Abstract
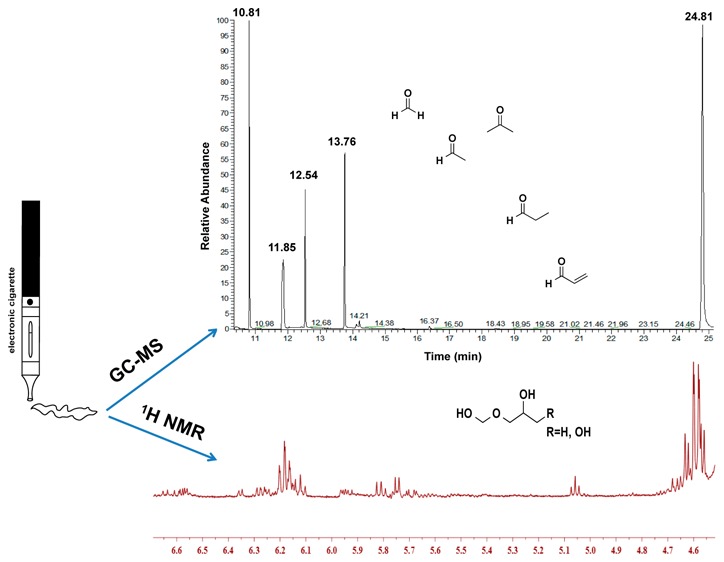
Acetaldehyde, acrolein, and formaldehyde are the principal toxic aldehydes present in cigarette smoke and contribute to the risk of cardiovascular disease and noncancerous pulmonary disease. The rapid growth of the use of electronic cigarettes (e-cigarettes) has raised concerns over emissions of these harmful aldehydes. This work determines emissions of these aldehydes in both free and bound (aldehyde–hemiacetal) forms and other carbonyls from the use of e-cigarettes. A novel silicon microreactor with a coating phase of 4-(2-aminooxyethyl)-morpholin-4-ium chloride (AMAH) was used to trap carbonyl compounds in the aerosols of e-cigarettes via oximation reactions. AMAH–aldehyde adducts were measured using gas chromatography–mass spectrometry. 1H nuclear magnetic resonance spectroscopy was used to analyze hemiacetals in the aerosols. These aldehydes were detected in the aerosols of all e-cigarettes. Newer-generation e-cigarette devices generated more aldehydes than the first-generation e-cigarettes because of higher battery power output. Formaldehyde–hemiacetal was detected in the aerosols generated from some e-liquids using the newer e-cigarette devices at a battery power output of 11.7 W and above. The emission of these aldehydes from all e-cigarettes, especially higher levels of aldehydes from the newer-generation e-cigarette devices, indicates the risk of using e-cigarettes.
1. Introduction
Low-molecular-weight aldehydes are estimated to be the most toxic constituents of tobacco products and tobacco smoke.1,2 Three toxic aldehydes—acetaldehyde, acrolein, and formaldehyde—have been ranked by the Institute of Medicine as the most significant cardiovascular (CV) toxins in tobacco smoke.3 These aldehydes are present in cigarette smoke (700–800 μg/cigarette in mainstream smoke),4 cigars, and waterpipes (hookah and narghile) and are also present in electronic cigarette (e-cigarette) aerosols.5−8 Although levels of some aldehydes are lower in e-cigarette aerosols than those in the smoke of conventional tobacco,5,8 a “safe level” of exposure has not been established.9 Many studies have shown that acute exposure to even low levels of acrolein can induce dyslipidemia,10 vascular injury,11 endothelial dysfunction,12 and platelet activation,13 whereas chronic exposures accelerate cardiovascular disease (CVD).14−18 Indeed, over 92% of the theoretical cardiopulmonary disease (noncancer) risk due to smoking is attributed to just the three aforementioned aldehydes, that is, acrolein, 88.5%; acetaldehyde, 2.4%; and formaldehyde, 0.4%.2 Animal experiments have indicated that acrolein plays a role in carcinogenesis,19,20 macrophage and neutrophil accumulation with the consequent production of proinflammatory cytokines and proteases,21 suppression of endothelial progenitor cells, and endothelial dysfunction.22−24 Thus, it is necessary to both detect and accurately measure the aldehydes from aerosols of e-cigarettes to evaluate their harm to the e-cigarette users and secondhand exposures.
The emission of aldehydes including acetaldehyde, acrolein, and formaldehyde from e-cigarettes has thus raised concern over the health risk of active and passive exposures.25 Because e-cigarettes are battery-powered devices that vaporize nicotine-containing solutions known as e-liquids,26,27 the delivered battery power output dictates the heating coil temperature of the atomizer and, therefore, the quantity of aldehydes generated from thermal decomposition.8,26 As e-cigarette industry promotes next-generation e-cigarette devices with sub-ohm resistance coil (∼0.5 Ω) atomizers, the battery power output is much higher than that of the first- and second-generation e-cigarette devices. Thus, the emission of aldehydes from new generations of e-cigarette devices needs to be studied. Additionally, there are hundreds of different e-liquid brands.27 e-Liquids are a mixture of humectants (propylene glycol and glycerin) and food-grade flavoring additives that give rise to different aldehydes when heated (e.g., glycerin makes acrolein, whereas propylene glycol forms acetaldehyde and formaldehyde).27 Thus, the quantity of the aldehydes in e-cigarette aerosols needs to be determined to best assess the potential toxicity of these compounds. Yet, because of their high reactivity, it is difficult to quantify volatile aldehydes (and other carbonyls), and current methods that depend solely on capture by 2,4-dinitrophenylhydrazine (DNPH)-impregnated silica gel cartridges are generally criticized to be inaccurate at the low levels encountered in cigarette smoke and e-cigarette aerosols.28 Moreover, a recent study reported that formaldehyde may form reversible formaldehyde–hemiacetal that may contribute to a proportion of the total aldehyde.26 Unfortunately, no study has measured both free aldehydes and aldehyde–hemiacetals in the aerosols of e-cigarettes.
