Abstract
Key points
A mathematical model of a small conductance Ca2 +‐activated potassium (SK) channel was developed and incorporated into a physiologically detailed ventricular myocyte model.
Ca2+‐sensitive K+ currents promote negative intracellular Ca2+ to membrane voltage (CAi 2+→ Vm) coupling.
Increase of Ca2+‐sensitive K+ currents can be responsible for electromechanically discordant alternans and quasiperiodic oscillations at the cellular level.
At the tissue level, Turing‐type instability can occur when Ca2+‐sensitive K+ currents are increased.
Abstract
Cardiac alternans is a precursor to life‐threatening arrhythmias. Alternans can be caused by instability of the membrane voltage (V m), instability of the intracellular Ca2+ () cycling, or both. V m dynamics and dynamics are coupled via Ca2+‐sensitive currents. In cardiac myocytes, there are several Ca2+‐sensitive potassium (K+) currents such as the slowly activating delayed rectifier current (I Ks) and the small conductance Ca2+‐activated potassium (SK) current (I SK). However, the role of these currents in the development of arrhythmias is not well understood. In this study, we investigated how these currents affect voltage and Ca2+ alternans using a physiologically detailed computational model of the ventricular myocyte and mathematical analysis. We define the coupling between V m and cycling dynamics (→V m coupling) as positive (negative) when a larger Ca2+ transient at a given beat prolongs (shortens) the action potential duration (APD) of that beat. While positive coupling predominates at baseline, increasing I Ks and I SK promote negative →V m coupling at the cellular level. Specifically, when alternans is Ca2+‐driven, electromechanically (APD–Ca2+) concordant alternans becomes electromechanically discordant alternans as I Ks or I SK increase. These cellular level dynamics lead to different types of spatially discordant alternans in tissue. These findings help to shed light on the underlying mechanisms of cardiac alternans especially when the relative strength of these currents becomes larger under pathological conditions or drug administrations.
Keywords: cardiac alternans, cardiac electrophysiology, cardiac potassium current, cardiac function
Key points
A mathematical model of a small conductance Ca2 +‐activated potassium (SK) channel was developed and incorporated into a physiologically detailed ventricular myocyte model.
Ca2+‐sensitive K+ currents promote negative intracellular Ca2+ to membrane voltage (CAi 2+→ Vm) coupling.
Increase of Ca2+‐sensitive K+ currents can be responsible for electromechanically discordant alternans and quasiperiodic oscillations at the cellular level.
At the tissue level, Turing‐type instability can occur when Ca2+‐sensitive K+ currents are increased.
Abbreviations
- AP
action potential
- APD
action potential duration
intracellular Ca2+
- DI
diastolic interval
- gks
slowly activating delayed rectifier conductance
- gsk
small conductance Ca2+‐activated potassium conductance
- IKs
slowly activating delayed rectifier current
- ICaL
L‐type Ca2+ current
- ISK
small conductance Ca2+‐activated potassium current
- NCX
Na+–Ca2+ exchanger
- PCL
pacing cycle length
- SR
sarcoplasmic reticulum
- Vm
membrane voltage
Introduction
Ventricular arrhythmia is a major cause of sudden cardiac death. It has been shown that a precursor to life‐threatening arrhythmia formation is the development of cardiac alternans, a sequence of paired long and short action potentials (APs) (Pastore et al. 1999; Garfinkel et al. 2000; Fox et al. 2002 b; Hayashi et al. 2007; Groenendaal et al. 2014). However, physiological and dynamical mechanisms are not fully understood (Weiss et al. 2006, 2011; Wilson et al. 2006; Laurita & Rosenbaum, 2008; Merchant & Armoundas, 2012; Sato & Clancy, 2013; Kanaporis & Blatter, 2015; Valdivia, 2015). At the cellular level, alternans can be caused by instability of membrane voltage (V m) due to steep action potential duration (APD) restitution (Nolasco & Dahlen, 1968; Hayashi et al. 2007), instability of intracellular calcium () cycling due to steep sarcoplasmic reticulum (SR) Ca2+ release dependence on Ca2+ load/refractoriness, or both (Chudin et al. 1999; Shiferaw et al. 2003, 2005; Picht et al. 2006; Groenendaal et al. 2014; Wang et al. 2014). Dynamical systems of V m and are coupled via Ca2+‐sensitive currents. We previously investigated the role of the major Ca2+‐sensitive currents, the L‐type Ca2+ current (I CaL) and sodium(Na+)–Ca2+ exchanger (NCX) (Shiferaw et al. 2005; Sato et al. 2006, 2007, 2013). However, the slowly activating delayed rectifier current (I Ks) is a Ca2+‐sensitive current, and recent experimental studies showed that the small conductance Ca2+‐activated potassium (SK) channels exist in cardiac myocytes and play an important role in regulating APs (Xu et al. 2002; Tuteja et al. 2005; Zhang et al. 2008; Li et al. 2009; Hsueh et al. 2013; Chang et al. 2013 a; Chang & Chen, 2015; Yu et al. 2015; Zhang et al. 2015). Yet, little is known about the role of these Ca2+‐sensitive K+ currents in the formation of alternans. In this study, we investigate dynamical effects of Ca2+‐sensitive K+ currents on V m and alternans and show how ion channel/current level modifications lead to various phenomena at cellular and tissue levels including electromechanically (APD–Ca2+) discordant alternans and spatially discordant alternans.
Methods
In order to investigate the dynamical and physiological mechanisms of alternans, we used a physiologically detailed mathematical model of AP and cycling of the ventricular myocyte developed by Shiferaw et al. (2005). Figure 1 A shows the schematic diagram of the currents and fluxes that regulate V m dynamics and cycling. The membrane potential is governed by
where V m is the membrane potential, C m is the cell capacitance and I represents the transmembrane currents. The details of the model are described in the online Supporting information, Data S1.
Figure 1. Physiologically detailed mathematical model.
