Abstract
Background
Parkinson's disease (PD) traditionally is characterized by tremor, rigidity, and bradykinesia, although cognitive impairment also is a common symptom. The clinical presentation of PD is heterogeneous and associated with different risk factors for developing cognitive impairment. PD patients with primary akinetic/rigidity (PDAR) are more likely to develop cognitive deficits compared to those with tremor-predominant symptoms (PDT). Because cognitive impairment in PD appears to be related to changes in the default mode network (DMN), this study tested the hypothesis that DMN integrity is different between PDAR and PDT subtypes.
Method
Resting state fMRI (rs-fMRI) and whole brain volumetric data were obtained from 17 PDAR, 15 PDT and 24 healthy controls (HCs) using a 3T scanner. PD patients were matched closely to HCs for demographic and cognitive variables, and showed no symptoms of dementia. Voxel-based morphometry (VBM) was used to examine brain gray matter (GM) volume changes between groups. Independent component analysis (ICA) interrogated differences in the DMN among PDAR, PDT, and HC.
Results
There was decreased activity in the left inferior parietal cortex (IPC) and the left posterior cingulate cortex (PCC) within the DMN between PDAR and both HC and PDT subjects, even after controlling for multiple comparisons, but not between PDT and HC. GM volume differences between groups were detected at a lower threshold (p < 0.001, uncorrected). Resting state activity in IPC and PCC were correlated with some measures of cognitive performance in PD but not in HC.
Conclusion
This is the first study to demonstrate DMN differences between cognitively comparable PDAR and PDT subtypes. The DMN differences between PD and HC appear to be driven by the PDAR subtype. Further studies are warranted to understand the underlying neural mechanisms and their relevance to clinical and cognitive outcomes in PDAR and PDT subtypes.
Keywords: Resting state fMRI, Parkinson's disease, Independent component analysis (ICA), Cognitive impairment
1. Introduction
Parkinson's disease (PD) is a progressive, neurodegenerative disease characterized by resting tremor, rigidity, bradykinesia, and gait disorder with substantial physical, psychological, and social implications (Dorsey et al., 2007; Nussbaum & Ellis, 2003). Although traditionally thought of as purely a movement disorder, there is growing recognition that cognition also is compromised in PD patients. For example, mild cognitive impairment (MCI) is observed in 15–20% of PD patients at the time of diagnosis, whereas 20–57% will display MCI within three to five years of diagnosis (Ferrer, 2011; Janvin, Larsen, Aarsland, & Hugdahl, 2006; Janvin, Larsen, Salmon, et al., 2006; Kehagia, Barker, & Robbins, 2010; Pagonabarraga & Kulisevsky, 2012). The presence of cognitive impairment is a risk factor for developing dementia that can impact dramatically patient morbidity and mortality (Berg et al., 2014; Kehagia et al., 2010; Pagonabarraga & Kulisevsky, 2012). The type of cognitive deficit, severity of cognitive dysfunction, and progression to dementia in PD, however, seem to be uncertain due to conflicting reports (Aarsland & Kurz, 2010; Ballard et al., 2006; Caviness et al., 2007).
The clinical phenotype of PD is known to be heterogeneous. Based on the clinical presentation of motor symptoms, PD patients can be categorized into two subtypes: 1) akinetic/rigidity predominant (PDAR) and 2) tremor-predominant (PDT) (Jankovic & Kapadia, 2001; Jankovic et al., 1990; Lewis et al., 2011; Louis et al., 1999; Rajput, Voll, Rajput, Robinson, & Rajput, 2009). This clinical heterogeneity also appears to be related to the risk of cognitive impairment such that PDAR patients demonstrate faster cognitive decline compared to PDT patients (Eggers et al., 2012; Helmich, Hallett, Deuschl, Toni, & Bloem, 2012; Lewis et al., 2011; Zaidel, Arkadir, Israel, & Bergman, 2009).
In recent years, the default-mode network (DMN) has been implicated as a key resting state network in cognitive processing (Raichle et al., 2001; Raichle & Snyder, 2007; Tessitore, Esposito, et al., 2012). DMN dysfunction has been observed in neurodegenerative disorders, including PD, supporting the idea that this network may be involved in the underlying neural mechanisms related to cognitive impairment (Agosta et al., 2012; Rocca et al., 2010; Tedeschi et al., 2012; Zhang et al., 2015; Zhou et al., 2010). As an example, a recent study reported functional variations in the DMN involving the medial temporal lobe (MTL) that were significantly correlated with memory scores in PD, suggesting that DMN changes may precede cognitive decline (Tessitore, Esposito, et al., 2012). No previous study, however, has investigated the DMN integrity in cognitively comparable PDAR and PDT subjects and directly compared them to each other or to healthy controls (HCs).
The goal of the current study was to test the hypothesis that the DMN is significantly different between cognitively comparable PDAR and PDT subtypes. We used group independent component analysis (ICA) to identify and assess the functional integrity of the DMN in PDAR, PDT, and HCs. Additionally, voxel-based morphometry (VBM) was used to assess whether any between-group differences in resting state functional connectivity were related to gray matter (GM) volume differences among the three groups.
2. Methods
2.1. Participants
Seventeen PDAR, 15 PDT, and 24 age-, gender-, and handedness-matched HC participants were selected from a pool of subjects that were part of an ongoing study (Table 1). PD and HC subjects were recruited from a tertiary care movement disorders clinic, the related spousal population, and the community via material approved by the Institutional Review Board/Human Subjects Protection Office (IRB/HSPO) of the Penn State Hershey Medical Center. Written informed consent was obtained from all participants according to IRB/HSPO guidelines. The study was conducted according to the principles approved by the Declaration of Helsinki (“World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects,” 2013).
Table 1.
Demographic information is shown as the mean ± standard deviation. p-Values were derived from Fisher's Exact test for Gender, whereas all other comparisons including Age, Education, Mini-Mental Status Examination (MMSE), and Hamilton Depression Rating Scale (HMD Scale) were derived from one-way analysis of variance. All analyses were considered statistically significant at p < .05.
| HC (n = 24) | PDAR (n = 17) | PDT (n = 15) | p-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| NC vs PDAR | NC vs PDT | PDAR vs PDT | ||||
| Age (yrs.) | 57.8 ± 7.4 | 59.1 ± 7.4 | 61.7 ± 6.7 | .836 | .236 | .569 |
| Gender (M:F) | 11:13 | 9:8 | 6:9 | .876 | .423 | .724 |
| Education (yrs.) | 15.8 ± 2.4 | 13.9 ± 2.1 | 15.2 ± 3.2 | .068 | .795 | .329 |
| MMSE | 29.6 ± .9 | 29.1 ± 1.2 | 29.7 ± .5 | .257 | .959 | .221 |
| HAM-D Scale | 3.7 ± 2.1 | 7.2 ± 3.3 | 6.9 ± 4.0 | .0003 | .078 | .215 |
| Disease duration (yrs.) | NA. | 3.7 ± 4.6 | 3.5 ± 3.5 | NA | NA | .91 |
| UPDRS III | 1.2 ± 2.6 | 20.8 ± 12.9 | 17.1 ± 9.5 | <.0001 | <.0001 | .27 |
| T/AR | NA. | .18 ± .22 | 2.63 ± 1.77 | NA. | NA. | <.0001 |
| H&Y stage | NA. | 1.7 ± .6 | 1.7 ± .6 | NA. | NA. | .96 |
| LEDD (mg/day) | NA. | 366 ± 229 | 354 ± 282 | NA. | NA. | .88 |
UPDRS = Unified Parkinson's Disease Rating Scale III; T/AR = mean tremor/mean akinetic/rigidity score; LEDD = Levodopa equivalent daily dosage.
PD subjects were diagnosed by a movement disorder specialist (XH) according to published criteria (Calne, Snow, & Lee, 1992). Except for two subjects who had very mild symptoms and were drug naïve, PD patients were treated with antiparkinsonian medications. Patients were negative for other neurological history, hypothyroidism, vitamin B12 and folate deficiency, and kidney and liver disease. Only right-handed PD subjects less than 70 years of age with a Mini-Mental Status Examination (MMSE) Score ≥ 24, and who took neither a centrally acting acetylcholinesterase inhibitor nor memantine were selected for inclusion. HC were free from any history of neurologic or psychiatric disorder, including previous head injury. In addition, both the HC and PD subjects were screened for MRI compatibility (e.g., metal implants, claustrophobia).
