Abstract
OBJECTIVES
Prior studies suggest that the composition of the vaginal microbiome may positively or negatively affect susceptibility to sexually transmitted infections (STI) and bacterial vaginosis (BV). Some female hormonal contraceptive methods also appear to positively or negatively influence STI transmission and BV. Therefore changes in the vaginal microbiome that are associated with different contraceptive methods may explain, in part, effects on STI transmission and BV.
STUDY DESIGN
We performed a retrospective study of 16S rRNA gene survey data of vaginal samples from a subset of participants from the Human Vaginal Microbiome Project at Virginia Commonwealth University. The subset included 682 women who reported using a single form of birth control that was condoms, combined oral contraceptives (COCs), depot medroxyprogesterone acetate (DMPA), or the levonorgestrel-releasing intrauterine system (LNG-IUS).
RESULTS
Women using COCs (adjusted odds ratio (aOR) 0.29, 95% CI [0.13, 0.64]), and DMPA (aOR 0.34, 95% CI [0.13, 0.89]), but not LNG-IUS (aOR 1.55, 95% CI [0.72, 3.35]) were less likely to be colonized by BV-associated bacteria relative to women who used condoms. Women using COCs (aOR, 1.94, 95% CI [1.25 3.02]) were more likely to be colonized by beneficial H2O2-producing Lactobacillus species compared with women using condoms, while women using DMPA (aOR 1.09, 95% CI [0.63, 1.86]) and LNG-IUS (aOR 0.74, 95% CI [0.48, 1.15]) were not.
CONCLUSIONS
Use of COCs is significantly associated with increased vaginal colonization by healthy lactobacilli and reduced BV-associated taxa.
IMPLICATIONS
COC use may positively influence gynecologic health through an increase in healthy lactobacilli and a decrease in BV-associated bacterial taxa.
Keywords: Bacterial vaginosis, vaginal microbiome, hormonal contraceptives
1. INTRODUCTION
The vaginal microbiome appears to play an important role in women’s health and in vitro evidence suggests that certain Lactobacillus species may help to protect the vagina from pathogens. For example, Lactobacillus crispatus inhibits infection of HeLa cells by Chlamydia trachomatis, and inhibits growth of Neisseria gonorrhoeae in a porcine model of vaginal mucosa [1,2]. In addition, a recent report suggests that L. crispatus alters cervicovaginal mucus in such a way that trapping of HIV particles in enhanced [3]. These lactobacilli produce lactic acid, which is bactericidal, and some produce bacteriocins and/or H2O2. There is some evidence that lactic acid is the major microbicidal component produced by vaginal lactobacilli and that H2O2 does not play an important role in killing bacteria in the anoxic conditions of the vagina[4,5]. However, whether H2O2 production plays an important role in controlling bacterial growth or whether it is just a marker, there is strong evidence that the presence of lactobacilli with the capacity to produce H2O2 is associated with vaginal “eubiosis” or a healthy microbiome [6,7].
Bacterial vaginosis (BV) is a dysbiosis of the vaginal microbiome characterized by a decrease in lactobacilli and an increase in anaerobic neutralophiles. BV predisposes women to acquisition of HIV and other STIs from their sexual contacts[8–11]. There is an association between susceptibility to STIs and the abundance of protective lactobacilli (reviewed in [12]), but also to damaging effects of the BV-associated taxa on the vaginal epithelium. Gardnerella vaginalis produces the cholesterol-dependent cytolysin, vaginolysin, which can lyse vaginal epithelial cells, and this could compromise the barrier effect of the vaginal mucosa. There is also evidence that sialidase and mucin-degrading enzymes produced by the BV-associated bacteria disrupt the protective mucus layer [13]. In addition to higher rates of HIV acquisition by women with BV, the rate of transmission of HIV from women with BV to their sexual partners is also higher. BV-associated bacteria enhance HIV replication and higher viral loads are found in cervico-vaginal secretions from women with BV, Candida vaginitis, and genital Herpes Simplex Virus (HSV) [10,14,15].
Estrogen induces the accumulation of glycogen in the vaginal epithelium and glycogen positively influences colonization by healthy lactobacilli[16,17]. When estrogen levels are high in the follicular phase of the menstrual cycle and during pregnancy, the abundance of lactobacilli tends to increase. It would follow that estrogen-containing hormonal contraceptives might influence the vaginal microbiome. In fact, a number of studies have shown reduced rates of BV in women using combined oral contraceptives (COCs)[18–20].
Results from studies of the effect of progestin-only contraceptives have been less clear. A longitudinal study of intrauterine systems that release the progestin levonorgestrel (LNG-IUS) found no influence of the systems on vaginal microbiomes and another study in baboons supported this finding[21,22]. Results from studies of changes in the vaginal microbiome in women using DMPA injections as a contraceptive method have been inconsistent. A systematic review of 36 prior studies found that BV rates were lower in women using DMPA[23], but another study found no effect of hormonal contraception on the composition of the vaginal microbiome[24]. Yet another report described a decrease in the prevalence of H2O2-producing lactobacilli after one year of DMPA use[25]. Prior studies have relied on conventional microbiological techniques or phylogenetic microarrays to identify vaginal microbes, but to date, comprehensive 16S rRNA gene survey data has not been reported for women using hormonal contraceptives. Herein, we performed a retrospective comparison of 16S gene profiles from women using condoms, COCs (excluding progestin-only mini-pills), LNG-IUS, and DMPA to determine whether hormonal contraceptives affect vaginal microbial community profiles or the abundance of vaginal lactobacilli.
2. MATERIALS AND METHODS
2.1 Participant recruitment
This was a retrospective study of subjects selected from the 4,306 women enrolled in the Vaginal Human Microbiome Project (VaHMP) at Virginia Commonwealth University (VCU). Participants recruited from outpatient clinics at the Virginia Commonwealth University Medical Center and the Virginia Department of Health following written, informed consent from 2009–2013. The Institutional Review Boards for Human Subjects Research at VCU (Panel B) and the Virginia Department of Health reviewed and approved this study. Participants filled out a detailed questionnaire that included questions about ethnicity, education, employment, health habits, dietary habits, and sexual history. Clinicians also filled out a diagnosis form at the time of each visit that included information about the purpose of each visit, and any diagnoses. Inclusion criteria for VaHMP included women age 18–44 years who were able to provide informed consent and who were willing or already scheduled to undergo a vaginal examination using a speculum. Inclusion criteria for the subset included in this study were use of a single contraceptive method that was condoms, COCs, DMPA, or LNG-IUS. Subjects were considered “healthy” at the time of a visit if the purpose of the visit was for an annual exam, they received no diagnosis, and were asymptomatic (e.g., no abnormal discharge). BV testing was performed only when indicated, and was based solely on Amsel’s criteria[26]. Trichomoniasis was diagnosed by wet mount microscopy. Chlamydia was diagnosed by PCR. Herpes simplex virus (HSV) was diagnosed by culture. Gonorrhea was diagnosed by culture. Molecular identification of human papillomavirus (HPV) was performed.
