Abstract
Hepatic ischemia-reperfusion injury (HIRI) is a major cause of hepatic failure and death after liver trauma, haemorrhagic shock, resection surgery and liver transplantation. AMP-activated protein kinase (AMPK) is an energy sensitive kinase that plays crucial roles in the regulation of metabolic homeostasis. In HIRI, ischemia induces the decline of ATP and the increased ratio of AMP/ATP, which promotes the phosphorylation and activation of AMPK. Three AMPK kinases, liver kinase B1 (LKB1), Ca2+/calmodulin-depedent protein kinase kinase β (CaMKKβ) and TGF-β-activated kinase-1 (TAK1), are main upstream kinases for the phosphorylation of AMPK. In addition to the changed AMP/ATP ratio, the activated CaMKKβ by increased intracelluar Ca2+ and the overproduction of reactive oxygen species (ROS) are also involved in the activation of AMPK during HIRI. The activated AMPK might provide protective benefits in HIRI via prevention of energy decline, inhibition of inflammatory response, suppression of hepatocyte apoptosis and attenuation of oxidative stress. Thus, AMPK might become a novel target for the pharmacological intervention of HIRI.
Keywords: AMP-activated protein kinase, hepatic ischemia-reperfusion injury, oxidative stress, inflammation, apoptosis
Hepatic ischemia-reperfusion injury (HIRI) happens when the blood supply to liver is interrupted and subsequently returned, resulting in robust oxidative stress and inflammatory response in liver [1]. HIRI is a major cause of hepatic failure and death after liver trauma, haemorrhagic shock, resection surgery and liver transplantation [2,3]. In HIRI, ischemia and hypoxia induce decline of ATP, a status of shortage of energy, thus directly or indirectly lead to hepatic damage [4]. Meanwhile, the fallen energy status activates several energy sensors such as AMP-activated protein kinase (AMPK) [5]. AMPK plays important roles in the maintenance of energy homeostasis via regulating energy metabolism [6]. In addition, there are increasing evidence indicated that AMPK also participated in the regulation of oxidative stress, inflammatory response and cellular apoptosis [7,8]. Recent studies have revealed that AMPK could provide beneficial effects in HIRI and AMPK is emerging as a novel target for pharmacological intervention of HIRI [9].
The structure of AMPK
AMPK is a heterotrimeric complexes of catalytic α subunit, regulatory β/γ subunits in all eukaryotic cells [10]. In mammals, there are several subunit isoforms including α1, α2, β1, β2, γ1, γ2 and γ3 [11]. For α subunit, there is a binding segment for β/γ subunit in the C-terminal domain, an auto-inhibitory domain (AID) in the middle position and an activation of kinase domain which contains the Thr172 residue in the N-terminal domain [12]. The C-terminal domain of β isoform is crucial for its interaction with α and γ subunits and a glycogen/carbohydrate-binding domain/motif (GBD/CBM) is located in the middle region of β isoform [13]. α1/α2 and β1/β2 isoforms are very similar in mammalian. AMPKγ subunits whose sizes vary from the length of N terminal domain, contain four cystathionine-β-synthase (CBS) which make up two Bateman domains in a series of tandem repeats (CBS1 and CBS2 in Bateman domains 1, CBS3 and CBS4 in Bateman domains 2) [14]. Binding AMP or ADP to the AMPKγ subunits is crucial for the sensing of lower energy status and the activation of AMPK [15].