To address the challenge of accurately measuring aldehydes in aerosols of e-cigarettes, we used a newly developed technology that combines an enhanced carbonyl trapping agent along with a microfabricated silicon microreactor to capture aldehydes via oximation reactions. This microreactor approach has demonstrated superior lower limit of detection in an efficient and quantitative trap of trace carbonyl compounds in air and exhaled breath.29,30 In addition, we investigated the contribution of aldehyde-specific hemiacetals to the total aldehyde presence in the aerosols, using nuclear magnetic resonance (NMR) spectroscopy. We report herein the measurements of toxic aldehydes and their respective hemiacetals in the aerosols generated from both the first-generation e-cigarettes with cartridges and a second-generation e-cigarette with a “tank-type” atomizer for vaporization of a number of popular e-liquids.
2. Materials and Methods
2.1. Materials
All reagents and solvents, including formaldehyde, 1,3,5-trioxane, cyclohexanone, acetone (acetone-d6), and poly-4-vinylpyridine (PVP), were purchased from Sigma-Aldrich. 4-(2-Aminooxyethyl)-morpholin-4-ium chloride (AMAH) was synthesized according to a published procedure.30 A popular first-generation e-cigarette blu with a fixed battery output voltage of 3.7 V along with four cartridges (coil resistance 3.0 Ω) was purchased online from Amazon. A newer-generation e-cigarette device consisting of an atomizer EVOD2 made by KangerTech (coil resistance 1.5 Ω) and an iTaste VV V3.0 battery with a digital voltage output of 3.3–5 V was purchased from a local e-cigarette store. Three popular e-liquids, EL01–EL03, were purchased from a local e-cigarette store. Each of these e-liquid products contained 10 mL of solution with a nicotine level of 6 mg/mL. Another three flavored e-liquids, EL04–EL06, were purchased from their manufacturer. These e-liquids contained 7 mL of solution with a nicotine level of 6 mg/mL. Table 1 lists the characteristics of these e-cigarette cartridges and e-liquids.
Table 1. Characteristics of e-Cigarette Cartridges and e-Liquids Used in This Study.
| product code | brand name | type | nicotine content (label) | manufacturer |
|---|---|---|---|---|
| EC01 | blu Classic Tobacco | cartridge | 16 mg | Imperial Tobacco |
| EC02 | blu Magnificent Menthol | cartridge | 16 mg | Imperial Tobacco |
| EC03 | blu Vivid Vanilla | cartridge | 16 mg | Imperial Tobacco |
| EC04 | blu Cherry Crush | cartridge | 16 mg | Imperial Tobacco |
| EL01 | eVo Black Diamond | e-liquid | 6 mg/mL | NicoPure Lab USA |
| EL02 | Smooththol | e-liquid | 6 mg/mL | NicQuid |
| EL03 | Perfected Vapes/Clearwater | e-liquid | 6 mg/mL | Delaware Vapor USA |
| EL04 | Halo Café Mocha | e-liquid | 6 mg/mL | Halo USA |
| EL05 | Halo Menthol Ice | e-liquid | 6 mg/mL | Halo USA |
| EL06 | Halo South Classic | e-liquid | 6 mg/mL | Halo USA |
2.2. Microreactors
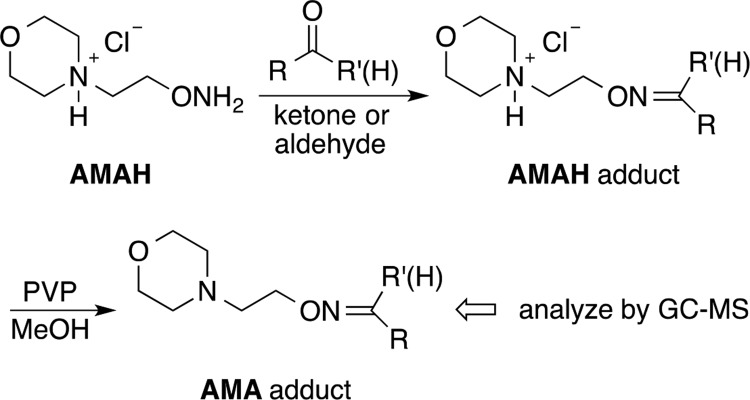
The silicon microreactors were fabricated from silicon wafers using standard microelectromechanical system fabrication techniques. The microreactors have been used for trapping carbonyl compounds in environmental air and exhaled breath.29,30 Detailed fabrication and characterization procedures of the microreactors for the capture of carbonyl compounds were published elsewhere.29,30 Each microreactor contains thousands of micropillars. The surface of the micropillars was functionalized with AMAH by infusing a solution of AMAH (32 μL, 1 × 10–6 mol) in methanol into a microreactor followed by evaporation of the solvent in a vacuum oven. Fused silica capillary tubes with 350 μm o.d. and 250 μm i.d. were connected to the inlet and outlet ports of the microreactor with a silica-based bonding agent, respectively. The use of AMAH-coated microreactors to capture carbonyl compounds followed by conversion of AMAH adducts to neutral analytes for gas chromatography–mass spectrometry (GC–MS) analysis have been published elsewhere.30
2.3. Generation of Aerosols from e-Cigarettes
Aerosols were generated from 10 puffs (puff duration of 4 s, puff volume of 91 mL/puff, and puff frequency of 2 puffs/min) and were collected in Tedlar bags using a software-controlled (FlexiWare) cigarette-smoking robot (SCIREQ, Montreal, Canada). The puff duration, puff volume, and puff frequency in this study are within the ranges used by e-cigarette users.31,32 To study the effect of puffing topography on the emission of aldehydes in aerosols, polypropylene syringes with a 60 mL capacity were also used to collect aerosols of e-cigarettes by manually varying puff duration and puff volume. Whereas the first-generation e-cigarette has a fixed battery power output of 4.6 W (voltage 3.7 V), the battery power output of the second-generation e-cigarette was tested at 9.1 W (3.7 V), 11.7 W (4.2 V), 14.7 W (4.7 V), and 16.6 W (5.0 V) for vaporization of e-liquids. Aerosol samples collected in Tedlar bags were evacuated through the microreactors by a vacuum pump at a flow rate of 3.5 mL/min for the capture of carbonyl compounds. For aerosols collected from the EL04, EL05, and EL06 e-liquids at the battery power output of 14.7 and 16.6 W, the aerosol samples were diluted 50 times with N2 and then were drawn through the microreactors because of much higher levels of generated aldehydes. After the evacuation process, the microreactor was eluted with 150 μL of MeOH followed by addition of an internal reference, AMAH–cyclohexanone (1 × 10–7 mol). Cyclohexanone was chosen because it is symmetrical (i.e., no geometrical isomers for the AMAH–cyclohexanone adduct) and was not detected in e-cigarette aerosols. Calibration curves of the internal standard against all detected aldehydes at different concentrations were established for quantitative measurements. PVP (5 mg) was added to the eluted solutions to neutralize the positively charged AMAH adducts to AMA (4-(2-aminooxyethyl)morpholine) adducts (Scheme 1).30 The suspensions were vortex-mixed for 30 s and allowed to stand for 30 min for the sedimentation of PVP particles, after which a 2 μL aliquot was used for GC–MS analysis.
