Abstract
Lamins are important constituents of the nuclear inner membrane and provide a platform for transcription factors and chromatin. Progerin, a C-terminal truncated lamin A mutant, causes premature aging termed Hutchinson-Gilford Progeria Syndrome (HGPS). Oxidative stress appears to be involved in the pathogenesis of HGPS, although the mechanistic role of progerin remains elusive. Here we examined whether nuclear lamins are important for a cellular antioxidant mechanism, and whether progerin compromises it. We investigated the activation of nuclear factor-E2-related factor 2 (Nrf2) which regulates various antioxidant genes including heme oxygenase-1 (HMOX1), following exposure to sodium arsenite or cadmium chloride in lamin knockdown human cell lines and primary HGPS human fibroblasts. Knocking down lamin A/C, or B, or all nuclear lamins simultaneously in three human cell lines (HaCaT, SW480, and K562) did not impair arsenite- or cadmium-induced activation of Nrf2. Progerin-expressing human primary HGPS fibroblasts showed lower basal levels of HMOX1 and NQO1 expression; however, in response to arsenic stress both normal and HGPS primary fibroblasts showed Nrf2 nuclear accumulation along with upregulation and phosphorylation of p62/SQSTM1 at Ser351, downregulation of Keap1, and comparable expression of an array of downstream Nrf2-regulated antioxidant genes. We also observed new forms of cleaved lamin A, B1 and B2 induced by cadmium stress although their roles in the Nrf2 antioxidant system need further investigation. These results suggest that the nuclear lamins and progerin have marginal roles in the activation of the antioxidant Nrf2 response to arsenic and cadmium.
Keywords: HGPS, Nrf2, Lamin, Progerin, Arsenic, Cadmium
INTRODUCTION
Oxidative stress is caused by metabolic imbalance between the formation and detoxification of oxidants and reactive oxygen species (ROS)[1]. ROS are produced constantly during cellular homeostatic activities such as mitochondrial oxidative metabolism, or produced urgently during bacterial invasion and inflammation. In addition to the endogenous ROS, cells have constant threats of contacting and handling exogenous sources of ROS from environmental chemicals. Arsenic (As) and cadmium (Cd) are major environmental and industrial metals that induce cellular toxicity at least in part through oxidative stress [2–4]. Chronic exposure to As is associated with the development of human tumors of the skin, lung, bladder and kidney [5] and the risk of diabetes [6]. Likewise, chronic Cd exposure causes kidney and bone damage, and development of lung, breast, and prostate cancers [7] In general, ROS are important signal transducers to stimulate cell defense mechanisms and appropriate physiological pathways [1], while ROS in excess or oxidative stress causes macromolecular damages or trigger aberrant cell signaling pathways [8] that are important but complex mechanisms associated with various human disorders including diabetes, cancer, neurodegeneration and aging [9].
To cope with oxidative stress and circumventing oxidative cell damage, cells evolved antioxidant defense systems. Along with important enzymes that directly detoxify ROS such as superoxide dismutases, catalase, and glutathione peroxidase [10], cells induce transcription of an array of phase II metabolism and detoxification genes, such as heme oxygenase-1 (HMOX1) and NAD(P)H quinone reductase-1 (NQO1), in response to exposure of electrophiles and oxidants. This is achieved through binding of Nrf2 (nuclear factor-E2-related factor 2) transcription factor to a highly conserved enhancer termed ARE (antioxidant responsive element) in these phase II detoxification genes [11]. Nrf2 is activated in the cytoplasm through liberation from the inhibitory Keap1-Cul3 E3 ubiquitin ligase complex when Keap1 is inactivated by conformational changes through either direct oxidation by oxidants or adduct formation by xenobiotics [12]. In addition, the stability of Nrf2 protein is regulated by the Keap1-independent E3 ligase βTrCP [13] along with autophagic degradation of Keap1 [14,15] via facilitated interaction with p62/SQSTM1 (Sequestosome-1) upon phosphorylation at Ser351 [16]. Nrf2 dissociated from Keap1 translocates to the nucleus and bind to ARE with a small Maf as a heterodimerization partner in concert with transcriptional coactivators and histone modification enzymes [17].
