Abstract
Background
Interneuronal pathology is implicated in many neuropsychiatric disorders, including autism spectrum disorder (ASD) and Tourette syndrome (TS). Interneurons of the striatum, including the parvalbumin-expressing fast-spiking interneurons (FSIs) and the large cholinergic interneurons (CINs), are affected in patients with TS and in preclinical models of both ASD and TS.
Methods
To test the causal importance of these neuronal abnormalities, we have recapitulated them in vivo in developmentally normal mice, using a combination transgenic-viral strategy for targeted toxin-mediated ablation.
Results
We find that conjoint ~40% depletion of FSIs and CINs in the dorsal striatum of male mice produces spontaneous stereotypy and marked deficits in social interaction. Strikingly, these behavioral effects are not seen in female mice; since ASD and TS have a marked male predominance, this observation reinforces the potential relevance of this finding to human disease. Neither of these effects is seen when only one or the other interneuronal population is depleted; ablation of both is required. Depletion of FSIs, but not of CINs, also produces anxiety-like behavior, as has been described previously. Behavioral pathology in males after conjoint FSI and CIN depletion is accompanied by increases in activity-dependent signaling in the dorsal striatum; these alterations were not observed after disruption of only one interneuron type, or in doubly-depleted females.
Conclusions
These data indicate that disruption of CIN and FSI interneurons in the dorsal striatum is sufficient to produce network and behavioral changes of potential relevance to ASD, in a sexually dimorphic manner.
Keywords: autism-spectrum disorder, striatum, interneuron, social preference, stereotypy, anxiety
INTRODUCTION
Interneuronal pathology is increasingly recognized as a contributor to a range of neuropsychiatric symptomatology (1–6), including in autism spectrum disorder (ASD) (1, 2). Much of this work has focused on the cerebral cortex; dysregulation of interneurons of the basal ganglia (7) has, until recently, received less attention.
ASD and Tourette syndrome (TS) are frequently comorbid (8, 9). ASD is characterized by social deficits, communication deficits, and stereotyped behaviors, frequently accompanied by intellectual disability (10, 11), and affects 1.0–2.6% of the population (12). TS is characterized by persistent motor and vocal tics and affects 0.5–1.0% of the population (13). Both TS and ASD are more common in males than females (11, 12, 14). Our understanding of the pathophysiology of ASD is growing rapidly (15), and convergent evidence implicates pathology in the basal ganglia circuitry (16–19). Comorbidity patterns and the fact that the basal ganglia circuitry is implicated in both TS and ASD suggests that the pathophysiology of these two disorders may overlap (9).
Post-mortem analysis has identified deficits in striatal cholinergic (CINs) and parvalbumin-expressing striatal fast-spiking interneurons (FSIs) interneurons in individuals with refractory TS (4, 5, 20–22). These two interneuron types both regulate GABAergic inhibition in the striatal microcircuitry (directly in the case of the FSIs and indirectly in the case of the CINs), but the subcellular targets of this inhibition and its effects on the microcircuitry differ (7, 23, 24), and deficits in these two interneuron populations may, therefore, produce interactive effects.
Patients with autism have a reduced number of FSIs in the medial prefrontal cortex, compared to controls (25). Comparable data on striatal interneurons is lacking, but preclinical studies suggest that striatal interneuronal abnormalities may also contribute to ASD. For example, FSIs are reduced in number in the CNTNAP2 knockout mouse, which recapitulates a rare genetic cause of autism (26). Parvalbumin (PV)-expressing interneurons are abnormal in the cortex in the BTBR T+tf/J model of idiopathic autism; similar abnormalities have been reported in the Shank3 knockout model of monogenetic autism and the MECP2 knockout model of Rett syndrome (27). Pathology of the striatal circuitry contributes to ASD-like behavioral abnormalities in both of these models (28, 29). Shank3 knockout mice have reduced PV expression in the striatum (30). MECP2 knockout mice have an increased number of PV interneurons in the striatum, suggesting a distinct but perhaps related pathology (31); restricting MECP2 knockout to PV interneurons, throughout the brain, produces motor, sensory, and social deficits (32). Deletion of the PV gene itself produces ASD-like behaviors, including communication deficits, abnormalities in reciprocal social interactions, and stereotyped behaviors (33).
There is also preclinical evidence for involvement of striatal cholinergic interneurons (CINs) in ASD pathophysiology, although it is more limited. The BTBR T+tf/J spontaneous genetic model exhibits lower levels of acetylcholine (ACh) in the prefrontal cortex; interestingly, ASD-relevant behavioral deficits in this model – abnormal social behaviors and cognitive inflexibility – are ameliorated following infusions of ACh directly into the striatum (34).
To address the causal relationship of striatal interneuron pathology to ASD-relevant effects, we specifically depleted CIN and FSI interneurons in the dorsal striatum in developmentally normal mice (21, 22). We report that conjoint depletion of both interneuronal populations produces abnormalities in both core behavioral domains of ASD: deficits in social interaction and enhancement in stereotypic behavior. These effects are seen only after depletion of both interneuron types, not of either one alone, and are seen only in males. These results suggest that striatal interneuronal pathology may contribute causally to ASD.
MATERIALS AND METHODS
Detailed Methods are provided in the Supplementary Material.
Animals
To target FSIs we used PV-cre transgenic mice, which have been previously described (35). To target CINs we used Chat tm1(cre)Lowl transgenic mice (www.jax.org:006410), as in our previous work (21). PV-cre homozygous/ChAT-cre hemizygous breeders; were bred with wild-type C57Bl/6 mice to produce PV-cre/ChAT-cre double-hemizygous mice for experimental use.
Viruses, surgical procedures and viral infusions, and interneuron depletion
We infused virus AAVrh10 EF-DIO-sDTR-FLAG (A46) into the dorsal striatum of transgenic mice, as previously described (21, 22); this virus expresses the simian diphtheria toxin receptor (sDTR) in cre-positive cells, rendering them susceptible to ablation after systemic diphtheria toxin (DT), and GFP in cre-negative cells. Control animals received the control AAVrh10-EF-mutDIO-sDTR-FLAG (C46) virus, which expresses GFP in all infected cells (22).
