Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
A point mutation in murine factor H (W1206R) impairs its interaction with host cells but does not affect its complement-regulating activity.
W1206R mutant mice develop complement-mediated systemic thrombotic angiopathy leading to renal failure, stroke, and retinopathy.
Abstract
Complement plays a key role in host defense, but its dysregulation can cause autologous tissue injury. Complement activation is normally controlled by regulatory proteins, including factor H (FH) in plasma and membrane cofactor protein (MCP) on the cell surface. Mutations in FH and MCP are linked to atypical hemolytic uremic syndrome, a type of thrombotic microangiopathy (TMA) that causes renal failure. We describe here that disruption of FH function on the cell surface can also lead to disseminated complement-dependent macrovascular thrombosis. By gene targeting, we introduced a point mutation (W1206R) into murine FH that impaired its interaction with host cells but did not affect its plasma complement-regulating activity. Homozygous mutant mice carrying this mutation developed renal TMA as well as systemic thrombophilia involving large blood vessels in multiple organs, including liver, lung, spleen, and kidney. Approximately 30% of mutant mice displayed symptoms of stroke and ischemic retinopathy, and 48% died prematurely. Genetic deficiency of complement C3 and factor D prevented both the systemic thrombophilia and renal TMA phenotypes. These results demonstrate a causal relationship between complement dysregulation and systemic angiopathy and suggest that complement activation may contribute to various human thrombotic disorders involving both the micro- and macrovasculature.
Introduction
Complement is an important innate immune system that protects the host from infection.1 However, if not properly regulated, activated complement has the potential to cause significant tissue injury. In humans and mammals, a number of cell surface and plasma proteins function to inhibit complement attack of host cells, and dysfunction in these proteins has been linked to complement-mediated diseases.2 Among the major complement regulators, factor H (FH) is an abundant plasma glycoprotein that inhibits alternative pathway (AP) complement activation.3,4 It regulates complement activation, both in plasma and on the cell surface, by accelerating decay of the AP C3 convertase C3bBb and by acting as a cofactor for factor I–mediated cleavage and inactivation of C3b.5 FH is composed of 20 short consensus repeat (SCR) domains. Its complement-regulating function resides in 4 N-terminal SCRs. The other SCRs, especially SCR 19 and 20 at the C terminus, are involved in binding host cells through interaction with surface-deposited C3b/C3d and host cell–specific constituents on the cell surface.6-9
Recent data from genetic studies in animals and humans have linked FH dysfunction to several complement-mediated pathologies. Mutations in the FH gene leading to absent or low-level FH protein in plasma impaired fluid phase and cell surface control of complement activation, and this caused secondary complement deficiency due to C3 and factor B consumption.10,11 Rare heterozygous mutations in human FH, mostly in SCR 19 and 20, are associated with atypical hemolytic uremic syndrome (aHUS), a prototype of thrombotic microangiopathy (TMA) that causes renal failure.12-14 A single-nucleotide polymorphism in the human FH gene (Y402H) in SCR 7 significantly increased the risk of age-related macular degeneration (AMD).15-18
In the present study, we introduced a single amino acid mutation (W1206R) to mouse FH by gene targeting in order to better understand the physiological role of FH as a cell surface complement regulator. The W1206R change in mouse FH corresponded to W1183R mutation in human FH found in multiple aHUS families.19,20 We found that the W1206R mutation in the mouse FH impaired its interaction with host cells but did not affect its plasma complement-regulating activity. Homozygous mutant mice carrying this mutation developed renal TMA as expected. Surprisingly, the mutant mice also developed systemic thrombophilia involving large blood vessels in multiple organs, including liver, lung, spleen, and kidney. Approximately 30% of mutant mice displayed symptoms of stroke and ischemic retinopathy, and approximately half died by 30 weeks of age. Mutant mice showed endothelial and vascular smooth muscle cell dysfunction with significantly elevated plasma levels of von Willebrand factor (VWF) and a lower ratio of high- to low-molecular-weight VWF multimers. Genetic deficiency of complement C3 and factor D prevented both the systemic thrombophilia and renal TMA phenotypes. These results demonstrate a causal relationship between complement dysregulation and systemic angiopathy and suggest that complement activation may contribute to various human thrombotic disorders involving both the micro- and macrovasculature.
Materials and methods
Production of recombinant mouse FH proteins
Recombinant mouse FH SCR 19-20 was produced in HEK cells as described previously.21 Production of full-length mouse FH protein followed a similar procedure, and experimental details are described in supplemental Methods (available at the Blood Web site).
Mice
All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Mutant mice were either generated using C57BL/6J embryonic stem cells or backcrossed onto C57BL/6J background. The source and generation of FD−/−, C3−/−, and DAF−/−Crry−/−C3−/− mice have been reported previously.21-23 The W1206R mutant mouse was generated by homologous recombination in embryonic stem cells following procedures previously described,11 and more detailed are provided in supplemental Methods. Wild-type (WT), heterozygous, and mutant mice used for comparative studies were all littermates from heterozygous breeding.
Survival curve
The survival curve was analyzed using GraphPad Prism (GraphPad, La Jolla, CA) as described previously.11
Serum AP complement activity assay
A lipopolysaccharide (LPS)–dependent enzyme-linked immunosorbent assay (ELISA) was used to measure total serum AP complement activity as published previously24 and described in detail in supplemental Methods.
Hemolysis assay
Hemolytic activity of WT or W1206R mutant mouse serum was assessed as reported previously11 using red blood cells (RBCs) from DAF−/−Crry−/−C3−/− mice and 50% test serum and is described in detail in supplemental Methods.
Measurement of serum Cr and BUN and CBC
Serum creatinine (Cr) was measured using a CRE-III plus kit (Kainos, Tokyo, Japan). Blood urea nitrogen (BUN) was measured using serum and urea nitrogen reagents (Sigma-Aldrich, St Louis, MO) as described previously.25 Complete blood count (CBC) was measured using an automated hematology analyzer (XT-2000iV, Sysmex) at the Translational Core Laboratory of the Children’s Hospital of Philadelphia.
Platelet aggregation assay
Washed mouse blood platelets were prepared as described previously.26 Platelets (0.5 mL, adjusted to 2 × 108 cells/mL) were incubated at 37°C with buffer or AYPGYK (250 μM), 2 MeSADP (50 nM), or collagen (5 mg/mL). Light transmission was monitored with a PAP4 light scattering platelet aggregometer (BioData Corporation, Horsham, PA) for 3 minutes.
Data collection and analysis
All data collection, including pathology scoring, was performed in a blinded manner. Mantel-Haenszel log-rank test, Student t test or 1-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test were used for statistical analysis as appropriate, and a P value of .05 was considered to be significant.
Additional experimental details are provided in supplemental Methods.