In this nuclear translocation event, Nrf2 needs to cross the nuclear membrane and find accessible ARE enhancer sequences for transcriptional activation of target genes. The nuclear membrane consists of a double lipid bilayer separated by perinuclear space and linked by nuclear pore complexes [18]. The nuclear face of the inner nuclear membrane builds nuclear lamina, composed of lamins A/C (A and C are alternative splicing products), B1, and B2 to organize and support chromatin, to anchor nuclear pore complexes and transcription factors, and to serve as a platform for protein traffic and signaling complexes [19,20]. The integrity of the nuclear lamina is important because lamin mutations primarily found in the human lamin A gene cause various inheritable diseases including premature aging (HGPS: Hutchinson-Gilford progeria syndrome), cardiomyopathy, and muscular dystrophy [21]. Human lamin A dominant mutants include progerin (an internal lamin A deletion of 50 amino acids causing HGPS) [22], point mutations R482W causing familial partial lipodystrophy [23] and L530P causing Emery-Dreifuss muscular dystrophy [23]. The pathogenesis due to lamin mutations appears to be associated with ROS toxicity and oxidative stress [24], although the mechanistic roles of lamins and progerin in cellular antioxidant defense and transcriptional response to the environment remain elusive.
The aim of this study was to test our hypothesis that nuclear lamins are critical determinants of the Nrf2-ARE-dependent gene transcription for cellular adaptive response to oxidative stress conditions. Given the fact that HGPS cells are prone to increased oxidative stress [24], we also investigated whether the lamin A dominant mutant progerin expressed in HGPS human primary fibroblasts impairs the nuclear accumulation of Nrf2 and/or Nrf2-regulated antioxidant gene expression.
MATERIALS AND METHODS
Cells and chemical reagents
SW480 human colon adenocarcinoma and HaCaT human keratinocytes were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Mediatech 35-010-CV) and 100 units/ml penicillin/100 μg/ml streptomycin (Mediatech). K562 human erythroleukemia cells were cultured in RPMI 1640 medium containing 25 mM Hepes [4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid, 10% FBS, and the antibiotics. Primary human normal fibroblasts (GM07492, passage 5 and GM08398, passage 8) and HGPS fibroblasts (AG11498, passages 6 and 10 and AG06917, no passage number provided) were obtained from Coriell Institute and cultured in MEM supplemented with 15% FBS and the antibiotics. Sodium arsenite, cadmium chloride, H2O2 (all purchased from Fisher Scientific), and cisplatin (Calbiochem), were dissolved and diluted with water, and etoposide (BioMol) was dissolved in methanol. tert-butylhydroquinone (t-BHQ, SIGMA) was dissolved in DMSO for 1M solution and diluted with water to make 25 μM solution. Cells were maintained at 37°C in a humidified 5% CO2 atmosphere.
Small interfering(si) RNA and transfection
100–300 pmol of non-targeting siRNA or 100 pmol each of siRNA (all from Qiagen) for lamin A/C, lamin B1, and/or lamin B2 were electroporated into 100 μl suspension of SW480, HaCaT, or K562 cells in siRNA transfection medium (Santa Cruz Biotechnology) using Gene Pulser X-Cell (BioRad) and incubated at room temperature for 10 min. Cells were resuspended in growth media, plated in 60 mm or 100 mm plates, and incubated for 36–48 hr to reach subconfluent cell density prior to treatment with sodium arsenite and other chemicals. The sequences of siRNAs used in this study are as follows;
siControl: AllStars Neg. Control siRNA, Cat. #1027281 (the sequence was not disclosed by Qiagen).