Activation of AMPK in HIRI
The phosphorylation of AMPKα catalytic subunit at Thr172 is a hallmark of AMPK activation [13]. AMPK is activated by metabolic stress when the intracellular AMP/ATP ratio and/or ADP/ATP ratio increases [5]. Binding AMP or ADP to AMPKγ subunit can change AMPK into a better substrate for its upstream kinases [7]. Three AMPK kinases, liver kinase B1 (LKB1), Ca2+/calmodulin-depedent protein kinase kinase β (CaMKKβ) and TGF-β-activated kinase-1 (TAK1, a member of the mitogen-activated protein kinase family) have been identified as the main upstream kinases to mediate the phosphorylation of AMPKα at Thr172 [16]. The phosphorylation of AMPK is reversed mainly by protein phosphatase-2A (PP2A) and protein phosphatase-2C (PP2C) [17]. In addition to promoting AMPK phosphorylation, AMP can also prevent AMPK against dephosphorylation and the subsequent deactivation (Figure 1) [18]. In addition, the endogenous hormones including ghrelin, cannabinoids, glucocorticoids, resistin, adiponectin also play pivotal regulatory roles in the activation of AMPK [19-23].
Figure 1.
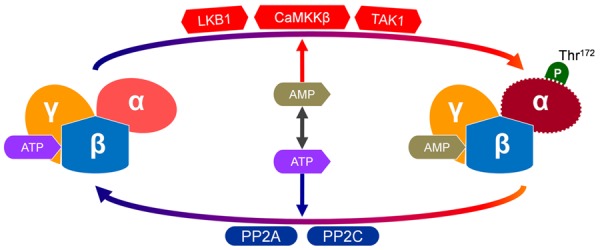
Activation of AMPK. AMPK is sensitive to AMP/ATP ratio, it can be activated when intracellular AMP increases. Binding AMP to AMPKγ subunit can change AMPK into a better substrate for its upstream kinases to phosphorylate and activate AMPK. The phosphorylation of AMPKα catalytic subunit at Thr172 is a hallmark of AMPK activation. Three AMPK kinases, liver kinase B1 (LKB1), Ca2+/calmodulin-depedent protein kinase kinase β (CaMKKβ) and TGF-β-activated kinase-1 (TAK1), are main upstream kinases for the phosphorylation of Thr172 in AMPKα. On the contrary, AMPK can be deactivated by protein phosphatase-2A (PP2A) and protein phosphatase-2C (PP2C).
There is evidence suggested that AMPK was activated in HIRI [24], but the underlying mechanisms largely remains unknown. Here, we will present several possible pathways (Figure 2). Firstly, AMPK might be activated in response to the changed ratio of AMP/ATP in liver during HIRI [9]. Secondly, the increase of intracelluar Ca2+ during HIRI could act as a second messenger and induce the activation of CaMKKβ [25], which is reported to be an upstream kinase of AMPK and be involved in the phosphorylation of AMPK [26]. Thirdly, increased reactive oxygen species (ROS), such as H2O2, were reported to be able to activate AMPK during HIRI because H2O2 could induce the oxidation of cysteine residues of the subunits of AMPK and then assist the phosphorylation of AMPK by increased AMP [27,28].
Figure 2.
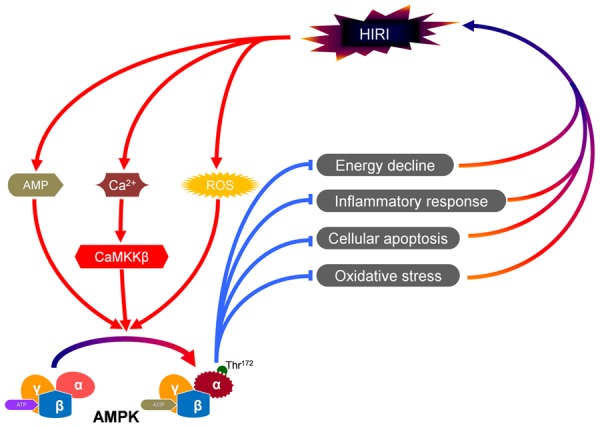
Pathophysiological significance of AMPK in HIRI. AMPK is activated in HIRI via several possible pathways. Firstly, AMPK might be activated in response to increased ratio of AMP/ATP. Secondly, the increased intracelluar Ca2+ during HIRI could induce the activation of CaMKKβ, an upstream kinase of AMPK. Thirdly, increased reactive oxygen species (ROS) might also be involved in the activation of AMPK. The activated AMPK might provide protective benefits via prevention of energy decline, inhibition of inflammatory response, suppression of hepatocyte apoptosis and attenuation of oxidative stress.