Results
Characterization of W1206R mutation in murine FH
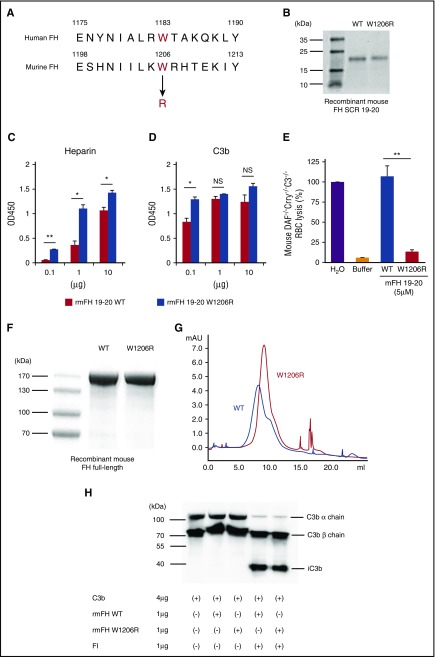
The W1183R mutation in SCR 20 of human FH is found in multiple aHUS patients.19,27,28 Based on sequence alignment, we identified W1206 as the murine equivalent of W1183 in human FH (Figure 1A). Previous studies using a recombinant protein corresponding to human FH SCR 19-20 showed that W1183R mutation increased its binding to C3b and heparin and abolished its interaction with RBCs.29,30 We used similar assays to test functional changes in the W1206R mutation in mouse FH. We expressed and purified recombinant mouse FH SCR 19-20 and W1206R variant in HEK cells (Figure 1B). We found that W1206R mutant exhibited an increased binding of mouse FH SCR 19-20 to plate-coated heparin (Figure 1C). At the lower concentration tested, it also showed increased binding to plate-coated C3b (Figure 1D). In a hemolytic assay, WT mouse FH SCR 19-20 caused lysis of complement-susceptible RBCs from DAF−/−Crry−/−C3−/− mice (Figure 1E) because it lacked the N-terminal complement-regulating SCRs and acted as a competitive inhibitor of natural FH in RBC binding. In contrast, W1206R mutant of FH SCR 19-20 failed to induce RBC lysis in the same assay (Figure 1E), suggesting that it lost the ability to interact with RBCs. We also expressed and studied full-length mouse FH and W1206R mutant (Figure 1F-G). Compared with WT mouse FH, W1206R mutant had a larger retention volume on a heparin column, suggesting that full-length FH carrying this mutation also had increased binding to heparin. Finally, we assessed FH function and confirmed that W1206R mutation did not affect the cofactor activity of murine FH in factor I–mediated cleavage of C3b (Figure 1H).
Figure 1.
W1206R mutation alters cell surface binding, but not cofactor activity, of mouse FH. (A) Alignment of amino acid sequences showing W1206 of mouse FH is equivalent to W1183 in human FH. (B) Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis of purified recombinant mouse FH SCR 19-20 and its W1206R variant (Coomassie blue staining). (C) ELISA plate-based heparin and (D) C3b-binding assay of WT and mutant mouse FH SCR 19-20. Heparin and C3b were coated onto plates at 0.1 to 10 μg per well, and recombinant mouse FH SCR 19-20 was added at 0.5 μg per well. (E) When added to 50% mouse serum, WT but not the W1206R mutant form of mouse FH SCR 19-20 caused lysis of complement-susceptible RBCs of DAF−/−Crry−/−C3−/− mice. Hypotonic lysis in pure water was used as reference control (100% lysis). (F) Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis of purified recombinant full-length mouse FH and its W1206R mutant variant (Coomassie blue). (G) Fast protein liquid chromatography analysis showing that the W1206R mutant FH had strong longer retention on a heparin column than WT mouse FH. Recombinant mouse FH proteins (100 μg) were loaded onto the heparin column in PBS and eluted off with 0.5 M NaCl in PBS. (H) Western blot analysis of products from a cofactor activity assay. The W1206R mutant did not affect the cofactor activity of mouse FH in factor I–mediated cleavage of C3b. Data shown in panels C-E represent mean ± standard deviation (SD) of the results. *P < .05; **P < .01; NS, not significant (Student t test).
Complement dysregulation in FH W1206R mutant mice
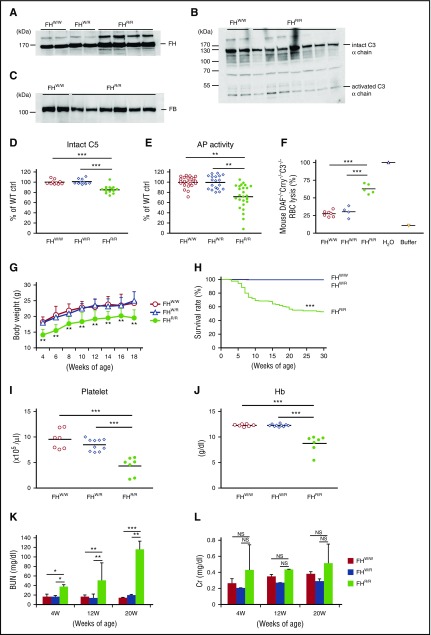
To assess the in vivo consequence of W1206R mutation in murine FH, we generated by gene targeting a mutant mouse (supplemental Figure 1). Western blot and ELISA analysis of plasma from WT (FHW/W), heterozygous (FHW/R), and homozygous (FHR/R) mice showed that mutant FH protein was stable and present at a higher level (115% by densitometry estimation of western blot) in FHR/R mice than normal FH in FHW/W mice (Figure 2A and supplemental Figure 2). In contrast, we detected lower levels of plasma intact C3, factor B (FB), and C5 in FHR/R mice (50%, 52%, and 86% of the FHW/W mouse level for C3 α-chain, FB, and C5, respectively, by densitometry or ELISA) (Figure 2B-D). Confirming the reduction in C3 and FB levels, plasma AP complement activity, as assessed by an LPS-based ELISA,24 was significantly lower in FHR/R mice (Figure 2E). Despite the reduction in C3, FB, and C5 levels, FHR/R but not FHW/W or FHW/R mouse serum caused marked lysis of complement-sensitive RBCs from DAF−/−Crry−/−C3−/− mice (Figure 2F). Thus, while W1206R mutation in murine FH caused a reduction in plasma complement levels, it led to increased complement activity on the cell surface due to impaired interaction of FH with host cells, a finding that is consistent with data from in vitro experiments using recombinant proteins (Figure 1).
Figure 2.