Scheme 1. Microreactor Oximation of AMAH with Carbonyl Compounds and Neutralization of Adducts with PVP before GC–MS Analyses.
2.4. GC–MS Analysis of Carbonyl Adducts
A Thermo Scientific GC–MS instrument equipped with an AI 1310 automatic sampler, a TRACE 1310 GC with a split/splitless injector, and an ITQ 1100 series ion trap MS was used for analysis. The GC had an Agilent J&W DB-17ms column (60 m × 0.25 mm × 0.25 μm film thickness). The flow rate of the carrier gas helium was 1.5 mL/min. The column temperature was 50 °C for 1 min, then increased by 10 °C/min up to 160 °C, and then to 200 °C by 2 °C/min. After that, the temperature was increased by 12 °C/min up to 280 °C and was held at 280 °C for 5 min. The total running time was 41 min. The samples were split-injected with a split flow of 15 mL/min and a split ratio of 10.
2.5. NMR Analysis of Hemiacetals
Ten puffs (puff volume of 35 mL, puff duration of 4 s, and puff frequency of 1 puff/min) of the aerosols generated by the first-generation e-cigarette and the second-generation e-cigarette were collected using a 60 mL polypropylene syringe with a very short rubber tube to connect the e-cigarette. After collection, the rubber tube and e-cigarette were immediately removed, and then the syringe was fitted with a stainless steel needle to transfer the aerosolized liquid into an NMR test tube in an ice bath. During the transfer, most of the aerosol condensed and was collected as liquid. Four hundred microliters of deuterated dimethyl sulfoxide-d6 (DMSO) was added into the NMR tube followed by the addition of a known amount of benzene (1.72 × 10–6 mol) as an internal standard. Then, 1H NMR spectra (referenced to tetramethylsilane (TMS)) were immediately taken at 400 MHz. To verify the formation of formaldehyde–hemiacetal in e-liquids, formaldehyde was also generated by heating 1,3,5-trioxane and 8 N sulfuric acid at 95 °C accordingly33 and then introduced as a gas into e-liquids. Formaldehyde–hemiacetal was quantified by relative integration against the known amount of benzene added as an internal standard.
2.6. Statistical Data Analysis
All measured amounts of carbonyl volatile organic compounds were analyzed using the Wilcoxon test to statistically determine the differences in the changes in puff voltage, puff volume, and puff duration of e-cigarettes. A p-value smaller than 0.05 was defined as statistically significant. The Wilcoxon tests were performed using Minitab version 16.0.
3. Results and Discussion
3.1. Measurement of Aldehydes in Aerosols of e-Cigarettes
To measure aldehydes and other carbonyl compounds in aerosols of e-cigarettes, we used a sensitive microreactor-capture approach with an AMAH coating as previously reported (Scheme 1).30 The use of microreactors with aminooxy coatings for chemoselective capture of trace carbonyl compounds via oximation reactions has been established.29,30Table 2 lists the measured amounts of the four most abundant carbonyl compounds in the aerosols of the blu e-cigarettes at the battery power output of 4.6 W and the newer-generation e-cigarette at the battery power output of 9.1 W at the same battery output voltage of 3.7 V. All tested e-cigarettes produced acetaldehyde, acrolein, and formaldehyde. The amounts of acetaldehyde and formaldehyde were much higher than that of acrolein in aerosols of all e-cigarettes. The new-generation e-cigarette with the tank-type atomizer (EVOD2) and iTaste VV V3.0 battery for vaporization of the six e-liquids produced much higher levels of aldehydes and acetone than did the blu e-cigarettes because of the higher battery power output. Formaldehyde and acrolein in 10-puff aerosols generated from the six e-liquids by the newer e-cigarette device ranged from 8.2 to 40.4 μg and 1.6 to 5.8 μg, respectively, which were lower than these aldehydes in mainstream smoke of conventional cigarettes measured using Health Canada intense puffing regime shown in Table 2 (formaldehyde 74 μg/cigarette and acrolein 120.4 μg/cigarette).34 The amounts of acetaldehyde and acetone in 10-puff aerosols of these e-liquids were much lower than those in mainstream smoke of conventional cigarettes.34 Acetone in tobacco cigarette smoke has a much less toxic effect than that of the three aldehydes.2,3 The levels of propionaldehyde and butyraldehyde in conventional cigarette smoke are much lower than the levels of formaldehyde and acetaldehyde, and the toxic effects of these two compounds were not documented.2
Table 2. Carbonyl Compounds Formed from e-Cigarettes and e-Liquidsa.
| product code | acetaldehyde μg/10 puffs | acrolein μg/10 puffs | formaldehyde μg/10 puffs | acetone μg/10 puffs |
|---|---|---|---|---|
| EC01 | 0.57 ± 0.03 | 0.05 ± 0.01 | 0.55 ± 0.03 | 4.97 ± 0.31 |
| EC02 | 0.49 ± 0.03 | 0.24 ± 0.01 | 0.62 ± 0.05 | 5.90 ± 0.36 |
| EC03 | 0.52 ± 0.04 | 0.07 ± 0.01 | 0.43 ± 0.05 | 6.21 ± 0.34 |
| EC04 | 0.15 ± 0.02 | 0.02 ± 0.002 | 0.18 ± 0.02 | 1.29 ± 0.16 |
| EL01 | 63.1 ± 3.5 | 1.6 ± 0.2 | 26.8 ± 2.6 | 9.3 ± 0.7 |
| EL02 | 23.3 ± 2.4 | 1.9 ± 0.5 | 8.2 ± 0.2 | 4.2 ± 0.9 |
| EL03 | 44.8 ± 3.3 | 2.0 ± 0.04 | 40.4 ± 0.4 | 12.5 ± 0.3 |
| EL04 | 13.3 ± 0.02 | 5.8 ± 0.8 | 15.2 ± 0.02 | 1.3 ± 0.2 |
| EL05 | 13.9 ± 5.3 | 2.1 ± 0.5 | 20.1 ± 3.7 | 2.9 ± 0.2 |
| EL06 | 15.2 ± 4.8 | 3.1 ± 0.4 | 21.8 ± 5.1 | 3.0 ± 0.5 |
| tobacco cigaretteb | 1240.3 ± 17.7 | 120.4 ± 14.7 | 74.0 ± 23.7 | 641.9 ± 71.2 |
Aerosol samples were generated at a battery power output of 4.6 W for the blu e-cigarettes EC01–EC04 and a battery power output of 9.1 W for e-liquids EL01–EL06 using an EVOD2 atomizer (puff volume of 91 mL and puff duration of 4 s). Each experiment was performed in triplicate, and the data are expressed as the average (±SD) of the measured values.