siLMNA-9: CAGGCAGTCTGCTGAGAGGAA, Cat. #SI00300643
siLMNA-10: CCCACCAAAGTTCACCCTGAA, Cat. #SI00300650
siLMNA-13: AACTGGACTTCCAGAAGAACA, Cat. #SI02654862
siLMNB1-2: AACGCGCTTGGTAGAGGTGGA, Cat. #SI00300671
siLMNB2-5: TCGGCAATAGCTCACCGTTTA, Cat. #SI02652244
Western blotting and antibodies
Whole cell lysates were prepared by suspending cells in phosphate buffered saline (PBS) containing 1% Triton X-100, 0.5% deoxycholic acid, and 0.1% sodium dodecyl sulfate (SDS). Cytoplasmic and nuclear fractions were isolated according to the nuclear extraction protocol from Thermo Fisher Scientific, procedures similar to a nuclear extraction kit from Active Motif. 10–30 μg of proteins as whole cell lysates or cytoplasmic and nuclear fractions were separated on 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a polyvinylidene difluoride (PVDF) membrane (Thermo Scientific), and incubated with a primary antibody at 4°C, overnight. The primary antibodies used in this study are lamin A/C (4C11, #4777, mouse monoclonal), lamin B1 (#9087, rabbit polyclonal), and lamin B2 (#9622, rabbit polyclonal) from Cell Signaling Technology; Nrf2 (sc-13032x), HMOX1 (sc-7695, sc-136960), NQO-1 (sc-32793), histone H1 (sc-8030), progerin (sc-81611) from Santa Cruz Biotechnology, Keap1 (Millipore, clone 144), p62/SQSTM1 (BD Transduction Laboratories), Phospho-Ser351 p62/SQSTM1 (Medical and Biological Laboratories). PVDF membranes were incubated with a horseradish peroxidase-conjugated secondary antibody at room temperature for 1–2 hr. Protein bands were visualized on X-ray blue films (Denville Scientific) using HyGlo (Denville Scientific) or ECL Prime (GE Healthcare) chemiluminescence detection reagents. Quantification of Western blot signals was performed by densitometric analysis with Image J software. Relative density of each signal band was measured by normalization to corresponding loading control signal band.
Immunofluorescence and microscopy
Cells plated on coverslips were treated with 10 μM sodium arsenite for 24 hr and fixed with 4 % formaldehyde for 15 min at room temperature. Nrf2 was detected with anti-Nrf2 antibody (sc-13032x) and Alexa Fluor 488 anti-Rabbit IgG (H+L) secondary antibody (Thermo Fisher scientific, A-11008). Nuclei were counterstained with Hoechst 33342. Images were captured using the Olympus BX41 fluorescence microscopy and DP80 camera with cellSens image capture software (Olympus).
ROS measurement
Cells were treated with 10 μM sodium arsenite for 24 hr, washed with PBS twice, and incubated in normal growth media containing 5 μM CM-H2DCFDA (Invitrogen) for 30 min at 37°C. Cells were washed with PBS twice, trypsinized, and suspended in Hank’s Balanced Salt solution (Sigma). To measure fluorescent DCF produced from oxidization of CM-H2DCFDA by intracellular ROS, cells were analyzed by BD Accuri C6 flow cytometry (BD Biosciences). Cells were kept in the dark on ice until they were used for the flow cytometry analysis.
A PCR array analysis focused on oxidative stress signaling pathway
Human primary normal (GM07492, passage 9–11) and HGPS (AG11498, passage 6–8) fibroblasts were untreated or treated with 10 μM sodium arsenite for 24 hr and total RNA was isolated with TRI Reagent RT (Molecular Research Center). RNA was reverse transcribed to cDNA according to the manual of the RT2 First Strand kit (Qiagen), and expression of 84 oxidative stress pathway genes in untreated and arsenite-treated normal and HGPS samples were analyzed according to the manual of the Human Oxidative Stress Plus Pathway RT2 Profiler PCR Array (Qiagen). PCR Array was performed with the CFX96 (Bio-Rad) at 95°C for 10 min once and 40 cycles of 95°C for 15 sec and 60°C for 1 min. The results were analyzed using the RT2 Profiler PCR Array Data Analysis 3.5 software provided by the Qiagen online web site. To normalize gene expression (2−ΔCt) and determine the fold change between groups (2−ΔΔCt), a housekeeping gene (β-actin) was used as an internal control.