2.2 Sampling and sample processing
Samples were taken using CultureSwab EZ (Becton Dickinson) by a physician from the mid-vaginal wall during a speculum examination. DNA was extracted from the swabs within 4 hours of collection using the Powersoil® kit (MoBio). The swabs were swirled directly in the Powerbead tubes supplied with the kit and processing was according to manufacturer’s instructions.
2.3 16S rRNA gene survey
The V1–V3 hypervariable regions of the bacterial 16S rRNA gene were amplified by PCR using barcoded primers. The 16S primers contain the A or B Titanium sequencing adapter (shown in italics), followed immediately by a unique variable (6–9 base) barcode sequence and finally the 5’ end of primer. The forward primer was a mixture (4:1) of the primers Fwd-P1 (5’ - CCATCTCATCCCTGCGTGTCTCCGACTCAG BBBBBB AGAGTTYGATYMTGGCTYAG) and Fwd-P2 (5’ - CCATCTCATCCCTGCGTGTCTCCGACTCAG BBBBBB AGARTTTGATCYTGGTTCAG). The reverse primer was Rev1B (5’ – CCTATCCCCTGTGTGCCTTGGCAGTCTCAG ATTACCGCGGCTGCTGG). PCR products were sequenced using the Roche 454 GS FLX Titanium platform. These data were generated as part of the Vaginal Human Microbiome Project[27]. Raw sequence data from the project is available from the Short Read Archive at NCBI (projectID phs000256)[27]. We used a deep sequencing approach with a median 24,030 reads/sample. All processed samples were represented by > 5,000 reads.
Reads that met the following criteria were processed: 1) valid primer and multiplex identifier sequences were observed; 2) less than 10% of base calls had a quality score less than 10; 3) the average quality score was greater than Q20; and 4) the read length was between 200 and 540 bases. Sequences were classified using a local installation of the RDP classifier (0.8 cutoff) and using STIRRUPS, an analysis platform that employs the USEARCH algorithm combined with a curated vaginal 16S rRNA gene database[28,29].
2.4 Statistical Analyses
Only non-pregnant women with populated age, ethnicity, and current clinical diagnosis fields were retained for analysis. STIs included trichomoniasis, chlamydia, HSV, gonorrhea, and HPV.
Sequencing read counts were converted to proportions for all samples to determine the percent of the total microbiome that each bacterial species contributed. Abundance was defined as the mean percentage of a particular species or group of species within a subject or within a group of subjects. The predominant taxon in a sample refers to the taxon for which the largest number of reads were assigned taxonomic classification with confidence (i.e. the highest percentage of reads in the sample).
Alpha diversity was measured using the inverse Simpson’s index. This defines diversity in a way that accounts for the relative abundance of taxa, such that a sample predominated by a single organism that contained a large number of minor taxa would have a lower alpha diversity than a sample with an equal number of taxa all at intermediate levels of abundance.
Linear models were fit and visualizations were created using the R Environment including package ggplot2 [30]. A linear model with a logit transformed response was fit for relating the percentage of BV-associated bacteria in a microbiome profile as a function of contraceptive method, age, ethnicity, and STI status. Logistic regression models were fit (1) relating the presence of H2O2-producing lactobacilli in a microbiome profile as a function of contraceptive method, age, ethnicity, and STI status, and (2) relating the presence of lactobacilli in a microbiome profile as a function of contraceptive method, age, ethnicity, and STI status. Lactobacillus was considered present in a sample if it comprised at least 1% of the microbiome profile. All linear models were initially fit with up to two-way interactions among predictors. Analysis of variance (ANOVA) was used to evaluate the overall significance of each effect and subsequently reduce the model, if necessary. All multiple comparisons were performed using Tukey’s HSD procedure.
Linear discriminant analysis effect size (LEfSe) applies a Kruskal-Wallis rank sum test for each bacterium, then uses linear discriminant analysis to estimate effect size[31]. The effect size is the contribution of a variable to the ability to distinguish two different groups.