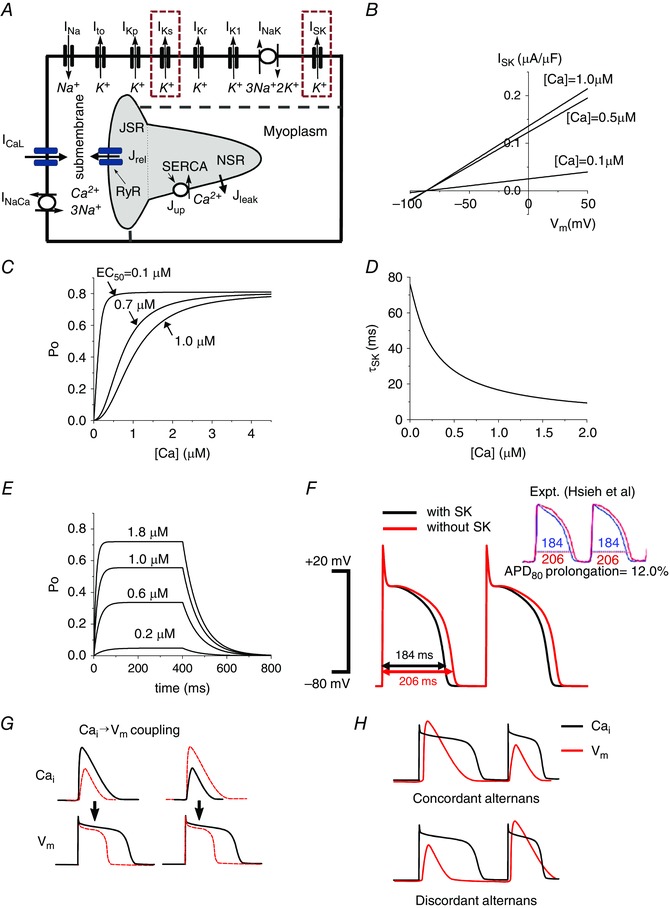
A, schematic diagram of the currents and fluxes that regulate V m dynamics and cycling. B, model of the SK channel: I SK vs. V m when [Ca2+] is 0.1, 0.5 and 1.0 μm. C, channel open probability as a function of intracellular Ca2+. D, inverse relationship between intracellular Ca2+ and the SK time constant (τSK2). E, open probability (P o) vs. time when various test [Ca2+] pulses are applied. [Ca2+] was changed from 0 μm to test [Ca2+] for 400 ms, and then changed to 0 μm. F, transmembrane voltage plotted against time demonstrating the decrease in APD from baseline (red) to inclusion of the SK channel (black). When g sk = 0.8 μS μF−1 and EC50 = 0.7 μm are chosen, the model shows 12% difference of APDs, which was shown by Hsieh et al. experimentally (Hsueh et al. 2013), by copyright permission of the American Heart Association, Inc. G, positive and negative →V m coupling. H, electromechanically concordant (large APD→large Ca2+ transient, small APD→small Ca2+ transient) alternans and discordant (large APD→small Ca2+ transient, small APD→large Ca2+ transient) alternans.
The formula of the Ca2+ dependence of I Ks from Mahajan et al. (2008) was incorporated into this model. I Ks is given by
where g ks is the maximum conductance of I Ks, q Ks is the Ca2+ dependence, x s1 and x s2 are the time‐dependent gating variables, E Ks is the reversal potential of I Ks, and K m controls the affinity of Ca2+. We varied g Ks and K m to explore the effects of I Ks on alternans.
The SK channel has been recently described in atrial and ventricular myocytes (Xu et al. 2002; Tuteja et al. 2005; Zhang et al. 2008; Li et al. 2009; Hsueh et al. 2013; Chang et al. 2013 a; Chang & Chen, 2015; Yu et al. 2015; Zhang et al. 2015). In this study we develop a novel computational model of the SK channel and integrate it with a physiologically detailed ionic model of a ventricular myocyte. We used the Ca2+ dependence formulation by Hirschberg et al. (1998). The governing equations for the SK channel are
where g sk is the maximum conductance, E K is the reversal potential, and EC50 controls the affinity of Ca2+. Several experimental studies have reported the EC50 of the SK channel in cardiac cells. Hongyuan et al. have reported that the EC50 of the SK channel in rat ventricles is 0.23–0.59 μm (Hongyuan et al. 2016). Chang et al. have reported that the EC50 of the SK channel in human ventricles is 0.35–0.6 μm (Chang et al. 2013 b). In this study, we varied the EC50 from 0.1 to 1.0 μm to cover the whole range of physiological and pathophysiological conditions. In experimental studies, the SK current (I SK) shows weak (or sometimes reverse) rectification (Lu et al. 2007; Zhang et al. 2008; Hsieh et al. 2013). Thus, in this study we chose a linear current–voltage relationship (Fig. 1 B). Rectification properties can affect our results quantitatively. However, they did not affect our results qualitatively. Ca2+ dependence and its time constant are plotted in Fig. 1 C and D. Figure 1 E shows the open probability (P o) of the SK channel when various test [Ca2+] pulses are applied. Some reported g sk values in ventricular myocytes are as high as 10 μS μF−1 (Lu et al. 2007; Zhang et al. 2008; Chang et al. 2013 b; Hongyuan et al. 2016), which would profoundly shorten APD. We have chosen a range of g sk more conservatively (from 0.4 to 4 μS μF−1), based on apamin effects on APD. Hsieh et al. showed a 12% prolongation of APD when apamin was applied (pacing cycle length, PCL = 300 ms, heart failure rabbit ventricular myocyte) (Hsueh et al. 2013). When g sk is 0.8 μS μF−1 and EC50 is 0.7 μm, the model also showed 12% difference between AP with I SK (Fig. 1 F black) and AP without I SK (Fig. 1 F red).
Tissue simulations were performed in a mono‐domain one‐dimensional cable. The governing equation for the membrane potential V m of a cell in tissue is
where C m is the membrane capacitance, I ion is the total ionic current through the membrane, and I coupling is the current that comes from the neighbouring cells through the gap junctions. This equation was solved by an operator splitting method (Qu & Garfinkel, 1999; Xie et al. 2004).
At the cellular level, alternans can be caused by instability of V m due to steep APD restitution. We call this V m‐driven alternans. To alter the steepness of the restitution slope, we varied the time constant of the voltage‐dependent inactivation of the L‐type Ca2+ channel (τf) (Shiferaw et al. 2005). Alternans can also be caused by instability of cycling due to a steep SR Ca2+ release vs. SR Ca2+ load relationship and Ca2+ restitution properties. We call this ‐driven alternans (Chudin et al. 1999; Shiferaw et al. 2003, 2005). To alter the instability of cycling, we varied the gain of the SR Ca2+ release function (u) (Shiferaw et al. 2005).