Data from Counts et al. (Health Canada Intense puffing regime).34
Previous studies of carbonyl compounds in aerosols of e-cigarettes have found that generation of aldehydes including acrolein, formaldehyde, and acetaldehyde was related to the battery power output of the e-cigarette device and thermal decomposition of humectants and flavoring chemicals in e-liquids.8,35−37 Several studies have investigated the mechanisms of thermal decomposition of propylene glycol and glycerin, which are the main constituents of e-liquids.37−39 Thermal decomposition of propylene glycol generates more acetone, more acetaldehyde, and less formaldehyde,37,39 whereas thermal decomposition of glycerin results in more acrolein and formaldehyde.37,38 A recent study have also found that thermal decomposition of some flavoring chemicals in e-liquids can exponentially increase the generation of aldehydes.40 Numerous studies of animal models have demonstrated the high sensitivity of pulmonary and CV systems to acrolein and formaldehyde at a relatively low level of inhalation.19−24 Therefore, newer-generation e-cigarettes with emissions of higher levels of formaldehyde and acrolein will need further animal studies to examine the health risk.
Because the puffing topography of e-cigarettes affects the formation of toxic aldehydes, we used a simple polypropylene syringe to imitate the characteristics of e-cigarette users by varying puff volumes and puff durations in the first-generation blu e-cigarettes with two different flavor cartridges, that is, Classic Tobacco and Magnificent Menthol. When puff volume was varied from 20 to 60 mL/puff at a fixed puff duration of 2 s, the amounts of acetaldehyde, acrolein, and formaldehyde were proportionally increased with the puff volume for both Classic Tobacco and Magnificent Menthol (Figures S1 and S2). For Magnificent Menthol, the amounts of acetaldehyde, acrolein, and formaldehyde were higher than those for Classic Tobacco at the same puff conditions (Figure S1). Varying puff duration from 1 to 6 s at a fixed puff volume of 35 mL/puff had a more complicated effect as the amount of acetaldehyde, acrolein, and formaldehyde increased when the duration was increased from 1 to 4 s but then declined as the puff duration was increased to 6 s for both Classic Tobacco and Magnificent Menthol (Figures S3 and S4). The maximum amounts of formaldehyde, acetaldehyde, and acrolein were generated at a puff duration of approximately 4 s/puff. Coincidently, several studies of puffing topography of e-cigarette users reported average puff durations between 3.5 and 4.3 s.31,32
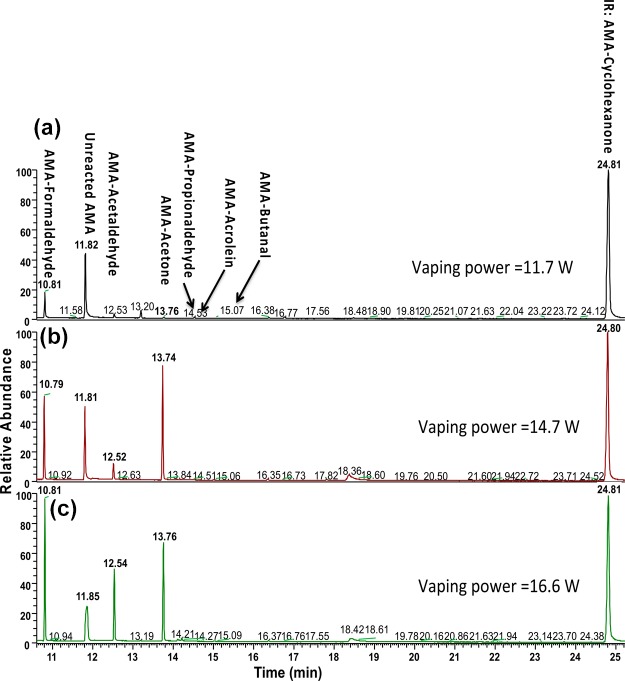
Because increasing the battery power output of newer e-cigarette devices increases the atomizer coil temperature that results in emissions of more aldehydes in aerosols of e-liquids,8,26,36 we investigated the effect of the battery power output of the newer e-cigarette device on the generation of carbonyl compounds in aerosols of e-liquids. EL04 and EL05 generated much more carbonyl compounds at a power output of 11.7 W (voltage 4.2 V) and above. Figure 1 shows GC–MS chromatograms of AMAH and its adducts from the aerosol samples collected from EL05 at the battery power output of 11.7 W (4.2 V), 14.7 W (4.7 V), and 16.6 W (5.0 V). Acetaldehyde, acrolein, formaldehyde, propionaldehyde, and butyraldehyde were detected. Increasing the power from 11.7 to 16.6 W resulted in dramatic increases in the levels of these aldehydes (Table 3). Similar results of dramatic increases in acetaldehyde, acrolein, and formaldehyde with increasing vaping power output to 9 and 10 W have been reported.36 High battery power output results in overheating of the coil and leads to excessive aldehyde generation by thermal decomposition of humectants (“dry puff” condition).36
Figure 1.
Representative GC–MS chromatograms of aldehydes. The newer e-cigarette device (iTaste) was used to vaporize e-liquid EL05 at a battery power output of (a) 11.7 W (4.2 V), (b) 14.7 W (4.7 V), and (c) 16.6 W (5.0 V).