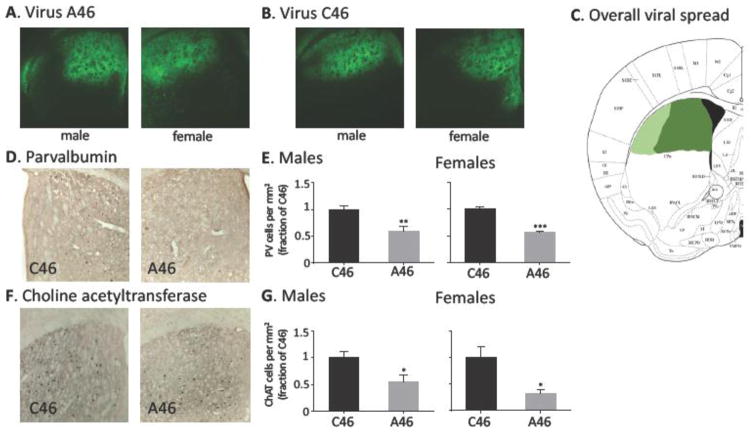
0.5 μl virus was infused bilaterally into the central dorsal striatum under ketamine/xylazine anesthesia as described previously (21), targeting coordinates AP +0.5mm, ML ±1.5mm, DV −2.7 mm (36). Typical viral spreads are shown in Figure 1A–C.
Figure 1. Specific depletion of FSIs and CINs by DT.
A. Typical viral spread in male and female A46-expressing mice. B. Typical viral spread in male and female C46-expressing control mice. C. Viral spread across all animals (dark green area expressed virus in most or animals; lighter green area expressed virus in a subset). D. PV-expressing striatal FSIs were identified by immunostaining and quantified in mice with targeted FSI/CIN depletion (A46) and negative controls (C46). E. PV-positive cells were reduced in density in A46 mice in both males (N = 7,7; t[12] = 3.49, p = 0.004) and females (N = 5 C46, 6 A46; t[9] = 5.52, p = 0.0005). F. ChAT-expressing striatal CINs were identified by immunostaining and quantified. G. ChAT-positive cells were reduced in density in A46 mice, in both males (N = 7,7; t[12] = 2.68, p = 0.02) and females (N = 5, 6; t[9] = 3.04, p = 0.016).
Two weeks after surgery, all mice received two injections of diphtheria toxin (DT, 15 or 30 μg/kg IP, one injection/day on 2 consecutive days). Behavioral analysis began 1 week after DT injection.
Drugs
Diphtheria toxin (DT, Calbiochem #322326) was dissolved in sterile saline and administered IP. Oxytocin (1 mg/kg, Tocris #1910) was dissolved in sterile saline solution and then administered IP.
Behavioral analysis
Locomotor activity was assessed using an open field apparatus (Omnitech Electronics) as described previously (37, 38).
Elevated plus maze, rotarod, and pre-pulse inhibition of startle were measured as described previously (21, 39, 40).
Social interaction
Social interaction quantifies the preference of a mouse to interact with an unfamiliar, conspecific ‘stranger’ mouse relative to an inanimate novel object (Duplo® blocks, black, of similar size to a mouse) (41). Stranger mice and control inanimate objects were placed within wire cylinders in an open field. Entries by the test mouse into and time within a social target zone (an annulus around the stranger mouse’s wire enclosure) and an equivalent nonsocial target zone were quantified.
Stereotypic behavior
Stereotypic time (grooming and sniffing) was scored from video using an automated system (Cleversys HomeCage Scan); we have previously shown that this measure correlates with manual scoring by a blinded observer (21). In an independent assay, stereotypical beam-breaks were defined as repetitive breaks of a single infrared beam within one second in the open field (42–44).
Operant behavior
Mice were food restricted to 85% body weight. Operant training was conducted in standard aluminum chambers (Med Associates) and performed in three phases. First, after shaping, mice learned to nose-poke in either of two noseports for a sucrose reward when both ports were illuminated. Next, mice learned a simple cue discrimination operant task: only one of the two noseports was illuminated, and mice learned to nose-poke at this port for a sucrose reward. Finally, the reinforcement schedule changed, such that the left noseport was always reinforced, irrespective of illumination, requiring mice to switch from using a cue to a spatial strategy. In all three phases, training continued until a predetermined behavioral criterion was reached. Trials to criterion was analyzed using general estimating equations with a Poisson loglinear distribution in SPSS, with virus (A46 vs C46) as the between-subjects factor and experimental phase as a within-subject factor.
Immunohistochemistry
Following behavioral testing, brains were removed, fixed, sliced, and immunostained as described previously (37, 38, 45, 46).
Immunopositive cells were counted from a single field in the dorsal striatum on each side in representative slices, as previously described (45, 46). N in these experiments reflects number of animals. To assay microglial activation, total Iba1 immunopositive area was quantified, as previously described (45).
Data analysis
All quantification was performed blind to experimental condition. Statistical analysis was performed using Prism 6.0 (GraphPad) or, for 3-way analyses, Minitab 17 (Minitab, Inc.) or SPSS 22.0 (IBM). Group values are presented as mean ± SEM. Pairwise comparisons were performed using Student’s t-test. More complex comparisons were performed using ANOVA with Sidak’s post-hoc. Differences between conditions were considered statistically significant at p < 0.05.
RESULTS
Conjoint depletion of CINs and FSIs
We used a viral system (21, 22) in double-transgenic ChAT-cre/PV-cre transgenic mice to achieve specific conjoint depletion of CINs and FSIs in the dorsal striatum of developmentally normal mice. Male and female mice were tested in parallel. Active (A46) or control (C46) virus was stereotaxically infused bilaterally into the dorsal striatum. 2 weeks later, all mice received DT (15 μg/kg per day x 2 days, IP). Approximately 4 weeks later, following behavioral testing, mice were sacrificed, and FSI and CIN interneuron density in the dorsal striatum was quantified. As shown in Figure 1, both PV-expressing FSIs and ChAT-expressing CINs were reduced by ~50% by this treatment, in male and female mice infused with the active DTR virus, A46, relative to the negative control virus, C46.
We produced separate cohorts of male mice in which only FSIs or only CINs were depleted (21, 22). To explore whether more complete depletion of single interneuron populations would recapitulate the effects seen after conjoint depletion of both populations, we produced additional cohorts of male mice in which DT dosage was doubled (30μg/kg for two consecutive days) (see Supplementary Figure S1); these were tested in key behavioral experiments, as detailed below.
Viral infusion and interneuronal apoptosis may produce inflammatory changes in the brain; however, we found no differences in microglial activation between the A46 and C46 groups, either 24 hours or several weeks after DT injection (Supplemental Figure S2).