Complement dysregulation, premature death and development of aHUS symptoms in FH W1206R mutant mice. (A) Western blot analysis of plasma FH levels in WT (FHW/W), heterozygous (FHW/R), and homozygous (FHR/R) mutant mice showing that mutant FH was stable and present at a slightly higher level in FHR/R mice. A polyclonal rabbit anti–mouse FH antibody was used to detect both WT and mutant FH. (B-C) Western blot analysis of plasma C3 (B) and FB (C) levels showing that both intact C3 and FB were lower in FHR/R mice than in FHW/W mice. (D) ELISA of plasma intact C5 showing that FHR/R mice had significantly lower C5 levels than FHW/W or FHW/R mice. (E) ELISA of LPS-induced AP complement activity in 10% mouse sera. Data are normalized to the average value of FHW/W mice (100%). (F) Hemolytic assay using 50% sera from FHW/W, FHW/R, and FHR/R mice and RBCs from DAF−/−Crry−/−C3−/− mice. Hypotonic lysis in pure water was used as reference control (100% lysis). (G) FHR/R mice had lower body weights than FHW/W or FHW/R mice (n = 10 in each group, all male mice). (H) Survival curves of FHW/W (n = 275), FHW/R (n = 310), and FHR/R (n = 147) mice up to 30 weeks of age. (I-J) CBC analysis showing FHR/R mice had thrombocytopenia (I) and anemia with low blood hemoglobin (Hb) levels (J). (K-L) BUN and serum Cr levels in mice at different ages. Serum samples were collected from the same mice at 4, 12, and 20 weeks of age (FHW/W n = 20, FHW/R n = 16, and FHR/R n = 19). Data shown in panels K-L are mean ± SD of the results. Each lane in panels A-C and each symbol in panels D-F,I-J represent an individual mouse (4-25 weeks old). Horizontal bars through the scatterplots in panels D-F,I-J indicate the average values in each group. *P < .05; **P < .01; ***P < .001; NS, not significant (Mantel-Haenszel log-rank test for panel H; 1-way ANOVA and Student t test for other panels).
Premature death and development of renal TMA in FH W1206R mutant mice
At weaning (3-4 weeks), most FHR/R mice were noticeably smaller and thinner compared with their FHW/W and FHW/R littermates. This deficiency in body weight persisted in a group of mice that we examined from 4 to 18 weeks of age (Figure 2G). We also followed a large cohort of FHW/W, FHW/R, and FHR/R mice up to 30 weeks of age for survival. Of 147 FHR/R mice genotyped, 48% died by 30 weeks, whereas none of the 275 FHW/W and 310 FHW/R mice died during this time period (Figure 2H). To investigate the cause of death, we studied FHR/R mice for signs of aHUS, which is characterized by thrombocytopenia, anemia, and kidney failure. A CBC revealed severe thrombocytopenia and anemia in FHR/R mice at 4 and 8 weeks of age (Figure 2I-J) but a normal white blood cell count (supplemental Figure 3A). Consistent with low hemoglobin (Hb) levels, we found significantly increased reticulocyte count in FHR/R mice (supplemental Figure 3B), likely the result of compensatory hematopoiesis. These results suggested that thrombocytopenia and anemia were caused by peripheral blood destruction rather than bone marrow production failure. We measured BUN and serum Cr levels as markers of kidney function at 4, 12, and 20 weeks of age. BUN in FHR/R mice was progressively increased with age (38 and 116 mg/dL at 4 and 20 weeks, respectively), and values were significantly higher than that of FHW/W and FHW/R mice within each age group (Figure 2K). Although Cr was also higher in FHR/R mice, it varied considerably among different FHR/R mice (Figure 2L).
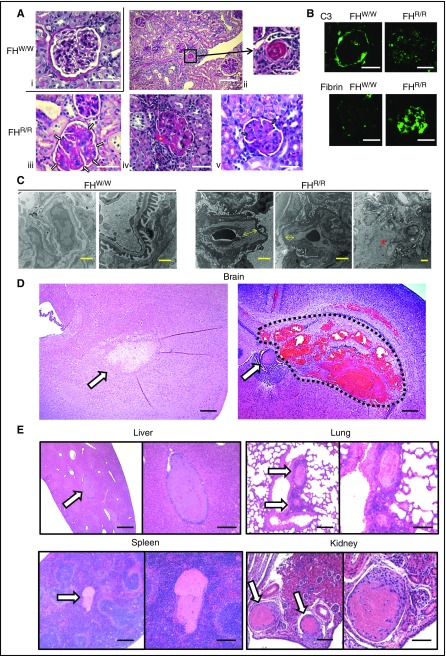
Periodic acid–Schiff (PAS) staining of kidney sections showed mesangial expansion and double contours of glomerular basement membrane with capillary microthrombi and endothelial swelling with narrowing of the capillary lumen in FHR/R mouse glomeruli (Figure 3A). Hyalinosis and thrombosis were also detected in interlobular arteries and arterioles of FHR/R mice (Figure 3A). We analyzed 30 FHR/R mice (1-30 weeks of age) and found that all contained aHUS-like pathological changes in the kidney, with 53%, 68%, 52%, and 78% of the glomeruli showing expanded matrix and endothelial swelling, microthrombi, large vein thrombi, and arteriolar hyalinosis, respectively (supplemental Tables 1 and 2). These pathological changes were not observed in age-matched FHW/W and FHW/R mice (Figure 3A; supplemental Table 1). Immunofluorescent staining of C3 and fibrin, markers of complement activation and vascular injury, respectively, showed positive glomerular deposition of both, with weak C3 staining showing a granular pattern in the mesangium and strong fibrin staining in capillary lesions (Figure 3B and supplemental Figure 4). Electron microscopy analysis revealed subendothelial expansion with fluffy granular material without immune-type deposits and mesangial interposition with basement membrane duplication (Figure 3C; supplemental Table 3A). These findings suggested that FHR/R mice developed severe aHUS that might have contributed to premature death.
Figure 3.
Light, immunofluorescence, and electron microscopy of kidney and other pathology in FH W1206R mutant mice. (A) PAS staining of kidney sections. (i) A normal-looking FHW/W mouse glomerulus. (ii-v) Representative FHR/R mouse kidney sections (from 30 mice analyzed) showed features of aHUS, including arteriolar thrombosis (ii, black square), capillary wall thickening and double contours in glomeruli (iii, white arrows), afferent arteriolar thrombosis in arteriole (iv, yellow asterisk), and expanded matrix and microthrombi in capillary lumen (v, blue arrows). Scale bars represent 25 μm (i-ii, left; iii-v) and 50 μm (ii, right). (B) Immunofluorescence staining of C3 and fibrin or fibrinogen. C3 staining was restricted to the Bowman’s capsule only in FHW/W mice, whereas weak C3 staining of a granular pattern was observed in the glomerulus of FHR/R mice. Strong fibrin and fibrinogen staining of a diffuse pattern was detected both in capillaries and in mesangial lesions of FHR/R mouse glomeruli, but not in FHW/W mouse glomeruli. Scale bar, 25 μm. (C) Electron microscopy of kidneys. A FHW/W mouse (15-week-old female, 1 of 2 WT mice analyzed by electron microscopy) kidney showed normal basement membrane and foot processes. In a representative FHR/R mouse (15-week-old female, 1 of 4 mutant mice analyzed by electron microscopy) kidney, the glomerular capillary wall showed subendothelial expansion with fluffy granular material (yellow arrow) and mesangiolysis with rarefaction of mesangial matrix (red asterisk), with no electron-dense deposits. Scale bars represent 2 μm (FHW/W, left, and FHR/R) and 500 nm (FHW/W, right). (D) hematoxylin and eosin staining of brain sections from a representative FHR/R mouse with neurological abnormalities. Ischemic changes (arrow, left panel), thrombus formation (arrow, right panel) or intracerebral hemorrhage (area circled by dotted line) were observed. Scale bar, 25 μm. (E) Hematoxylin and eosin staining of liver, lung, spleen, and kidney sections from representative FHR/R mice showed thrombi (arrows) in large blood vessels. Scale bars represent 25 μm (left panel of each organ) and 50 μm (right panel of each organ).