Table 3. Effect of Varying Battery Power Output on the Generation of Aldehydes in the Aerosols from e-Liquid EL05a.
| voltage (W) | acetaldehyde (μg) | acrolein (μg) | formaldehyde (μg) | acetone (μg) | propionaldehyde (μg) | butyraldehyde (μg) |
|---|---|---|---|---|---|---|
| 11.7 | 22.71 ± 3.35 | 1.22 ± 0.82 | 129.55 ± 9.66 | 11.46 ± 0.50 | 0.57 ± 0.41 | 0.49 ± 0.32 |
| 14.7 | 134.30 ± 7.8 | 3.18 ± 0.71 | 386.77 ± 11.00 | 984.92 ± 50.10 | 3.37 ± 1.52 | 4.95 ± 8.55 |
| 16.6 | 532.10 ± 60.2 | 16.21 ± 0.30 | 819.81 ± 76.80 | 808.72 ± 72.6 | 17.92 ± 0.90 | 13.60 ± 0.53 |
A total of 10 puffs of aerosol was collected at the puff volume of 91 mL and puff duration of 4 s. Each experiment was performed in triplicate, and the data are expressed as the average (±SD) of the measured values.
3.2. Measurement of Hemiacetals in Aerosols of e-Cigarettes
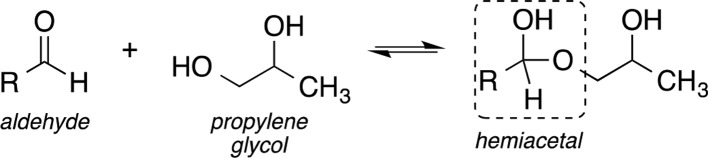
Hemiacetals are formed when alcohols, such as propylene glycol in e-liquids, add reversibly to the carbonyl functional group of aldehydes, as shown in Scheme 2. The reaction between formaldehyde and propylene glycol or glycerin of e-cigarette liquids during vaporization is, therefore, thought to form measurable formaldehyde–hemiacetal, as detected using 1H NMR spectroscopy.26
Scheme 2. Reversible Formation of a Hemiacetal through the Reaction of an Aldehyde and an Alcohol.
A recent report have suggested that emission of formaldehyde in e-cigarette aerosols is higher than a direct measurement of formaldehyde because a portion of formaldehyde is sequestered in the form of a hemiacetal, which prompted more health concerns over using e-cigarettes.26 Unfortunately, this work on the measurement of formaldehyde–hemiacetal did not measure free or unreacted formaldehyde or any other aldehydes in the aerosols of the e-cigarettes. The work was generally criticized for using unrealistic battery output power (voltage of 5 V) for the measurement of formaldehyde–hemiacetal.41 The reported formaldehyde–hemiacetal in aerosols was generated from e-liquid EL04. Thus, there is a need to measure total aldehydes in the aerosols of other e-cigarettes, especially for newer-generation e-cigarettes with variable battery power output and highly variable e-liquid compositions that could influence hemiacetal formation (e.g., humectant and flavoring chemicals as well as nicotine concentration). To quantify the fraction of aldehydes that reacted with propylene glycol and/or glycerin to form hemiacetals in the aerosols of e-cigarettes, we collected aerosolized e-liquids in NMR tubes containing DMSO-d6 using the newer e-cigarette device. We initially attempted to detect the formation of a formaldehyde-derived hemiacetal in aerosols of all e-cigarettes in Table 1. No formaldehyde–hemiacetal signal was detected in any of the aerosols generated from the first-generation blu e-cigarettes with all tested puff volume and puff duration scenarios (data not shown). No hemiacetal was detectable in the aerosols of EL01, EL02, and EL03 at any battery power output from 9.1 to 16.6 W as shown in Figure 2a. In a positive control experiment, formaldehyde gas was introduced into EL01 e-liquid, and under this condition, a triplet signal at δ 6.18 ppm and a doublet signal at δ 4.61 ppm were observed, confirming the formation of a formaldehyde–hemiacetal in this e-liquid (Figure 2b). Thus, the lack of detectable formaldehyde–hemiacetal in aerosols of the first-generation e-cigarette and e-liquids EL01, EL02, and EL03 was likely related to the generally low amount of formaldehyde present in these aerosols, even at higher battery power output.
Figure 2.
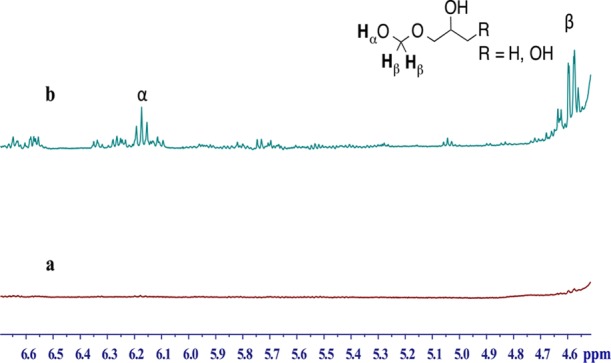
1H NMR spectra (DMSO-d6) for detection of hemiacetals: (a) e-liquid EL01 vaporization at the battery power output of 16.6 W (no hemiacetal detected) and (b) e-liquid EL01 spiked with formaldehyde.