Coupling of Ca2+ on the APD (→V m coupling) is defined as positive (negative) if a large Ca2+ transient prolongs (shortens) the APD of the same beat (Shiferaw et al. 2005; Weiss et al. 2006) (Fig. 1 G). In our previous study (Shiferaw et al. 2005), we controlled →V m coupling with a varying relative contribution of I CaL and NCX by changing the Ca2+‐induced inactivation strength (γ). As γ is increased, I CaL dominates and →V m coupling becomes more negative. The →V m coupling is positive when γ is 0.7. By fixing γ = 0.7 and varying Ca2+‐sensitive K+ currents, we demonstrate that Ca2+‐sensitive K+ currents can change the →V m coupling.
V m and alternans can be electromechanically concordant (a Long–Short–Long–Short APD sequence corresponding to a Large–Small–Large–Small Ca2+ transient sequence) or discordant (a Long–Short–Long–Short APD sequence corresponding to a Small–Large–Small–Large Ca2+ transient sequence) (Fig. 1 H). These phenomena depend on the underlying instability mechanisms (V m‐driven or ‐driven) and the coupling between V m and cycling.
Results
I SK is an outward current during the AP. Introduction of the SK channel, while keeping all other parameters constant as in Shiferaw et al. (2005) was shown to shorten the APD (Fig. 1 F) similar to other outward currents.
Introduction of I SK increases the area of stable APs with three distinct modes of oscillations at the stability boundary
By varying the instability factors of V m (τf) and cycling (u), we plotted the stability diagram (Fig. 2 A and B) for the pacing cycle length (PCL) of 300 ms. Without I SK (Fig. 2 A), the →V m coupling is positive and alternans was always electromechanically concordant regardless of the instability mechanism. When I SK was introduced (g sk = 4 μS μF−1, EC50 = 0.7 μm), the area of the stable APs (i.e. periodic APs) was increased, evident from Fig. 2 A and B. In addition, three distinct modes of oscillations, electromechanically concordant alternans, quasiperiodic oscillations and electromechanically discordant alternans, occurred at the stability boundary, as labelled in Fig. 2 B (as C, D, E). The relation between peak [Ca2+]i and APD was plotted, with (C) corresponding to electromechanically concordant alternans, (D) representing electromechanically discordant alternans as seen by the negative relation between peak [Ca2+]i and APD, while (E) shows the quasiperiodic oscillation with corresponding orbit in peak [Ca2+]i–APD plane (Fig. 2 E right panel). From our previous study (Shiferaw et al. 2005) three modes of oscillations implies that the →V m coupling is negative.
Figure 2. Effects of I SK at the cellular level.
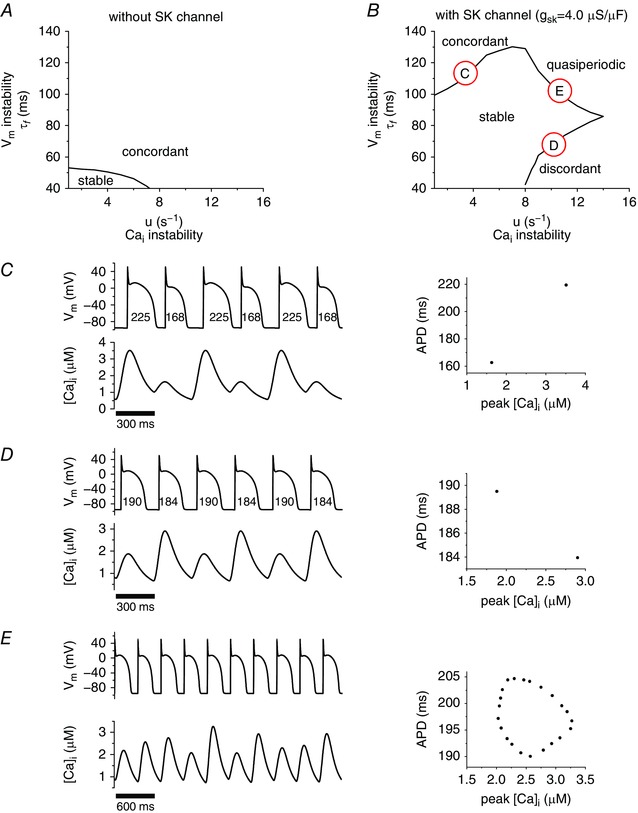
Stability boundaries were numerically determined for both the baseline system and the baseline plus SK, as seen in A and B, respectively. A demonstrates one mode of instability, namely concordant alternans, while B shows three distinct modes of instability; concordant alternans (C), discordant alternans (D), and quasiperiodic oscillation (E).
Affinity of [Ca2+] also affects V m– dynamics
Figure 3 A shows how the stability boundary curves (at PCL = 300 ms) shift with increasing I SK conductance (g sk). Figure 3 B shows how these curves shift as the I SK [Ca2+]i dependence (EC50) is altered from 0.1 to 1.0 μm. When Ca2+ affinity is high (lower EC50), I SK shortens both short AP and long AP regardless of the amplitude of the Ca2+ transient. However, when Ca2+ affinity becomes lower (higher EC50), I SK shortens only when the amplitude of the Ca2+ transient is large. This means that the change in the APD becomes larger even when the change in the amplitude of the Ca2+ transient is the same. This promotes negative →V m coupling (ΔAPD vs. Δpeak [Ca2+]i will become steeper).
Figure 3. Effects of the maximum conductance and Ca2+ affinity of I SK .