3. RESULTS
3.1 The dominant vaginal microbial taxa differ in women using different contraceptive methods
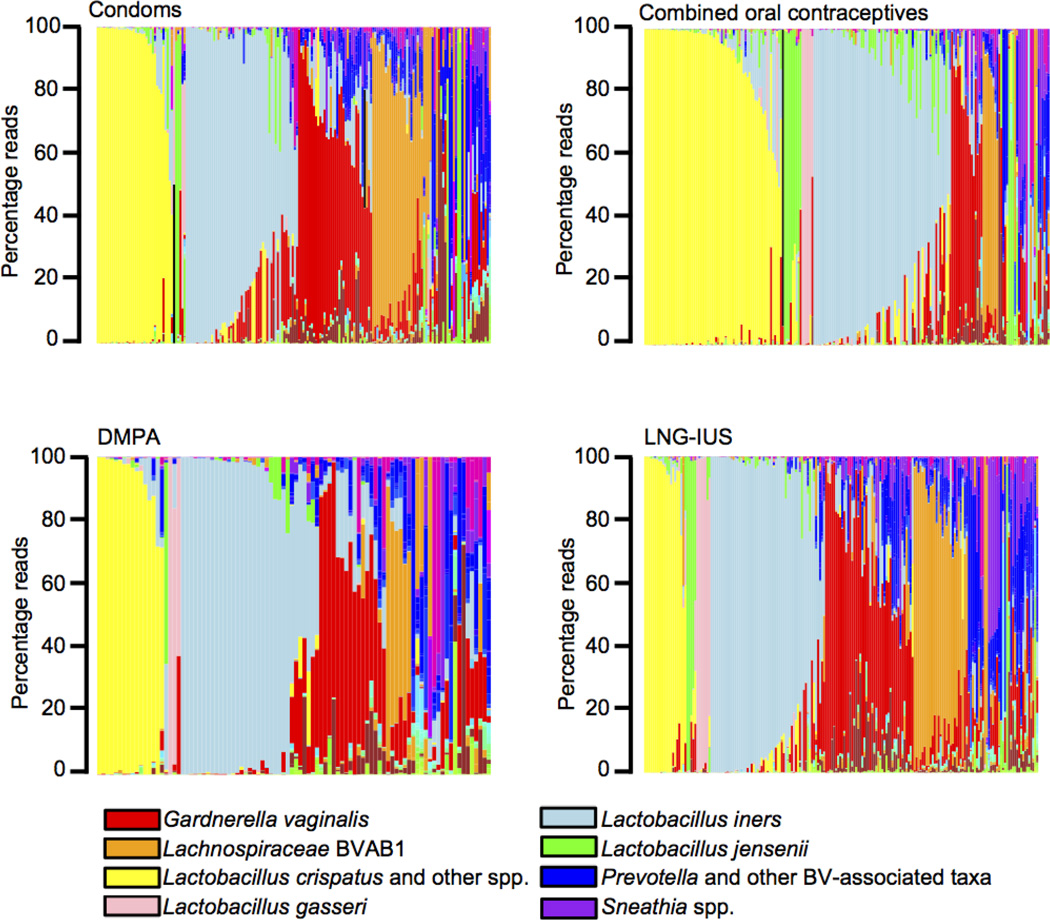
A total of 682 non-pregnant women who reported use of a single contraceptive method were included in this study (Table 1). Figure 1 displays the 16S rRNA survey data of vaginal microbiomes ordered first by predominant species comprising at least 30% of a sample and second, by abundance of the predominant species. The percentage of subjects with vagitypes dominated by Lactobacillus species was greatest in the COC users. A vagitype dominated by the genus Lachnospiraceae (coded orange in Figure 1), which is predominantly bacterial vaginosis associated bacteria 1 (BVAB1) was rare in COC users. It was more common in women using DMPA, but was still rare in DMPA users relative to women using condoms and LNG-IUS. In addition, the alpha diversity, measured using the inverse Simpson’s index, within the vaginal microbiomes of COC users (mean=1.78, std. dev.=1.28) was significantly lower (as determined via a Tukey multiple comparison procedure) than that of DMPA (mean=2.52, std.dev.=2.13; p=0.0051), LNG-IUS (mean=2.51, std. dev.=2.09; p=0.0003), and condom (mean=2.27, std. dev.=1.71; p=0.035) users.
Table 1.
Information about study participants
| Condoms | COCs | DMPA | LNG-IUS | |
|---|---|---|---|---|
| Total number | 186 | 206 | 94 | 196 |
| Ethnicity | ||||
| African American |
109 (59%) | 62 (30%) | 78 (83%) | 121 (62%) |
| Asian | 5 (3%) | 5 (2%) | 0 | 1 (0.5%) |
| Caucasian | 48 (26%) | 115 (56%) | 9 (10%) | 62 (32%) |
| Hispanic | 16 (9%) | 16 (8%) | 4 (4%) | 4 (2%) |
| Other | 8 (4%) | 8 (4%) | 3 (3%) | 8 (4%) |
|
Duration of method1 |
||||
| <1 months | 7(3%) | 11(11%) | 16(8%) | |
| 1–3 months | 21(10%) | 9(10%) | 15(8%) | |
| 3–6 months | 9(4%) | 7(7%) | 12(6%) | |
| 6–12 months | 12(6%) | 9(10%) | 17(9%) | |
| 12–24 months | 39(19%) | 22(23%) | 61(31%) | |
| >24 months | 115(56%) | 30(32%) | 71(36%) | |
| NR3 | 3(1%) | 6(6%) | 3(2%) | |
|
Number sex partners2 |
||||
| 0 | 5 (3%) | 11 (5%) | 5 (5%) | 2 (1%) |
| 1 | 79 (42%) | 149 (72%) | 54 (57%) | 123 (63%) |
| 2 | 45 (24%) | 21 (10%) | 15 (16%) | 30 (15%) |
| 3–5 | 21 (11%) | 9 (4%) | 6 (6%) | 17 (9%) |
| 6–10 | 1 (0%) | 2 (1%) | 1 (1%) | 2 (1%) |
| 11–20 | 2(1%) | 0 (0%) | 1(1%) | 0 (0%) |
| NR3 | 33 (18%) | 14 (7%) | 12(13%) | 22 (11%) |
|
Frequency of sex in past year |
||||
| 0 | 4 (2%) | 14 (7%) | 6 (6%) | 4 (2%) |
| <1/month | 22 (12%) | 17 (8%) | 3 (3%) | 18 (9%) |
| 1–3/month | 48 (26%) | 44 (21%) | 21 (22%) | 48 (24%) |
| 1/week | 37 (20%) | 31 (15%) | 20 (21%) | 29 (15%) |
| 2–6/week | 59 (32%) | 91 (44%) | 34 (36%) | 85 (43%) |
| 1/day | 8 (4%) | 8(4%) | 8 (9%) | 8 (4%) |
| NR3 | 9 (4%) | 1 | 2 (2%) | 4 (2%) |
|
Douche >1/month |
19 (10%) | 10 (5%) | 14 (15%) | 23 (12%) |
| BV Diagnosis4 | 32 (17%) | 16 (8%) | 12 (13%) | 23 (12%) |
| STI Diagnosis4 | 7 (4%) | 1 (0.5%) | 8 (9%) | 10 (5%) |
Self-reported duration of current hormonal contraceptive method
Number of sexual partners over lifetime
NR= Not reported
Clinical diagnosis at time of sampling
Figure 1. Microbial community profiles of women using different methods of contraception.
Stacked bar plots showing vaginal microbial community profiles from 186 women who reported only using condoms, 206 women who reported only using combined oral contraceptives, 94 women who reported using only DMPA injections, and 196 women who reported using only LNG-IUS for contraception. The profiles are grouped by the most abundant species and are ordered by decreasing proportion of the dominant bacterium. An abbreviated color code showing the most abundant taxa is shown. Complete color codes for bacterial taxa appear in Supplementary Data file.