The beneficial actions of AMPK in HIRI
The beneficial effects of AMPK in ischemia-reperfusion have been observed in heart and kidney [29,30]. In rats with HIRI, administration of AMPK activator AICAR preserved ATP content, decreased lactate accumulation, suppressed hepatocyte apoptosis and alleviated hepatic injury [9]. Adiponectin is an important adipocytokine that involved in energy metabolism and other important physiological or pathological processes [23,31]. There is increasing evidence suggests that the biological activities of adiponectin largely depend on AMPK [32-34]. Recent research found that treatment with adiponectin suppressed the elevation of aminotransferase and the degree of histological abnormalities, these beneficial effects were associated with enhanced activation of AMPK while inhibition of AMPK abolished the protective effects of adiponectin [35]. These data also support the protective actions of AMPK in HIRI.
The potential mechanism underlying the benefits of AMPK
Although the beneficial effects of AMPK in HIRI and ischemia-reperfusion injury in other organs have been confirmed by various researchers, the underlying mechanisms largely remains unknown. It is well-established that the primary role of AMPK is maintenance the balance of energy metabolism [36], therefore, preventing the decline of ATP might be the basic mechanism contributes to the protective benefits of AMPK in HIRI [9]. In addition, AMPK also have pivotal regulatory roles in inflammatory response, oxidative stress and cellular apoptosis [37-39], these actions of AMPK could also provide beneficial effects in HIRI (Figure 2).
Maintenance of energy homeostasis
It was reported that ischemia-reperfusion could induce marked reduction in hepatic ATP level [9]. Preconditioning, a well documented approach against ischemia injury [40], stimulated the activation of hepatic AMPK, suppressed ATP decline and attenuated HIRI, treatment with AMPK activator could also maintain ATP level and provide beneficial effects [9]. These data suggested that preservation of ATP level might be closely associated with the protective effects of AMPK in HIRI [41]. The central roles of AMPK in maintenance of energy balance have been widely recognized. AMPK preserve ATP level via switching on catabolic pathways to produce ATP and shutting off anabolic pathways to prevent ATP consumption [42].
Suppression of inflammation
In addition to metabolic regulation, AMPK is also involved in several energy-intensive physiological and pathological processes such as inflammation [43,44]. It was reported that transfection with constitutively active AMPKα significantly suppressed LPS-induced production of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) in macrophages, whereas inhibition of AMPK by RNA interference dramatically enhanced the expression of TNF-α and IL-6 in LPS-stimulated macrophages [45,46]. Additionally, the anti-inflammatory actions of AMPK activators have extensively confirmed in vitro and in vivo [47,48].
Ischemia-reperfusion injury is usually accompanied with severe activation and infiltration of leukocytes. In HIRI, the activation of liver kupffer cell, neutrophils, T-lymphocytic and monocytes results in the generation of pro-inflammatory cytokines and matrix metalloproteinases (MMPs), these inflammatory mediators would greatly aggravate hepatic injury in HIRI [49]. It has been suggested that activation of AMPK in HIRI could suppress the expression of adhesion molecules, reduce the infiltration of leukocytes and decrease the level of pro-inflammatory cytokines [35]. Thus, suppression of inflammatory response might contribute to the protective benefits of AMPK in HIRI.
Modulation of apoptosis
AMPK is also an important regulator involved in determining the fate of cells. It was recently reported that activation of AMPK suppressed glucose deprivation-induced apoptosis in neurons, hyperglycemia-induced apoptosis in endothelial cells and free fatty acids-induce apoptosis in retinal pericytes [50-52]. In rats with HIRI, administration of AMPK activator AICAR significantly suppressed the apoptosis of hepatocytes [9]. In addition, the suppressive effects of adiponectin on the cleavage of caspase-3 and the percentage of TUNEL-positive cells in rats with HIRI could be reversed by AMPK inhibitor [35]. The above data suggests that the protective benefits of AMPK in HIRI might also attribute to its anti-apoptotic activities.