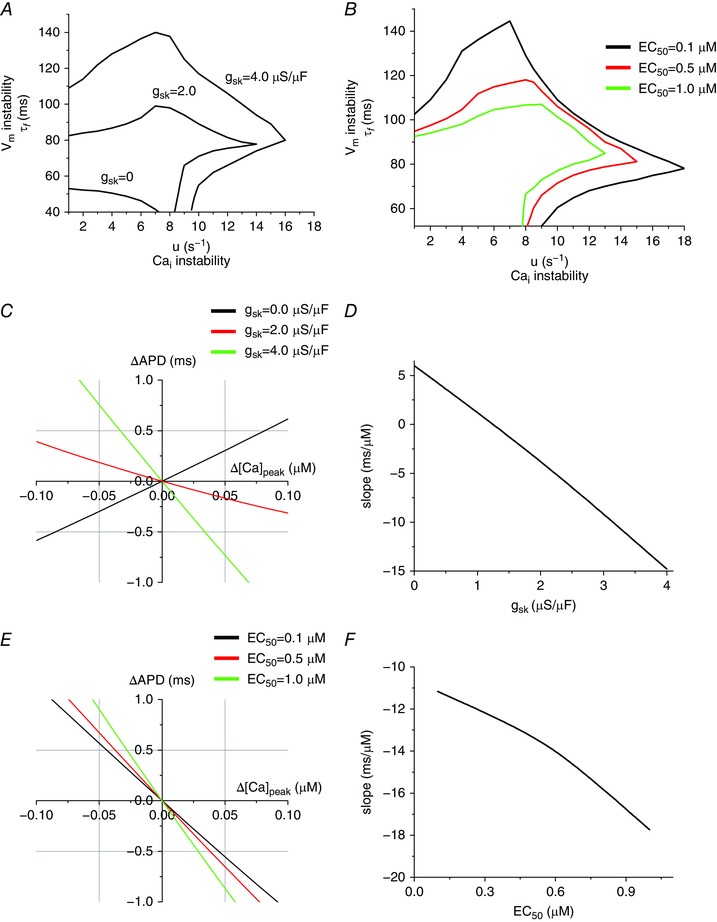
A, stability boundaries plotted for three g sk, with values of 0, 2.0 and 4.0 μS μF−1. B, stability boundaries plotted for three EC50 values of 1.0, 0.5 and 0.1 μm corresponding to the black, red and green curves, respectively. C, the slope of ΔAPD vs. Δ[Ca2+]peak indicates the →V m coupling. When g sk = 0 μS μF−1, the →V m coupling is positive, while when g sk = 4.0 μS μF−1, the →V m coupling is negative. EC50 is 0.7 μm. D, slope (ΔAPD/Δ[Ca2+]peak) vs. g sk. E, ΔAPD vs. Δ[Ca2+]peak when EC50 is varied. g sk is 4.0 μS μF−1. F, slope (ΔAPD/Δ[Ca2+]peak) vs. EC50.
To test this idea of coupling change, we plotted ΔAPD vs. peak [Ca2+]i (Fig. 3 C) for small changes in [Ca2+]i. Peak [Ca2+]i was varied by changing initial (diastolic) SR Ca2+ load. Without I SK the positive slope indicates positive →V m coupling, but as g sk increases the slope flattens and by g sk = 4 μS μF−1, the →V m coupling becomes substantially negative (Fig. 3 C). The sign was changed around g sk = 1 μS μF−1 (Fig. 3 D). Higher Ca2+ affinity also makes the →V m coupling more negative (Fig. 3 E and F).
I Ks is also Ca2+ sensitive. As expected, qualitatively similar results are seen with increasing I Ks as were seen with I SK. When the maximum conductance of I Ks (g ks) is reduced by half, we observed electromechanically concordant alternans at the stability boundaries (Fig. 4 A). On the other hand, when g ks is increased to 300%, we observed three modes of oscillations (Fig. 4 B). These modes are plotted in Fig. 4 C (electromechanically concordant alternans), Fig. 4 D (electromechanically discordant alternans), and Fig. 4 E (quasiperiodic oscillations).
Figure 4. Effects of I Ks at the cellular level.
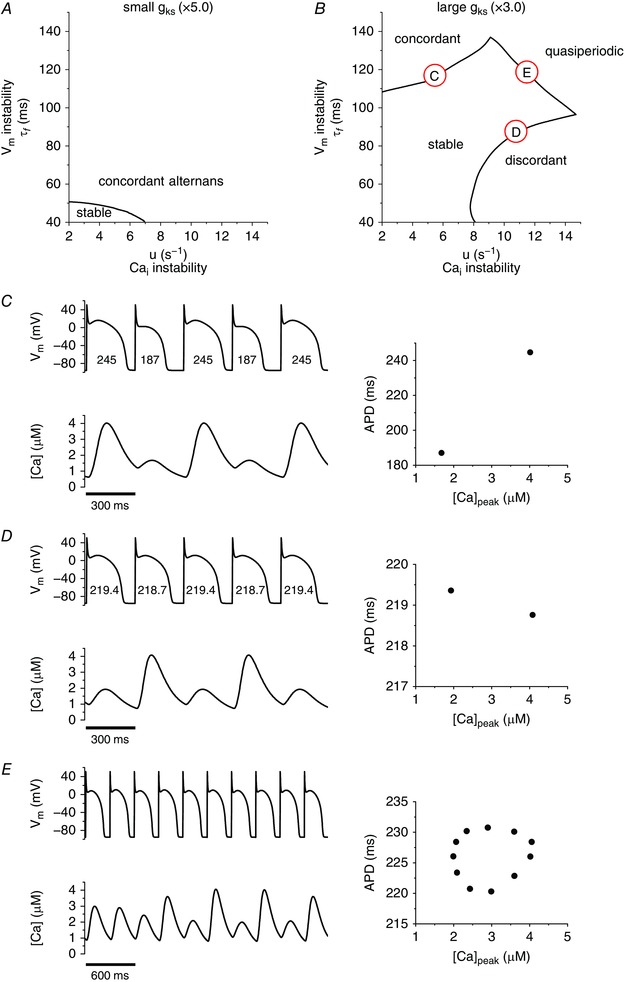
A, stability diagram when g ks is small (50% of the original value, the original g ks is 0.0245 mS μF−1). Alternans is always electromechanically concordant. B, stability diagram when g ks is large (300% of the original value). In this case, there are three distinct modes of instability; electromechanically concordant alternans (C), electromechanically discordant alternans (D), and quasiperiodic oscillation (E).
Both the maximum conductance (g ks) and Ca2+ sensitivity affect the stability boundaries and the modes of oscillations
When g ks was increased from 50 to 300%, it not only increased the stable area but also induced three modes (Fig. 5 A). On the other hand, when Ca2+ sensitivity was decreased, it suppressed electromechanically concordant and discordant alternans but promoted quasiperiodic oscillations (Fig. 5 B). This indicates that Ca2+ sensitivity changed only the coupling without changing V m and instabilities. Figure 5 C and D shows positive →V m coupling when I Ks is small (g ks × 0.5) and negative →V m coupling when I Ks is large (g ks × 3).
Figure 5. Effects of the maximum conductance and Ca2+ affinity of I Ks .