2.2. Clinical and neuropsychological assessment
For both motor and cognitive tests, PD subjects were assessed in a practically defined “off” state after withholding all medications overnight [∼12 h (Langston et al., 1992)]. Unified PD Rating Scale III (UPDRS-III) scores were recorded for all subjects and verified by a second rater from a video recording of the original assessment. Disease severity was recorded using Hoehn and Yahr staging (Hoehn & Yahr, 1967). Levodopa equivalent daily dosage (LEDD) was calculated based on previously published criteria (Tomlinson et al., 2010).
All subjects were administered a standardized neuropsychological test battery to assess overall cognitive health and specific cognitive domains (Lee et al., 2013), along with the Hamilton Depression Scale [HAM-D (Hamilton, 1960)] and the Dementia Rating Scale, Second Edition [DRS-2 (Brown et al., 1999)]. The following five cognitive domains were examined:
Executive function was assessed with the Design Fluency Test (DesFlu), the Verbal Fluency Test (VerbFlu), Stroop color-word interference test, and Visual Verbal Test (Fig. S1, Sup. Material).
Spatial cognition
Visuospatial abilities were evaluated using Benton's Judgment of Line Orientation (JoLO) and the Construction subtest of the Dementia Rating Scale (DSR 2) provided a quantitative measure of constructional praxis.
Visuospatial learning/memory was measured with the Brief Visuospatial Memory Test-Revised (BVMT-R) yielding total learning, delayed recall, and delayed recognition discrimination index scores.
Verbal learning/memory was assessed using the Hopkins Verbal Learning Test-Revised (HVLT-R) yielding total learning, delayed recall, and delayed recognition discrimination index scores.
Attention/working memory
The Digit Span test measured the maximal span of immediately recalled digit sequences, with Spatial Span providing a non-verbal analog. The Letter-Number Sequencing Test required subjects to register and mentally manipulate sequences of intermixed letters and numbers, generating a measure of maximal working memory load (Figure S1, Sup. Material).
None of the subjects were demented based on clinical and neuropsychological analyses. All were independent in daily activities and had MMSE scores >26. There was one HC who had mild cognitive change in two domains [i.e., z-scores 1.5 standard deviation (SD) below the normative mean]. Four PDAR subjects met criteria for mild cognitive impairment (PD-MCI).
2.3. PD subtype classification
PD subjects were classified as either PDAR or PDT using the modified ratio developed by Schiess et al. based on the UPDRS-III (Table S1, Sup. Material), with a numerical ratio derived from the mean tremor and akinetic-rigidity scores (Lewis et al., 2011; Schiess, Zheng, Soukup, Bonnen, & Nauta, 2000). Tremor was assessed using a nine-item scale that included history of left or right arm tremor (two items), rest tremor of the face/lips/chin and each limb (five items), as well as postural tremor of the right and left upper extremities (two items). The 14-item akinetic-rigidity scale assessed passive range of motion rigidity of the neck and each extremity (five items), rapid opening/closing of hands (two items), finger tapping (two items), rising from a chair (one item), posture and postural instability (two items), gait (one item), and body bradykinesia (one item). Each item was rated 0—4 with 0 representing absence of symptoms (or normal activity) and 4 the presence of significant symptoms or impairment. The mean of each scale was calculated and then the ratio (tremor/akinetic-rigidity score) determined. Using this method, PDAR subjects will have a ratio ≤.8, whereas PDT subjects will have a ratio ≥.9. In the current study, the average ratio for PDAR subjects was .18 ± .22 (range 0–.73) and for PDT subjects 2.63 ± 1.77 (range .9–7.14; Table 1). The DMN is considered to consist of a narrative introduction into experience (Catani, Dell'acqua, & Thiebaut de Schotten, 2013). Therefore, we measured visual hallucination (VH) using the Neuropsychiatric Inventory (NI) and none of the PD patients reported VH.
2.4. Volumetric/functional MR image acquisition and analysis protocols
Volumetric and resting state data were acquired using a 3T Siemens, Trio MRI scanner with an eight channel phased array head coil. To minimize discomfort, MRI images were obtained from PD subjects while on medication (i.e., “on” state). To ensure that subjects were fully awake during fMRI scanning, they were instructed to relax and keep their eyes open. A high-resolution, T1-weighted, 3D gradient-echo sequence (MPRAGE sequence) was used to acquire anatomical images for functional overlay using the following parameters: TR = 2300 msec; TE = 2.98 msec; flip angle = 9°; FOV = 256 × 256 mm2; matrix size = 256 × 256; slice thickness = 1 mm (no slice gap); number of slices = 160; voxel size 1 × 1 × 1 mm3. The resting state data was acquired using an EPI imaging sequence with the following parameters: TR = 2000 msec; TE = 30 msec; flip angle = 90°; FOV = 240 × 240 mm2; matrix size = 80 × 80; voxel size = 3.0 mm × 3.0 mm × 3.0 mm; number of time points = 230.
2.4.1. Functional MRI preprocessing and ICA decomposition
Functional MRI data pre-processing and subsequent statistical testing were performed using the DPARSFA (version 2.1) (www.restfmri.net) and REST (version 1.8) (www.restfmri.net) toolboxes. Briefly, the following steps were completed: 1) removal of the first 10 time points; 2) slice time correction; 3) realignment; 4) unified segmentation using 3D-T1 images and spatial normalization using the deformation parameters, and 5) time course de-trending and spatial smoothing. REST software was used to estimate motion parameters for all subjects to ensure that they were within 3.0 mm maximum translation in any of the x, y, or z directions or 3.0° of maximum rotation about the three axes. Only one healthy HC and one PDT had the maximum translation between 2.5 mm–3.0 mm. Although, ICA has a remarkable ability to detect and remove motion-related artifacts, excessive head motion during fMRI was corrected using the REST software before performing the ICA decomposition described below (Liao, Krolik, & McKeown, 2005).
ICA decomposition was performed using the subject order-independent group ICA (SOI-GICA) method as implemented in the Stable and Consistent Group ICA Toolbox (http://www.nitrc.org/projects/cogicat/, MICA version beta 1.2) after combining data from all 58 subjects (Zhang et al., 2010). This methodology incorporates a three-step principal component analysis to decompose the dataset into 20 IC components. The SOI-GICA algorithm was run 100 times to obtain highly robust and consistent results. The DMN was identified via visual inspection and found to be highly consistent with previous studies in terms of network nodes (Raichle et al., 2001). Individual DMNs were obtained by back-reconstruction and converted into calibrated z-maps before group comparisons (Zhang et al., 2010).
We also performed a fractional amplitude of low-frequency fluctuations (fALFFs) analysis of the resting-state fMRI signal using the REST software in order to investigate the intensity of regional spontaneous brain activity (Zou et al., 2008).
2.4.2. VBM analysis and group comparison
1-weighted volumetric data processing was done using SPM8 (Wellcome Trust Center for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm) and DPARSFA (version 2.1) using methods as implemented in the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html) with default parameters. Briefly, the warped GM segments were affine-transformed into MNI space, scaled and smoothed with a Gaussian kernel of 8-mm (full-width at half maximum) before generating GM volume maps. These smoothed GM volume maps then were analyzed statistically using a general linear model with total intracranial volume (TIV) and age as covariates. Whole brain and regions of interest (ROI: based on the results of the whole-brain resting state fMRI analyses) differences were assessed using statistical inference performed at the voxel level with a family-wise error (FWE) correction for multiple comparisons (p < .05). Additionally, functional findings were corrected using markers of GM volume in each group. Finally, we also performed an independent cortical thickness analysis using the Free Surfer software to further investigate differences in structural markers between HC and PD.