Conjoint striatal FSI and CIN depletion produces elevated anxiety in male but not female mice
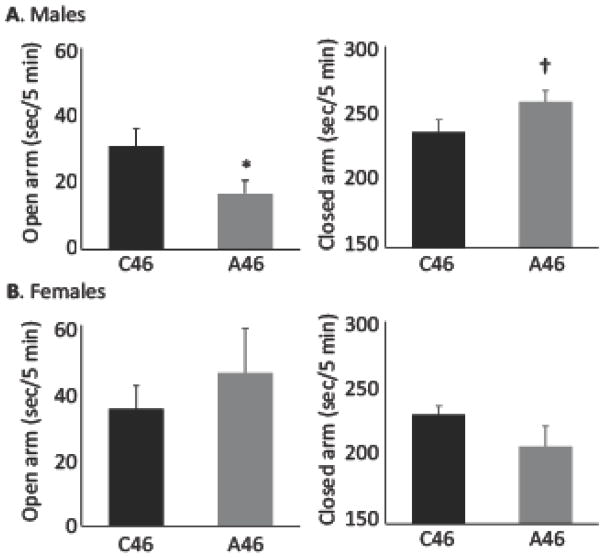
Consistent with previous results (22), male mice with conjoint depletion of FSIs and CINs in the dorsal striatum (Figure 2A), or depletion of only FSIs (Supplemental Figure S3), showed increased anxiety-like behavior in the elevated plus maze, whereas mice in which only CINs were depleted did not (Supplementary Figure S3) (21). In contrast, female mice with conjoint depletion of FSIs and CINs showed no increased anxiety-like behavior in the elevated plus maze; indeed, they showed a trend towards reduced anxiety-like behavior (Figure 2B). An omnibus ANOVA analysis confirmed this sex difference: mixed ANOVA, three-way Arm x Sex x Virus interaction (F[1,35] = 6.04, p = 0.019); main effect of Arm (F[1,35] = 779, p < 0.0001; Arm x Sex interaction (F[1,35] = 9.75, p = 0.004). Lower-order ANOVAs revealed a significant Sex x Virus interaction for closed arm occupancy (F[1,35] = 5.6, p = 0.023) and a trend for open arm occupancy (F[1,35] = 2.8, p = 0.10). These results confirm the ability of FSI depletion in the dorsal striatum, alone or conjointly with CIN depletion, to produce elevated anxiety-like behavior in male mice (22), but also reveal an unexpected sexual dimorphism.
Figure 2. Elevated anxiety in male but not female mice after conjoint FSI and CIN depletion.
A. Male mice with conjoint FSI and CIN depletion in the dorsal striatum exhibited elevated anxiety in the elevated plus maze. Open arm occupancy: t[19] = 2.26, p = 0.036; closed arm occupancy: t[19] = 1.85, p = 0.081; n = 10 C46, 11 A46. See also Supplementary Figure S2. B. Female mice with conjoint FSI and CIN depletion, in contrast, showed no significant change in anxiety. Open arm occupancy: t[16] = 0.75, p = 0.46; closed arm occupancy: t[16] = 1.51, p = 0.15; n = 9, 9.
Conjoint FSI and CIN depletion does not affect a range of control behaviors
Conjoint interneuron depletion did not alter locomotion in an unfamiliar open field environment in either male or female mice; there was nominally reduced locomotion in depleted males, but it did not approach statistical significance (Supplementary Figure S4A). There was no alteration in PPI after conjoint FSI and CIN depletion in either male or female mice (Supplementary Figure S5). We also tested these mice in an operant learning paradigm that assays reward-motivated learning (to nose-poke for food), cue discrimination, and strategy shifting; there was no difference in performance between A46 and C46 male mice in any phase, indicating intact reinforcement-driven learning, sensory processing, and cognitive flexibility (Supplemental Figure S6).
Conjoint FSI and CIN depletion produces spontaneous stereotypy in male but not in female mice
Depletion of FSIs or CINs alone does not produce stereotypy at baseline (21, 22). We tested whether conjoint depletion of both interneuron types could produce spontaneous stereotypy. Stereotypy was measured using an automated system that quantifies stereotypic behaviors from video; we have previously shown that automatic scoring is highly correlated (r > 0.8) with manual scoring from video (21).
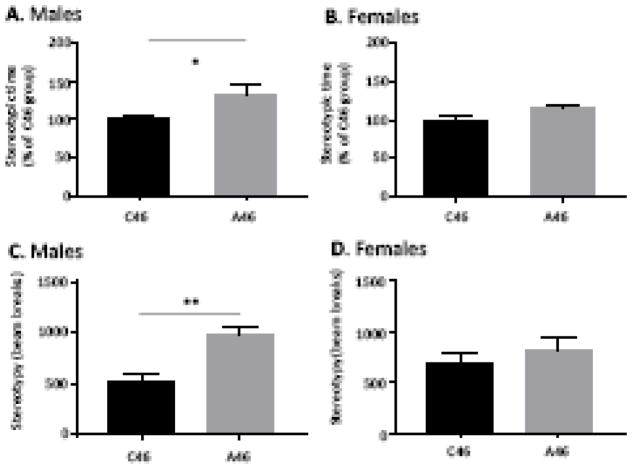
This analysis revealed elevated spontaneous grooming and related stereotypic movements in male mice after conjoint interneuron depletion (Figure 3A). In contrast, there was no spontaneous stereotypy in an identical analysis after depletion of either FSIs or CINs in isolation (Supplementary Figure S7A), consistent with previous results (21, 22). More extensive depletion of FSIs or CINs did not produce spontaneous stereotypy (Supplemental Figure S7B), indicating that this is an interactive rather than an additive effect of conjoint interneuron depletion. Ablation of CIN and FSI did not affect stereotypic behavior in female mice (Figure 3B).
Figure 3. Elevated stereotypy in male but not female mice after conjoint FSI and CIN depletion.
A. Male mice with conjoint FSI and CIN depletion in the dorsal striatum exhibited elevated spontaneous stereotypic behaviors. t[19] = 2.23, p = 0.038, n= 11 C46, n=12 A46. B. In contrast, female mice with conjoint FSI and CIN depletion in the dorsal striatum showed no elevation in stereotypic behaviors. t[16] = 0.81, p – 0.43, n=9 C46,n=9 A46. C. Increased stereotypic beam breaks confirms aberrant behavior. t[19]=3.7, p = 0.014, n= 11 C46, n=12 A46. D. No differences were found in females when stereotypic beam breaks were quantified. t[16]=0.82, p = 0.43, n= 9 C46, n=9 A46
To confirm the presence of elevated spontaneous stereotypic behavior in conjointly depleted males, we quantified repetitive beam breaks in the open field, a measure of repetitive low-amplitude movements that has been used to capture stereotypy in a number of contexts (42–44, 47). Stereotypy by this independent measure was also increased after conjoint FSI and CIN depletion in males (Figure 3C), but not in females (Figure 3D).