Regulation of oxidative stress
Severe oxidative stress induced by ischemia-reperfusion is another crucial tache in the development of HIRI [53]. Several studies have also revealed the important roles of AMPK in antioxidant defenses. It was reported that activation of AMPK induced the expression heme oxygenase-1 (HO-1), a representative anti-oxidative enzyme, via E2-related factor 2 (Nrf2)-dependent manner [54,55]. AMPK could also increase the expression of manganese superoxide dismutase and catalase via phosphorylation and activation of forkhead box O1 (FoxO1) [56,57]. In addition to the enhanced antioxidant capacity, AMPK also suppressing ROS generation via inhibiting the NAD(P)H oxidase [58,59]. These anti-oxidative activities of AMPK might also result in beneficial effects in HIRI.
Conclusions and prospects
AMPK is a critical enzyme involved in metabolic regulation and other energy-associated processes. Ischemia-reperfusion is a typical situation with severe disturbance of energy metabolism. There is increasing evidence suggests that AMPK is activated during ischemia-reperfusion and activated AMPK plays crucial roles against ischemia-reperfusion injury [39,60]. The protective benefits of AMPK activator, such as AICAR, have been confirmed in HIRI and ischemia-reperfusion injury in other organs [9,30]. In addition, the widely used first-line anti-diabetic drug metformin is an indirect activator of AMPK and most of the hypoglycemic actions of metformin depend on AMPK [61,62]. Interestingly, administration of metformin also prevented ischemia-reperfusion injury, including HIRI [63-67]. Therefore, AMPK might become a novel target for the pharmacological intervention of HIRI and ischemia-reperfusion injury in other organs.
Acknowledgements
This work was supported by the grant from the National Nature Science Foundation of China (No. 81370179, 81600465, 81671953), the grant from Chongqing Municipal Education Commission (No. KJ1400235) and the Training Program of Chongqing Medical University (No. 201419).
Disclosure of conflict of interest
None.
References
- 1.Saidi RF, Kenari SK. Liver ischemia/reperfusion injury: an overview. J Invest Surg. 2014;27:366–379. doi: 10.3109/08941939.2014.932473. [DOI] [PubMed] [Google Scholar]
- 2.Karatzas T, Neri AA, Baibaki ME, Dontas IA. Rodent models of hepatic ischemia-reperfusion injury: time and percentage-related pathophysiological mechanisms. J Surg Res. 2014;191:399–412. doi: 10.1016/j.jss.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Nastos C, Kalimeris K, Papoutsidakis N, Tasoulis MK, Lykoudis PM, Theodoraki K, Nastou D, Smyrniotis V, Arkadopoulos N. Global consequences of liver ischemia/reperfusion injury. Oxid Med Cell Longev. 2014;2014:906965. doi: 10.1155/2014/906965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans ZP, Mandavilli BS, Ellett JD, Rodwell D, Fariss MW, Fiorini RN, Schnellmann RG, Schmidt MG, Chavin K. Vitamin E succinate enhances steatotic liver energy status and prevents oxidative damage following ischemia/reperfusion. Transplant Proc. 2009;41:4094–4098. doi: 10.1016/j.transproceed.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardie DG, Schaffer BE, Brunet A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016;26:190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertoldo MJ, Faure M, Dupont J, Froment P. AMPK: a master energy regulator for gonadal function. Front Neurosci. 2015;9:235. doi: 10.3389/fnins.