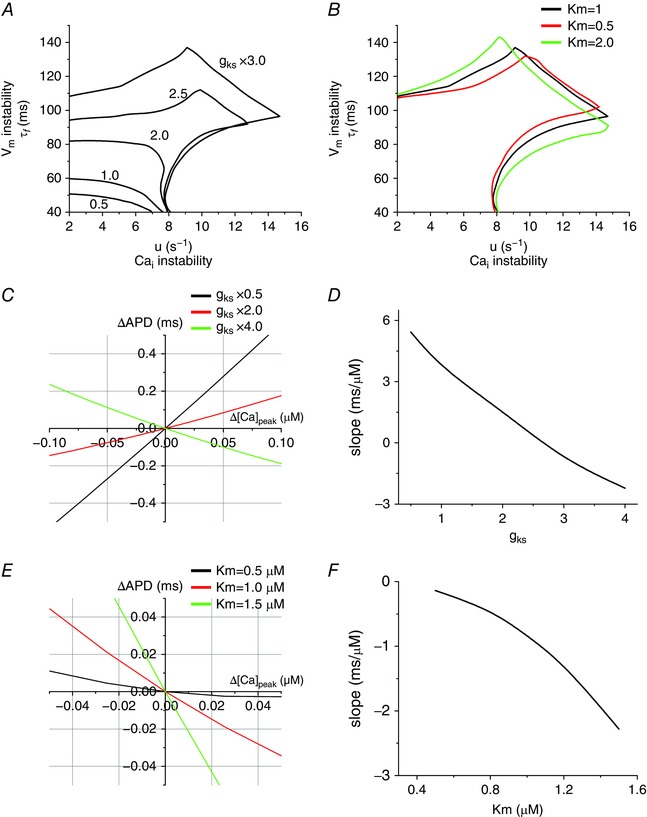
A, stability boundaries plotted for multiple g ks, with values of 50, 100, 200, 250 and 300% of the original value (0.0245 mS μF−1). B, stability boundaries plotted for three K m, with values of 1.0, 0.5 and 2.0 μm corresponding to the black, red and green curves, respectively. C, ΔAPD vs. Δ[Ca2+]peak when g ks is varied. D, slope (ΔAPD/Δ[Ca2+]peak) vs. g ks. E, ΔAPD vs. Δ[Ca2+]peak when K m is varied. F, slope (ΔAPD/Δ[Ca2+]peak) vs. K m.
From these single cell simulations, we summarize as follows. If alternans is ‐driven (small τf and large u, along the abscissa in Figs 2, 3, 4), increasing the maximum conductance of I SK or I Ks promotes electromechanically discordant alternans (Fig. 6 A). On the other hand, if alternans is V m‐driven (large τf and small u, along ordinate in Figs 2, 3, 4), electromechanically concordant alternans remains electromechanically concordant even when the maximum conductance of I SK or I Ks is increased (Fig. 6 B).
Figure 6. Summary of the effects of Ca2+ ‐sensitive K+ currents.
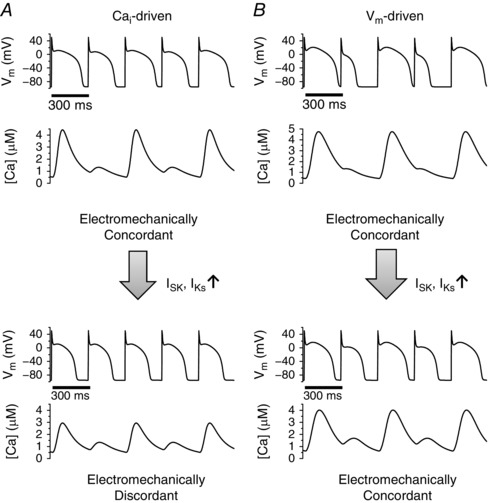
A, if alternans is ‐driven, Ca2+‐sensitive K+ currents promote electromechanically discordant alternans. B, if alternans is V m‐driven, electromechanically concordant alternans remains electromechanically concordant even when Ca2+‐sensitive K+ currents are increased.
At the tissue level, increasing I SK or I Ks leads to different types of spatially discordant alternans
In tissue, cellular level instability mechanisms lead to different alternans. We paced the left‐most five cells of the 6 cm (400 cell) homogeneous cable. First, we paced the cable at a PCL of 600 ms until it reached the steady state. At this PCL, there is no alternans. Then, the PCL was decreased to 300 ms. Alternans gradually developed. When all cells in the cable reached the steady state, we plotted APD and peak [Ca2+]i along the cable (Fig. 7).
Figure 7. Effects of I SK and I Ks at the tissue level.
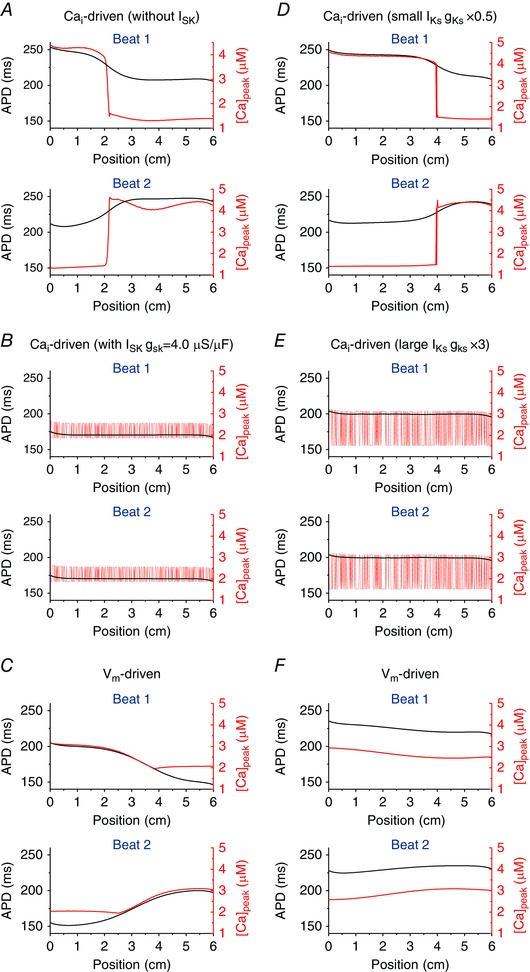
A, Ca2+‐driven alternans without I SK. The mechanism of spatially discordant alternans is competition between synchronization due to diffusive coupling and desynchronization due to stochasticity. B, Ca2+‐driven alternans with I SK. The mechanism of spatially discordant alternans is Turing‐type instability. C, V m‐driven alternans. The mechanism of spatially discordant alternans is interaction between APD and conduction velocity restitution. When alternans is V m‐driven, changing the magnitude of I SK does not change the mechanism of spatially discordant alternans. In this simulation, g sk is 4.0 μS μF−1. D, Ca2+‐driven alternans when g ks is small (50% of the original value). The mechanism of spatially discordant alternans is competition between synchronization due to diffusive coupling and desynchronization due to stochasticity. E, Ca2+‐driven alternans when g ks is large (300% of the original value). The mechanism of spatially discordant alternans is Turing‐type instability. F, V m‐driven alternans. The mechanism of spatially discordant alternans is interaction between APD and conduction velocity restitution. When alternans is V m‐driven, changing the magnitude of I Ks does not change the mechanism of spatially discordant alternans. In this simulation, g ks is 300% of the original value.