Social deficits in male but not in female mice after conjoint FSI and CIN depletion
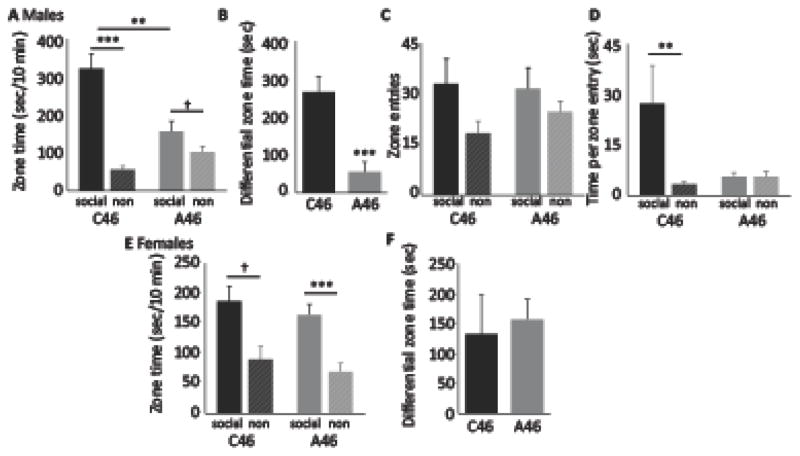
We next tested social interaction, a core ASD-relevant phenotype (48). Mice were placed in an environment containing both an unfamiliar same-sex mouse and a novel object target, and their bias towards the social target was quantified. Male mice with conjoint depletion of FSIs and CINs showed a marked reduction in social preference, with reduced time in the social target zone and increased time in the nonsocial (novel object) target zone (Figure 4A, 4B). Although interneuron-depleted mice showed much less dwell time in the social target zone (and more in the non-social target zone), the number of entries into the social target zone was not significantly different from controls (Figure 4C). Correspondingly, the time spent in the social target zone per entry was much greater than that in the non-social target zone for control mice, but not for interneuron depleted animals (Figure 4D): depleted mice entered the social target zone just as often as controls, but did not stay there. There was no significant effect of single interneuron depletion on social interaction, after either standard or more complete interneuron depletion (Supplementary Figure S8).
Figure 4. Reduced social interaction in males but not females after conjoint dorsal striatal FSI and CIN depletion.
A. Male mice with conjoint FSI and CIN depletion in the dorsal striatum exhibited markedly reduced preference for the social target zone, both in absolute terms and relative to the non-social (novel object) target zone. 2-way ANOVA: main effect of zone, F[1,19] = 37.7, p < 0.001; main effect of virus, F[1,19] = 6.4, p = 0.021; interaction, F[1.29] = 16.5, p = 0.001. Post-hoc t-test: *** p < 0.001, ** p < 0.005; † p < 0.1. B. This effect is clearly shown by plotting the difference in occupancy between the social and non-social target zones for each group; this difference was very large for control but dramatically reduced in interneuron-depleted mice (though still significantly greater than zero, which would indicate no bias, at trend level). Between-group comparison, t[19] = 4.06, p = 0.001; one-sample t-test for depleted group: t[10] = 1.90, p = 0.087. C. Entries into the social target zone were modestly greater than entries into the non-social target zone; this bias was only slightly and non-significantly blunted in interneuron-depleted animals. 2-way ANOVA: main effect of zone, F[1,19] = 7.2, p = 0.015; main effect of virus, F[1,19] = 0.15, p = 0.71; interaction, F[1,19] = 0.97, p = 0.34. D. Time spent in each target zone was dramatically greater for the social target zone in control mice; this bias was completely lost in interneuron-depleted animals. 2-way ANOVA: main effect of zone, F[1,19] = 5.5, p = 0.030; main effect of virus, F[1,19] 2.99, p = 0.10; interaction, F[1,19] = 5.4, p = 0.031. ** Wilcoxon: p < 0.01. E. In female mice there was a significant, though less dramatic, bias towards the social target zone, but it was not affected by interneuronal depletion. 2-way ANOVA: main effect of zone, F[1,16] = 17.6, p = 0.001; main effect of virus, F[1,16] = 3.04, p = 0.1; interaction, F[1,16] = 0.063, p = 0.8. F. There was a significant bias towards exploration of the social target zone, relative to the non-social target zone, in both control and depleted female mice, with no difference between the groups.
Social preference in female mice was not affected by conjoint FSI and CIN depletion (Figure 4E, F). This sexually dimorphic effect was confirmed in an ANOVA analysis across all data, with zone as a within-subject factor and sex and virus as between-subject factors: the three-way Zone x Sex x Virus interaction was significant (F[1,35] = 10.45, p = 0.003), as were main effects of Zone (F[1,35] = 52.5, p < 0.001) and Virus (F[1,35] = 8.8, p = 0.005), and 2-way interactions between Zone and Sex (F[1,35] = 4.3, p = 0.046) and between Zone and Virus (F[1,35] = 8.54, p = 0.006).
The peptide hormone oxytocin has been observed to have pro-social effects and has been investigated in preclinical models of autism (49, 50) and in patients, albeit with mixed results (51, 52). In a separate cohort of male mice with conjoint FSI/CIN depletion, we replicated both spontaneous stereotypy and social preference phenotypes. Pretreatment with oxytocin (1mg/kg) produced a modest nominal mitigation of the behavioral abnormalities, but these effects failed to reach statistical significance in primary analyses (Supplementary Figure S9).
Sexually dimorphic alterations in dorsal striatal neural activity after interneuron depletion
To identify mechanistic correlates of these behavioral effects, we examined activity-dependent markers of several different signaling pathways in striatal medium spiny neurons (MSNs) after conjoint FSI and CIN depletion, as in our recent work (38, 46).