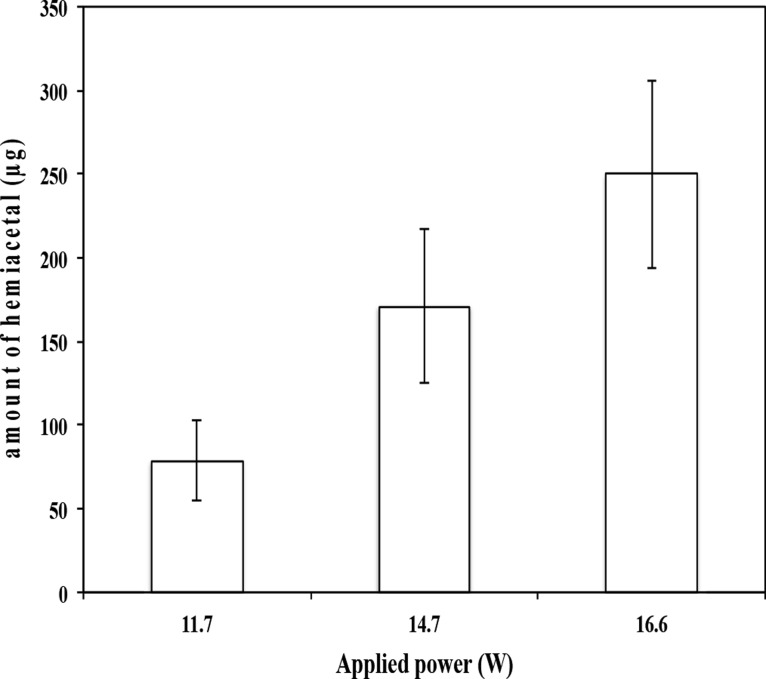
Formaldehyde hemiacetal in aerosols of EL05 was detected at the battery power output from 11.7 to 16.6 W using NMR. No other aldehyde hemiacetal such as acetaldehyde hemiacetal and acrolein hemiacetal was detected. Figure 3 shows that there was an increase in peak intensity of the hemiacetal as the e-cigarette battery power output was increased from 11.7 to 16.6 W. The limit of detection of formaldehyde–hemiacetal using NMR was determined to be 7.1 μg/10 puffs. The formaldehyde–hemiacetal level was below the limit of detection at a battery power output of 9.1 W. The amounts of formaldehyde–hemiacetal in aerosol increased as the power was increased, and the calculated amounts (mean ± SD) of hemiacetal were based on the internal standard (Figure 4). At a battery power output of 11.7 W, 78.6 ± 23.8 μg/10 puffs of formaldehyde hemiacetal was measured, whereas at 16.6 W, 250.4 ± 56.1 μg/10 puffs of the hemiacetal was measured. We were able to estimate the amount of the bound formaldehyde to be 22.2 ± 6.7 and 70.7 ± 15.8 μg from the measured formaldehyde–hemiacetal at 11.7 and 16.6 W, respectively. These amounts of formaldehyde could be released from the reversible reaction of formaldehyde–hemiacetal. Given the puff volume of 35 mL of this work, we could estimate that the formaldehyde in formaldehyde–hemiacetal could be approximately 44.6% of free formaldehyde at the power output of 11.7 W and approximately 22.4% of free formaldehyde at the power output of 16.6 W. Higher formaldehyde levels of 380 (puff volume of 50 mL for 10 puffs) was reported from formaldehyde–hemiacetal at the e-cigarette voltage output of 5 V for vaporization of EL04 e-liquid.26
Figure 3.
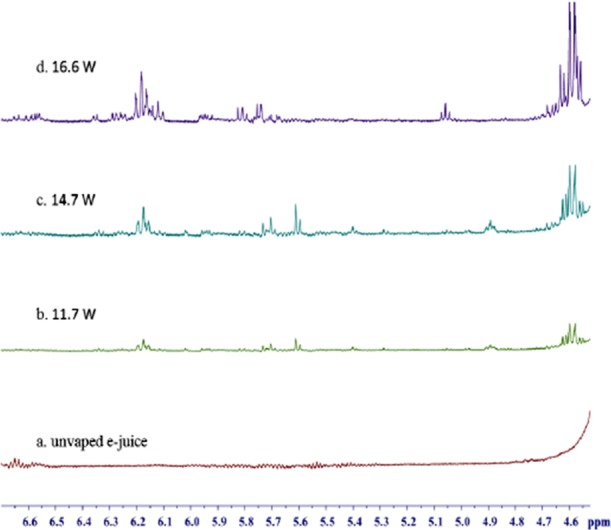
1H NMR spectra (DMSO-d6) of hemiacetals: (a) unvaporized e-liquid EL05; the tank-type e-cigarette (iTaste) was used to vaporize the e-liquid EL05 at a battery power output of (b) 11.7 W (4.2 V), (c) 14.7 W (4.7 V), and (d) 16.6 W (5.0 V).
Figure 4.
Relationship between the amounts of formaldehyde–hemiacetal in 10 puffs of aerosols collected from e-liquid EL05 at the battery power output of 11.7, 14.7, and 16.6 W.
The effects of tobacco products on CVD risk are distinct from those on other organ systems. Because of their low capacity to detoxify xenobiotics,42 CV tissues appear to be relatively more sensitive to smoking. As a result, effects of smoking on CV tissues appear at exposure levels far lower than those that cause other diseases, such as cancer.43 Moreover, in contrast to cancer, substantial CVD risk is associated even with very light smoking, such that 80% of the risk of smoking >20 cigarettes per day is associated with <3 cigarettes per day.44 These published data suggest that even though there may be a decrease in the risk of other diseases with e-cigarette use, substantial harm to the CV system could occur because of high sensitivity of this target system to aldehydes. The emission of aldehydes including acrolein, acetaldehyde, and formaldehyde in aerosols of e-cigarettes as measured in this study supports the need to maintain an appropriate level of concern over active and passive exposures to harmful emissions of e-cigarettes and calls for more research on the newer-generation e-cigarettes and their potentially harmful effects.
Acknowledgments
M.A.O. thanks the University of Louisville School of Interdisciplinary and Graduate Studies for a graduate research fellowship. We acknowledge assistance of Whitney Theis, MS, for technical support. Research reported in this publication was supported by grant numbers P50HL120163 from the National Heart, Lung and Blood Institute (NHLBI) and FDA Center for Tobacco Products (CTP), HL122676, GM 103492 from NIH, and CBET:1159829 from NSF. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.6b00489.
The effects of puff volume and puff duration on the amounts of aldehydes in aerosols of the first-generation e-cigarette (PDF)
Author Present Address
∥ Department of Chemical Engineering, Virginia Commonwealth University, Richmond, VA 23284, USA (Y.C.).
Author Present Address
⊥ Institute of Microelectronics, Chinese Academy of Sciences, Beijing 100029, P.R. China (M.L.).