Real-time quantitative PCR (RT-qPCR) and primers
Significant gene expression changes detected in the oxidative stress pathway PCR-array between untreated and arsenite-treated normal (and HGPS) fibroblasts were verified individually by SYBR Green RT-qPCR. cDNAs were synthesized with iScript cDNA Synthesis Kit (Rio-Rad), and mixed with iTaq Universal SYBR Green Supermix (Bio-Rad) and specific primers for human β-actin (HHK-1, Real Time Primers, LLC, PA), or the other primers as listed below (Sigma). RT-qPCR was performed with the CFX96 qPCR System (Bio-Rad) and data was normalized with β-actin as an internal control gene. The following human primer sequences were used (5′ to 3′):
GCLC forward primer: GGCACAAGGACGTTCTCAAGT
GCLC reverse primer: CAGACAGGACCAACCGGAC
GCLM forward primer: TGTCTTGGAATGCACTGTATCTC
GCLM reverse primer: CCCAGTAAGGCTGTAAATGCTC
HMOX1 forward primer: CCACCAACAAAGTGCAAGATTC
HMOX1 reverse primer: TCACATGGCATAAAGCCCTACAG
TXNRD1 forward primer: ATATGGCAAGAAGGTGATGGTCC
TXNRD1 reverse primer: GGGCTTGTCCTAACAAAGCTG
NQO1 forward primer: GAAGAGCACTGATCGTACTGGC
NQO1 reverse primer: GGATACTGAAAGTTCGCAGGG
PRDX1 forward primer: CCACGGAGATCATTGCTTTCA
PRDX1 reverse primer: AGGTGTATTGACCCATGCTAGAT
SOD1 forward primer: GGTGGGCCAAAGGATGAAGAG
SOD1 reverse primer: CCACAAGCCAAACGACTTCC
SQSTM1 forward primer: GCACCCCAATGTGATCTGC
SQSTM1 reverse primer: CGCTACACAAGTCGTAGTCTGG
TXN forward primer: GTGAAGCAGATCGAGAGCAAG
TXN reverse primer: CGTGGCTGAGAAGTCAACTACTA
Statistical analysis
Data were analyzed statistically by one-way factorial analysis of variance (ANOVA), followed by the post hoc Tukey test.
RESULTS
The effects of arsenic and cadmium on expression and cleavage of lamins
It was reported previously that expression of lamin B1 was induced by oxidative stress, causing nuclear shape alterations and senescence [25] or cytoprotection against oxidative cell damage [26,27]. Conversely, expression of lamin B1 was downregulated by progerin expression in HGPS cells [28,29]. To investigate expression of all nuclear lamins under oxidative stress conditions that can elicit the cellular Nrf2 antioxidant response, first of all, we treated SW480 human colon carcinoma cells with varying concentrations (1–100 μM) of sodium arsenite and cadmium chloride and determined that the nuclear accumulation of Nrf2 was observed at 10–100 μM of sodium arsenite and 10–50 μM cadmium chloride (Supplementary Fig. 1A). Then SW480 cells were treated with 50 μM sodium arsenite or cadmium chloride for up to 8 hr prior to the appearance of cytotoxicity and analyzed expression of lamins A/C, B1, and B2 by Western blotting (Fig. 1A). As expected, Nrf2 protein was accumulated in the nucleus by 5 hr of treatment with arsenite, and to a lesser extent with cadmium (Fig. 1B). We observed that nuclear lamins B1 and C were increased by arsenite treatment for 2.5, 5, and 8 hr, while lamin B2 was increased only transiently at 2.5 hr after arsenite treatment (Fig. 1A). In contrast, cadmium chloride induced cleavages of lamin B1 to 19 kDa and lamin B2 to 40 kDa in a time-dependent manner (Fig. 1A). As the lamin B1 and B2 antibodies used in this study have the epitope containing Lys415 and Leu75, respectively (Fig. 1B), it is unlikely that cadmium-induced 19 kDa lamin B1 and 40 kDa B2 are as the result of the cleavage at a conserved aspartic acid at the positions 231 (B1) and 225 (B2) (Fig. 1C) as previously reported [30–32]. Likewise, if lamin A (the mature lamin A 646 amino acids, 72 kDa) is cleaved at the conserved aspartic acid at the position 230 (Fig. 1B) as previously reported [30,33,34], it should give rise to two fragments of N-terminal 230 and C-terminal 416 amino acids. However, cadmium chloride treatment gave rise to only a slightly smaller lamin A band in Western blotting between the lamin A and lamin C (Fig. 1A). This slightly shorter form of lamin A was induced only by treatment with cadmium but not some other oxidative and DNA damaging agents such as cisplatin (10 and 25 μM), etoposide (25 μM), hydrogen peroxide (100 μM), or sodium arsenite (50 μM) (Fig. 1C). Progerin is a C-terminal 50-amino acid truncated form of pre-lamin A (664 amino acids) due to C to T mutation at codon 608 (Gly608Gly), which does not affect the Glycine codon 608 but create a new 150-nucleotide splicing site [22]. Thus, the migration of progerin (614 amino acids) on SDS-PAGE is also observed between the mature lamin A (646 amino acids) and lamin C (572 amino acids). To determine whether the cadmium-induced slightly smaller lamin A is cross-reactive with a progerin antibody, nuclear extracts from cadmium-treated SW480 were analyzed by Western blotting with lamin A and progerin antibodies (Fig. 1D). Cadmium treatment gave rise to the shorter lamin A band between lamin A and lamin C (Fig. 1D, top) but it was not detected with anti-progerin antibody that recognizes the spliced junction of progerin (Fig. 1D, bottom). As the progerin antibody clearly detected progerin expressed in human HGPS fibroblasts (Fig. 1D, bottom), cadmium-induced smaller form of lamin A may be the product of a new proteolytic cleavage of lamin A at the C-terminal region between 572 and 646 amino acids. Of note, these new cleavages of lamins were observed only in SW480 cells treated with cadmium chloride in our experimental conditions (see below and discussion).