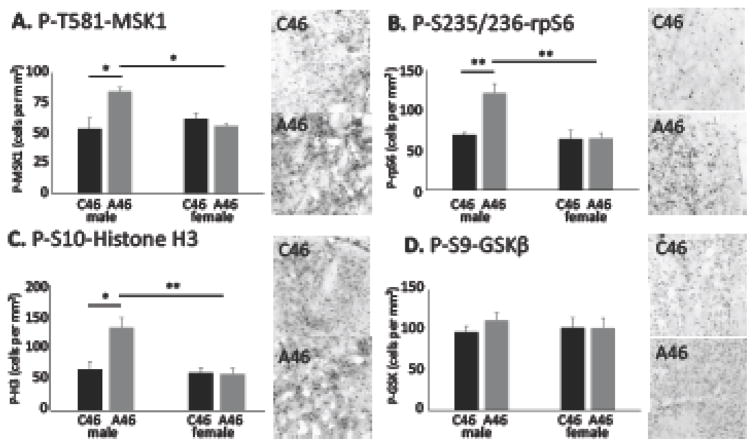
Mitogen and stress activated kinase 1 (MSK1) is a marker of MAPK signaling activity in striatal MSNs (53, 54). Phosphorylation of MSK1 was elevated in male mice after conjoint FSI and CIN depletion, but this effect was not observed in female mice (Figure 5A) or in male mice with only FSI or only CIN depletion (Supplementary Figure S10A).
Figure 5. Elevated activity-dependent neural signaling in dorsal striatum of male mice after conjoint FSI/CIN depletion.
A. Immunostaining for P-T581-MSK1 showed an increase in immuonpositive cells in dorsal striatum in males with conjoint interneuron depletion, relative to C46-injected controls, but not in females. 2-way ANOVA: main effect of virus, F[1,21] = 3.31, p = 0.08; main effect of sex, F[1,21] = 2.2, p = 0.15; interaction, F[1,21] = 6.96, = 0.015; n = 7 C46 males, n=7 A46 males, n=5 C46 females, n=6 A46 males. B. Immunostaining for P-S235/236-rpS6, a target of MAPK and many other signaling pathways in neurons, showed an increase in immunopositive cells in males with conjoint interneuron depletion but not in females. 2-way ANOVA: main effect of virus, F[1,21] = 6.58, p = 0.018; main effect of sex, F[1,21] = 8.82, p = 0.007; interaction, F[1,21] = 6.05, p = 0.023. Ns as in A. C. Immunostaining for P-S10-histone 3, which is phosphorylated in response to neural activity, showed a similar increase in in males with conjoint interneuron depletion but not in females. 2-way ANOVA: main effect of virus, F[1,21] = 4.68, p = 0.042; main effect of sex, F[1,21] = 7.1, p = 0.015; interaction, F[1,21] = 5.27, p = 0.032. Ns as in A. D. Immunostaining for P-S9-GSKβ showed no alterations in either male or female mice after interneuron depletion. 2-way ANOVA: main effect of virus, F[1,19] = 0.30, p = 0.59; main effect of sex, F[1,19] = 0.03, p = 0.86; interaction, F[1,19] = 0.32, p = 0.58. Tukey’s post-hoc: * p < 0.05, ** p < 0.01.
A similar pattern was seen in phosphorylation of the translational regulator rpS6, which is a target of MAPK signaling as well as of other activity-regulated neural signaling pathways (55). Again, elevated phosphorylation was seen in male mice after conjoint depletion of FSIs and CINs, but not in females (Figure 5B) or after single ablation of either FSIs or CINs (Supplementary Figure S10B). The same pattern was seen in phosphorylation of histone H3 at P-S10, an activity-regulated epigenetic marker that is a target of MSK1 (56) (Figure 5C, Supplementary Figure S10C).
AKT-GSKβ signaling is an important regulator of striatal function, especially in dopamine D2 receptor-expressing MSNs (42, 57), and has been implicated in preclinical studies of TS and other neuropsychiatric conditions (46, 58). However, depletion of FSIs and CINs had no effect on phosphorylation of AKT-GSKβ signaling, as measured by phosphorylation of GSKβ at S9 (Figure 5D).
DISCUSSION
ASD is heterogeneous and derives from multiple, interacting developmental and environment influences. Mechanistic studies in animals can identify abnormalities that are sufficient to produce ASD-relevant effects, complementing correlational studies in patients. There is evidence that aberrant striatal interneuronal function can contribute to ASD (29–31, 34), as well as to TS (4, 5, 20–22). We show that depletion of CINs and FSIs produces behavioral abnormalities in core domains of ASD symptomatology (10), similar to what is seen in models of rare genetic forms of autism (28, 59). Our results indicate that striatal interneuron disruption is sufficient to cause ASD-relevant behavioral abnormalities.
Most strikingly, conjoint interneuron depletion leads to a marked deficit in social interaction in males. Social deficits are a defining symptom of ASD (10, 11) and have been documented in a number of mouse models that are based on rare but high-penetrance ASD-associated mutations (26, 28). In our social preference assay, interneuron-depleted mice enter the social target zone just as often as controls, but they do not remain there nearly as long (Figure 4C). This suggests intact exploration of the environment, including the social zone, but no differential interaction with the social target. Performance in an instrumental learning task that tests both sensory and contextual/spatial discrimination is intact (Supplemental Figure S6), suggesting that the preference deficit is specific to social situations. These results, in conjunction with previous preclinical studies (34), focus attention on the relevance of dorsal striatal pathology in the development of ASD-relevant social deficits.
The second core symptom domain in ASD consists of repetitive behaviors or restricted interests (10). Restricted interest is difficult to model in a mouse, but repetitive behaviors are readily assayed, and they, too, are elevated in a number of genetic models of ASD with etiologic validity (26, 28). We find an elevation of stereotypic behaviors after dorsal striatal FSI and CIN depletion, extending the parallel to ASD and to well-established models.
It is striking that these two behavioral effects emerge only after conjoint depletion of both FSIs and CINs, and not after similar or greater depletion of either population alone (Supplementary Figure S7, S8). Although FSIs and CINs modulate the striatal microcircuitry in qualitatively different ways (7), they both can directly or indirectly inhibit MSNs (23, 24). It is possible that either interneuron population can compensate for a deficit in the other under normal circumstances, such that dysregulated baseline striatal activity and corresponding behavioral effects emerge only when both are depleted. However, our results suggest that these sources of MSN inhibition are not redundant; the key behavioral effects do not emerge after depletion of only FSIs or only CINs, even at higher levels of depletion. Reduction of both populations appears to be required for these effects.
We also observed elevated anxiety after interneuron depletion; this is attributable to FSI depletion. We have previously shown that depletion of FSIs in the dorsal striatum of male mice produces elevated anxiety-like behavior (22), while depletion of CINs does not (21); we replicate these findings here (Supplemental Figure S3). Anxiety is not a defining symptom of ASD; but comorbid anxiety is seen in ~40% of youth with the diagnosis (60, 61).