2.5. Statistical analyses
Demographic (age and education) and clinical factors (HAM-D, MMSE, LEDD, and UPDRS III) were compared using simple oneway analysis of variance (ANOVA). The sex ratio between groups was compared using Fisher's Exact Test.
Cognitive test scores were converted to standardized z-scores. One-way analysis of covariance (ANCOVA) was used to examine group differences in each cognitive domain with adjustment for HAM-D scores, where the individual cognitive domain was the dependent variable and group was the independent variable. One-way ANCOVA also was used to test for group differences in individual cognitive tests with HAM-D scores as a covariate. The Tukey–Kramer method was used to correct for multiple group (3 groups) comparisons. Since multiple cognitive tests were compared, results were corrected for the multiple comparisons of five test domains with a false discovery rate (FDR) at the .05 level. We report raw p-values but indicate whether the results were also significant at the FDR = .05 level.
Finally, an explorative correlation analysis was performed to test the significance of the relationship between ICA z-scores and the neuropsychological test scores in the five cognitive domains described above.
3. Results
3.1. Demographic and cognitive comparisons
There were no significant differences in age, MMSE, sex ratio, or education among PDAR, PDT, and HC (p > .068; see Table 1 and Table S1, Sup. Material). PD subjects had significantly higher UPDRS III scores (p < .0001) compared to HC. PDAR subjects had significantly higher HAM-D scores than HC (p = .0003), whereas PDT subjects showed a trend for higher HAM-D values (p = .078).
None of the neuropsychological domains (executive function, spatial cognition, verbal and visuospatial learning/memory, or attention) showed any difference among the three groups after FDR correction (p > .075; for individual subtests, see Table 2 and Fig. S1, Sup. Material).
Table 2.
Neuropsychological test scores and analyses. Neuropsychological test results are shown as the mean ± standard deviation. All test scores were converted to standard z-scores. Higher z-scores indicate better performance. One-way analysis of covariance (ANCOVA) was conducted with Group as the independent variable and each cognitive domain as the dependent variable, with the Hamilton Depression score as a covariate. Individual neuropsychological subtests also were tested using ANCOVA with Hamilton Depression score as a covariate. The results of the five domains were considered significant at FDR (false discovery rate) corrected p < .05. We report raw p-values. None of the tests were significant after FDR correction.
| Test | HC (n = 24) | PDAR (n = 17) | PDT (n = 15) | p-value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| HC vs PDAR | HC vs PDT | PDAR vs PDT | ||||
| Executive function | ||||||
| Z-scores | .39 ± .40 | .05 ± .59 | .09 ± .37 | .075 | .223 | .788 |
| CWInt Inhibition | .65 ± .70 | .29 ± 1.04 | .53 ± .85 | .866 | .999 | .882 |
| Inhibition Errors | .01 ± .76 | −.49 ± 1.03 | .33 ± .58 | .409 | .360 | .032 |
| CWInt Switch | .65 ± 1.00 | −.08 ± 1.34 | .62 ± .63 | .226 | .998 | .202 |
| Switch Errors | .46 ± .64 | −.08 ± .91 | .47 ± .57 | .195 | .976 | .133 |
| VVT_Total | .20 ± .80 | −.21 ± 1.22 | —.33 ± 1.68 | .971 | .661 | .826 |
| VVT_Switch | .05 ± .87 | −.36 ± 1.15 | —.59 ± 1.77 | .978 | .524 | .686 |
| DesFlu Switch | .69 ± .89 | .55 ± .70 | .22 ± .91 | .786 | .204 | .600 |
| DesFlu Total Correct | .82 ± 1.18 | .12 ± .76 | .18 ± .81 | .286 | .234 | 1.00 |
| DesFlu Total Design | 1.07 ± 1.30 | .59 ± .85 | .80 ± 1.42 | .739 | .897 | .943 |
| DesFlu Design Accuracy | −.36 ± .92 | −.75 ± 1.12 | −.80 ± 1.43 | .531 | .452 | .998 |
| VerbFlu Letter | .01 ± .85 | −.43 ± 1.14 | −.41 ± .85 | .101 | .191 | .900 |
| VerbFlu Category | .46 ± .92 | .29 ± .90 | .08 ± 1.02 | .429 | .245 | .959 |
| Spatial cognition | ||||||
| Z scores | .15 ± .71 | −.31 ± .79 | −.03 ± .58 | .183 | .755 | .505 |
| JoLO | .30 ± 1.42 | −.51 ± 1.50 | .05 ± 1.17 | .252 | .742 | .632 |
| DRS2 Construction | 0 | −.12 ± .33 | 0 | .243 | .997 | .214 |
| Learning/memory | ||||||
| Spatial Memory | .03 ± .84 | −.61 ± 1.35 | −41 ± 1.48 | .695 | .768 | .985 |
| BVMT Learning | .00 ± 1.10 | −.51 ± 1.24 | −.48 ± 1.40 | .828 | .693 | .981 |
| BVMT Delayed Recall | .21 ± 1.24 | −.27 ± 1.20 | −.01 ± 1.32 | .975 | .999 | .977 |
| BVMT Discrimination Indexa | −.10 ± 1.29 | −1.05 ± 2.24 | −.74 ± 2.14 | .558 | .715 | .951 |
| Verbal Memory | −.57 ± 1.15 | −.55 ± 1.00 | −.43 ± .66 | .982 | .959 | .898 |
| HVLT Total Learning | .49 ± 1.15 | −.64 ± 1.03 | −.78 ± .92 | .717 | .571 | .983 |
| HVLT Delayed Recall | −.65 ± 1.25 | −.68 ± 1.09 | −.42 ± .98 | .967 | .877 | .760 |
| HVLT Discrimination Indexa | −.57 ± 1.47 | −.33 ± 1.31 | −.09 ± .62 | .896 | .544 | .846 |
| Attention/working memory | ||||||
| Z-scores | .62 ± .68 | .29 ± .50 | .41 ± .64 | .301 | .576 | .845 |
| Letter Number Sequencing | .43 ± .75 | .33 ± .88 | .09 ± .82 | .818 | .357 | .765 |
| Spatial Span | .44 ± .90 | .22 ± .90 | .44 ± 1.16 | .913 | .990 | .855 |
| Digit Span | .42 ± .68 | .20 ± .71 | .29 ± .74 | .301 | .642 | .791 |
CWInt = Color-Word Interference Test; VVT = Visual Verbal Test; DesFlu = Design Fluency; VerbFlu = Verbal Fluency; JoLO = Judgment of Line Orientation; BVMT-R = Brief Visuospatial Memory Test-Revised; HVLT-R = Hopkins Verbal Learning Test-Revised.
Discrimination index scores are an index of recognition memory load on calculation of number of Hits –number of False Alarms.
3.2. GM comparisons among groups
The VBM analysis revealed no volume differences in any brain region among PD subgroups and HCs at a p < .05 FWE-corrected for multiple comparisons. At a more liberal threshold of p < .001 (uncorrected; cluster size <10), however, GM volume differences were detected within the functionally defined DMN. This finding, therefore, was further investigated using an independent cortical thickness analysis (using Free Surfer). There were no differences in cortical thickness among PDAR, PDT and HC in brain regions that are part of the DMN.
3.3. DMN network comparisons
The group ICA method reliably identified the DMN in HC and PD subgroups as reported previously and shown in Fig. 1. The regions encompassed in the DMN network were: 1) the posterior cingulate cortex (PCC), extending dorsally into the precuneus; 2) the bilateral inferior parietal cortex (IPC, left and right); 3) the medial prefrontal cortex (mPFC)/anterior cingulate cortex; and 4) the medial/lateral temporal lobe (MTL).
Fig. 1.