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonioli L, Colucci R, Pellegrini C, Giustarini G, Sacco D, Tirotta E, Caputi V, Marsilio I, Giron MC, Nemeth ZH, Blandizzi C, Fornai M. The AMPK enzyme-complex: from the regulation of cellular energy homeostasis to a possible new molecular target in the management of chronic inflammatory disorders. Expert Opin Ther Targets. 2016;20:179–191. doi: 10.1517/14728222.2016.1086752. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg GR, Schertzer JD. AMPK promotes macrophage fatty acid oxidative metabolism to mitigate inflammation: implications for diabetes and cardiovascular disease. Immunol Cell Biol. 2014;92:340–345. doi: 10.1038/icb.2014.11. [DOI] [PubMed] [Google Scholar]
- 9.Peralta C, Bartrons R, Serafin A, Blazquez C, Guzman M, Prats N, Xaus C, Cutillas B, Gelpi E, Rosello-Catafau J. Adenosine monophosphate-activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology. 2001;34:1164–1173. doi: 10.1053/jhep.2001.29197. [DOI] [PubMed] [Google Scholar]
- 10.Liu WY, Jiang RS. Advances in the research of AMPK and its subunit genes. Pak J Biol Sci. 2013;16:1459–1468. doi: 10.3923/pjbs.2013.1459.1468. [DOI] [PubMed] [Google Scholar]
- 11.Bright NJ, Thornton C, Carling D. The regulation and function of mammalian AMPK-related kinases. Acta Physiol (Oxf) 2009;196:15–26. doi: 10.1111/j.1748-1716.2009.01971.x. [DOI] [PubMed] [Google Scholar]
- 12.Oakhill JS, Scott JW, Kemp BE. Structure and function of AMP-activated protein kinase. Acta Physiol (Oxf) 2009;196:3–14. doi: 10.1111/j.1748-1716.2009.01977.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanz P. AMP-activated protein kinase: structure and regulation. Curr Protein Pept Sci. 2008;9:478–492. doi: 10.2174/138920308785915254. [DOI] [PubMed] [Google Scholar]
- 14.Novikova DS, Garabadzhiu AV, Melino G, Barlev NA, Tribulovich VG. AMP-activated protein kinase: structure, function, and role in pathological processes. Biochemistry (Mosc) 2015;80:127–144. doi: 10.1134/S0006297915020017. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: mechanisms of action and physiological activities. Exp Mol Med. 2016;48:e224. doi: 10.1038/emm.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo GL, Russo M, Ungaro P. AMP-activated protein kinase: a target for old drugs against diabetes and cancer. Biochem Pharmacol. 2013;86:339–350. doi: 10.1016/j.bcp.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 17.Salminen A, Kaarniranta K, Kauppinen A. Age-related changes in AMPK activation: role for AMPK phosphatases and inhibitory phosphorylation by upstream signaling pathways. Ageing Res Rev. 2016;28:15–26. doi: 10.1016/j.arr.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5’-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Nishi M, Doi A, Shono T, Furukawa Y, Shimada T, Furuta H, Sasaki H, Nanjo K. Ghrelin inhibits insulin secretion through the AMPK-UCP2 pathway in beta cells. FEBS Lett. 2010;584:1503–1508. doi: 10.1016/j.febslet.2010.02.069. [DOI] [PubMed] [Google Scholar]
- 20.Vara D, Salazar M, Olea-Herrero N, Guzman M, Velasco G, Diaz-Laviada I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagy. Cell Death Differ. 2011;18:1099–1111. doi: 10.1038/cdd.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nader N, Ng SS, Lambrou GI, Pervanidou P, Wang Y, Chrousos GP, Kino T. AMPK regulates metabolic actions of glucocorticoids by phosphorylating the glucocorticoid receptor through p38 MAPK. Mol Endocrinol. 2010;24:1748–1764. doi: 10.1210/me.2010-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai CH, Tsai HC, Huang HN, Hung CH, Hsu CJ, Fong YC, Hsu HC, Huang YL, Tang CH. Resistin promotes tumor metastasis by down-regulation of miR-519d through the AMPK/p38 signaling pathway in human chondrosarcoma cells. Oncotarget. 