Stroke, retinopathy, and multiorgan thrombosis in FH W1206R mutant mice
We found that ∼30% of FHR/R mice exhibited severe neurological abnormalities, including hindlimb paralysis and circular movement (supplemental Video 1). Histological examination of these mice consistently showed ischemic brain injury and the presence of thrombi and/or intracerebral hemorrhage in the brain (Figure 3D). These findings suggested that FHR/R mice were prone to developing thrombotic and hemorrhagic strokes, which promoted us to further examine signs of thrombosis and vascular injury in other organs. By histology, we found thrombi in medium and large blood vessels in all organs examined, including liver, lung, spleen, and kidney (Figure 3E; supplemental Table 2). Most of these thrombi were found in veins (eg, portal veins of the liver, splenic veins, and interlobular and renal veins of the kidney) and pulmonary arteries (Figure 3E). Portal vein thrombosis was particularly prevalent and was present in the liver of >80% of FHR/R mice on histology examination. Using ultrasonography, we were also able to detect a large clot (2.9 × 1.3 mm, sagittal view) in the portal vein of a 20-week-old male FHR/R mouse (supplemental Video 2).
We assessed the correlations among stroke and ischemic brain injury, renal pathology, and organ thrombosis in 14 FHR/R mice with symptoms of stroke and 11 FHR/R mice without. As expected, we detected ischemic brain injury only in mice with symptoms of stroke (supplemental Table 2). On the other hand, TMA-like renal pathology was detected in all mice regardless of the neurological phenotype. We found that mice with symptoms of stroke were more likely to have multiorgan thrombosis, but portal vein thrombosis appeared to be common and was less correlated with stroke symptoms (supplemental Table 2).
To understand the chronology of thrombus formation and renal pathology development, we examined by histology of embryonic day 15.0 fetuses and 1-week-old FHR/R mice. Fetuses were collected from heterozygous breeders and sectioned sagittally as a whole, whereas liver and kidney were dissected from postnatal 1-week-old FHR/R mice for histology. We found no thrombus formation in any of the fetal organs, nor were any pathological features detected in the fetal kidney (supplemental Figure 5A; supplemental Table 3C). On the other hand, we detected mesangial expansion in glomeruli in all 3 of the 1-week-old FHR/R mice examined and kidney thrombi in 1 and liver thrombi in 2 of the 3 mice (supplemental Figure 5B; supplemental Table 3C).
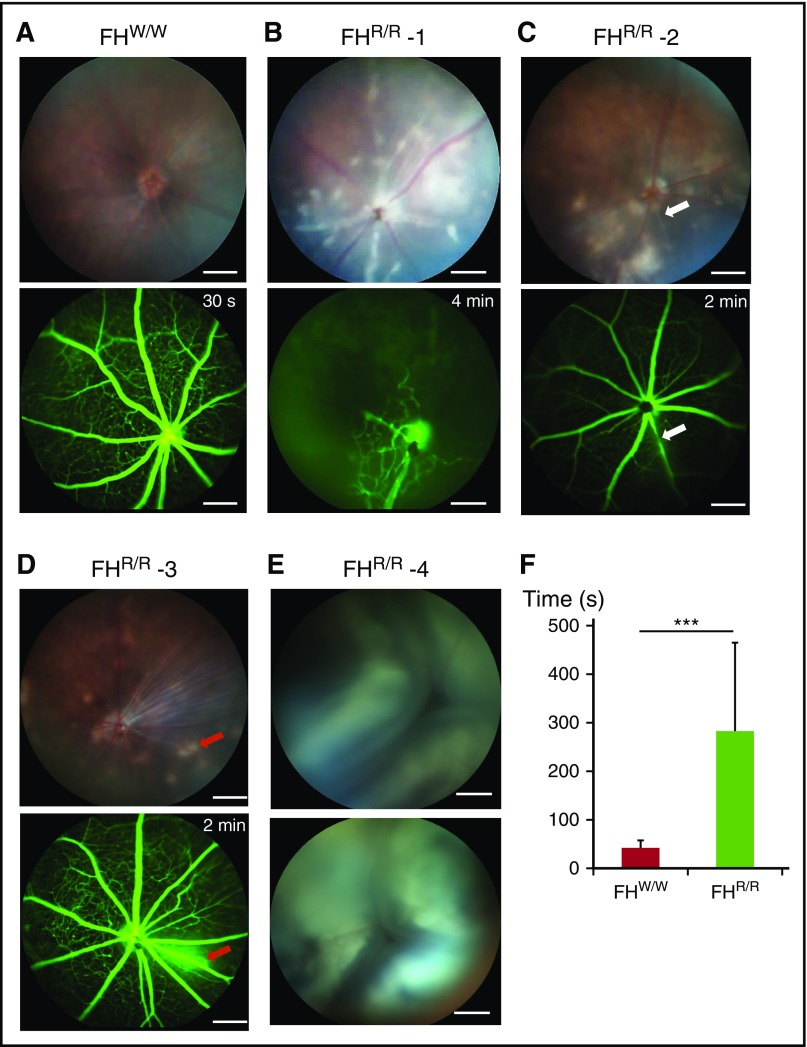
In addition to thrombosis in internal organs, we observed blood vessel occlusion in the eyes of 70% of FHR/R mice (n = 21, 5-10 weeks old) (supplemental Table 3B). Using in vivo retinal photography, we found signs of ischemic injury, including retinal edema, whitening, and cotton wool spots in most of the mutant mice examined (Figure 4A-D; supplemental Table 3B). Using fluorescein angiography, we observed dropout of retinal vasculature, central and branch retinal artery occlusions, and perivascular leakage (Figure 4A-D; supplemental Video 3). In a fluorescein angiography test, the time required for the dye to fill the retinal vasculature in FHR/R mice was significantly increased (45 vs 300 seconds for FHW/W and FHR/R mice, respectively, Figure 4F). Approximately 9% of FHR/R mice (2 out of 23 mice examined by funduscopy, 5-10 weeks old) also had retinal detachments (Figure 4E), most likely resulting from exudation secondary to infarctions in the choroidal vasculature. These were visible grossly in some mice as leukocoria (white pupil).
Figure 4.