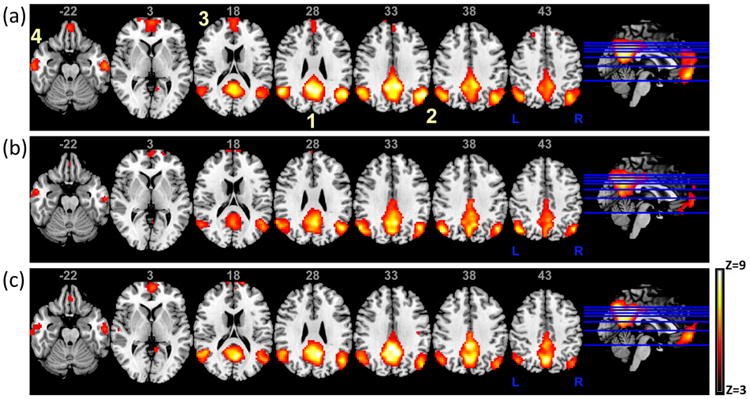
The DMN for (a) healthy controls (HC), (b) PDAR (akinetic/rigidity-predominant) and (c) PDT (tremor-predominant) subjects. The DMN as shown above is consistent with previously reported results and highlights the possible differences in activity between PDT/PDAR subtypes and HCs. Images are in radiological orientation. 1) posterior cingulate cortex (PCC), extending dorsally into the precuneus; 2) bilateral inferior parietal cortex (left and right IPC); 3) medial prefrontal cortex (mPFC)/anterior cingulate cortex and 4) medial/lateral temporal lobe (MTL).
Using ANCOVA, resting state fMRI activity differed among PDAR, PDT, and HCs in the left IPC and left PCC within the DMN (Fig. 2a). Specifically, PDAR subjects showed significantly lower activation in the left IPC and PCC regions compared both to PDT and HC subjects, whereas PDT and HCs were not different from each other. PDAR subjects also displayed significantly lower activation in the right IPC compared to PDT and HC subjects (Fig. 2b). These fMRI activity differences did not change when the functional data were corrected for GM volume markers. To further validate our findings, we also performed a simulation using leave-one-out assessments to demonstrate the robustness of group differences, i.e., PDAR, PDT and HC, in the presence of outliers in our data. The results demonstrated that 98% and 80% of the time, group differences between HCs and PD subjects were detected at a p < .005 in the IPC and PCC, respectively.
Fig. 2.
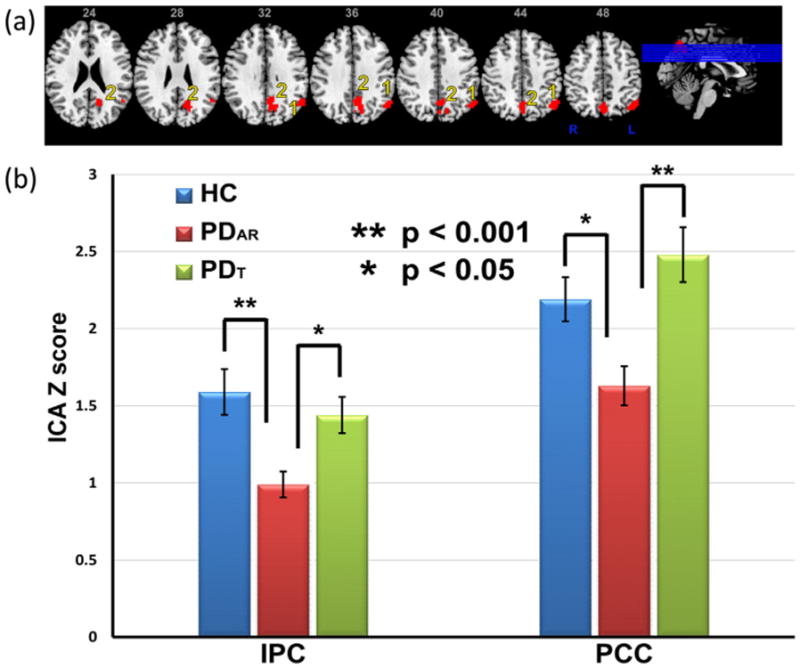
(a). Areas showing differences in activity in PD patients and healthy controls (HCs). Images are in radiological orientation. 1) left inferior parietal cortex (IPC) and 2) left posterior cingulate cortex (PCC). (b) Bar graph showing differences in ICA z-scores between HC, PDAR and PDT subjects. Data represent the mean ± SEM.
The group differences persisted even after excluding subjects who could be classified as PD-MCI. The effects of depression and dopaminergic medication (i.e., LEDD and/or the presence of a long-acting dopamine agonist) on DNM activity also were investigated in the above mentioned group comparisons. When HAM-D scores were entered as covariates in the ANCOVA, the pattern of group differences persisted (i.e., in the IPC and PCC) at the same corrected p < .05. When LEDD was included as a covariate, similar patterns of activation differences were found, although the differences in IPC activity survived only at a corrected p < .051.
Finally, marginally significant fALFF differences in posterior brain regions of the DMN were detected at an uncorrected threshold of p < .05. These differences did not survive any statistical correction.
3.4. Correlations between DMN regions and cognitive scores
There was a significant positive correlation between individual ICA z-scores in the PCC and spatial memory scores in PDAR subjects (r = .58, p = .018, N = 17) and a negative correlation of the IPC with spatial memory (r = −.59, p = .012) after co-varying for age. For PDT subjects, there also was a positive correlation between the PCC and spatial cognition scores (r = .56, p < .038, N = 15) and a trend for a negative correlation with verbal memory scores (r = −.53, p < .054) after co-varying for age. These exploratory correlation analyses were not corrected for multiple comparisons (Fig. S2, Sup. Material). No correlations between brain regions and cognitive scores were significant in HC subjects.
4. Discussion
The current study demonstrated that there was decreased activity in the left IPC and left PCC within the DMN of the PDAR sample when compared to PDT subjects, despite comparable cognitive performance and no statistically discernible GM volume differences among subjects (Ashburner & Friston, 2001; Bookstein, 2001; Winkler et al., 2012). The SOI-GICA method, performed on a concatenated dataset of PDAR, PDT, and HC, not only identified the DMN but also was sensitive enough to detect activity differences between the respective groups. To our knowledge, this is the first study to compare DMNs between cognitively comparable PDAR and PDT subtypes. Significant correlations also were observed between DMN activity and spatial memory performance, suggesting that DMN measures may relate to cognitive functions in a meaningful way. Thus, our data support differential DMN activity in cognitively comparable PDAR and PDT subjects. Future studies with larger sample sizes are needed to confirm this finding, and longitudinal methodologies are required to clarify the role of early DMN functional disruption in predicting future cognitive performance in PDAR.
ICA does not provide information about the amplitude of brain activity in brain regions within networks such as the DMN. Therefore, we investigated the intensity of regional spontaneous brain activity using fractional ALFF (fALFF), a measure that is less sensitive to physiological noise (Zou et al., 2008). Although differences in posterior regions of the DMN (similar to the ones shown in Fig. 2) were detected, none survived statistical correction for multiple comparisons.
In the current study, we did not detect VBM group differences after multiple comparisons correction FWE (Ashburner & Friston, 2001; Bookstein, 2001; Winkler et al., 2012). A trend, however, was detected within the functionally defined DMN when the statistical threshold was relaxed to an uncorrected p < .001 (cluster size <10). A subsequent power analysis revealed that our study cohort may be underpowered to detect significant GM differences between HC and PD, suggesting that GM volume differences may be detected with larger sample sizes. Nevertheless, using resting state fMRI data from the same cohort, we did detect significant functional differences in the DMN, even after controlling for GM volume effects, suggesting superior sensitivity of our method to detect differences between PDAR and PDT.
4.1. Inconsistent literature on DMN integrity in PD
Previous studies investigating DMN integrity in PD, including task-related deactivation approaches, have reported conflicting results, with some studies reporting differences and others no difference (Delaveau et al., 2010; van Eimeren, Monchi, Ballanger, & Strafella, 2009; Ibarretxe-Bilbao et al., 2011; Krajcovicova, Mikl, Marecek, & Rektorova, 2012). For example, two studies using a similar methodology to ours (i.e., ICA of resting state data) did not detect any differences in DMN integrity between PD patients and HC, although one reported differences in connectivity among several resting state networks (Baggio et al., 2014; Krajcovicova et al., 2012). Our study results suggest that not taking into account PD subtypes may account partially for the observed discrepancies in the literature. A previous study investigated DMN activity in PD subgroups based on whether they had VH or not and found that those with VH had increased activity in the DMN whereas those without had decreased DMN activity (Franciotti et al., 2015). In the present study, we investigated PD subtypes depending on their predominant motor symptom presentation, i.e., PDAR and PDT. We found that PDT participants did not demonstrate significant differences in DMN activity when compared to HC, whereas PDAR subjects had significantly lower activation in the DMN compared to both groups. The results from Franciotti et al. and our group suggest that not taking into account PD subtypes may explain partially the observed discrepancies in the literature.