When alternans are ‐driven (small τf and large u), if these currents are small, the →V m coupling is positive and the mechanism of spatially discordant alternans is due to competition between synchronization due to diffusive electrical coupling and desynchronization due to Ca2+‐related stochasticity (Sato et al. 2013). The mechanism of spatially discordant alternans does not depend on the details of the ionic currents. This occurs whenever the cellular level instability mechanism is ‐driven and the →V m coupling is positive. In this case, the spatial scale of phase reversal of alternans is short (Sato et al. 2007) (Fig. 7 A and D), where spatially discordant alternans is shown. However, when I SK or I Ks becomes large, the →V m coupling becomes negative and the mechanism of spatially discordant alternans is due to Turing‐type instability (instability due to electrotonic coupling) (Sato et al. 2006) (Fig. 7 B and E). The mechanism of this spatially discordant alternans is also model independent and requires only ‐driven instability and negative →V m coupling. If alternans is V m‐driven, the mechanism of spatially discordant alternans is due to interaction between APD and conduction velocity restitution (Echebarria & Karma, 2002, 2007)(Fig. 7 C and F). In this case, the spatial scales of phase reversal of alternans is large (e.g. vs. that in Fig. 7 A and D) (Sato et al. 2007).
Discussion
In this study, we have shown that Ca2+‐sensitive K+ currents I Ks and I SK promote negative →V m coupling, which creates three modes of instability at the cellular level and Turing‐type instability at the tissue level.
In 1968, Nolasco and Dahlen used APD restitution, which is the relationship between APD and the previous diastolic interval (DI), APD(n + 1) = Function (F) (DI(n)), to demonstrate that the formation of alternans occurs when the slope of the APD restitution curve exceeds unity (Nolasco & Dahlen, 1968). This interpretation provides a model for the relationship of V m and APD stability. However, this one‐dimensional map cannot explain the existence of three distinct modes (electromechanically concordant/discordant alternans and quasiperiodicity) of instability (Shiferaw et al. 2005), which have been shown experimentally (Rubenstein & Lipsius, 1995; Gilmour et al. 1997; Walker & Rosenbaum, 2003).
One possible mechanism for these multiple modes is the interactions between V m and cycling. cycling can be unstable when the myocyte is Ca2+ overloaded or RyRs are sensitized. cycling can also be unstable when the cell is rapidly paced. In fact, Chudin et al. have demonstrated that transients exhibit alternans even with AP clamp waveform (i.e. APs are periodic) (Chudin et al. 1999). This implies that cycling has its own non‐linear dynamics (Dilly & Lab, 1988; Hall et al. 1999; Hall & Gauthier, 2002; Fox et al. 2002 a; Pruvot et al. 2004; Picht et al. 2006; Wang et al. 2014).
In the present study, we used a computational model which shows both non‐linearities of V m and cycling. These two non‐linear systems are coupled via Ca2+‐sensitive currents. As the myocyte experiences a large Ca2+ transient, the open probability of the Ca2+‐sensitive K+ channel will increase, increasing outward K+ current. This larger Ca2+ transient also promotes Ca2+‐dependent inactivation of L‐type Ca2+ channels, limiting inward Ca2+ current, so both K+ and Ca2+ current effects tend to promote negative →V m coupling. However, Ca2+‐dependence increases in inward current via NCX (due to changes in electrochemical driving force) promoting positive →V m coupling. Net changes in the competition between these Ca2+‐dependent currents produces the transition from positive to negative →V m coupling. Any increase in Ca2+‐dependent K+ current (I Ks or I SK) would tend to shift the coupling negative. Moreover, increasing either I Ks or I SK reveals three modes of instability. As the →V m coupling becomes more negative (with rising I Ks or I SK), the slope of ΔAPD vs. ΔCa2+ is negative, and three distinct modes of alternans are induced: (1) V m‐driven electromechanically concordant alternans (large τf, small u), (2) ‐driven electromechanically discordant alternans (small τf, large u), and (3) quasiperiodic oscillation (large τf, large u)). All three of these modes of instability have been observed in both voltage and Ca2+ recordings (Dilly & Lab, 1988; Hall et al. 1999; Hall & Gauthier, 2002; Fox et al. 2002 a; Pruvot et al. 2004).
Another important point of this study is that we introduced a novel model of the SK channel. Using this model, we demonstrate that its Ca2+ dependence (Hirschberg et al. 1998) is responsible for the observed existence of three distinct modes of instability.
Typical healthy myocytes show electromechanically concordant alternans during fast pacing. We found that as the maximum conductance of I SK was increased, electromechanically concordant alternans became electromechanically discordant when alternans is ‐driven. These findings shed light on the underlying mechanisms of cardiac alternans, especially for failing hearts since I SK was shown to be up‐regulated in ventricular myocytes in heart failure (Yu et al. 2015). In this study, we used a ventricular AP model. Alternans have also been observed in atrial cells (Kanaporis & Blatter, 2015). We expect I SK to have the same dynamical effects on alternans in atrial cells, and may be even more impactful there because of higher basal density of I SK in atrial vs. ventricular myocytes (Xu et al. 2003). Finally, our study also provides insights into the non‐linearities of cardiac tissue behaviour and a potential link between molecular processes within the cell to the development of disorders of the organ itself.
Additional information
Competing interests
None declared.