Retinopathy in FH W1206R mutant mice. (A) A 5-week-old male FHW/W mouse showed normal appearance of retina on funduscopy and rapid dye distribution across retinal blood vessels (<30 seconds) on fluorescein angiography. (B) A 10-week-old male FHR/R mouse exhibited retinal whitening and cotton wool spots by funduscopy and widespread nonperfusion of retinal vasculature, with dilation of the few perfused vessels by fluorescein angiography. This appearance is consistent with a central retinal artery occlusion with limited perfusion provided by a cilioretinal artery. (C) A 5-week-old FHR/R mouse showed retinal whitening by funduscopy and stenosis of vessel (white arrow) by fluorescein angiography. (D) A 10-week-old FHR/R mouse exhibited multiple hypopigmented spots on funduscopy and dye leakage (red arrows) by fluorescein angiography. (E) A 5-week-old male FHR/R mouse had detached retina in both eyes by funduscopy. (F) Time lapse analysis of the arteriovenous phase in fluorescein angiography shows delayed perfusion of retinal blood vessels in FHR/R mice (n = 21) compared with FHW/W mice (n = 10). Data are presented mean ± SD. ***P < .001 (Student t test). In panels B-E, the time label on fluorescein angiography designates the time point at which the picture was taken after dye injection. Scale bars, 200 μm.
Study of vascular and coagulation phenotypes of FH W1206R mutant mice
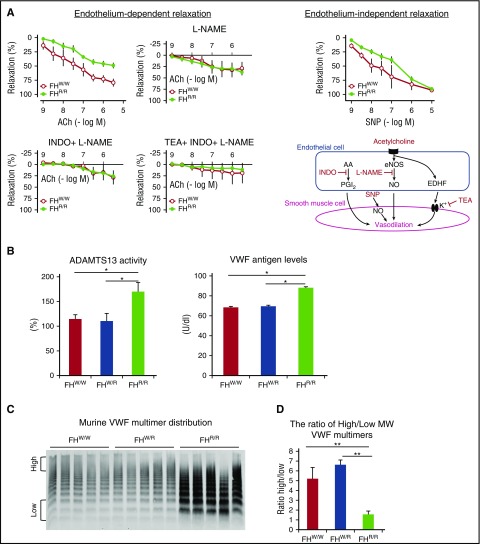
To test the hypothesis that vascular injury in FHR/R mice contributed to thrombotic or organ dysfunctional phenotypes, we used myography to measure endothelial cell–dependent and independent relaxations of small mesenteric artery fragments from FHW/W and FHR/R mice. Administration of acetylcholine, an agonist of endothelium-dependent relaxation pathways mediated by nitric oxide, prostaglandin I2, and endothelium-derived hyperpolarizing factor, demonstrated a clear difference between FHW/W and FHR/R mice (Figure 5A). The FHR/R mouse blood vessel fragment had reduced dilation in response to acetylcholine stimulation. This difference disappeared with the addition of nitro-L-arginine methyl ester or combinations of nitro-L-arginine methyl ester, indomethacin, and tetraethylammonium, respective inhibitors for endothelial nitric oxide synthase in the nitric oxide pathway, cyclooxygenase in the prostaglandin I2 pathway, and potassium channel in the endothelium-derived hyperpolarizing factor pathway (Figure 5A). Administration of sodium nitroprusside (SNP), an endothelium-independent vasodilator that can directly act on vascular smooth muscle cells, showed no significant difference in blood vessel relaxation at low or high doses, but a detectible difference was observed at medium doses. These data suggested that endothelium-dependent and independent relaxations of smooth muscle cells were impaired in FHR/R mice. Although we have not characterized blood pressure changes in the mutant mice, many aHUS patients are known to develop hypertension,31,32 which can be caused by thrombotic microangiopathy, including impairment in smooth muscle cell function.
Figure 5.
Vasculopathy and changes in plasma ADAMTS13 and VWF profiles in FH W1206R mutant mice. (A) Endothelium-dependent and independent relaxation of small mesenteric arteries was impaired in FHR/R mice. The endothelium-dependent nature of the phenotype was established by the use of inhibitors of nitric oxide synthase (L-NAME), COX (INDO), and KCa (TEA). An endothelium-independent component of the phenotype was indicated by the use of SNP, which acts directly on vascular smooth muscle cells. Three vessel segments per mouse were analyzed (n = 3 mice). (B) ADAMTS13 activity and plasma VWF levels were significantly higher in FHR/R mice than in FHW/W or FHR/R mice (n = 7 per group for ADAMTS13; n = 3 per group for VWF). (C) Western blot of plasma VWF shows that FHR/R mice had higher levels of total VWF (n = 5; each lane represents 1 mouse) and more abundant lower-molecular-weight (MW) multimers. (D) The ratio of high- to low-molecular-weight VWF multimers, as defined in panel C and quantitated using ImageJ software, was significantly lower in FHR/R mice. Data are from 8-week-old FHW/W, FHW/R, and FHR/R mice. Data in panels B,D represent mean ± SD of results from all mice. *P < .05; **P < .01; ***P < .001 (one-way ANOVA and Student t test). AA, arachidonic acid; eNOS, endothelial nitric oxide synthase; MW, molecular weight; NO, nitric oxide; PGI2, prostaglandin I2.
Thrombotic thrombocytopenic purpura (TTP) is another prototype of TMA that shares many clinical features with aHUS but is primarily caused by severe deficiency of ADAMTS13, a plasma metalloprotease that cleaves von Willebrand factor (VWF).33-36 Under normal conditions, ultralarge VWF multimers are secreted from Weibel-Palade bodies and cleaved into small multimers by ADAMTS13. Severe deficiency of plasma ADAMTS13 activity results in accumulation of ultralarge VWF multimers on endothelial cell surface or in circulation.37 To confirm that the pathogenic mechanism in FHR/R mice is distinct from that of TTP, we measured ADAMTS13 activity, VWF antigen concentrations, and VWF multimer distribution in FHR/R mice and FHW/W controls. Contrary to TTP conditions in humans and in mice, we detected increased plasma ADAMTS13 activity (Figure 5B), increased plasma VWF antigen levels, and a lower ratio of high- to low-molecular-weight VWF multimers in FHR/R mice (Figure 5B-D).
In an attempt to understand the thrombotic phenotype, we used intravital microscopy to examine FeCl3-induced thrombus formation in mesenteric arteries and veins of FHW/W and FHR/R mice.38,39 This experiment was conducted under relatively gentle conditions in order to maximize the chance of picking up a prothrombotic phenotype in the mutant mice. We also tested platelet sensitivity to agonist stimulation in an aggregation assay and by fluorescence-activated cell sorting analysis of 2 activation markers, αIIbβ3 and P-selectin. Interestingly, we did not find FHR/R mice with preexisting thrombophilia and aHUS phenotypes to be more sensitive than FHW/W mice to FeCl3-induced thrombus formation under the experimental setting used, nor did we observe platelet hypersensitivity to agonist stimulation in these mice (supplemental Figure 6).