3.2 The association of specific bacterial taxa with method of contraception
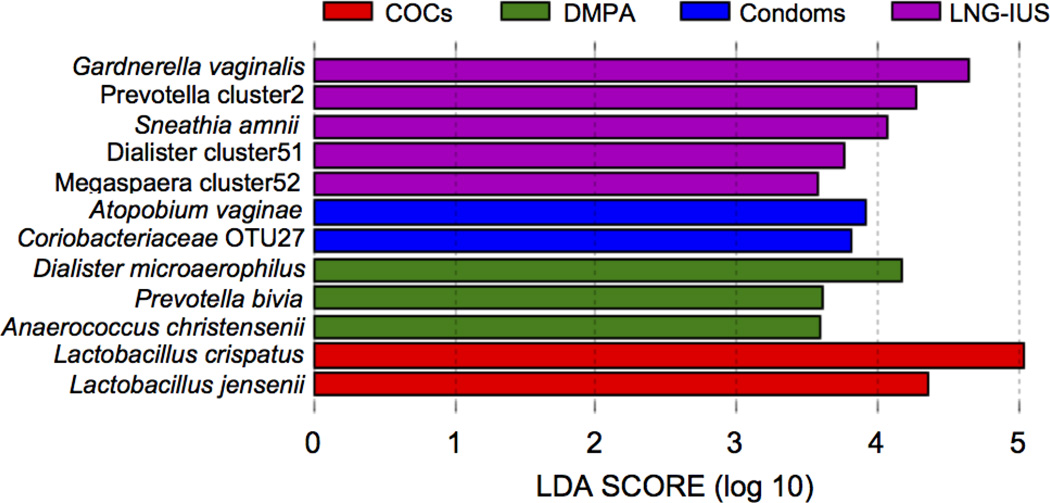
A search for significant associations between specific bacterial species and contraceptive method was performed. Figure 2 indicates the linear discriminant analysis (LDA) effect sizes (LEfSe) for bacterial taxa associated with each of the contraceptive methods. A number of taxa typically associated with a dysbiotic vaginal microbiome, including Prevotella cluster 2, which includes a number of unnamed Prevotella species, Sneathia amnii, Dialister, and Megasphaera species were significantly more abundant in women using LNG-IUS. Gardnerella vaginalis, which has a strong association with BV, and Lachnospiraceae OTUs 33 and 27 were more abundant in women who used condoms rather than a hormonal contraceptive. Atopobium vaginae, another major BV-associated species, and other taxa associated with vaginal dysbiosis, including Dialister microaerophilus, Prevotella bivia, Prevotella amnii, and Anaerococcus christensenii were more abundant in women using DMPA. In women using COCs, L. crispatus and Lactobacillus jensenii were more abundant.
Figure 2. Association between bacterial taxa and contraceptive method.
LEfSe analysis of microbial profiles detected significant associations between the abundance of a number of bacterial taxa and contraceptive method when all methods were compared.
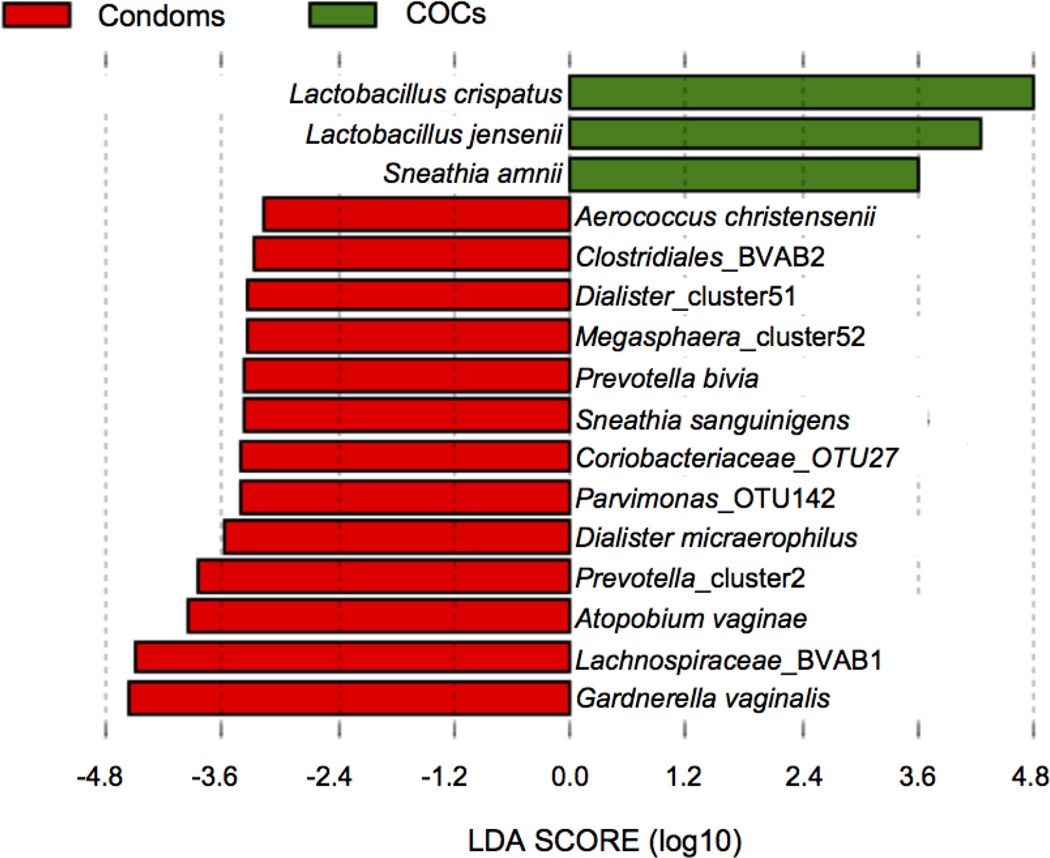
When each of the hormonal contraceptive methods was compared directly to condom use, significantly lower abundance of BVAB1, BVAB2, G. vaginalis, Prevotella species, Atopobium vaginae, Sneathia sanguinigens, and other BV-associated taxa in COC users relative to condom users was found, whereas L. crispatus and L. jensenii were, again, more abundant in the COC group (Figure 3). A significantly higher abundance of L. iners in the DMPA group relative to the condom group was detected. The abundance of L. crispatus was higher in condom users than in women using LNG-IUS, but Peptoniphilus indolicus and Finegoldia magna were more abundant in the LNG-IUS group relative to the condom group.
Figure 3. Association between bacterial taxa and COC use.
LEfSe analysis of microbial profiles detected significant differences in the abundance of a number of bacterial taxa in the COC group versus the condom group. Taxa that were more abundant in the condom users are shown in red and taxa that were more abundant in the COC users are shown in green.
