
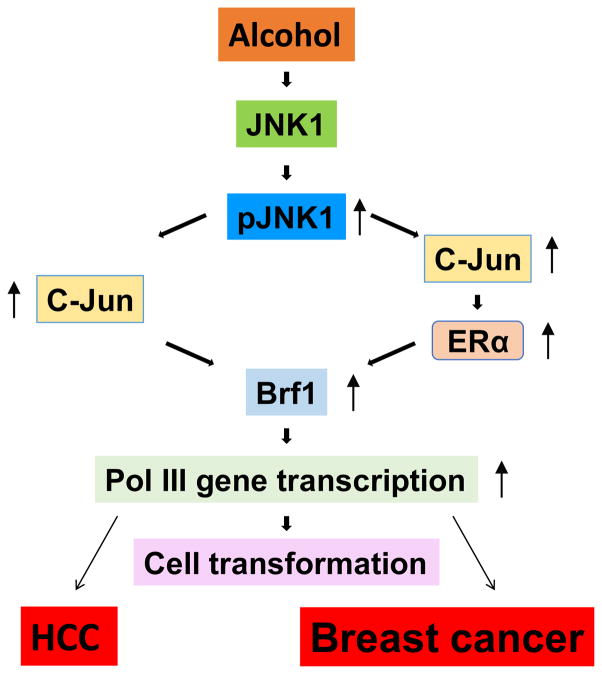
Alcohol has been shown to induce JNK activation (8,32) and the JNKs play an important role in regulating Pol III genes (33–34). However, it remains to be determined whether JNKs mediate alcohol-induced deregulation of Pol III genes. Ethanol induces strong activation of JNK1, but only has a small effect on JNK2, in the HepG2-ADH cells (7). Ethanol also dramatically activated JNK1 in ER+ MCF-7 cells (8). Earlier studies have demonstrated that JNK1 and JNK2 have different function in Pol III gene transcription (33–34). JNK1 positively, but JNK2 negatively, modulates Pol III genes (33). This suggests that alcohol-activated JNK1 may play an important role in Pol III gene transcription and cell transformation. Further analysis indicates that a JNK inhibitor, SP600125 significantly reduces the induction of pre-tRNALeu and 5S rRNA caused by alcohol. JNK1 siRNA, which specifically reduces cellular level of JNK1 (7–8), dramatically decreases alcohol-induced Pol III gene transcription (7–8). In contrast, increasing cellular level of JNK1 by its expression construct elevates alcohol-induced Pol III gene transcription in both HepG2-ADH cells and MCF-7 cells (7–8). Furthermore, alcohol increases the rates of transformation of liver and breast cells (7–8), repressed JNK1 and Brf1 expression, decreased transcription of Pol III genes and reduced the rates of colony formation of AML-12 and MCF-10 cells. Together, these studies support the idea that alcohol activates JNK1 to elevate Brf1 expression and Pol III gene transcription to promote cell transformation and tumor formation, which is a common mechanism of alcohol-induced deregulation of Pol III genes to bring about greater phenotypic changes (Fig. 1).
Fig. 1. Schematic illustration of a common mechanism of alcohol-associated cancers.
Alcohol increases Brf1 expression through JNK1 and c-Jun pathway, which in turn upregulates Pol III gene transcription to promote cell transformation and tumor development caused by alcohol.
A rapidly increasing number of Americans are developing and dying from liver cancer, despite the fact that viral hepatitis – a primary cause of liver cancer – is preventable and treatable (35). This implies that alcohol consumption may be a key issue in causing HCC (13). Emerging evident indicates that alcohol consumption is tightly associated with breast cancer. This association involves the estrogen receptor, which is over-expressed in approximately 70–80% of breast cancer cases (36). Alcohol intake is known to promote mammary tumorigenesis (37–38). However, the mechanism of alcohol-associated cancers is not clear. Recent studies have demonstrated that alcohol induces deregulation of Pol III genes to promote cell transformation and tumor formation of liver and breast. The novel findings suggest a common mechanism of alcohol-associated human cancers and provide a possibility that inhibition of Brf1 expression may be a potential approach to repress alcohol-promoted cell transformation and tumor development.
Highlights.
Alcohol causes deregulation of RNA Pol III genes;
Alcohol induces activation of JNK1 and increases c-Jun expression to upregulate Brf1 expression and Pol III gene transcription;
Repressing Brf1 expression reduces the rates of alcohol-promoted transformation of liver and breast cells.
Acknowledgments
We want to thank Drs. Daniel Levy, Michael Stallcup and Neil Kaplowitz (University of Southern California) for scientific discussions. This work was supported by National Institutes of Health grants AA017288, AA021114 and AA02324 to S.Z.