4.2. Cognitive impairment and its relationship to DMNchanges in PD
Several of the cognitive deficits in PD (i.e., executive function and working-memory) have been ascribed to a failure in frontal–-striatal and basal ganglia circuits (Jankovic et al., 1990). Other cognitive deficits in non-demented PD patients, however, have implicated dysfunctional posterior cortical circuitry. Thus, the pattern of PD-related cognitive decline suggests that pathology may be occurring in at least two co-existing networks: a relatively slowly declining frontal striatal circuitry and a more rapidly declining posterior–cortical circuitry (Jankovic et al., 1990; Pagonabarraga & Kulisevsky, 2012).
Our data clearly supported decreased activity in posterior cortical regions, specifically the PCC and IPC within the DMN in PDAR. The above mentioned trajectories of cognitive decline in PD, however, are not completely independent from each other. Thus, deactivation in the DMN could reflect disinhibition in brain regions related to cognition and cause deficits in the ability to redirect attentional processes [i.e., from self-reflection to goal-directed behavior (Greicius, Krasnow, Reiss, & Menon, 2003; Raichle et al., 2001; Raichle & Snyder, 2007)]. Since the dynamic regulation of disinhibition and shifting attention to external goals is crucial for effective executive task performance, it is possible that a dysfunctional DMN could underlie some of the observed executive function (i.e., planning and set shifting) deficits in PD. This hypothesis has been validated in other neurodegenerative disorders such as Alzheimer disease (AD) (Agosta et al., 2012; Rocca et al., 2010; Tedeschi et al., 2012; Zhou et al., 2010). Therefore, further studies are needed to establish the specific contributions of these two networks in the neuropathology of cognitive impairment in PD (Greicius et al., 2003; Raichle et al., 2001; Raichle & Snyder, 2007).
4.3. The dissociation between functional and structural changes in PD
Anatomic as well as functional imaging studies suggest that posteromedial cortices within the DMN are strongly connected to the temporal lobe hippocampal memory system (Kobayashi & Amaral, 2007; Ward et al., 2014). Since the overall rate of cognitive decline in PD supports a rapidly declining posterior-cortical network, the observed decreased activity in the DMN in the absence of any significant local regional brain atrophy might provide an explanation for why PDAR are more likely to develop cognitive deficits (Pagonabarraga & Kulisevsky, 2012). This hypothesis derives further support from the observation that PD with dementia (PDD) generally is associated with diffuse cortical and subcortical Lewy body pathology in MTL, prefrontal, and parietal cortices, regions that all are part of the distributed memory network (Braak, Rub, Jansen Steur, Del Tredici, & de Vos, 2005; Poppenk & Moscovitch, 2011; Ranganath, 2010). The lack of statistically significant GM volume loss within the DMN in our patient groups suggested that the occurrence of significant regional brain atrophy might be lower in the absence of comorbid cognitive impairment in PD, a result also consistent with recent AD literature (Sheline et al., 2010). This inference, however, should be interpreted with caution because our study may be underpowered to detect significant GM differences between HCs and PD. Taken together, our results suggested that functional changes in the DMN may be sensitive in detecting possible cognitive decline risks in PD (Tessitore, Amboni, et al., 2012).
4.4. Compensatory mechanisms and the role of fMRI in the early detection of cognitive decline in PD
Studying early signs of cognitive impairment in PD is difficult because patients are able to mask such change by using compensatory brain mechanisms until a critical threshold is reached (Appel-Cresswell, de la Fuente-Fernandez, Galley, & McKeown, 2010). Therefore, a cognitively unbiased approach such as resting state fMRI may be an ideal approach to capture the heterogeneity of cognitive impairment in PD subtypes (Tessitore, Esposito, et al., 2012). The results of our correlation analyses appear to corroborate some sort of compensatory activity within the DMN in PDAR: positive as well as negative correlations were observed between spatial memory performance and resting state IPC and PPC activity. Recent studies suggest a role for parietal regions within the DMN in allocating visuospatial attention during anticipation and retrieval of information, the latter in concert with MTL structures (Sestieri, Corbetta, Romani, & Shulman, 2011; Small et al., 2003). Although not significant, we found that activity in IPC and PCC within the DMN was negatively correlated only in PDAR (data not shown). As such, it would be reasonable to hypothesize that compensatory mechanisms may come into play in an attempt to maintain a comparable cognitive performance in PDAR patients. With caution, it is possible to state that other resting state networks related to cognition (i.e., the executive and asymmetric fronto-parietal networks) may become active in an attempt to maintain an efficient cognitive performance in PDAR subjects at early stages of PD. In order to assert this possibility, an effect of disease duration on results must be established, an analysis that has not been performed in this study. With disease progression, behavioral differences may emerge when other networks can no longer compensate for the disrupted IPC and PCC functions in the DMN. Therefore, it is conceivable that the observed reduced activity of the PCC and IPC within the DMN potentially may play a role in the development of cognitive decline in PD and may precede structural abnormalities (i.e., atrophy of frontal parietal cortices and MTLs) already described in patients with PD-MCI and/or PDD (Song et al., 2011).
4.5. DMN as a possible predictor of cognitive decline in PD
Longitudinal studies clearly have shown that posterior cortical regions are good predictors of future dementia when compared to dopamine-related deficits in PD (Williams-Gray et al., 2009; Williams-Gray, Foltynie, Brayne, Robbins, & Barker, 2007). As an example, a longitudinal PET study revealed that reduced glucose metabolism in occipital and posterior cingulate regions heralded future conversion to dementia (Bohnen et al., 2011). Since posterior cortical regions are connected intimately to MTL regions, hippocampal neurodegeneration also has been associated with the initial stages of cognitive decline in PD, with more severe cognitive impairment associated with additional atrophy in MTL structures (Weintraub et al., 2011). As such, there is renewed emphasis on the importance of detecting posterior cortical changes as predictors of dementia in PD.
The reason DMN changes in PDAR subjects occurred only in left structures (left IPC and PCC) is unclear. There is evidence to support increased dopamine turnover in the dominant hemisphere (de la Fuente-Fernandez, Kishore, Calne, Ruth, & Stoessl, 2000). This dominance is hypothesized to produce increased oxidative stress (Jenner, 2003), excitatory neurotoxicity (Choi, 1988), and dopamine metabolites (Jenner, 2003). Since our PD subjects were right-handed, the left hemisphere would be the dominant hemisphere for our cohort. Additional studies are needed to delineate the neural underpinnings of this finding.
PD patients also demonstrate increased susceptibility for iron in the nigra contralateral to the most affected side (Azuma et al., 2016). Additionally, increased spectroscopy asymmetry has been observed in PD subjects compared to controls (Seraji-Bozorgzad et al., 2015). PD subjects also demonstrate decreased left FA values that are correlated with UPDRS motor scores (Prakash, Sitoh, Tan, & Au, 2012). In general the left nigra also seems to be vulnerable (Scherfler et al., 2012). A meta-analysis of handedness and side of PD onset indicated increased onset on the dominant hand in both left and right handers (van der Hoorn, Burger, Leenders, & de Jong, 2012). Taken together, these studies seem to suggest a left hemisphere vulnerability in PD.