Role of AP complement in disease pathogenesis of FH W1206R mutant mice
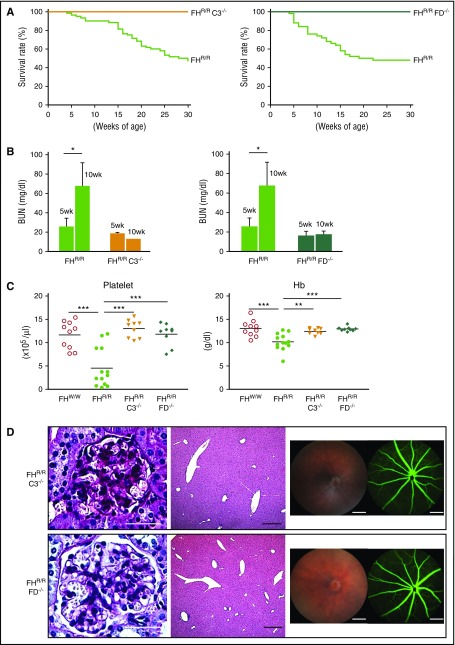
Since intact C3 and FB levels were decreased and plasma AP complement activity was lower in FHR/R mice, we tested the hypothesis that dysregulation of AP complement on the cell surface was responsible for disease development in FHR/R mice. We crossed FHR/R mice with C3-knockout (C3−/−) and factor D–knockout (FD−/−) mice and generated FHR/RC3−/− and FHR/RFD−/− mice. We found that both FHR/RC3−/− and FHR/RFD−/− mice lived normally, with none developing stroke symptoms, whereas their FHR/R littermates showed the neurological phenotype and high mortality rate observed earlier (Figure 6A). Likewise, while FHR/R littermates increased their BUN from 5 to 10 weeks of age, the levels of BUN in FHR/RC3−/− and FHR/RFD−/− mice were normal and remained unchanged with age (Figure 6B). Furthermore, unlike their FHR/R littermates, FHR/RC3−/− and FHR/RFD−/− mice had normal platelet counts and Hb levels (Figure 6C), and displayed no evidence of renal pathology, nor was thrombus formation detected in liver and other organs (Figure 6D; supplemental Table 4). Finally, funduscopy showed completely normal-looking retina, and fluorescein angiography revealed normal retinal blood flow in FHR/RC3−/− and FHR/RFD−/− mice (Figure 6D; supplemental Table 4). Thus, stroke, thrombophilia, retinopathy, and aHUS in FHR/R mice were all mediated by AP complement.
Figure 6.
Disease phenotype in FH W1206R mutant mice is mediated by AP complement. (A) FHR/R mice with C3 or FD deficiency survived normally, whereas their FHR/R littermates with normal C3 and FD had high mortality (FHR/RC3−/− n = 64, FHR/RC3+/+ n = 38, FHR/RFD−/− n = 60, and FHR/RFD+/+ n = 36). (B) BUN of FHR/R mice (n = 10) was significantly increased between 5 and 10 weeks of age, whereas BUN in FHR/RC3−/− (n = 6) and FHR/RFD−/− (n = 7) mice remained the same at 5 and 10 weeks of age. (C) CBC analysis showed that FHR/RC3−/− (n = 9) and FHR/RFD−/− (n = 9) mice had normal blood platelet counts and Hb levels, whereas their FHR/R littermates (n = 13) had thrombocytopenia and anemia compared with FHW/W mice (n = 13). (D) Representative pictures of kidney (PAS staining) and liver (hematoxylin and eosin staining) histology, retinal funduscopy, and fluorescence angiography showing that there was no glomerular injury in the kidney or thrombosis in the liver and eye of FHR/RC3−/− and FHR/RFD−/− mice and no retinopathy. Scale bars in panel D represent 25 μm (kidney), 50 μm (liver), and 200 μm (eye). Data in panel B represent mean ± SD. Horizontal bars across scatterplots in panel C represent average values. *P < .05; **P < .01; and ***P < .001 (Mantel-Haenszel log-rank test for panel A; 1-way ANOVA and Student t test for other panels).
Discussion
We have shown in this study that a single amino acid mutation in SCR 20 of mouse FH led to AP complement dysregulation on the cell surface and produced an unexpectedly severe phenotype of renal TMA as well as systemic thrombophilia involving large blood vessels. Mutant mice failed to thrive, had lower body weights, and developed multiorgan thrombi, stroke, retinopathy, and progressive renal disease. Our in vitro experiments demonstrated that the W1206R mutation altered mouse FH binding to heparin and C3b, and impaired its interaction with RBCs but did not affect the cofactor activity for factor I–mediated cleavage of C3b. Accordingly, the mutant FH is expected to have retained its fluid-phase AP complement-regulating activity. Thus, unlike mice with FH knockout or hypomorphic mutation10,11,40 in which fluid-phase AP complement is uncontrollably activated and consumed, complement dysregulation is expected to have occurred only on the cell surface in FHR/R mice, and cell surface complement activation and consumption likely accounted for the observed lower plasma C3, FB, and C5 levels. The mechanism for slightly elevated plasma FH in the mutant mice remains to be determined. The W1206R mutation may have increased FH messenger RNA and/or protein stability and half-lives. Additionally, lower tissue binding and retention of mutant FH due to its impaired interaction with host cells may have led to a higher plasma level.
The extent of thrombophilia in FHR/R mice was notable, and a direct causal relationship was found between complement dysregulation and thrombotic angiopathy. Apart from renal failure, occlusion of vital organs such as the brain, lung, and heart likely contributed to the premature mortality of FHR/R mice. Indeed, we observed sudden death of FHR/R mice of different ages on numerous occasions. The onset of organ thrombosis and renal injury appeared to be fast, occurring within the first week of life, but not during fetal stage in a setting where the mother was heterozygous for the W1206R mutation and thus had functional FH in maternal blood which could have crossed the placenta.41,42 An alternative explanation for the lack of phenotype in fetuses is that AP complement was not yet fully functional. The precise mechanisms for the observed macrovascular thrombophilia phenotype remain to be determined but several hypotheses may be tested in future studies. In the absence of FH protection on the cell surface, terminal complement activation could have damaged vascular endothelial and smooth muscle cells, creating a prothrombotic state of the vessel wall. Platelets and neutrophils or monocytes could also have been activated by membrane attack complex and C5a, respectively, leading to the release of tissue factor from the stimulated leukocytes,43,44 platelet and leukocyte aggregation, and clot formation both in capillaries and in large blood vessels.45,46 Notably, however, when tested in vitro and in vivo, platelet function in mutant mice with systemic thrombophilia and aHUS disease appeared to be impaired rather than increased. The thrombocytopenia phenotype suggested that platelets were being consumed, shortening their survival in the circulation. Given that, the in vivo results of FeCl3-induced experimental clotting study likely reflected both the thrombocytopenia and an as-yet-unexplained defect in platelet function found in the in vitro studies.
TTP is a form of TMA that is caused by genetic or acquired ADAMTS13 deficiency leading to formation of ultralarge VWF multimers.37 Many studies have explored a possible mechanistic overlap between TTP and aHUS.47-49 Complement dysregulation may occur in TTP patients and exacerbate the TMA phenotype,50-52 and several recent studies have also demonstrated an interaction between FH and VWF.53,54 It is clear, however, that the pathogenic mechanism of FHR/R mice is distinct from that of TTP. Instead of lower ADAMTS13 activity, we detected higher ADAMTS13 activity in FHR/R mice, possibly as a feedback response to increased VWF release from damaged endothelium and activated platelets. Thus, despite higher plasma VWF levels, the relative ratio of ultralarge to smaller VWF multimers was lower in FHR/R mice. Supporting a complement-dependent mechanism, we showed that AP complement deficiency by either C3 or FD deletion completely prevented disease manifestations in FHR/R mice.