3.3 The abundance of H2O2-producing Lactobacillus species is higher in COC users
Stacked bar plots (Figure 1) of the microbial profiles suggested differences in Lactobacillus abundance depending on contraceptive method. To further investigate this association, we analyzed the abundance of H2O2-producing Lactobacillus species, including L. crispatus, Lactobacillus gasseri, and L. jensenii in women who used different contraceptives. African American women were more likely to use DMPA and Caucasian women were more likely to use COCs (Supplementary Figure 2). A chi-square test of independence revealed that ethnicity and contraceptive method are indeed dependent (p < 0.0001). Therefore, ethnicity was included as a covariate in the models for predicting the abundance of H2O2-producing lactobacilli and BV-associated bacteria to confirm that associations among abundance and contraceptive method were not influenced by the unequal distribution of ethnicities among the contraceptive groups.
We also analyzed the relationship between age and contraceptive (Supplementary Figure 3). Method and age were associated with each other (p=0.0081; Tukey multiple comparison indicates age difference between DMPA versus condoms and DMPA versus LNG-IUS). Age was therefore also included in the models.
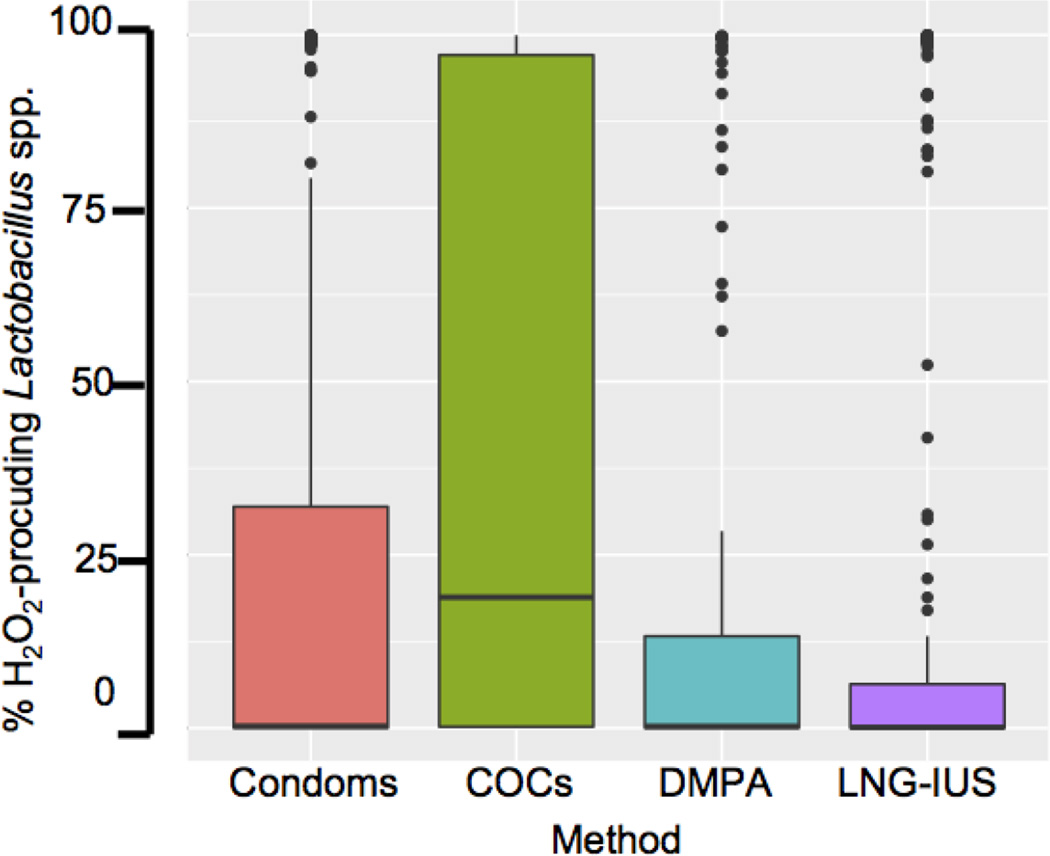
Caucasians were more likely (aOR 3.2, 95% CI [2.23, 4.72] p=7.96e-10) to be colonized by H2O2-producing Lactobacillus species compared with African Americans. Figure 4 is a boxplot of H202-producing Lactobacillus abundance by contraceptive method. Even when adjusting for ethnicity, there was an association between colonization by H2O2-producing Lactobacillus species with COC use (p=.003). A logistic regression model fit indicates that women using COCs were more likely (AOR 1.94, 95% CI [1.25, 3.02]) to be colonized with H2O2 producing Lactobacillus species compared with women who used condoms. Women using DMPA (aOR 1.09, 95% CI [0.63, 1.86]) or LNG-IUS (0.74, 95% CI [0.48, 1.15]) were no more or less likely to be colonized by H2O2-producing Lactobacillus than women using condoms. The likelihood of colonization by H2O2-producing Lactobacillus in 1) condom users versus DMPA users and 2) condom users versus LNG-IUS users did not reach statistical significance.
Figure 4. Relationship between the percentage of H2O2-producing Lactobacillus species and contraceptive method.
The analysis includes healthy women and women with BV. Subjects were grouped based on self-reported contraceptive method. Within each group, the proportion of H2O2-producing Lactobacillus species, including L. crispatus, L. gasseri, and L. jensenii was plotted. The boxes indicate the interquartile range, and the horizontal line in each box indicates the median.
The number of women in this study who had an STI was low and no associations with contraceptive method were detected; however, women with a diagnosis of an STI (Table 1) were less likely to have H2O2-producing Lactobacillus (aOR 0.3, 95% CI [0.08, 0.81] p=0.03) relative to those without an STI.