In the current study, we observed left IPC and PCC vulnerability in PD. The PCC is a highly connected and metabolically active brain region that is functionally important for proper learning and memory functioning (Leech & Sharp, 2014). Patients with attention deficit hyperactivity disorder (ADHD) have shown abnormalities in PCC activity and a study by Burgess et al. also has highlighted the importance of left PCC in multi-tasking (Burgess, Veitch, de Lacy Costello, & Shallice, 2000; Greicius, 2008). Similarly, posterior parietal cortex or IPC is implicated in conscious motor intention and awareness generation (Desmurget & Sirigu, 2009, 2012). Lesions of the left IPC in humans are associated with limb apraxia, a syndrome involving difficulty copying or producing gestures and movements to command (Singh-Curry & Husain, 2009). Although IPC and PCC are clearly affected in PD there is no consensus as to the exact roles they play in PD pathophysiology. Therefore, it remains to be investigated whether the reduced posterior activity in the DMN including its laterality is related to structural changes that underlie cortical pathology critical for developing dementia in PD, such as synucleinopathy or Alzheimer's-type pathology (Baggio et al., 2014; Compta et al., 2011).
4.6. Limitations of the study
There are several study limitations. Although our fMRI results survived statistical correction for multiple comparisons, our findings should be considered preliminary because of the relatively small size of the study cohort. The exploratory correlation analyses were not corrected for multiple comparisons and thus need to be replicated with a larger study cohort. Although ICA is a robust technique to detect group differences, our unbalanced sample sizes can be considered as another study limitation. Cross-validation of the current finding(s) using larger and independent datasets also is warranted (Fox, Spreng, Ellamil, Andrews-Hanna, & Christoff, 2015). PD patients were assessed in practically-defined “on” and “off” (withholding medication for ∼12 h) states and thus the potentially confounding effects of chronic dopaminergic medications could not be accounted for completely in this study. The lack of longitudinal fMRI and cognitive data in our PD subjects also is a limitation, underscoring the need for follow-up analyses of functional changes in the DMN in predicting cognitive performance in PD. Nevertheless, we hypothesize that the observed reduced activity in posterior regions of the DMN in PDAR may constitute a significant risk factor for progressive cognitive decline and, ultimately, dementia. Based on the current results, this hypothesis is necessarily speculative. Lastly, with the expansion of knowledge about PD pathophysiology, there are certainly other ways to subtype PD patients, i.e., according to different genetic backgrounds and/or clinical presentations. Our study, however, focused on PDAR and PDT, one of the first clinical subtypes described (Jankovic et al., 1990). Future studies, therefore, are warranted to understand the relevance of resting state functional connectivity differences between PD subtypes other than PDAR and PDT.
4.7. Summary and conclusions
PDAR subjects displayed significant changes in DMN functional activity compared to PDTand HC subjects, specifically in posterior IPC and PCC regions. These changes in resting state activation also were associated with cognitive performance. Because of the relatively small sample size, the absence of significant GM volume differences between HCs and PD does not imply that there is no effect of PD on GM volume. Nevertheless, the current results agree with several lines of evidence suggesting that PDAR subjects have a different underlying pathophysiology from that of PDT subjects (Jankovic & Kapadia, 2001; Marjama-Lyons & Koller, 2000; Spiegel et al., 2007; Vingerhoets, Schulzer, Calne, & Snow, 1997; Zaidel et al., 2009; Zetusky, Jankovic, & Pirozzolo, 1985). Our results clarify the potential profile of DMN dysfunction in early stage PDAR and raise the possibility that changes in posterior DMN regions may increase the risk for progressive cognitive deficits. Future studies aimed at validating the current findings and longitudinal investigation of the functional integrity of DMN and other cognitively-relevant resting state networks in cognitively unimpaired PD patients will provide further insights into the mechanisms underlying cognitive decline evolution in PD (Christopher et al., 2015).
Supplementary Material
Acknowledgments
This work was supported by the NINDS grant NS060722, the HMC GCRC (NIH M01RR10732), GCRC Construction Grants (C06RR016499), and the Department of Radiology, Pennsylvania State University College of Medicine.
Footnotes
The authors report no conflict of interest.
Supplementary data: Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.cortex.2016.04.021.
References
- Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson's disease. Brain Pathology. 2010;20(3):633–639. doi: 10.1111/j.1750-3639.2009.00369.x. BPA369 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M. Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiology of Aging. 2012;33(8):1564–1578. doi: 10.1016/j.neurobiolaging.2011.06.007. http://dx.doi.org/10.1016/j.neurobiolaging.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Appel-Cresswell S, de la Fuente-Fernandez R, Galley S, McKeown MJ. Imaging of compensatory mechanisms in Parkinson's disease. Current Opinion in Neurology. 2010;23(4):407–412. doi: 10.1097/WCO.0b013e32833b6019. http://dx.doi.org/10.1097/WCO.0b013e32833b6019. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Why voxel-based morphometry should be used. NeuroImage. 2001;14(6):1238–1243. doi: 10.1006/nimg.2001.0961. http://dx.doi.org/10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- Azuma M, Hirai T, Yamada K, Yamashita S, Ando Y, Tateishi M, et al. Lateral asymmetry and spatial difference of iron deposition in the substantia nigra of patients with Parkinson disease measured with quantitative susceptibility mapping. AJNR American Journal of Neuroradiology. 2016 doi: 10.3174/ajnr.A4645. http://dx.doi.org/10.3174/ajnr.A4645. [DOI] [PMC free article] [PubMed]
- Baggio HC, Segura B, Sala-Llonch R, Marti MJ, Valldeoriola F, Compta Y, et al. Cognitive impairment and resting-state network connectivity in Parkinson's disease. Human Brain Mapping. 2014 doi: 10.1002/hbm.22622. http://dx.doi.org/10.1002/hbm.22622. [DOI] [PMC free article] [PubMed]
- Ballard C, Ziabreva I, Perry R, Larsen JP, O'Brien J, McKeith I, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67(11):1931–1934. doi: 10.1212/01.wnl.0000249130.63615.cc. http://dx.doi.org/10.1212/01.wnl.0000249130.63615.cc. [DOI] [PubMed] [Google Scholar]
- Berg D, Postuma RB, Bloem B, Chan P, Dubois B, Gasser T, et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson's disease. Movement Disorders. 2014;29(4):454–462. doi: 10.1002/mds.25844. http://dx.doi.org/10.1002/mds.25844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Koeppe RA, Minoshima S, Giordani B, Albin RL, Frey KA, et al. Cerebral glucose metabolic features of Parkinson disease and incident dementia: longitudinal study. Journal of Nuclear Medicine. 2011;52(6):848–855. doi: 10.2967/jnumed.111.089946. http://dx.doi.org/10.2967/jnumed.111.089946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images. NeuroImage. 2001;14(6):1454–1462. doi: 10.1006/nimg.2001.0770. http://dx.doi.org/10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Jansen Steur EN, Del Tredici K, de Vos RA. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology. 2005;64(8):1404–1410. doi: 10.1212/01.WNL.0000158422.41380.82. http://dx.doi.org/10.1212/01.WNL.0000158422.41380.82. [DOI] [PubMed] [Google Scholar]