2015;6:258–270. doi: 10.18632/oncotarget.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo Y, Liu M. Adiponectin: a versatile player of innate immunity. J Mol Cell Biol. 2016;8:120–128. doi: 10.1093/jmcb/mjw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin Y, Li Z, Wang Z, Li Y, Zhao J, Mulholland M, Zhang W. Ghrelin contributes to protection of hepatocellular injury induced by ischaemia/reperfusion. Liver Int. 2014;34:567–575. doi: 10.1111/liv.12286. [DOI] [PubMed] [Google Scholar]
- 25.Evankovich J, Zhang R, Cardinal JS, Zhang L, Chen J, Huang H, Beer-Stolz D, Billiar TR, Rosengart MR, Tsung A. Calcium/calmodulin-dependent protein kinase IV limits organ damage in hepatic ischemia-reperfusion injury through induction of autophagy. Am J Physiol Gastrointest Liver Physiol. 2012;303:G189–198. doi: 10.1152/ajpgi.00051.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Wang G, Yao J, Li Z, Zu G, Feng D, Shan W, Li Y, Hu Y, Zhao Y, Tian X. miR-34a-5p inhibition alleviates intestinal ischemia/reperfusion-induced reactive oxygen species accumulation and apoptosis via activation of SIRT1 signaling. Antioxid Redox Signal. 2016;24:961–973. doi: 10.1089/ars.2015.6492. [DOI] [PubMed] [Google Scholar]
- 28.Emerling BM, Weinberg F, Snyder C, Burgess Z, Mutlu GM, Viollet B, Budinger GR, Chandel NS. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic Biol Med. 2009;46:1386–1391. doi: 10.1016/j.freeradbiomed.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paiva MA, Goncalves LM, Providencia LA, Davidson SM, Yellon DM, Mocanu MM. Transitory activation of AMPK at reperfusion protects the ischaemic-reperfused rat myocardium against infarction. Cardiovasc Drugs Ther. 2010;24:25–32. doi: 10.1007/s10557-010-6222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Decleves AE, Sharma K, Satriano J. Beneficial effects of AMP-activated protein kinase agonists in kidney ischemia-reperfusion: autophagy and cellular stress markers. Nephron Exp Nephrol. 2014 doi: 10.1159/000368932. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruan H, Dong LQ. Adiponectin signaling and function in insulin target tissues. J Mol Cell Biol. 2016;8:101–109. doi: 10.1093/jmcb/mjw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding W, Zhang Q, Dong Y, Ding N, Huang H, Zhu X, Hutchinson S, Gao X, Zhang X. Adiponectin protects the rats liver against chronic intermittent hypoxia induced injury through AMP-activated protein kinase pathway. Sci Rep. 2016;6:34151. doi: 10.1038/srep34151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Boer MP, Meijer RI, Richter EA, van Nieuw Amerongen GP, Sipkema P, van Poelgeest EM, Aman J, Kokhuis TJ, Koolwijk P, van Hinsbergh VW, Smulders YM, Serne EH, Eringa EC. Globular adiponectin controls insulin-mediated vasoreactivity in muscle through AMPKalpha2. Vascul Pharmacol. 2016;78:24–35. doi: 10.1016/j.vph.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Zhao L, Fu Z, Wu J, Aylor KW, Barrett EJ, Cao W, Liu Z. Globular adiponectin ameliorates metabolic insulin resistance via AMPK-mediated restoration of microvascular insulin responses. J Physiol. 2015;593:4067–4079. doi: 10.1113/JP270371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang C, Liao Y, Li Q, Chen M, Zhao Q, Deng R, Wu C, Yang A, Guo Z, Wang D, He X. Recombinant adiponectin ameliorates liver ischemia reperfusion injury via activating the AMPK/eNOS pathway. PLoS One. 2013;8:e66382. doi: 10.1371/journal.pone.0066382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grahame Hardie D. AMP-activated protein kinase: a key regulator of energy balance with many roles in human disease. J Intern Med. 2014;276:543–559. doi: 10.1111/joim.