Perhaps our most dramatic finding is that social impairments, stereotypic behaviors, and anxiety-like behavior are observed only in male mice. ASD has a 5:1 male predominance (11); partially for this reason, most behavioral studies in mouse models have examined only male animals (28, 59, 62). The ability of an identical cellular depletion to produce such divergent effects in male and female mice draws attention to the importance of examining pathophysiological processes in both sexes.
Behavioral experiments were not timed with respect to estrous cycle in this study. Repetitive behaviors described as ‘compulsive’ can vary with the estrous cycle (63). Any such effect in our model would necessarily be subtle, however, for there to be no evident spread of the data or trend-level effects in the current analysis. The theoretical possibility of a hormone dependent modulation of these behavioral effects does not weaken our core conclusion: that depletion of CINs and FSIs generates differential effects on striatal circuitry and ASD-relevant behaviors in males and females.
The mechanisms underlying this striking sexual dimorphism, hormonal or otherwise, remain to be established. As a first step in this direction, we tested the ability of oxytocin to mitigate behavioral effects of interneuronal depletion in males. Oxytocin has prosocial effects and has been explored as a therapeutic agent in preclinical and clinical studies of ASD (49–52, 64). Pretreatment with oxytocin produced a modest but nonsignificant reduction in both stereotypy and social deficits in interneuron-depleted male mice (Supplemental Figure S9). Other hormonal effects may contribute to the sexual dimorphism. For example, estradiol modulates striatal DA release and CIN activity (65, 66). Allopregnanolone, a metabolite of progesterone, enhances GABAergic neurotransmission through positive allosteric modulation of GABAA receptors (67). These or related mechanisms in females may mitigate the consequences of interneuronal depletion; this is an important topic for future study.
Depletion of CINs and FSIs in the dorsal striatum produced a marked effect on activity-dependent signaling in dorsal striatal MSNs in males. Interneuron depletion increased phosphorylation of rpS6, a regulator of activity-dependent translation, at P-235/236 (55, 68). Elevated phosphorylation was also seen at MSK1 T581, a marker of the MAPK cascade. Finally, phosphorylation of the MSK1 target H3 S10, a marker of epigenetic changes (69, 70), was also elevated. Together, these molecular changes suggest increased activity of dorsal striatal MSNs after interneuron depletion. Alterations in markers of both activity-regulated translation and epigenetic regulation suggest mechanisms whereby changes in neuronal activity may lead to long-lasting alterations in network function.
Aberrant signaling suggestive of elevated MSN activity after interneuron depletion is unsurprising, as local interneurons are a major source of inhibitory tone on MSNs. FSIs are major source of feed-forward inhibition in the striatum (7, 24). CINs can indirectly regulate MSN inhibition, through their regulation of GABAergic NPY-neuroglia form interneurons and/or the more recently identified 5HT3a-expressing GABAergic interneurons (7, 23, 71, 72).
Targeted depletion of specific interneurons in a developmentally normal adult is, of course, not a faithful recapitulation of the mechanisms of pathophysiology in patients with ASD, which is a neurodevelopmental condition (although it may have later onset associated with neuroinflammation in some cases; (73)). Furthermore, alterations in striatal interneurons, where they have been documented in clinical or preclinical studies, may reflect changes in cell phenotype that alter cell function and detection (such as reduced PV), rather than frank cell loss (30). Our experiments do not sek to fully recapitulate the pathophysiology of ASD, but rather to test the hypothesis that disruption of these specific interneurons in the dorsal striatum is sufficient to produce disease-relevant network and behavioral effects, independent of developmental disruptions. Such hypotheses cannot be tested in clinical studies or in brain-wide knockouts (26, 28, 33, 74) (or even brain-wide, cell type-specific manipulations (32)), as there is always the possibility of neuroanatomically off-target or developmental effects.
In sum, these studies provide compelling evidence that conjoint disruption of PV-expressing FSIs and cholinergic CINs in the dorsal striatum is sufficient to produce social deficits and stereotypic behavior, with potential relevance to the understanding of ASD. These effects are seen only in male mice, increasing the face validity of this manipulation for ASD, which has a much higher incidence in males (11). The consequences of interneuron ablation are independent of developmental disruptions, as the manipulation is performed in developmentally normal young adult mice. These behavioral phenotypes are not due to primary pathology elsewhere in the brain, as the viral manipulation is targeted specifically to the dorsal striatum. Future studies should examine the effects of reversible interneuronal dysregulation, and the mechanisms whereby female mice are protected from the pathogenic effects of interneuron disruption.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01MH091861 (CP), postdoctoral fellowship support from the Tourette Syndrome Association (MX, LRF), the Brain and Behavior Research Fund (MR), and the Allison Family Foundation (CP). This work was also funded in part by the State of Connecticut, Department of Mental Health and Addiction Services though its support of the Ribicoff Research Facilities at the Connecticut Mental Health Center, but this publication does not express the views of the Department of Mental Health and Addiction Services or the State of Connecticut. The views and opinions expressed are those of the authors. We gratefully acknowledge Stacey Wilber for assistance with mouse breeding and genotyping, Marina Picciotto for helpful comments on the manuscript, and our colleagues in the Division of Molecular Psychiatry at Yale University for many helpful conversations.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chattopadhyaya B, Cristo GD. GABAergic circuit dysfunctions in neurodevelopmental disorders. Frontiers in psychiatry. 2012;3:51. doi: 10.3389/fpsyt.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, Nutt DJ. GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neuroscience and biobehavioral reviews. 2012;36:2044–2055. doi: 10.1016/j.neubiorev.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biological psychiatry. 2015;77:1031–1040. doi: 10.1016/j.biopsych.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci U S A. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2007;32:471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreitzer AC. Physiology and pharmacology of striatal neurons. Annual review of neuroscience. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- 8.Canitano R, Vivanti G. Tics and Tourette syndrome in autism spectrum disorders. Autism: the international journal of research and practice. 2007;11:19–28. doi: 10.1177/1362361307070992. [DOI] [PubMed] [Google Scholar]