In addition to its implication in TMA, FH dysfunction has also been linked to the pathogenesis of dry AMD. A common single-nucleotide polymorphism encoding a Y402H change in SCR 7 of human FH significantly increases AMD risk.15-18 A rare mutation, R1210C, in SCR 20 of human FH originally found in some aHUS patients,55,56 has been reported to be a high penetrant mutation for dry AMD,57-59 but there is no known connection between retinopathy and the W1183R mutation in human FH. By funduscopy, we found severe retinal injury in a high percentage of FHR/R mice. We have not characterized the specific pathology of retinopathy in these mice, and whether retinal injury is secondary to vascular occlusion only or there is also direct complement-mediated attack on retinal cells remains to be investigated.
In summary, by creating a mouse with a FH point mutation that impaired FH activity on cell surface only, we have revealed a surprising role of FH in preventing macrovascular thrombosis as well as TMA and renal disease Our data suggest that dysfunction or variation of FH activity on the cell surface could be implicated in various human thrombotic disorders such as stroke and retinal arterial or venous occlusion, in addition to primary or secondary TMA. The FHR/R mouse will be a useful model to study the interaction between complement and thrombotic pathways and to test novel anti-complement therapies for related human diseases.
Acknowledgments
The authors are grateful for the chimeric mouse production and electron microscopy services provided by the Transgenic and Chimeric Mouse Facility and the Electron Microscopy Core of the Perelman School of Medicine, University of Pennsylvania, respectively, and for the mouse CBC analysis provided by the Clinical and Translational Research Center of the Children’s Hospital of Philadelphia.
This work is supported by National Institutes of Health, National Institute of Allergy and Immunological Diseases grants AI1174190, AI44970, AI085596, and National Institutes of Health, National Eye Institute grant EY023709.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: W.-C.S., T.M., and Y.U. designed experiments and interpreted data; Y.U., I.M., D.S., D.G., L.Z., Z.C., S.G., J.B., Y.M., and T.M. performed experiments; M.P. performed pathological analysis; S.S. and Y.W. contributed reagent generation and mouse colony maintenance; W.-C.S. and Y.U. prepared figures and wrote the manuscript; and H.W., X.L.Z., L.B., M.P., and J.D. interpreted data and critically edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wen-Chao Song, Department of Systems Pharmacology and Translational Therapeutics, Perelman School of Medicine, University of Pennsylvania, Room 1254 BRBII/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: songwe@mail.med.upenn.edu.
References
- 1.Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20(1):34-50. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344(14):1058-1066. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez de Córdoba S, Esparza-Gordillo J, Goicoechea de Jorge E, Lopez-Trascasa M, Sánchez-Corral P. The human complement factor H: functional roles, genetic variations and disease associations. Mol Immunol. 2004;41(4):355-367. [DOI] [PubMed] [Google Scholar]
- 4.Józsi M, Zipfel PF. Factor H family proteins and human diseases. Trends Immunol. 2008;29(8):380-387. [DOI] [PubMed] [Google Scholar]
- 5.Fearon DT. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci USA. 1978;75(4):1971-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira VP, Herbert AP, Hocking HG, Barlow PN, Pangburn MK. Critical role of the C-terminal domains of factor H in regulating complement activation at cell surfaces. J Immunol. 2006;177(9):6308-6316. [DOI] [PubMed] [Google Scholar]
- 7.Józsi M, Oppermann M, Lambris JD, Zipfel PF. The C-terminus of complement factor H is essential for host cell protection. Mol Immunol. 2007;44(10):2697-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkinson JP, Goodship TH. Complement factor H and the hemolytic uremic syndrome. J Exp Med. 2007;204(6):1245-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp A, Hebecker M, Svobodová E, Józsi M. Factor h: a complement regulator in health and disease, and a mediator of cellular interactions. Biomolecules. 2012;2(1):46-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickering MC, Cook HT, Warren J, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31(4):424-428. [DOI] [PubMed] [Google Scholar]
- 11.Lesher AM, Zhou L, Kimura Y, et al. Combination of factor H mutation and properdin deficiency causes severe C3 glomerulonephritis. J Am Soc Nephrol. 2013;24(1):53-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caprioli J, Bettinaglio P, Zipfel PF, et al. ; Itaslian Registry of Familial and Recurrent HUS/TTP. The molecular basis of familial hemolytic uremic syndrome: mutation analysis of factor H gene reveals a hot spot in short consensus repeat 20. J Am Soc Nephrol. 2001;12(2):297-307. [DOI] [PubMed] [Google Scholar]
- 13.Pérez-Caballero D, González-Rubio C, Gallardo ME, et al. Clustering of missense mutations in the C-terminal region of factor H in atypical hemolytic uremic syndrome. Am J Hum Genet. 2001;68(2):478-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards A, Buddles MR, Donne RL, et al. Factor H mutations in hemolytic uremic syndrome cluster in exons 18-20, a domain important for host cell recognition. Am J Hum Genet. 2001;68(2):485-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419-421. [DOI] [PubMed] [Google Scholar]
- 17.Edwards AO, Ritter R III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421-424. [DOI] [PubMed] [Google Scholar]
- 18.Zareparsi S, Branham KE, Li M, et al. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am J Hum Genet. 2005;77(1):149-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remuzzi G, Ruggenenti P, Codazzi D, et al. Combined kidney and liver transplantation for familial haemolytic uraemic syndrome. Lancet. 2002;359(9318):1671-1672. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira VP, Herbert AP, Cortés C, et al. The binding of factor H to a complex of physiological polyanions and C3b on cells is impaired in atypical hemolytic uremic syndrome. J Immunol. 2009;182(11):7009-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barata L, Miwa T, Sato S, Kim D, Mohammed I, Song WC. Deletion of Crry and DAF on murine platelets stimulates thrombopoiesis and increases factor H-dependent resistance of peripheral platelets to complement attack. J Immunol. 2013;190(6):2886-2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, Ma M, Ippolito GC, Schroeder HW Jr, Carroll MC, Volanakis JE. Complement activation in factor D-deficient mice. Proc Natl Acad Sci USA. 2001;98(25):14577-14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miwa T, Zhou L, Kimura Y, Kim D, Bhandoola A, Song WC. Complement-dependent T-cell lymphopenia caused by thymocyte deletion of the membrane complement regulator Crry. Blood. 2009;113(12):2684-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura Y, Miwa T, Zhou L, Song WC. Activator-specific requirement of properdin in the initiation and amplification of the alternative pathway complement. Blood. 2008;111(2):732-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada K, Miwa T, Liu J, Nangaku M, Song WC. Critical protection from renal ischemia reperfusion injury by CD55 and CD59. J Immunol. 2004;172(6):3869-3875. [DOI] [PubMed] [Google Scholar]