3.4 The relationship between BV-associated bacterial taxa and contraceptive method
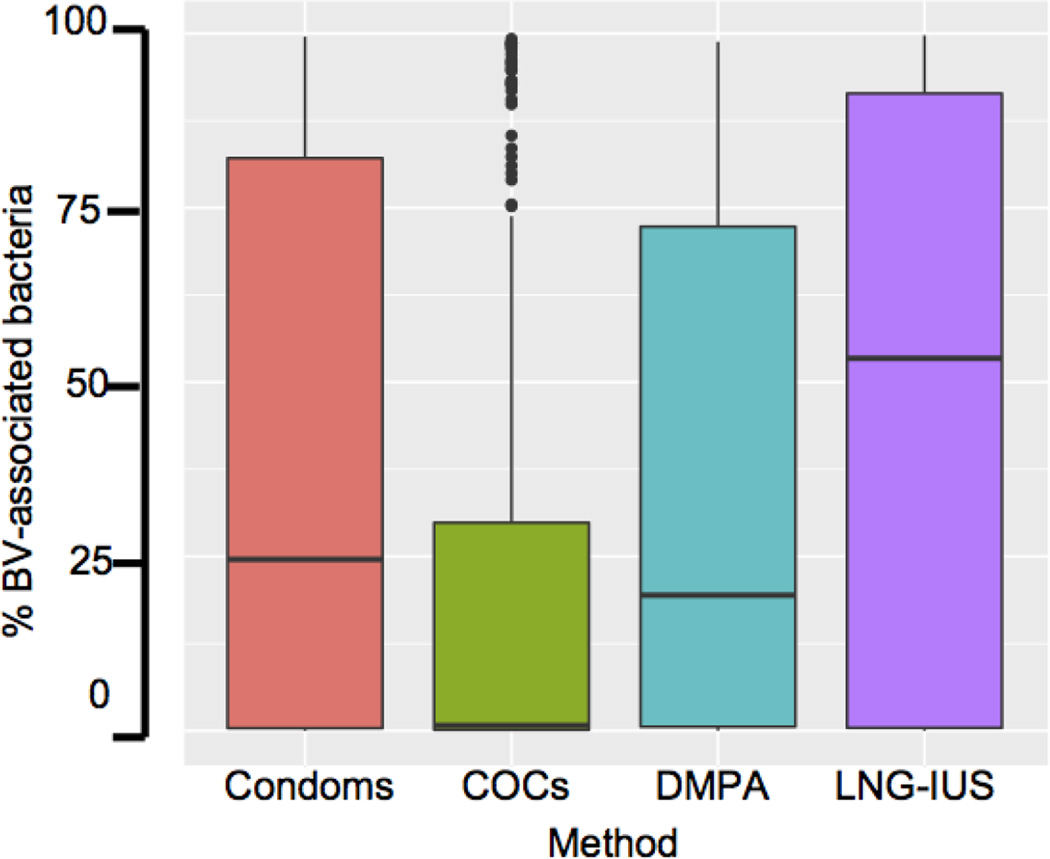
We analyzed the prevalence of a group of bacteria that has been previously reported to have a strong association with BV, including Ureaplasma, Mycoplasma, Fusobacterium, Leptotrichia, Gardnerella, Sneathia, Prevotella, BVAB1, BVAB2, BVAB3, Atopobium, Mobiluncus, and Megasphaera, in the different contraceptive user groups [32,33]. Caucasians (aOR 0.08, 95% CI [0.04, 0.15] p=1.49e-13) were less likely to be colonized by BV-associated bacteria relative to the African American group. Women using COC (aOR 0.29, 95% CI [0.13, 0.64] p=0.002) or DMPA (aOR 0.34, 95% CI [0.13, 0.89] p=0.028) were less likely to be colonized by BV-associated bacteria relative to women using condoms (Figure 5). However, the likelihood that women using LNG-IUS (aOR 1.55 times, 95% CI [0.72, 3.35]) were colonized by BV-associated bacteria did not differ from women using condoms. Because there was a trend towards higher levels of BV-associated bacteria in LNG-IUS users, we performed a Tukey multiple comparison test to determine whether there was a significant difference when comparing the LNG-IUS group to the COC group and the DMPA group. The amount of BV-associated bacteria in women using LNG-IUS was higher than in women using COC (p<0.0001), but there was not a significant difference in the level of BV-associated bacteria in women using LNGIUS relative to DMPA (p=0.21).
Figure 5. Relationship between the percentage of BV-associated bacteria and contraceptive method.
The analysis includes healthy women and women with BV. Subjects were grouped based on self-reported contraceptive method. Within each group, the proportion of BV-associated bacteria was plotted. The boxes indicate the interquartile range, and the horizontal line in each box indicates the median.
4. DISCUSSION
Vaginal microbiomes can be categorized based on the predominant species and these categories have been referred to previously by our group as vagitypes [34] and by others as community state types (CSTs)[35]. This study suggests that use of COCs tends to favor a L. crispatus CST and disfavor a high diversity CST. H2O2-producing Lactobacillus species correlate with vaginal health, and while Lactobacillus iners, which does not produce H2O2, can dominate the health vaginal microbiome[36], it does not exhibit a strong negative association with BV [37] and temporal analysis of the vaginal microbiome suggests that vaginal microbiomes that are dominated by L. iners are more likely to switch to a BV-like profile[38]. In the COC group, the abundance of H2O2-producing lactobacilli was significantly higher and BV-associated bacteria were significantly lower compared with condom users. This could be due to the effect that estrogen has on glycogen accumulation in vaginal epithelial cells, as glycogen has been linked with dominance by lactobacilli, however, further studies would be needed to affirm this as the basis for the association. These findings support prior studies showing a protective effect of COC use against BV [18,19,39] and expand the previous studies through the use of 16S rRNA survey data, which enabled the identification of specific bacterial taxa associated with the use of COCs and other contraceptive methods.
LEfSe is an algorithm designed to detect biomarkers, such as bacterial species, that are associated with a specific state, from a rich set of variables. The algorithm weighs the effect size of linear discriminants to quantitatively assess the relative effects that different discriminants have in defining that particular state. By assigning a quantitative value to the effect size, LEfSe was designed not only to detect statistically significant discriminants, but to produce a relative measure of biologic relevance as well. Specifically, LEfSe analysis identified Atopobium vaginae, Sneathia spp., and Lachnospiraceae BVAB1, as taxa negatively associated with COC use and L. crispatus and L. jensenii, as taxa positively associated with COC use when compared with condom use. The proportion of DMPA users with Lactobacillus-dominated vagitypes was larger when compared to condom users; however, the overall abundance of H2O2-producing lactobacilli was not significantly greater. DMPA use was associated with lower overall levels of BV-associated bacteria, in agreement with prior studies reporting decreased numbers of G. vaginalis [40] and lower rates of BV in DMPA users [23,39,41,42]. However, DMPA use was also associated with higher levels of some BV-associated species, including A. vaginae and P. bivia.