- 9.Clarke RA, Lee S, Eapen V. Pathogenetic model for Tourette syndrome delineates overlap with related neurodevelopmental disorders including Autism. Transl Psychiatry. 2012;2:e158. doi: 10.1038/tp.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Association AP. Diagnostic and Statistical Manual of Mental Disorders. Washington DC: American Psychiatric Association; 2013. [Google Scholar]
- 11.CDC. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years. Surveillance Summaries. 2014:1–21. [Google Scholar]
- 12.Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, et al. Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry. 2011;168:904–912. doi: 10.1176/appi.ajp.2011.10101532. [DOI] [PubMed] [Google Scholar]
- 13.Scahill L, Dalsgaard S. Prevalence and methods for population screening. In: Martno D, Leckman JF, editors. Tourette Syndrome. New York: Oxford University Press; 2013. [Google Scholar]
- 14.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. Jama. 2014;311:1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krumm N, O’Roak BJ, Shendure J, Eichler EE. A de novo convergence of autism genetics and molecular neuroscience. Trends Neurosci. 2014;37:95–105. doi: 10.1016/j.tins.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuccillo MV. Striatal Circuits as a Common Node for Autism Pathophysiology. Front Neurosci. 2016;10:27. doi: 10.3389/fnins.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delmonte S, Gallagher L, O’Hanlon E, McGrath J, Balsters JH. Functional and structural connectivity of frontostriatal circuitry in Autism Spectrum Disorder. Front Hum Neurosci. 2013;7:430. doi: 10.3389/fnhum.2013.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, et al. Aberrant striatal functional connectivity in children with autism. Biological psychiatry. 2011;69:847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ha S, Sohn IJ, Kim N, Sim HJ, Cheon KA. Characteristics of Brains in Autism Spectrum Disorder: Structure, Function and Connectivity across the Lifespan. Exp Neurobiol. 2015;24:273–284. doi: 10.5607/en.2015.24.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lennington JB, Coppola G, Kataoka-Sasaki Y, Fernandez TV, Palejev D, Li Y, et al. Transcriptome Analysis of the Human Striatum in Tourette Syndrome. Biol Psychiatry. 2016;79:372–382. doi: 10.1016/j.biopsych.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu M, Kobets A, Du JC, Lennington J, Li L, Banasr M, et al. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proc Natl Acad Sci U S A. 2015;112:893–898. doi: 10.1073/pnas.1419533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu M, Li L, Pittenger C. Ablation of fast-spiking neurons in the dorsal striatum, recapitulating abnormalities seen post-mortem in Tourette syndrome, produces anxiety and elevated grooming. Neuroscience. 2016;324:321–329. doi: 10.1016/j.neuroscience.2016.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faust TW, Assous M, Tepper JM, Koos T. Neostriatal GABAergic Interneurons Mediate Cholinergic Inhibition of Spiny Projection Neurons. J Neurosci. 2016;36:9505–9511. doi: 10.1523/JNEUROSCI.0466-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gittis AH, Nelson AB, Thwin MT, Palop JJ, Kreitzer AC. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J Neurosci. 2010;30:2223–2234. doi: 10.1523/JNEUROSCI.4870-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashemi E, Ariza J, Rogers H, Noctor SC, Martinez-Cerdeno V. The Number of Parvalbumin-Expressing Interneurons Is Decreased in the Medial Prefrontal Cortex in Autism. Cereb Cortex 2016 [Google Scholar]
- 26.Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gogolla N, Takesian AE, Feng G, Fagiolini M, Hensch TK. Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron. 2014;83:894–905. doi: 10.1016/j.neuron.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao YT, Goffin D, Johnson BS, Zhou Z. Loss of MeCP2 function is associated with distinct gene expression changes in the striatum. Neurobiol Dis. 2013;59:257–266. doi: 10.1016/j.nbd.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filice F, Vorckel KJ, Sungur AO, Wohr M, Schwaller B. Reduction in parvalbumin expression not loss of the parvalbumin-expressing GABA interneuron subpopulation in genetic parvalbumin and shank mouse models of autism. Mol Brain. 2016;9:10. doi: 10.1186/s13041-016-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kao FC, Su SH, Carlson GC, Liao W. MeCP2-mediated alterations of striatal features accompany psychomotor deficits in a mouse model of Rett syndrome. Brain Struct Funct. 2015;220:419–434. doi: 10.1007/s00429-013-0664-x. [DOI] [PubMed] [Google Scholar]
- 32.Ito-Ishida A, Ure K, Chen H, Swann JW, Zoghbi HY. Loss of MeCP2 in Parvalbumin-and Somatostatin-Expressing Neurons in Mice Leads to Distinct Rett Syndrome-like Phenotypes. Neuron. 2015;88:651–658. doi: 10.1016/j.neuron.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wohr M, Orduz D, Gregory P, Moreno H, Khan U, Vorckel KJ, et al. Lack of parvalbumin in mice leads to behavioral deficits relevant to all human autism core symptoms and related neural morphofunctional abnormalities. Transl Psychiatry. 2015;5:e525. doi: 10.1038/tp.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karvat G, Kimchi T. Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2014;39:831–840. doi: 10.1038/npp.2013.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanahira C, Higo S, Watanabe K, Tomioka R, Ebihara S, Kaneko T, et al. Parvalbumin neurons in the forebrain as revealed by parvalbumin-Cre transgenic mice. Neurosci Res. 2009;63:213–223. doi: 10.1016/j.neures.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. Amsterdam; Boston: Elsevier Academic Press; 2004. Compact. [Google Scholar]