- 26.Pickens B, Mao Y, Li D, et al. Platelet-delivered ADAMTS13 inhibits arterial thrombosis and prevents thrombotic thrombocytopenic purpura in murine models. Blood. 2015;125(21):3326-3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann HP, Salzmann M, Bohnert-Iwan B, et al. Haemolytic uraemic syndrome and mutations of the factor H gene: a registry-based study of German speaking countries. J Med Genet. 2003;40(9):676-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunders RE, Abarrategui-Garrido C, Frémeaux-Bacchi V, et al. The interactive Factor H-atypical hemolytic uremic syndrome mutation database and website: update and integration of membrane cofactor protein and Factor I mutations with structural models. Hum Mutat. 2007;28(3):222-234. [DOI] [PubMed] [Google Scholar]
- 29.Morgan HP, Schmidt CQ, Guariento M, et al. Structural basis for engagement by complement factor H of C3b on a self surface. Nat Struct Mol Biol. 2011;18(4):463-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roumenina LT, Loirat C, Dragon-Durey MA, Halbwachs-Mecarelli L, Sautes-Fridman C, Fremeaux-Bacchi V. Alternative complement pathway assessment in patients with atypical HUS. J Immunol Methods. 2011;365(1-2):8-26. [DOI] [PubMed] [Google Scholar]
- 31.Loirat C, Frémeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet J Rare Dis. 2011;6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan BS, Ruebner RL, Spinale JM, Copelovitch L. Current treatment of atypical hemolytic uremic syndrome. Intractable Rare Dis Res. 2014;3(2):34-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276(44):41059-41063. [DOI] [PubMed] [Google Scholar]
- 34.Plaimauer B, Zimmermann K, Völkel D, et al. Cloning, expression, and functional characterization of the von Willebrand factor-cleaving protease (ADAMTS13). Blood. 2002;100(10):3626-3632. [DOI] [PubMed] [Google Scholar]
- 35.Dong JF, Moake JL, Nolasco L, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100(12):4033-4039. [DOI] [PubMed] [Google Scholar]
- 36.Tsai HM. Thrombotic thrombocytopenic purpura and the atypical hemolytic uremic syndrome: an update. Hematol Oncol Clin North Am. 2013;27(3):565-584. [DOI] [PubMed] [Google Scholar]
- 37.Zheng XL. ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annu Rev Med. 2015;66:211-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ni H, Denis CV, Subbarao S, et al. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest. 2000;106(3):385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckly A, Hechler B, Freund M, et al. Mechanisms underlying FeCl3-induced arterial thrombosis. J Thromb Haemost. 2011;9(4):779-789. [DOI] [PubMed] [Google Scholar]
- 40.Pickering MC, de Jorge EG, Martinez-Barricarte R, et al. Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. J Exp Med. 2007;204(6):1249-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffman MC, Rumer KK, Kramer A, Lynch AM, Winn VD. Maternal and fetal alternative complement pathway activation in early severe preeclampsia. Am J Reprod Immunol. 2014;71(1):55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savaris RF, Becker C, Guedes Neto EP. Maternal plasma levels of complement Factor H in miscarriage and in normal pregnancy: a cohort study. J Reprod Immunol. 2016;114:1-5. [DOI] [PubMed] [Google Scholar]
- 43.Karpman D, Ståhl AL, Arvidsson I, et al. Complement interactions with blood cells, endothelial cells and microvesicles in thrombotic and inflammatory conditions. Adv Exp Med Biol. 2015;865:19-42. [DOI] [PubMed] [Google Scholar]
- 44.Schneider AE, Sándor N, Kárpáti É, Józsi M. Complement factor H modulates the activation of human neutrophil granulocytes and the generation of neutrophil extracellular traps. Mol Immunol. 2016;72:37-48. [DOI] [PubMed] [Google Scholar]
- 45.Ritis K, Doumas M, Mastellos D, et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177(7):4794-4802. [DOI] [PubMed] [Google Scholar]
- 46.Kourtzelis I, Markiewski MM, Doumas M, et al. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood. 2010;116(4):631-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Remuzzi G, Galbusera M, Noris M, et al. ; Italian Registry of Recurrent and Familial HUS/TTP. Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome. von Willebrand factor cleaving protease (ADAMTS13) is deficient in recurrent and familial thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Blood. 2002;100(3):778-785. [DOI] [PubMed] [Google Scholar]
- 48.Veyradier A, Obert B, Haddad E, et al. Severe deficiency of the specific von Willebrand factor-cleaving protease (ADAMTS 13) activity in a subgroup of children with atypical hemolytic uremic syndrome. J Pediatr. 2003;142(3):310-317. [DOI] [PubMed] [Google Scholar]
- 49.Licht C, Stapenhorst L, Simon T, Budde U, Schneppenheim R, Hoppe B. Two novel ADAMTS13 gene mutations in thrombotic thrombocytopenic purpura/hemolytic-uremic syndrome (TTP/HUS). Kidney Int. 2004;66(3):955-958. [DOI] [PubMed] [Google Scholar]
- 50.Noris M, Bucchioni S, Galbusera M, et al. ; International Registry of Recurrent and Familial HUS/TTP. Complement factor H mutation in familial thrombotic thrombocytopenic purpura with ADAMTS13 deficiency and renal involvement. J Am Soc Nephrol. 2005;16(5):1177-1183. [DOI] [PubMed] [Google Scholar]
- 51.Phillips EH, Westwood JP, Brocklebank V, et al. The role of ADAMTS-13 activity and complement mutational analysis in differentiating acute thrombotic microangiopathies. J Thromb Haemost. 2016;14(1):175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan X, Kremer Hovinga JA, Shirotani-Ikejima H, et al. Genetic variations in complement factors in patients with congenital thrombotic thrombocytopenic purpura with renal insufficiency. Int J Hematol. 2016;103(3):283-291. [DOI] [PubMed] [Google Scholar]
- 53.Nolasco L, Nolasco J, Feng S, Afshar-Kharghan V, Moake J. Human complement factor H is a reductase for large soluble von Willebrand factor multimers--brief report. Arterioscler Thromb Vasc Biol. 2013;33(11):2524-2528. [DOI] [PubMed] [Google Scholar]
- 54.Rayes J, Roumenina LT, Dimitrov JD, et al. The interaction between factor H and VWF increases factor H cofactor activity and regulates VWF prothrombotic status. Blood. 2014;123(1):121-125. [DOI] [PubMed] [Google Scholar]
- 55.Manuelian T, Hellwage J, Meri S, et al. Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J Clin Invest. 2003;111(8):1181-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez-Barricarte R, Pianetti G, Gautard R, et al. ; European Working Party on the Genetics of HUS. The complement factor H R1210C mutation is associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2008;19(3):639-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raychaudhuri S, Iartchouk O, Chin K, et al. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat Genet. 2011;43(12):1232-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferrara D, Seddon JM. Phenotypic characterization of complement factor H R1210C rare genetic variant in age-related macular degeneration. JAMA Ophthalmol. 2015;133(7):785-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Recalde S, Tortajada A, Subias M, et al. Molecular basis of factor H R1210C association with ocular and renal diseases. J Am Soc Nephrol. 2016;27(5):1305-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]