The Centre for the AIDS Programme of Research in South Africa (CAPRISA), which includes women from the KwaZulu-Natal province in South Africa where a large percentage of women of reproductive age are infected with HIV, studies biologic determinants associated with increased risk for HIV acquisition. Studies coming from this program have recently yielded data implicating specific vaginal bacterial species in HIV acquisition (in particular P. bivia) and in HIV prophylaxis failure (in particular G. vaginalis), although a full report of these data has not yet been published [43]. Although on the surface some clinical significance might be attached to the increased P. bivia abundance associated with DMPA use, the association of P. bivia with HIV acquisition and with DMPA use needs to be confirmed with larger sample sets before any conclusions about clinical risk can be drawn. It should also be noted that when compared with condom use alone, DMPA was not associated with an increase in P. bivia abundance. As a retrospective study, the use of an analysis to test the power of our results would not be appropriate. However, the estimate based on the available data, and tests applied, indicated increased abundance of P. bivia and A. vaginae, but no other detrimental effects of DMPA use on the vaginal microbiome.
The amount of BV-associated bacteria detected in women using an LNG-IUS device was significantly greater than in the COC group. Furthermore, LEfSe analysis detected an association between LNG-IUS use and several BV-associated species including G. vaginalis and S. amnii. Prior studies suggest that LNG-IUS use does not alter the vaginal microbiome, however one of these studies looked at changes that had occurred by 12 weeks of use a much shorter duration than the average duration of use for women in this study[21]. The other study used a primate model and the healthy primate vaginal microbiome is not dominated by lactobacilli as is the healthy human vaginal microbiome [22]. Additional prospective analysis would be needed to confirm the association between use of the LNG-IUS device and colonization with BV-associated bacteria. However, the results from this study suggest that there may be a negative effect of LNG-IUS use on the vaginal microbiome.
This study did not detect a significant association between a contraceptive method and infection with a sexually transmitted pathogen but this may have been because the numbers of subjects in each group who reported an STI were quite low. The numbers were also too low to analyze different STIs separately. There was, however, a significant negative association between the abundance of H2O2-producing lactobacilli and positive STI status.
The major strength of this study is the use of comprehensive 16S rRNA survey data to investigate the association between particular species and contraceptive method as this is the first study of this kind. Another strength of this study is the relatively large cohort; 682 women from over 4,000 women who participated in the Vaginal Human Microbiome Project met the criteria for inclusion. We chose condom use as a control rather than no contraceptive method to increase the similarity between the demographics and sexual history of the participants, and we feel that this is a strength of the study. Other strengths include the wide-ranging health and habit history collected for each subject, the examination of multiple forms of hormonal contraception, and the ethnic diversity of the cohort. Weaknesses of the study include its retrospective nature and the lack of samples prior from subjects prior to initiation of a particular hormonal contraceptive method.
5. CONCLUSION
This study comprehensively details characteristics of the human vaginal microbiome that are associated with different forms of hormonal contraception, relative to condom use, through the use of 16S rRNA survey. Findings from the CAPRISA study suggest that the association between BV and HIV susceptibility may be based upon a discrete subset of bacterial species, making speciation of the vaginal microbial flora, and characterization of the effects of contraceptive methods on the abundance of these species, very important. Use of COCs increased abundance of healthy vaginal flora and decreased the abundance of BV-associated bacteria. DMPA use did not increase the abundance of BV-associated taxa. It was associated with an increase in P. bivia when compared with all methods, but this was not significant when it was compared with condom use alone. DMPA use also lacked the apparent protective effect of an increase in beneficial organisms seen in COC users.
Supplementary Material
Acknowledgments
FUNDING
This work was supported by National Institutes of Health [grants 4UH3AI083263, “The Vaginal Microbiome: Disease, Genetics and the Environment”, P60 MD002256, the “VCU NIMHD Comprehensive Center of Excellence”, and U54 DE023786-01 “A Multi-'omic Analysis of the Vaginal Microbiome during Pregnancy”].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTERESTS
The Authors declare that there is no conflict of interest.
REFERENCES
- 1.Nardini P, Ñahui Palomino RA, Parolin C, Laghi L, Foschi C, Cevenini R, et al. Lactobacillus crispatus inhibits the infectivity of Chlamydia trachomatis elementary bodies, in vitro study. Sci Rep. 2016;6:29024. doi: 10.1038/srep29024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breshears LM, Edwards VL, Ravel J, Peterson ML. Lactobacillus crispatus inhibits growth of Gardnerella vaginalis and Neisseria gonorrhoeae on a porcine vaginal mucosa model. BMC Microbiol. 2015;15:276. doi: 10.1186/s12866-015-0608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nunn KL, Wang Y-Y, Harit D, Humphrys MS, Ma B, Cone R, et al. Enhanced Trapping of HIV-1 by Human Cervicovaginal Mucus Is Associated with Lactobacillus crispatus-Dominant Microbiota. mBio. 2015;6:e01084-1015. doi: 10.1128/mBio.01084-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Hanlon DE, Moench TR, Cone RA. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PloS One. 2013;8:e80074. doi: 10.1371/journal.pone.0080074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Hanlon DE, Moench TR, Cone RA. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis. 2011;11:200. doi: 10.1186/1471-2334-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis Off Publ Infect Dis Soc Am. 1993;16(Suppl 4):S273–S281. doi: 10.1093/clinids/16.supplement_4.s273. [DOI] [PubMed] [Google Scholar]
- 7.Eschenbach DA, Davick PR, Williams BL, Klebanoff SJ, Young-Smith K, Critchlow CM, et al. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 1989;27:251–256. doi: 10.1128/jcm.27.2.251-256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myer L, Denny L, Telerant R, Souza Mde, Wright TC, Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect Dis. 2005;192:1372–1380. doi: 10.1086/462427. [DOI] [PubMed] [Google Scholar]