- 37.Rapanelli M, Frick L, Pogorelov V, Ohtsu H, Bito H, Pittenger C. Histamine H3R receptor activation in the dorsal striatum triggers stereotypies in a mouse model of tic disorders. Translational Psychiatry. doi: 10.1038/tp.2016.290. (in press) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rapanelli M, Frick LR, Horn KD, Schwarcz RC, Pogorelov V, Nairn AC, et al. The Histamine H3 Receptor Differentially Modulates Mitogen-activated Protein Kinase (MAPK) and Akt Signaling in Striatonigral and Striatopallidal Neurons. J Biol Chem. 2016;291:21042–21052. doi: 10.1074/jbc.M116.731406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baldan Ramsey LC, Xu M, Wood N, Pittenger C. Lesions of the dorsomedial striatum disrupt prepulse inhibition. Neuroscience. 2011;180:222–228. doi: 10.1016/j.neuroscience.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castellan Baldan L, Williams KA, Gallezot JD, Pogorelov V, Rapanelli M, Crowley M, et al. Histidine decarboxylase deficiency causes tourette syndrome: parallel findings in humans and mice. Neuron. 2014;81:77–90. doi: 10.1016/j.neuron.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xuan IC, Hampson DR. Gender-dependent effects of maternal immune activation on the behavior of mouse offspring. PLoS One. 2014;9:e104433. doi: 10.1371/journal.pone.0104433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 45.Frick L, Rapanelli M, Abbasi E, Ohtsu H, Pittenger C. Histamine regulation of microglia: Gene-environment interaction in the regulation of central nervous system inflammation. Brain Behav Immun. 2016;57:326–337. doi: 10.1016/j.bbi.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rapanelli M, Frick LR, Pogorelov V, Ota KT, Abbasi E, Ohtsu H, et al. Dysregulated intracellular signaling in the striatum in a pathophysiologically grounded model of Tourette syndrome. Eur Neuropsychopharmacol. 2014;24:1896–1906. doi: 10.1016/j.euroneuro.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo X, Hamilton PJ, Reish NJ, Sweatt JD, Miller CA, Rumbaugh G. Reduced expression of the NMDA receptor-interacting protein SynGAP causes behavioral abnormalities that model symptoms of Schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:1659–1672. doi: 10.1038/npp.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Penagarikano O, Lazaro MT, Lu XH, Gordon A, Dong H, Lam HA, et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7:271ra278. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teng BL, Nonneman RJ, Agster KL, Nikolova VD, Davis TT, Riddick NV, et al. Prosocial effects of oxytocin in two mouse models of autism spectrum disorders. Neuropharmacology. 2013;72:187–196. doi: 10.1016/j.neuropharm.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Molecular psychiatry. 2016;21:1225–1231. doi: 10.1038/mp.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ooi YP, Weng SJ, Kossowsky J, Gerger H, Sung M. Oxytocin and Autism Spectrum Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmacopsychiatry. 2016 doi: 10.1055/s-0042-109400. [DOI] [PubMed] [Google Scholar]
- 53.Park HY, Kang YM, Kang Y, Park TS, Ryu YK, Hwang JH, et al. Inhibition of adenylyl cyclase type 5 prevents L-DOPA-induced dyskinesia in an animal model of Parkinson’s disease. J Neurosci. 2014;34:11744–11753. doi: 10.1523/JNEUROSCI.0864-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deak M, Clifton AD, Lucocq LM, Alessi DR. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–4441. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biever A, Valjent E, Puighermanal E. Ribosomal Protein S6 Phosphorylation in the Nervous System: From Regulation to Function. Front Mol Neurosci. 2015;8:75. doi: 10.3389/fnmol.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Day JJ, Sweatt JD. Epigenetic treatments for cognitive impairments. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37:247–260. doi: 10.1038/npp.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beaulieu JM. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J Psychiatry Neurosci. 2012;37:7–16. doi: 10.1503/jpn.110011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 59.Mei Y, Monteiro P, Zhou Y, Kim JA, Gao X, Fu Z, et al. Adult restoration of Shank3 expression rescues selective autistic-like phenotypes. Nature. 2016;530:481–484. doi: 10.1038/nature16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Steensel FJ, Bogels SM, Perrin S. Anxiety disorders in children and adolescents with autistic spectrum disorders: a meta-analysis. Clin Child Fam Psychol Rev. 2011;14:302–317. doi: 10.1007/s10567-011-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White SW, Oswald D, Ollendick T, Scahill L. Anxiety in children and adolescents with autism spectrum disorders. Clin Psychol Rev. 2009;29:216–229. doi: 10.1016/j.cpr.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brunner D, Kabitzke P, He D, Cox K, Thiede L, Hanania T, et al. Comprehensive Analysis of the 16p11.2 Deletion and Null Cntnap2 Mouse Models of Autism Spectrum Disorder. PLoS One. 2015;10:e0134572. doi: 10.1371/journal.pone.0134572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flaisher-Grinberg S, Albelda N, Gitter L, Weltman K, Arad M, Joel D. Ovarian hormones modulate ‘compulsive’ lever-pressing in female rats. Horm Behav. 2009;55:356–365. doi: 10.1016/j.yhbeh.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Penagarikano O. Oxytocin in Animal Models of Autism Spectrum Disorder. Dev Neurobiol. 2016 doi: 10.1002/dneu.22449. [DOI] [PubMed] [Google Scholar]
- 65.Tozzi A, de Iure A, Tantucci M, Durante V, Quiroga-Varela A, Giampa C, et al. Endogenous 17beta-estradiol is required for activity-dependent long-term potentiation in the striatum: interaction with the dopaminergic system. Front Cell Neurosci. 2015;9:192. doi: 10.3389/fncel.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shams WM, Sanio C, Quinlan MG, Brake WG. 17beta-Estradiol infusions into the dorsal striatum rapidly increase dorsal striatal dopamine release in vivo. Neuroscience. 2016;330:162–170. doi: 10.1016/j.neuroscience.2016.05.049. [DOI] [PubMed] [Google Scholar]
- 67.Yunes R, Casas S, Gaglio E, Cabrera R. Progesterone Exerts a Neuromodulatory Effect on Turning Behavior of Hemiparkinsonian Male Rats: Expression of 3 alpha -Hydroxysteroid Oxidoreductase and Allopregnanolone as Suggestive of GABAA Receptors Involvement. Parkinsons Dis. 2015;2015:431690. doi: 10.1155/2015/431690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Biever A, Puighermanal E, Nishi A, David A, Panciatici C, Longueville S, et al. PKA-dependent phosphorylation of ribosomal protein S6 does not correlate with translation efficiency in striatonigral and striatopallidal medium-sized spiny neurons. J Neurosci. 2015;35:4113–4130. doi: 10.1523/JNEUROSCI.3288-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sawicka A, Seiser C. Histone H3 phosphorylation - a versatile chromatin modification for different occasions. Biochimie. 2012;94:2193–2201. doi: 10.1016/j.biochi.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matamales M, Girault JA. Signaling from the cytoplasm to the nucleus in striatal medium-sized spiny neurons. Front Neuroanat. 2011;5:37. doi: 10.3389/fnana.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsaki G, Deisseroth K, et al. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neurosci. 2012;15:123–130. doi: 10.1038/nn.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silberberg G, Bolam JP. Local and afferent synaptic pathways in the striatal microcircuitry. Curr Opin Neurobiol. 2015;33:182–187. doi: 10.1016/j.conb.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 73.Hrdlicka M, Dudova I. Controversies in autism: is a broader model of social disorders needed? Child Adolesc Psychiatry Ment Health. 2013;7:9. doi: 10.1186/1753-2000-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.