Abbreviations
- Brf1
TFIIB-relate factor 1
- Pol III genes
RNA polymerase III-dependent genes
- HCC
hepatocellular carcinoma
- TBP
TATA-box binding protein
- ER
estrogen receptor
- ADH
alcohol dehydrogenase
- Tam
tamoxifen
- HCV
Hepatitis C virus
- HBV
Hepatitis B virus
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol 100, A Review of Human Carcinogens. Vol. 2011. Lyon, France: International Agency for Research on Cancer; 2011. [Accessed November 2, 2011]. http://monographs.iarc.fr/ENG/Monographs/PDFs/index.php. [Google Scholar]
- 2.Cogliani VJ, Baan R, Straif K, Crosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Wild CP. Preventable 33. exposures associated with human cancers. J Natl Cancer Inst. 2011;103:1827–1839. doi: 10.1093/jnci/djr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connor J. Alcohol consumption as a cause of cancer. Addiction. 2016 Jul 21; doi: 10.1111/add.13477. [DOI] [Google Scholar]
- 4.Scoccianti C, Straif K, Romieu I. Recent evidence on alcohol and cancer epidemiology. Future Oncol. 2013;(9):1315–22. doi: 10.2217/fon.13.94. [DOI] [PubMed] [Google Scholar]
- 5.Allen NE1, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, Green J. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101(5):296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 6.White RJ. RNA polymerase III transcription and cancer. Oncogene. 2001;23:3208–3216. doi: 10.1038/sj.onc.1207547. [DOI] [PubMed] [Google Scholar]
- 7.Zhong S, Machida K, Tsukamoto H, Johnson DL. Alcohol induces RNA polymerase III-dependent transcription through c-Jun by coregulating TBP and Brf1 expression. J Biol Chem. 2011;286:2393–2401. doi: 10.1074/jbc.M110.192955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, Jin J, Zhong Q, Yu XL, Levy D, Zhong S. ERa mediates alcohol-induced deregulation of Pol III genes in breast cancer cells. Carcinogenesis. 2013;34:28–37. doi: 10.1093/carcin/bgs316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong Q, Shi G, Zhang Q, Lu L, Levy D, Zhong S. Tamoxifen represses alcohol-induced transcription of RNA polymerase III-dependent genes. Oncotarget. 2014;5:12410–12417. doi: 10.18632/oncotarget.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longnecker MP. Alcohol Health Res World. 1992;16:223–229;. [PMC free article] [PubMed] [Google Scholar]
- 11.Seitz HK, Poschi G, Simanowske UA. Alcohol and cancer. Recent Dev Alcohol. 1998;14:67–95. doi: 10.1007/0-306-47148-5_4. [DOI] [PubMed] [Google Scholar]
- 12.Bangnardi V, Blangiardo M, LaVecchia C, Corrao G. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer. 2001;85:1700–1705. doi: 10.1054/bjoc.2001.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan JM, Govindarajan S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer. 2004;101:1009–1017. doi: 10.1002/cncr.20427. [DOI] [PubMed] [Google Scholar]
- 14.Lieber C. Hepatic, metabolic, and nutritional disorders of alcoholism: from pathogenesis to therapy. Crit Rev Clin Lab Sci. 2000;37:551–584. doi: 10.1080/10408360091174312. [DOI] [PubMed] [Google Scholar]
- 15.American Cancer Society. American Cancer Society, Cancer Facts and Figures. Author; Atlanta: 2005. Available at http://www.cancer.org/downloads/STT/CAFF2005f4PWSecured.pdf. [Google Scholar]
- 16.Hamajima N, Hirose K, Tajima K, Rohan T, et al. Alcohol, tobacco and breast cancer--collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87(11):1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacMahon B. Epidemiology and the causes of breast cancer. Int J Cancer. 2006;118(10):2373–8. doi: 10.1002/ijc.21404. Review. [DOI] [PubMed] [Google Scholar]
- 18.Petri AL, Tjønneland A, Gamborg M, Johansen D, Høidrup S, Sørensen TI, Grønbæk MM. Alcohol intake, type of beverage, and risk of breast cancer in pre- and postmenopausal women. Alcohol Clin Exp Res. 2004;28:1084–1090. doi: 10.1097/01.alc.0000130812.85638.e1. [DOI] [PubMed] [Google Scholar]
- 19.Singletary K, Gapstur S. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA. 2001;286:2143–2151. doi: 10.1001/jama.286.17.2143. [DOI] [PubMed] [Google Scholar]
- 20.Singletary K, McNary M, Odoms A, Nelshoppen J, Wallig M. Ethanol consumption and DMBA-induced mammary carcinogenesis in rats. Nutr Cancer. 1991;16:13–23. doi: 10.1080/01635589109514136. [DOI] [PubMed] [Google Scholar]
- 21.Singletary K, Nelshoppen J, Wallig M. Enhancement by chronic ethanol intake of N-methyl-N-nitrosourea-induced rat mammary tumorigenesis. Carcinogenesis. 1995;16:959–964. doi: 10.1093/carcin/16.4.959. [DOI] [PubMed] [Google Scholar]