- 9.Myer L, Kuhn L, Stein ZA, Wright TC, Denny L. Intravaginal practices, bacterial vaginosis, and women’s susceptibility to HIV infection: epidemiological evidence and biological mechanisms. Lancet Infect Dis. 2005;5:786–794. doi: 10.1016/S1473-3099(05)70298-X. [DOI] [PubMed] [Google Scholar]
- 10.Cohn JA, Hashemi FB, Camarca M, Kong F, Xu J, Beckner SK, et al. HIV-inducing factor in cervicovaginal secretions is associated with bacterial vaginosis in HIV-1-infected women. J Acquir Immune Defic Syndr 1999. 2005;39:340–346. doi: 10.1097/01.qai.0000146599.47925.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 12.Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R, et al. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol. 2015;6:164. doi: 10.3389/fphys.2015.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olmsted SS, Meyn LA, Rohan LC, Hillier SL. Glycosidase and proteinase activity of anaerobic gram-negative bacteria isolated from women with bacterial vaginosis. Sex Transm Dis. 2003;30:257–261. doi: 10.1097/00007435-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 14.van de Wijgert JHHM, Morrison CS, Cornelisse PGA, Munjoma M, Moncada J, Awio P, et al. Bacterial vaginosis and vaginal yeast, but not vaginal cleansing, increase HIV-1 acquisition in African women. J Acquir Immune Defic Syndr 1999. 2008;48:203–210. doi: 10.1097/QAI.0b013e3181743936. [DOI] [PubMed] [Google Scholar]
- 15.Sha BE, Zariffard MR, Wang QJ, Chen HY, Bremer J, Cohen MH, et al. Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J Infect Dis. 2005;191:25–32. doi: 10.1086/426394. [DOI] [PubMed] [Google Scholar]
- 16.Ayre WB. The glycogen-estrogen relationship in the vaginal tract. J Clin Endocrinol Metab. 1951;11:103–110. doi: 10.1210/jcem-11-1-103. [DOI] [PubMed] [Google Scholar]
- 17.Spear GT, French AL, Gilbert D, Zariffard MR, Mirmonsef P, Sullivan TH, et al. Human α-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J Infect Dis. 2014;210:1019–1028. doi: 10.1093/infdis/jiu231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradshaw CS, Walker J, Fairley CK, Chen MY, Tabrizi SN, Donovan B, et al. Prevalent and incident bacterial vaginosis are associated with sexual and contraceptive behaviours in young Australian women. PloS One. 2013;8:e57688. doi: 10.1371/journal.pone.0057688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradshaw CS, Vodstrcil LA, Hocking JS, Law M, Pirotta M, Garland SM, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis Off Publ Infect Dis Soc Am. 2013;56:777–786. doi: 10.1093/cid/cis1030. [DOI] [PubMed] [Google Scholar]
- 20.Vodstrcil LA, Hocking JS, Law M, Walker S, Tabrizi SN, Fairley CK, et al. Hormonal contraception is associated with a reduced risk of bacterial vaginosis: a systematic review and meta-analysis. PloS One. 2013;8:e73055. doi: 10.1371/journal.pone.0073055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson JC, Turok DK, Dermish AI, Nygaard IE, Settles ML. Vaginal microbiome changes with levonorgestrel intrauterine system placement. Contraception. 2014;90:130–135. doi: 10.1016/j.contraception.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Hashway SA, Bergin IL, Bassis CM, Uchihashi M, Schmidt KC, Young VB, et al. Impact of a hormone-releasing intrauterine system on the vaginal microbiome: a prospective baboon model. J Med Primatol. 2014;43:89–99. doi: 10.1111/jmp.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Wijgert JHHM, Verwijs MC, Turner AN, Morrison CS. Hormonal contraception decreases bacterial vaginosis but oral contraception may increase candidiasis: implications for HIV transmission. AIDS Lond Engl. 2013;27:2141–2153. doi: 10.1097/QAD.0b013e32836290b6. [DOI] [PubMed] [Google Scholar]
- 24.Borgdorff H, Verwijs MC, Wit FWNM, Tsivtsivadze E, Ndayisaba GF, Verhelst R, et al. The impact of hormonal contraception and pregnancy on sexually transmitted infections and on cervicovaginal microbiota in african sex workers. Sex Transm Dis. 2015;42:143–152. doi: 10.1097/OLQ.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell CM, McLemore L, Westerberg K, Astronomo R, Smythe K, Gardella C, et al. Long-term effect of depot medroxyprogesterone acetate on vaginal microbiota, epithelial thickness and HIV target cells. J Infect Dis. 2014;210:651–655. doi: 10.1093/infdis/jiu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 27.Fettweis JM, Alves JP, Borzelleca JF, Brooks JP, Friedline CJ, Gao Y, et al. The Vaginal Microbiome: Disease, Genetics and the Environment. Nat Preced. 2011 [Google Scholar]
- 28.Fettweis JM, Serrano MG, Sheth NU, Mayer CM, Glascock AL, Brooks JP, et al. Species-level classification of the vaginal microbiome. BMC Genomics. 2012;13(Suppl 8):S17. doi: 10.1186/1471-2164-13-S8-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ggplot2 - Elegant Graphics for Data Analysis. Springer: Hadley Wickham; [accessed July 13, 2016]. n.d. http://www.springer.com/us/book/9780387981413. [Google Scholar]
- 31.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brotman RM. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest. 2011;121:4610–4617. doi: 10.1172/JCI57172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 34.Huang B, Fettweis JM, Brooks JP, Jefferson KK, Buck GA. The changing landscape of the vaginal microbiome. Clin Lab Med. 2014;34:747–761. doi: 10.1016/j.cll.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2:4. doi: 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamrakar R, Yamada T, Furuta I, Cho K, Morikawa M, Yamada H, et al. Association between Lactobacillus species and bacterial vaginosis-related bacteria, and bacterial vaginosis scores in pregnant Japanese women. BMC Infect Dis. 2007;7:128. doi: 10.1186/1471-2334-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UME, Zhong X, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4:132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rifkin SB, Smith MR, Brotman RM, Gindi RM, Erbelding EJ. Hormonal contraception and risk of bacterial vaginosis diagnosis in an observational study of women attending STD clinics in Baltimore, MD. Contraception. 2009;80:63–67. doi: 10.1016/j.contraception.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Roxby AC, Fredricks DN, Odem-Davis K, Ásbjörnsdóttir K, Masese L, Fiedler TL, et al. Changes in Vaginal Microbiota and Immune Mediators in HIV-1-Seronegative Kenyan Women Initiating Depot Medroxyprogesterone Acetate. J Acquir Immune Defic Syndr 1999. 2016;71:359–366. doi: 10.1097/QAI.0000000000000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pettifor A, Delany S, Kleinschmidt I, Miller WC, Atashili J, Rees H. Use of injectable progestin contraception and risk of STI among South African women. Contraception. 2009;80:555–560. doi: 10.1016/j.contraception.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riggs M, Klebanoff M, Nansel T, Zhang J, Schwebke J, Andrews W. Longitudinal association between hormonal contraceptives and bacterial vaginosis in women of reproductive age. Sex Transm Dis. 2007;34:954–959. [PubMed] [Google Scholar]
- 43.Cohen J. Vaginal microbiome affects HIV risk. Science. 2016;353:331–331. doi: 10.1126/science.353.6297.331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.