- Brown GG, Rahill AA, Gorell JM, McDonald C, Brown SJ, Sillanpaa M, et al. Validity of the Dementia Rating Scale in assessing cognitive function in Parkinson's disease. Journal of Geriatric Psychiatry and Neurology. 1999;12(4):180–188. doi: 10.1177/089198879901200403. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Veitch E, de Lacy Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38(6):848–863. doi: 10.1016/s0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson's disease. Annals of Neurology. 1992;32(Suppl.):S125–S127. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell'acqua F, Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour [Research Support, Non-U.S. Gov't Review] Neuroscience and Biobehavioral Reviews. 2013;37(8):1724–1737. doi: 10.1016/j.neubiorev.2013.07.001. http://dx.doi.org/10.1016/j.neubiorev.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Caviness JN, Driver-Dunckley E, Connor DJ, Sabbagh MN, Hentz JG, Noble B, et al. Defining mild cognitive impairment in Parkinson's disease. Movement Disorders. 2007;22(9):1272–1277. doi: 10.1002/mds.21453. http://dx.doi.org/10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Christopher L, Duff-Canning S, Koshimori Y, Segura B, Boileau I, Chen R, et al. Salience network and parahippocampal dopamine dysfunction in memory-impaired Parkinson disease. Annals of Neurology. 2015;77(2):269–280. doi: 10.1002/ana.24323. http://dx.doi.org/10.1002/ana.24323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compta Y, Parkkinen L, O'Sullivan SS, Vandrovcova J, Holton JL, Collins C, et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain. 2011;134(Pt 5):1493–1505. doi: 10.1093/brain/awr031. http://dx.doi.org/10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaveau P, Salgado-Pineda P, Fossati P, Witjas T, Azulay JP, Blin O. Dopaminergic modulation of the default mode network in Parkinson's disease. European Neuropsychopharmacology. 2010;20(11):784–792. doi: 10.1016/j.euroneuro.2010.07.001. http://dx.doi.org/10.1016/j.euroneuro.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Sirigu A. A parietal-premotor network for movement intention and motor awareness. Trends in Cognitive Sciences. 2009;13(10):411–419. doi: 10.1016/j.tics.2009.08.001. http://dx.doi.org/10.1016/j.tics.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Sirigu A. Conscious motor intention emerges in the inferior parietal lobule. Current Opinion in Neurobiology. 2012;22(6):1004–1011. doi: 10.1016/j.conb.2012.06.006. http://dx.doi.org/10.1016/j.conb.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. doi: 10.1212/01.wnl.0000247740.47667.03. http://dx.doi.org/10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- Eggers C, Pedrosa DJ, Kahraman D, Maier F, Lewis CJ, Fink GR, et al. Parkinson subtypes progress differently in clinical course and imaging pattern. PLoS One. 2012;7(10):e46813. doi: 10.1371/journal.pone.0046813. http://dx.doi.org/10.1371/journal.pone.0046813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eimeren T, Monchi O, Ballanger B, Strafella AP. Dysfunction of the default mode network in Parkinson disease: a functional magnetic resonance imaging study. Archives of Neurology. 2009;66(7):877–883. doi: 10.1001/archneurol.2009.97. http://dx.doi.org/10.1001/archneurol.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I. Neuropathology and neurochemistry of nonmotor symptoms in Parkinson's disease. Parkinson's Disease. 2011;2011:708404. doi: 10.4061/2011/708404. http://dx.doi.org/10.4061/2011/708404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KC, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. NeuroImage. 2015;111:611–621. doi: 10.1016/j.neuroimage.2015.02.039. http://dx.doi.org/10.1016/j.neuroimage.2015.02.039. [DOI] [PubMed] [Google Scholar]
- Franciotti R, Delli Pizzi S, Perfetti B, Tartaro A, Bonanni L, Thomas A, et al. Default mode network links to visual hallucinations: a comparison between Parkinson's disease and multiple system atrophy. Movement Disorders. 2015;30(9):1237–1247. doi: 10.1002/mds.26285. http://dx.doi.org/10.1002/mds.26285. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Kishore A, Calne DB, Ruth TJ, Stoessl AJ. Nigrostriatal dopamine system and motor lateralization. Behavioural Brain Research. 2000;112(1–2):63–68. doi: 10.1016/s0166-4328(00)00165-0. [DOI] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Current Opinion in Neurology. 2008;21(4):424–430. doi: 10.1097/WCO.0b013e328306f2c5. http://dx.doi.org/10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. http://dx.doi.org/10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmich RC, Hallett M, Deuschl G, Toni I, Bloem BR. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain. 2012;135(Pt 11):3206–3226. doi: 10.1093/brain/aws023. http://dx.doi.org/10.1093/brain/aws023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- van der Hoorn A, Burger H, Leenders KL, de Jong BM. Handedness correlates with the dominant Parkinson side: a systematic review and meta-analysis. Movement Disorders. 2012;27(2):206–210. doi: 10.1002/mds.24007. http://dx.doi.org/10.1002/mds.24007. [DOI] [PubMed] [Google Scholar]
- Ibarretxe-Bilbao N, Zarei M, Junque C, Marti MJ, Segura B, Vendrell P, et al. Dysfunctions of cerebral networks precede recognition memory deficits in early Parkinson's disease. NeuroImage. 2011;57(2):589–597. doi: 10.1016/j.neuroimage.2011.04.049. http://dx.doi.org/10.1016/j.neuroimage.2011.04.049. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Kapadia AS. Functional decline in Parkinson disease. Archives of Neurology. 2001;58(10):1611–1615. doi: 10.1001/archneur.58.10.1611. [DOI] [PubMed] [Google Scholar]
- Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40(10):1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson's disease: progression to dementia. Movement Disorders. 2006a;21(9):1343–1349. doi: 10.1002/mds.20974. http://dx.doi.org/10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- Janvin CC, Larsen JP, Salmon DP, Galasko D, Hugdahl K, Aarsland D. Cognitive profiles of individual patients with Parkinson's disease and dementia: comparison with dementia with lewy bodies and Alzheimer's disease. Movement Disorders. 2006b;21(3):337–342. doi: 10.1002/mds.20726. http://dx.doi.org/10.1002/mds.20726. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson's disease. Annals of Neurology. 2003;53(Suppl. 3):S26–S36. doi: 10.1002/ana.10483. http://dx.doi.org/10.1002/ana.10483. discussion S36–S28. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurology. 2010;9(12):1200–1213. doi: 10.1016/S1474-4422(10)70212-X. http://dx.doi.org/10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: III. Cortical efferents. The Journal of Comparative Neurology. 2007;502(5):810–833. doi: 10.1002/cne.21346. http://dx.doi.org/10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- Krajcovicova L, Mikl M, Marecek R, Rektorova I. The default mode network integrity in patients with Parkinson's disease is levodopa equivalent dose-dependent. Journal of Neural Transmission. 2012;119(4):443–454. doi: 10.1007/s00702-011-0723-5. http://dx.doi.org/10.1007/s00702-011-0723-5. [DOI] [PubMed] [Google Scholar]
- Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, et al. Core assessment program for intracerebral transplantations (CAPIT) Movement Disorders. 1992;7(1):2–13. doi: 10.1002/mds.870070103. http://dx.doi.org/10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- Lee EY, Sen S, Eslinger PJ, Wagner D, Shaffer ML, Kong L, et al. Early cortical gray matter loss and cognitive correlates in non-demented Parkinson's patients. Parkinsonism & Related Disorders. 2013;19(12):1088–1093. doi: 10.1016/j.parkreldis.2013.07.018. http://dx.doi.org/10.1016/j.parkreldis.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(Pt 1):12–32. doi: 10.1093/brain/awt162. http://dx.doi.org/10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MM, Du G, Sen S, Kawaguchi A, Truong Y, Lee S, et al. Differential involvement of striato- and cerebello-thalamo-cortical pathways in tremor- and akinetic/rigid-predominant Parkinson's disease. Neuroscience. 2011;177:230–239. doi: 10.1016/j.neuroscience.2010.12.060. http://dx.doi.org/10.1016/j.neuroscience.2010.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao R, Krolik JL, McKeown MJ. An information-theoretic criterion for intrasubject alignment of FMRI time series: motion corrected independent component analysis. IEEE Transactions on Medical Imaging. 2005;24(1):29–44. doi: 10.1109/tmi.2004.837791. [DOI] [PubMed] [Google Scholar]
- Louis ED, Tang MX, Cote L, Alfaro B, Mejia H, Marder K. Progression of parkinsonian signs in Parkinson disease. Archives of Neurology. 1999;56(3):334–337. doi: 10.1001/archneur.56.3.334. [DOI] [PubMed] [Google Scholar]
- Marjama-Lyons J, Koller W. Tremor-predominant Parkinson's disease. Approaches to treatment. Drugs & Aging. 2000;16(4):273–278. doi: 10.2165/00002512-200016040-00003. [DOI] [PubMed] [Google Scholar]