- 22.Watabili T, Okii Y, Tokiyasu T, Yoshimura S, Yoshida S, Akane A, Shikata N, Tsubura A. Long-term ethanol consumption in ICR mice causes mammary tumor in females and liver fibrosis in males. Alcohol Clin Exp Res. 2000;24:117S–22S. [PubMed] [Google Scholar]
- 23.Deandrea S, Talamini R, Foschi R, Montella M, Dal Maso L, Falcini F, La Vecchia C, Franceschi S, Negri E. Alcohol and breast cancer risk defined by estrogen and progesterone receptor status: a case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17:2025–2028. doi: 10.1158/1055-9965.EPI-08-0157. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki R, Orsini N, Mignone L, Saji S, Wolk A. Alcohol intake and risk of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis of epidemiological studies. Int J Cancer. 2008;122:1832–1841. doi: 10.1002/ijc.23184. [DOI] [PubMed] [Google Scholar]
- 25.Johnson SA, Dubeau L, Kawalek M, Dervan A, Schonthal AH, Dang CV, Johnson DL. Increased expression of TATA-binding protein, the central transcription factor, can contribute to oncogenesis. Mol Cell Biol. 2003;23:3043–3051. doi: 10.1128/MCB.23.9.3043-3051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson SA, Dubeau L, Johnson DL. Enhanced RNA polymerase III-dependent transcription is required for oncogenic transformation. J Biol Chem. 2008;283(28):19184–19191. doi: 10.1074/jbc.M802872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang QS, Zhong Q, Evans AG, Levy D, Zhong S. Phosphorylation of histone H3 serine 28 modulates RNA polymerase III-dependent transcription. Oncogene. 2011;30:3943–3952. doi: 10.1038/onc.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong Q1, Xi S, Liang J, Shi GG, Huang Y, Zhang YM, Levy D, Zhong S. The significance of Brf1 overexpression in human hepatocellular carcinoma. Oncotarget. 2016;7(5):6243–6254. doi: 10.18632/oncotarget.6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang HD, Yuh CH, Dang CV, Johnson D. The hepatitis B virus increases the cellular level of TATA-binding protein which mediates transactivation of RNA polymerase III genes. Mol Cell Biol. 1995;15:6720–6728. doi: 10.1128/mcb.15.12.6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang HD, Trivedi A, Johnson D. Regulation of RNA polymerase I-dependent promoters by the Hepatitis B virus X protein, activated Ras, and the TATA-binding protein. Mol Cell Biol. 1998;18:7086–7094. doi: 10.1128/mcb.18.12.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jordan VC. Foyrteeth Gaddum Memorial Lecture: A current view of tamoxifen for the treatment and prevention of breast cancer. Br J Pharmacol. 1993;110:507–517. doi: 10.1111/j.1476-5381.1993.tb13840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luedemann CE, Bord E, Qin G, Zhu Y, Goukassian D, Losordo DW, Kishore R. Ethanol modulation of TNF-alpha biosynthesis and signaling in endothelial cells: synergistic augmentation of TNF-alpha mediated endothelial cell dysfunctions by chronic ethanol. Alcohol Clin Exp Res. 2005;29:930–938. doi: 10.1097/01.alc.0000171037.90100.6b. [DOI] [PubMed] [Google Scholar]
- 33.Zhong S, Fromm J, Johnson D. TBP is differentially regulated by JNK1 and JNK2 through Elk-1, controlling c-Jun expression and cell proliferation. Mol Cell Biol. 2007;27:54–64. doi: 10.1128/MCB.01365-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong S, Johnson D. The JNKs differentially regulate RNA polymerase III transcription by coordinately modulating the expression of all TFIIIB subunits. Proc Natl Acad Sci U S A. 2009;106:12682–12687. doi: 10.1073/pnas.0904843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward JW. CDC statement on liver cancer and hepatitis. 2016 Mar 9; http://www.cdc.gov/nchhstp/newsroom/2016/cancer-hep-statement.html.
- 36.Deandrea S, Talamini R, Foschi R, Montella M, Dal Maso L, Falcini F, La Vecchia C, Franceschi S, Negri E. Alcohol and breast cancer risk defined by estrogen and progesterone receptor status: a case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17:2025–2028. doi: 10.1158/1055-9965.EPI-08-0157. [DOI] [PubMed] [Google Scholar]
- 37.Wang S, Xu M, Li F, Wang X, Bower KA, Frank JA, Lu Y, Chen G, Zhang Z, Ke Z, Shi X, Luo J. Ethanol promotes mammary tumor growth and angiogenesis: the involvement of chemoattractant factor MCP-1. Breast Cancer Res Treat. 2011;133:1037–1048. doi: 10.1007/s10549-011-1902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong AW, Dunlap SM, Holcomb VB, Nunez NP. Alcohol Promotes Mammary Tumor Development via the Estrogen Pathway in Estrogen Receptor Alpha-Negative HER2/neu Mice. Alcohol Clin Exp Res. 2012;36:577–587. doi: 10.1111/j.1530-0277.2011.01654.x. [DOI] [PubMed] [Google Scholar]