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 38.Wu SB, Wu YT, Wu TP, Wei YH. Role of AMPK-mediated adaptive responses in human cells with mitochondrial dysfunction to oxidative stress. Biochim Biophys Acta. 2014;1840:1331–1344. doi: 10.1016/j.bbagen.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 39.Qi D, Young LH. AMPK: energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol Metab. 2015;26:422–429. doi: 10.1016/j.tem.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suyavaran A, Thirunavukkarasu C. Preconditioning methods in the management of hepatic ischemia reperfusion-induced injury: update on molecular and future perspectives. Hepatol Res. 2017;47:31–48. doi: 10.1111/hepr.12706. [DOI] [PubMed] [Google Scholar]
- 41.Huynh MK, Kinyua AW, Yang DJ, Kim KW. Hypothalamic AMPK as a regulator of energy homeostasis. Neural Plast. 2016;2016:2754078. doi: 10.1155/2016/2754078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross FA, MacKintosh C, Hardie DG. AMP-activated protein kinase: a cellular energy sensor that comes in 12 flavours. FEBS J. 2016;283:2987–3001. doi: 10.1111/febs.13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang CH, Shen YJ, Lai CJ, Kou YR. Inflammatory role of ROS-sensitive AMP-activated protein kinase in the hypersensitivity of lung vagal C fibers induced by intermittent hypoxia in rats. Front Physiol. 2016;7:263. doi: 10.3389/fphys.2016.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren J, Xu X, Wang Q, Ren SY, Dong M, Zhang Y. Permissive role of AMPK and autophagy in adiponectin deficiency-accentuated myocardial injury and inflammation in endotoxemia. J Mol Cell Cardiol. 2016;93:18–31. doi: 10.1016/j.yjmcc.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5’-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carroll KC, Viollet B, Suttles J. AMPKalpha1 deficiency amplifies proinflammatory myeloid APC activity and CD40 signaling. J Leukoc Biol. 2013;94:1113–1121. doi: 10.1189/jlb.0313157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jhun BS, Jin Q, Oh YT, Kim SS, Kong Y, Cho YH, Ha J, Baik HH, Kang I. 5-Aminoimidazole-4-carboxamide riboside suppresses lipopolysaccharide-induced TNF-alpha production through inhibition of phosphatidylinositol 3-kinase/Akt activation in RAW 264.7 murine macrophages. Biochem Biophys Res Commun. 2004;318:372–380. doi: 10.1016/j.bbrc.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 48.Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, Tsuruta Y, Abraham E. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L497–504. doi: 10.1152/ajplung.90210.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Golen RF, Reiniers MJ, Olthof PB, van Gulik TM, Heger M. Sterile inflammation in hepatic ischemia/reperfusion injury: present concepts and potential therapeutics. J Gastroenterol Hepatol. 2013;28:394–400. doi: 10.1111/jgh.12072. [DOI] [PubMed] [Google Scholar]
- 50.Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- 51.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 52.Cacicedo JM, Benjachareonwong S, Chou E, Yagihashi N, Ruderman NB, Ido Y. Activation of AMP-activated protein kinase prevents lipotoxicity in retinal pericytes. Invest Ophthalmol Vis Sci. 2011;52:3630–3639. doi: 10.1167/iovs.10-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elias-Miro M, Jimenez-Castro MB, Rodes J, Peralta C. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radic Res. 2013;47:555–568. doi: 10.3109/10715762.2013.811721. [DOI] [PubMed] [Google Scholar]