- Nussbaum RL, Ellis CE. Alzheimer's disease and Parkinson's disease. The New England Journal of Medicine. 2003;348(14):1356–1364. doi: 10.1056/NEJM2003ra020003. http://dx.doi.org/10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- Pagonabarraga J, Kulisevsky J. Cognitive impairment and dementia in Parkinson's disease. Neurobiology of Disease. 2012;46(3):590–596. doi: 10.1016/j.nbd.2012.03.029. http://dx.doi.org/10.1016/j.nbd.2012.03.029. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Moscovitch M. A hippocampal marker of recollection memory ability among healthy young adults: contributions of posterior and anterior segments. Neuron. 2011;72(6):931–937. doi: 10.1016/j.neuron.2011.10.014. http://dx.doi.org/10.1016/j.neuron.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Prakash BD, Sitoh YY, Tan LC, Au WL. Asymmetrical diffusion tensor imaging indices of the rostral substantia nigra in Parkinson's disease. Parkinsonism & Related Disorders. 2012;18(9):1029–1033. doi: 10.1016/j.parkreldis.2012.05.021. http://dx.doi.org/10.1016/j.parkreldis.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. http://dx.doi.org/10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. http://dx.doi.org/10.1016/j.neuroimage.2007.02.041. discussion 1097–1089. [DOI] [PubMed] [Google Scholar]
- Rajput AH, Voll A, Rajput ML, Robinson CA, Rajput A. Course in Parkinson disease subtypes: a 39-year clinicopathologic study. Neurology. 2009;73(3):206–212. doi: 10.1212/WNL.0b013e3181ae7af1. http://dx.doi.org/10.1212/WNL.0b013e3181ae7af1. [DOI] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20(11):1263–1290. doi: 10.1002/hipo.20852. http://dx.doi.org/10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Valsasina P, Absinta M, Riccitelli G, Rodegher ME, Misci P, et al. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology. 2010;74(16):1252–1259. doi: 10.1212/WNL.0b013e3181d9ed91. http://dx.doi.org/10.1212/WNL.0b013e3181d9ed91. [DOI] [PubMed] [Google Scholar]
- Scherfler C, Seppi K, Mair KJ, Donnemiller E, Virgolini I, Wenning GK, et al. Left hemispheric predominance of nigrostriatal dysfunction in Parkinson's disease. Brain. 2012;135(Pt 11):3348–3354. doi: 10.1093/brain/aws253. http://dx.doi.org/10.1093/brain/aws253. [DOI] [PubMed] [Google Scholar]
- Schiess MC, Zheng H, Soukup VM, Bonnen JG, Nauta HJ. Parkinson's disease subtypes: clinical classification and ventricular cerebrospinal fluid analysis. Parkinsonism & Related Disorders. 2000;6(2):69–76. doi: 10.1016/s1353-8020(99)00051-6. [DOI] [PubMed] [Google Scholar]
- Seraji-Bozorgzad N, Bao F, George E, Krstevska S, Gorden V, Chorostecki J, et al. Longitudinal study of the substantia nigra in Parkinson disease: a high-field (1) H-MR spectroscopy imaging study. Movement Disorders. 2015;30(10):1400–1404. doi: 10.1002/mds.26323. http://dx.doi.org/10.1002/mds.26323. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. The Journal of Neuroscience. 2011;31(12):4407–4420. doi: 10.1523/JNEUROSCI.3335-10.2011. http://dx.doi.org/10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D'Angelo G, et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. The Journal of Neuroscience. 2010;30(50):17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Curry V, Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47(6):1434–1448. doi: 10.1016/j.neuropsychologia.2008.11.033. http://dx.doi.org/10.1016/j.neuropsychologia.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam MM. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. NeuroImage. 2003;18(3):633–641. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Song SK, Lee JE, Park HJ, Sohn YH, Lee JD, Lee PH. The pattern of cortical atrophy in patients with Parkinson's disease according to cognitive status. Movement Disorders. 2011;26(2):289–296. doi: 10.1002/mds.23477. http://dx.doi.org/10.1002/mds.23477. [DOI] [PubMed] [Google Scholar]
- Spiegel J, Hellwig D, Farmakis G, Jost WH, Samnick S, Fassbender K, et al. Myocardial sympathetic degeneration correlates with clinical phenotype of Parkinson's disease. Movement Disorders. 2007;22(7):1004–1008. doi: 10.1002/mds.21499. http://dx.doi.org/10.1002/mds.21499. [DOI] [PubMed] [Google Scholar]
- Tedeschi G, Trojsi F, Tessitore A, Corbo D, Sagnelli A, Paccone A, et al. Interaction between aging and neurodegeneration in amyotrophic lateral sclerosis. Neurobiology of Aging. 2012;33(5):886–898. doi: 10.1016/j.neurobiolaging.2010.07.011. http://dx.doi.org/10.1016/j.neurobiolaging.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Amboni M, Esposito F, Russo A, Picillo M, Marcuccio L, et al. Resting-state brain connectivity in patients with Parkinson's disease and freezing of gait. Parkinsonism & Related Disorders. 2012;18(6):781–787. doi: 10.1016/j.parkreldis.2012.03.018. http://dx.doi.org/10.1016/j.parkreldis.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Esposito F, Vitale C, Santangelo G, Amboni M, Russo A, et al. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology. 2012;79(23):2226–2232. doi: 10.1212/WNL.0b013e31827689d6. http://dx.doi.org/10.1212/WNL.0b013e31827689d6. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Movement Disorders. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. http://dx.doi.org/10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Vingerhoets FJ, Schulzer M, Calne DB, Snow BJ. Which clinical sign of Parkinson's disease best reflects the nigrostriatal lesion? Annals of Neurology. 1997;41(1):58–64. doi: 10.1002/ana.410410111. http://dx.doi.org/10.1002/ana.410410111. [DOI] [PubMed] [Google Scholar]
- Ward AM, Schultz AP, Huijbers W, Van Dijk KR, Hedden T, Sperling RA. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Human Brain Mapping. 2014;35(3):1061–1073. doi: 10.1002/hbm.22234. http://dx.doi.org/10.1002/hbm.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Doshi J, Koka D, Davatzikos C, Siderowf AD, Duda JE, et al. Neurodegeneration across stages of cognitive decline in Parkinson disease. Archives of Neurology. 2011;68(12):1562–1568. doi: 10.1001/archneurol.2011.725. http://dx.doi.org/10.1001/archneurol.2011.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, et al. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(Pt 11):2958–2969. doi: 10.1093/brain/awp245. http://dx.doi.org/10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130(Pt 7):1787–1798. doi: 10.1093/brain/awm111. http://dx.doi.org/10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Sabuncu MR, Yeo BT, Fischl B, Greve DN, Kochunov P, et al. Measuring and comparing brain cortical surface area and other areal quantities. NeuroImage. 2012;61(4):1428–1443. doi: 10.1016/j.neuroimage.2012.03.026. http://dx.doi.org/10.1016/j.neuroimage.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. http://dx.doi.org/10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- Zaidel A, Arkadir D, Israel Z, Bergman H. Akineto-rigid vs. tremor syndromes in Parkinsonism. Current Opinion in Neurology. 2009;22(4):387–393. doi: 10.1097/WCO.0b013e32832d9d67. http://dx.doi.org/10.1097/WCO.0b013e32832d9d67. [DOI] [PubMed] [Google Scholar]
- Zetusky WJ, Jankovic J, Pirozzolo FJ. The heterogeneity of Parkinson's disease: clinical and prognostic implications. Neurology. 1985;35(4):522–526. doi: 10.1212/wnl.35.4.522. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wei L, Hu X, Xie B, Zhang Y, Wu GR, et al. Akinetic-rigid and tremor-dominant Parkinson's disease patients show different patterns of intrinsic brain activity. Parkinsonism & Related Disorders. 2015;21(1):23–30. doi: 10.1016/j.parkreldis.2014.10.017. http://dx.doi.org/10.1016/j.parkreldis.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zuo XN, Ma SY, Zang YF, Milham MP, Zhu CZ. Subject order-independent group ICA (SOI-GICA) for functional MRI data analysis. NeuroImage. 2010;51(4):1414–1424. doi: 10.1016/j.neuroimage.2010.03.039. http://dx.doi.org/10.1016/j.neuroimage.2010.03.039. [DOI] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain: A journal of Neurology. 2010;133(Pt 5):1352–1367. doi: 10.1093/brain/awq075. http://dx.doi.org/10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. Journal of Neuroscience Methods. 2008;172(1):137–141. doi: 10.1016/j.jneumeth.2008.04.012. http://dx.doi.org/10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