Author Contributions
The manuscript was written through contributions of all authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Vorhees D. J.; Herger-Bernays W.; McClean M. D.. Human Health Risk Associated with Cigarette Smoke: The Link between Smoke Constituents and Additives; Menzie Inc.: Boston, MA, 1997. [Google Scholar]
- Haussmann H.-J. Use of hazard indices for a theoretical evaluation of cigarette smoke composition. Chem. Res. Toxicol. 2012, 25, 794–810. 10.1021/tx200536w. [DOI] [PubMed] [Google Scholar]
- Secondhand Smoke Exposure and Cardiovascular Effects; The National Academies: Washington, DC, 2010; p 228. [PubMed]
- Ghilarducci D. P.; Tjeerdema R. S. Fate and effects of acrolein. Rev. Environ. Contam. Toxicol. 1995, 144, 95–146. 10.1007/978-1-4612-2550-8_2. [DOI] [PubMed] [Google Scholar]
- Goniewicz M. L.; Knysak J.; Gawron M.; Kosmider L.; Sobczak A.; Kurek J.; Prokopowicz A.; Jablonska-Czapla M.; Rosik-Dulewska C.; Havel C.; Jacob P.; Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobac. Contr. 2014, 23, 133–139. 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholon J. F.; Becquemin M. H.; Annesi-Maesano I.; Dautzenberg B. Electronic cigarettes: A short review. Respiration 2013, 86, 433–438. 10.1159/000353253. [DOI] [PubMed] [Google Scholar]
- Cheng T. Chemical evaluation of electronic cigarettes. Tobac. Contr. 2014, 23, ii11–ii17. 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider L.; Sobczak A.; Fik M.; Knysak J.; Zaciera M.; Kurek J.; Goniewicz M. L. Carbonyl Compounds in Electronic Cigarette Vapors: Effects of Nicotine Solvent and Battery Output Voltage. Nicotine Tob. Res. 2014, 16, 1319–1326. 10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K. E.; Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: A systematic review. Ther. Adv. Drug Saf. 2014, 5, 67–86. 10.1177/2042098614524430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin D. J.; Barski O. A.; Lesgards J.-F.; Juvan P.; Rezen T.; Rozman D.; Prough R. A.; Vladykovskaya E.; Liu S.; Srivastava S.; Bhatnagar A. Acrolein consumption induces systemic dyslipidemia and lipoprotein modification. Toxicol. Appl. Pharmacol. 2010, 243, 1–12. 10.1016/j.taap.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin D. J.; Bhatnagar A.; Cowley H. R.; Johnson G. H.; Wiechmann R. J.; Sayre L. M.; Trent M. B.; Boor P. J. Acrolein generation stimulates hypercontraction in isolated human blood vessels. Toxicol. Appl. Pharmacol. 2006, 217, 277–288. 10.1016/j.taap.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin D. J.; Haberzettl P.; Prough R. A.; Bhatnagar A. Glutathione-S-transferase P protects against endothelial dysfunction induced by exposure to tobacco smoke. Am. J. Physiol.: Heart Circ. Physiol. 2009, 296, H1586–H1597. 10.1152/ajpheart.00867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithu S. D.; Srivastava S.; Siddiqui M. A.; Vladykovskaya E.; Riggs D. W.; Conklin D. J.; Haberzettl P.; O’Toole T. E.; Bhatnagar A.; D’Souza S. E. Exposure to acrolein by inhalation causes platelet activation. Toxicol. Appl. Pharmacol. 2010, 248, 100–110. 10.1016/j.taap.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S.; Sithu S. D.; Vladykovskaya E.; Haberzettl P.; Hoetker D. J.; Siddiqui M. A.; Conklin D. J.; D’Souza S. E.; Bhatnagar A. Oral exposure to acrolein exacerbates atherosclerosis in apoE-null mice. Atherosclerosis 2011, 215, 301–308. 10.1016/j.atherosclerosis.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole T. E.; Zheng Y.-T.; Hellmann J.; Conklin D. J.; Barski O.; Bhatnagar A. Acrolein activates matrix metalloproteinases by increasing reactive oxygen species in macrophages. Toxicol. Appl. Pharmacol. 2009, 236, 194–201. 10.1016/j.taap.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.-W.; Guo Y.; Vondriska T. M.; Zhang J.; Zhang S.; Tsai L. L.; Zong N. C.; Bolli R.; Bhatnagar A.; Prabhu S. D. Acrolein consumption exacerbates myocardial ischemic injury and blocks nitric oxide-induced PKCε signaling and cardioprotection. J. Mol. Cell. Cardiol. 2008, 44, 1016–1022. 10.1016/j.yjmcc.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Ismahil M. A.; Hamid T.; Haberzettl P.; Gu Y.; Chandrasekar B.; Srivastava S.; Bhatnagar A.; Prabhu S. D. Chronic oral exposure to the aldehyde pollutant acrolein induces dilated cardiomyopathy. Am. J. Physiol.: Heart Circ. Physiol. 2011, 301, H2050–H2060. 10.1152/ajpheart.00120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJarnett N.; Conklin D. J.; Riggs D. W.; Myers J. A.; O’Toole T. E.; Hamzeh I.; Wagner S.; Chugh A.; Ramos K. S.; Srivastava S.; Higdon D.; Tollerud D. J.; DeFilippis A.; Becher C.; Wyatt B.; McCracken J.; Abplanalp W.; Rai S. N.; Ciszewski T.; Xie Z.; Yeager R.; Prabhu S. D.; Bhatnagar A. Acrolein Exposure is Associated with Increased Cardiovascular Disease Risk. J. Am. Heart Assoc. 2014, 3, e000934 10.1161/jaha.114.000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J.-M.; Gao Y.-T.; Wang R.; Chen M.; Carmella S. G.; Hecht S. S. Urinary levels of volatile organic carcinogen and toxicant biomarkers in relation to lung cancer development in smokers. Carcinogenesis 2012, 33, 804–809. 10.1093/carcin/bgs026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. F.; Maier C. S. Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 2008, 52, 7–25. 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto N.; Volpi G.; Pastore F.; Facchinetti F. Acrolein effects in pulmonary cells: Relevance to chronic obstructive pulmonary disease. Ann. N.Y. Acad. Sci. 2012, 1259, 39–46. 10.1111/j.1749-6632.2012.06531.x. [DOI] [PubMed] [Google Scholar]