- 54.Liu XM, Peyton KJ, Shebib AR, Wang H, Korthuis RJ, Durante W. Activation of AMPK stimulates heme oxygenase-1 gene expression and human endothelial cell survival. Am J Physiol Heart Circ Physiol. 2011;300:H84–93. doi: 10.1152/ajpheart.00749.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zimmermann K, Baldinger J, Mayerhofer B, Atanasov AG, Dirsch VM, Heiss EH. Activated AMPK boosts the Nrf2/HO-1 signaling axis--A role for the unfolded protein response. Free Radic Biol Med. 2015;88:417–426. doi: 10.1016/j.freeradbiomed.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Awad H, Nolette N, Hinton M, Dakshinamurti S. AMPK and FoxO1 regulate catalase expression in hypoxic pulmonary arterial smooth muscle. Pediatr Pulmonol. 2014;49:885–897. doi: 10.1002/ppul.22919. [DOI] [PubMed] [Google Scholar]
- 57.Yun H, Park S, Kim MJ, Yang WK, Im DU, Yang KR, Hong J, Choe W, Kang I, Kim SS, Ha J. AMP-activated protein kinase mediates the antioxidant effects of resveratrol through regulation of the transcription factor FoxO1. FEBS J. 2014;281:4421–4438. doi: 10.1111/febs.12949. [DOI] [PubMed] [Google Scholar]
- 58.Alba G, El Bekay R, Alvarez-Maqueda M, Chacon P, Vega A, Monteseirin J, Santa Maria C, Pintado E, Bedoya FJ, Bartrons R, Sobrino F. Stimulators of AMP-activated protein kinase inhibit the respiratory burst in human neutrophils. FEBS Lett. 2004;573:219–225. doi: 10.1016/j.febslet.2004.07.077. [DOI] [PubMed] [Google Scholar]
- 59.Song P, Zou MH. Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems. Free Radic Biol Med. 2012;52:1607–1619. doi: 10.1016/j.freeradbiomed.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahn YJ, Kim H, Lim H, Lee M, Kang Y, Moon S, Kim HS, Kim HH. AMP-activated protein kinase: implications on ischemic diseases. BMB Rep. 2012;45:489–495. doi: 10.5483/bmbrep.2012.45.9.169. [DOI] [PubMed] [Google Scholar]
- 61.Sliwinska A, Drzewoski J. Molecular action of metformin in hepatocytes: an updated insight. Curr Diabetes Rev. 2015;11:175–181. doi: 10.2174/1573399811666150325233108. [DOI] [PubMed] [Google Scholar]
- 62.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 63.Asghari A, Akbari G, Meghdadi A, Mortazavi P. Protective effect of metformin on testicular ischemia/reperfusion injury in rats. Acta Cir Bras. 2016;31:411–416. doi: 10.1590/S0102-865020160060000008. [DOI] [PubMed] [Google Scholar]
- 64.Wang M, Weng X, Guo J, Chen Z, Jiang G, Liu X. Metformin alleviated EMT and fibrosis after renal ischemia-reperfusion injury in rats. Ren Fail. 2016;38:614–621. doi: 10.3109/0886022X.2016.1149770. [DOI] [PubMed] [Google Scholar]
- 65.Cahova M, Palenickova E, Dankova H, Sticova E, Burian M, Drahota Z, Cervinkova Z, Kucera O, Gladkova C, Stopka P, Krizova J, Papackova Z, Oliyarnyk O, Kazdova L. Metformin prevents ischemia reperfusion-induced oxidative stress in the fatty liver by attenuation of reactive oxygen species formation. Am J Physiol Gastrointest Liver Physiol. 2015;309:G100–111. doi: 10.1152/ajpgi.00329.2014. [DOI] [PubMed] [Google Scholar]
- 66.Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis. 2015;30:747–754. doi: 10.1007/s11011-014-9632-2. [DOI] [PubMed] [Google Scholar]
- 67.Barreto-Torres G, Parodi-Rullan R, Javadov S. The role of PPARalpha in metformin-induced attenuation of mitochondrial dysfunction in acute cardiac ischemia/reperfusion in rats. Int J Mol Sci. 2012;13:7694–7709. doi: 10.3390/ijms13067694. [DOI] [PMC free article] [PubMed] [Google Scholar]