Author contributions
All authors contributed ideas and discussion. M.K. and D.S. performed computer simulations and mathematical analysis. All authors wrote the manuscript, approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by National Institutes of Health grant R00‐HL111334, American Heart Association Grant‐in‐Aid 16GRNT31300018 and Amazon AWS Cloud Credits for Research program (D.S.); R01‐HL105242 (D.M.B.); and R01‐HL085844 (N.C.).
Translational perspective
Recent experimental studies showed that I SK becomes extremely large in failing hearts. Thus, understanding the role of the SK channel in alternans dynamics is potentially important to develop new drugs and therapies for heart failure. In this study, we investigated the role of Ca2+‐sensitive K+ channels (I SK and I Ks) on V m and Ca2+ dynamics. An increase of Ca2+‐sensitive K+ currents can be responsible for electromechanically discordant alternans and quasiperiodic oscillations at the cellular level and Turing‐type spatially discordant alternans in tissue. These results provide theoretical bases to understand and interpret experimental and clinical results.
Supporting information
Disclaimer: Supporting information has been peer‐reviewed but not copyedited.
Data S1: Details of the model.
Linked articles This article is highlighted by a Perspective by Huang. To read this Perspective, visit https://doi.org/10.1113/JP273815.
References
- Chang P‐C & Chen P‐S (2015). SK channels and ventricular arrhythmias in heart failure. Trends Cardiovasc Med 25, 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P‐C, Hsieh Y‐C, Hsueh C‐H, Weiss JN, Lin S‐F & Chen P‐S (2013. a). Apamin induces early afterdepolarizations and torsades de pointes ventricular arrhythmia from failing rabbit ventricles exhibiting secondary rises in intracellular calcium. Heart Rhythm 10, 1516–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PC, Turker I, Lopshire JC, Masroor S, Nguyen BL, Tao W, Rubart M, Chen PS, Chen Z & Ai T (2013. b). Heterogeneous upregulation of apamin‐sensitive potassium currents in failing human ventricles. J Am Heart Assoc 2, e004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudin E, Goldhaber J, Garfinkel A, Weiss J & Kogan B (1999). Intracellular Ca2+ dynamics and the stability of ventricular tachycardia. Biophys J 77, 2930–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilly SG & Lab MJ (1988). Electrophysiological alternans and restitution during acute regional ischaemia in myocardium of anaesthetized pig. J Physiol 402, 315–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echebarria B & Karma A (2002). Instability and spatiotemporal dynamics of alternans in paced cardiac tissue. Phys Rev Lett 88, 208101. [DOI] [PubMed] [Google Scholar]
- Echebarria B & Karma A (2007). Amplitude equation approach to spatiotemporal dynamics of cardiac alternans. Phys Rev E Stat Nonlin Soft Matter Phys 76, 051911. [DOI] [PubMed] [Google Scholar]
- Fox JJ, Bodenschatz E & Jr Gilmour RF (2002. a). Period‐doubling instability and memory in cardiac tissue. Phys Rev Lett 89, 138101. [DOI] [PubMed] [Google Scholar]
- Fox JJ, McHarg JL & Jr Gilmour RF (2002. b). Ionic mechanism of electrical alternans. Am J Physiol Heart Circ Physiol 282, H516–H530. [DOI] [PubMed] [Google Scholar]
- Garfinkel A, Kim YH, Voroshilovsky O, Qu Z, Kil JR, Lee MH, Karagueuzian HS, Weiss JN & Chen PS (2000). Preventing ventricular fibrillation by flattening cardiac restitution. Proc Natl Acad Sci USA 97, 6061–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour RF, Otani NF & Watanabe MA (1997). Memory and complex dynamics in cardiac Purkinje fibers. Am J Physiol Heart Circ Physiol 272, H1826–H1832. [DOI] [PubMed] [Google Scholar]
- Groenendaal W, Ortega FA, Krogh‐Madsen T & Christini DJ (2014). Voltage and calcium dynamics both underlie cellular alternans in cardiac myocytes. Biophys J 106, 2222–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GM, Bahar S & Gauthier DJ (1999). Prevalence of rate‐dependent behaviors in cardiac muscle. Phys Rev Lett 82, 2995–2998. [Google Scholar]
- Hall GM & Gauthier DJ (2002). Experimental control of cardiac muscle alternans. Phys Rev Lett 88, 198102. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Shiferaw Y, Sato D, Nihei M, Lin S‐F, Chen P‐S, Garfinkel A, Weiss JN & Qu Z (2007). Dynamic origin of spatially discordant alternans in cardiac tissue. Biophys J 92, 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg B, Maylie J, Adelman JP & Marrion NV (1998). Gating of recombinant small‐conductance Ca‐activated K+ channels by calcium. J Gen Physiol 111, 565–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongyuan B, Xin D, Jingwen Z, Li G & Yajuan N (2016). Apamin‐sensitive small conductance calcium‐activated potassium channels were negatively regulated by captopril in volume‐overload heart failure rats. J Membr Biol 249, 429–436. [DOI] [PubMed] [Google Scholar]
- Hsieh YC, Chang PC, Hsueh CH, Lee YS, Shen C, Weiss JN, Chen Z, Ai T, Lin SF & Chen PS (2013). Apamin‐sensitive potassium current modulates action potential duration restitution and arrhythmogenesis of failing rabbit ventricles. Circ Arrhythm Electrophysiol 6, 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh C‐H, Chang P‐C, Hsieh Y‐C, Reher T, Chen P‐S & Lin S‐F (2013). Proarrhythmic effect of blocking the small conductance calcium activated potassium channel in isolated canine left atrium. Heart Rhythm 10, 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaporis G & Blatter LA (2015). The mechanisms of calcium cycling and action potential dynamics in cardiac alternans. Circ Res 116, 846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurita KR & Rosenbaum DS (2008). Cellular mechanisms of arrhythmogenic cardiac alternans. Prog Biophys Mol Biol 97, 332–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Timofeyev V, Tuteja D, Xu D, Lu L, Zhang Q, Zhang Z, Singapuri A, Albert TR, Rajagopal AV, Bond CT, Periasamy M, Adelman J & Chiamvimonvat N (2009). Ablation of a Ca2+‐activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol 587, 1087–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Zhang Q, Timofeyev V, Zhang Z, Young JN, Shin H‐S, Knowlton AA & Chiamvimonvat N (2007). Molecular coupling of a Ca2+‐activated K+ channel to L‐type Ca2+ channels via α‐actinin2. Circ Res 100, 112–120. [DOI] [PubMed] [Google Scholar]