- Conklin D. J.; Haberzettl P.; Prough R. A.; Bhatnagar A. Glutathione-S-transferase P protects against endothelial dysfunction induced by exposure to tobacco smoke. Am. J. Physiol.: Heart Circ. Physiol. 2009, 296, H1586–H1597. 10.1152/ajpheart.00867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat L. A.; Haberzettl P.; Hellmann J.; Baba S. P.; Bertke M.; Lee J.; McCracken J.; O’Toole T. E.; Bhatnagar A.; Conklin D. J. Acrolein inhalation prevents vascular endothelial growth factor-induced mobilization of Flk-1+/Sca-1+ cells in mice. Arterioscler., Thromb., Vasc. Biol. 2011, 31, 1598–1606. 10.1161/atvbaha.111.227124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp R. O. Jr.; Andjelkovich D. A.; Kligerman A. D.; Morgan K. T.; Heck H. D.; Feron V. J. A critical review of the literature on acrolein toxicity. Crit. Rev. Toxicol. 1985, 14, 309–380. 10.3109/10408448509037461. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A.; Whitsel L. P.; Ribisl K. M.; Bullen C.; Chaloupka F.; Piano M. R.; Robertson R. M.; McAuley T.; Goff D.; Benowitz N. Electronic cigarettes: A policy statement from the American Heart Association. Circulation 2014, 130, 1418–1436. 10.1161/cir.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. P.; Luo W.; Pankow J. F.; Strongin R. M.; Peyton D. H. Hidden Formaldehyde in E-Cigarette Aerosols. N. Engl. J. Med. 2015, 372, 392–394. 10.1056/nejmc1413069. [DOI] [PubMed] [Google Scholar]
- Erickson B. E. Boom in E-Cigarettes Sparks Debate. Chem. Eng. News 2015, 93, 10–13. 10.1021/cen-09307-cover. [DOI] [Google Scholar]
- Ho S. S. H.; Ho K. F.; Liu W. D.; Lee S. C.; Dai W. T.; Cao J. J.; Ip H. S. S. Unsuitability of using the DNPH-coated solid sorbent cartridge for determination of airborne unsaturated carbonyls. Atmos. Environ. 2011, 45, 261–265. 10.1016/j.atmosenv.2010.09.042. [DOI] [Google Scholar]
- Li M.; Biswas S.; Nantz M. H.; Higashi R. M.; Fu X.-A. Preconcentration and analysis of trace volatile carbonyl compounds. Anal. Chem. 2012, 84, 1288–1293. 10.1021/ac2021757. [DOI] [PubMed] [Google Scholar]
- Knipp R. J.; Li M.; Fu X.-A.; Nantz M. H. A Versatile Probe for Chemoselective Capture and Analysis of Carbonyl Compounds in Exhaled Breath. Anal. Methods 2015, 7, 6027–6033. 10.1039/c5ay01576f. [DOI] [Google Scholar]
- Robinson R. J.; Hensel E. C.; Morabito P. N.; Roundtree K. A. Electronic cigarette topography in the natural environment. PLoS One 2015, 10, e0129296 10.1371/journal.pone.0129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S. E.; Hoffman A. C. Electronic cigarettes: Abuse liability, topography and subjective effects. Tobac. Contr. 2014, 23, ii23–ii29. 10.1136/tobaccocontrol-2013-051489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. F.; Chadwick A. F. Trioxane as a Source of Formaldehyde. Ind. Eng. Chem. 1947, 39, 974–977. 10.1021/ie50452a011. [DOI] [Google Scholar]
- Counts M. E.; Morton M. J.; Laffoon S. W.; Cox R. H.; Lipowicz P. J. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul. Toxicol. Pharmacol. 2005, 41, 185–227. 10.1016/j.yrtph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Bekki K.; Uchiyama S.; Ohta K.; Inaba Y.; Nakagome H.; Kunugita N. Carbonyl compounds generated from electronic cigarettes. Int. J. Environ. Res. Public Health 2014, 11, 11192–11200. 10.3390/ijerph111111192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K. E.; Voudris V.; Poulas K. E-cigarettes generate high levels of aldehydes only in ‘dry puff’ conditions. Addiction 2015, 110, 1352–1356. 10.1111/add.12942. [DOI] [PubMed] [Google Scholar]
- Sleiman M.; Logue J. M.; Montesinos V. N.; Russell M. L.; Litter M. I.; Gundel L. A.; Destaillats H. Emissions from electronic cigarettes: Key parameters affecting the release of harmful chemicals. Environ. Sci. Technol. 2016, 50, 9644–9651. 10.1021/acs.est.6b01741. [DOI] [PubMed] [Google Scholar]
- Laino T.; Tuma C.; Curioni A.; Jochnowitz E.; Stolz S. A revisited picture of the mechanism of glycerol dehydration. J. Phys. Chem. A 2011, 115, 3592–3595. 10.1021/jp201078e. [DOI] [PubMed] [Google Scholar]
- Laino T.; Tuma C.; Moor P.; Martin E.; Stolz S.; Curioni A. Mechanisms of propylene glycol and triacetin pyrolysis. J. Phys. Chem. A 2012, 116, 4602–4609. 10.1021/jp300997d. [DOI] [PubMed] [Google Scholar]
- Khlystov A.; Samburova V. Flavoring compounds dominate toxic aldehyde production during e-cigarette vaping. Environ. Sci. Technol. 2016, 50, 13080–13085. 10.1021/acs.est.6b05145. [DOI] [PubMed] [Google Scholar]
- Nitzkin J. L.; Farsalinos K.; Siegel M. More on hidden formaldehyde in e-cigarette aerosols. N. Engl. J. Med. 2015, 372, 1575–1576. 10.1056/nejmc1502242. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A. Cardiovascular pathophysiology of environmental pollutants. Am. J. Physiol.: Heart Circ. Physiol. 2004, 286, H479–H485. 10.1152/ajpheart.00817.2003. [DOI] [PubMed] [Google Scholar]
- Pope C. A.; Burnett R. T.; Krewski D.; Jerrett M.; Shi Y.; Calle E. E.; Thun M. J. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: Shape of the exposure-response relationship. Circulation 2009, 120, 941–948. 10.1161/circulationaha.109.857888. [DOI] [PubMed] [Google Scholar]
- Pope C. A.; Eatough D. J.; Gold D. R.; Pang Y.; Nielsen K. R.; Nath P.; Verrier R. L.; Kanner R. E. Acute exposure to environmental tobacco smoke and heart rate variability. Environ. Health Perspect. 2001, 109, 711–716. 10.1289/ehp.01109711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.