- Mahajan A, Shiferaw Y, Sato D, Baher A, Olcese R, Xie LH, Yang MJ, Chen PS, Restrepo JG, Karma A, Garfinkel A, Qu Z & Weiss JN (2008). A rabbit ventricular action potential model replicating cardiac dynamics at rapid heart rates. Biophys J 94, 392–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant FM & Armoundas AA (2012). Role of substrate and triggers in the genesis of cardiac alternans, from the myocyte to the whole heart: implications for therapy. Circulation 125, 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolasco JB & Dahlen RW (1968). A graphic method for the study of alternation in cardiac action potentials. J Appl Physiol 25, 191–196. [DOI] [PubMed] [Google Scholar]
- Pastore JM, Girouard SD, Laurita KR, Akar FG & Rosenbaum DS (1999). Mechanism linking T‐wave alternans to the genesis of cardiac fibrillation. Circulation 99, 1385–1394. [DOI] [PubMed] [Google Scholar]
- Picht E, DeSantiago J, Blatter LA & Bers DM (2006). Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res 99, 740–748. [DOI] [PubMed] [Google Scholar]
- Pruvot EJ, Katra RP, Rosenbaum DS & Laurita KR (2004). Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res 94, 1083–1090. [DOI] [PubMed] [Google Scholar]
- Qu Z & Garfinkel A (1999). An advanced algorithm for solving partial differential equation in cardiac conduction. IEEE Trans Biomed Eng 46, 1166–1168. [DOI] [PubMed] [Google Scholar]
- Rubenstein DS & Lipsius SL (1995). Premature beats elicit a phase reversal of mechanoelectrical alternans in cat ventricular myocytes: a possible mechanism for reentrant arrhythmias. Circulation 91, 201–214. [DOI] [PubMed] [Google Scholar]
- Sato D, Bers DM & Shiferaw Y (2013). Formation of spatially discordant alternans due to fluctuations and diffusion of calcium. PLoS One 8, e85365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato D & Clancy CE (2013). Cardiac electrophysiological dynamics from the cellular level to the organ level. Biomed Eng Comput Biol 5, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato D, Shiferaw Y, Garfinkel A, Weiss JN, Qu Z & Karma A (2006). Spatially discordant alternans in cardiac tissue: role of calcium cycling. Circ Res 99, 520–527. [DOI] [PubMed] [Google Scholar]
- Sato D, Shiferaw Y, Qu Z, Garfinkel A, Weiss JN & Karma A (2007). Inferring the cellular origin of voltage and calcium alternans from the spatial scales of phase reversal during discordant alternans. Biophys J 92, L33–L35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiferaw Y, Sato D & Karma A (2005). Coupled dynamics of voltage and calcium in paced cardiac cells. Phys Rev E Stat Nonlin Soft Matter Phys 71, 021903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiferaw Y, Watanabe MA, Garfinkel A, Weiss JN & Karma A (2003). Model of intracellular calcium cycling in ventricular myocytes. Biophys J 85, 3666–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja D, Xu D, Timofeyev V, Lu L, Sharma D, Zhang Z, Xu Y, Nie L, Vazquez AE, Young JN, Glatter KA & Chiamvimonvat N (2005). Differential expression of small‐conductance Ca2+‐activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol 289, H2714–H2723. [DOI] [PubMed] [Google Scholar]
- Valdivia HH (2015). Mechanisms of cardiac alternans in atrial cells: intracellular Ca2+ disturbances lead the way. Circ Res 116, 778–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ML & Rosenbaum DS (2003). Repolarization alternans: implications for the mechanism and prevention of sudden cardiac death. Cardiovasc Res 57, 599–614. [DOI] [PubMed] [Google Scholar]
- Wang L, Myles RC, De Jesus NM, Ohlendorf AK, Bers DM & Ripplinger CM (2014). Optical mapping of sarcoplasmic reticulum Ca2+ in the intact heart: ryanodine receptor refractoriness during alternans and fibrillation. Circ Res 114, 1410–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JN, Karma A, Shiferaw Y, Chen P‐S, Garfinkel A & Qu Z (2006). From pulsus to pulseless: the saga of cardiac alternans. Circ Res 98, 1244–1253. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Nivala M, Garfinkel A & Qu Z (2011). Alternans and arrhythmias: from cell to heart. Circ Res 108, 98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LD, Wan X & Rosenbaum DS (2006). Cellular alternans. Ann N Y Acad Sci 1080, 216–234. [DOI] [PubMed] [Google Scholar]
- Xie F, Qu Z, Yang J, Baher A, Weiss JN & Garfinkel A (2004). A simulation study of the effects of cardiac anatomy in ventricular fibrillation. J Clin Invest 113, 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Dong PH, Zhang Z, Ahmmed GU & Chiamvimonvat N (2002). Presence of a calcium‐activated chloride current in mouse ventricular myocytes. Am J Physiol Heart Circ Physiol 283, H302–H314. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tuteja D, Zhang Z, Xu D, Zhang Y, Rodriguez J, Nie L, Tuxson HR, Young JN, Glatter KA, Vázquez AE, Yamoah EN & Chiamvimonvat N (2003). Molecular identification and functional roles of a Ca2+‐activated K+ channel in human and mouse hearts. J Biol Chem 278, 49085–49094. [DOI] [PubMed] [Google Scholar]
- Yu C‐C, Corr C, Shen C, Shelton R, Yadava M, Rhea IB, Straka S, Fishbein MC, Chen Z, Lin S‐F, Lopshire JC & Chen P‐S (2015). Small conductance calcium‐activated potassium current is important in transmural repolarization of failing human ventricles. Circulation 8, 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Timofeyev V, Lu L, Li N, Singapuri A, Long MK, Bond CT, Adelman JP & Chiamvimonvat N (2008). Functional roles of a Ca2+‐activated K+ channel in atrioventricular nodes. Circ Res 102, 465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XD, Lieu DK & Chiamvimonvat N (2015). Small‐conductance Ca2+‐activated K+ channels and cardiac arrhythmias. Heart Rhythm 12, 1845–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supporting information has been peer‐reviewed but not copyedited.
Data S1: Details of the model.