Figure 1. New cleavage patterns of lamins A, B1, and B2 after exposure to cadmium chloride.
A) 30 μg of nuclear extracts from SW480 cells treated with 50 μM sodium arsenite (As) or 50 μM cadmium chloride (Cd) for 2.5 hr, 5 hr, or 8 hr were loaded on 10% SDS-PAGE and expression of Nrf2 and lamins were analyzed by Western blotting. Cleaved lamins A, B1, and B2 are indicated by arrows. Expression of Histone H1 is shown as a loading control of each sample. B) N-terminal or C-terminal epitopes of lamin antibodies used in Fig. 1A are indicated with thick arrows. The positions of conserved Aspartic acid in human lamins known to be cleaved by Caspase 6 are shown. The total amino acid numbers of human mature lamin A, C, B1, and B2 are also shown at their C-terminus. C) 30 μg of nuclear extracts from SW480 cells treated with 50 μM sodium arsenite, 50 μM cadmium chloride, 10 μM and 25 μM cisplatin, 25 μM etoposide, and 100 μM H2O2 for 5 hr or 8 hr were loaded on 10% SDS-PAGE and expression of Nrf2 and lamin A/C were analyzed by Western blotting. The arrow indicates the cleaved lamin A. D) 10 μg of the cytoplasmic and nuclear fractions of human HGPS fibroblasts (AG11498) treated with 25 μM tBHQ or 10 μM sodium arsenite (As) for 22 hr, 25 μg of cadmium chloride-treated SW480 nuclear fractions (used in Fig. 1C), and 10 μg of the nuclear fraction of another HGPS fibroblasts (AG06917) were loaded on 10% SDS-PAGE and the cleaved lamin A (arrow) and progerin were detected by Western blotting.
Knockdown of lamins A and B had marginal effects on Nrf2 nuclear accumulation and HMOX1 induction following arsenic and cadmium exposure
To investigate the possible involvement of lamins A/C and B in the Nrf2-ARE antioxidant system, siRNAs for lamin A/C or lamin B1 and B2 were transfected into HaCaT cells, and they were treated with sodium arsenite or cadmium chloride for 3–4 hr. The whole cell lysates isolated from lamin A/C knockdown cells (Fig. 2A) or cytoplasm/nuclear fractions from lamins B1 plus B2 knockdown cells (Fig. 2B) were analyzed by Western blotting for expression of Nrf2 and Nrf2-regulated heme oxygenase-1 (HMOX1). Sodium arsenite is a potent activator of Nrf2 [35] and an inducer of HMOX1 through Nrf2 activation and histone modifications in HaCaT cells [36] and human primary fibroblasts [37]. Under the lamin A/C knockdown conditions with three different siRNAs, cells still significantly induced Nrf2, like control siRNA transfected cells, after treatment with sodium arsenite (Fig. 2A). Consistently, Nrf2-regulated HMOX1 expression was induced in both control and lamin A/C deficient cells (Fig. 2A). NQO1 is another well-characterized Nrf2-regulated gene in various cell types [11] but we observed no induction by arsenite but similar NQO1 levels between siControl and lamin A/C knockdown HaCaT cells (Fig. 2A). Similar results were obtained in lamins B1 and B2 deficient HaCaT cells, in which Nrf2 was equivalently accumulated in the nucleus after arsenite or cadmium treatment in both control and lamins B1, B2 deficient cells (Fig. 2B). Likewise, induced expression and subcellular localization of HMOX1 in the cytoplasm and nucleus were normal in lamins B1 and B2 deficient HaCaT cells (Fig. 2B).
Figure 2. The knockdown effects of lamin A/C or lamin B on nuclear accumulation of Nrf2 and HMOX1 expression induced by arsenite or cadmium exposure.
A) HaCaT cells were transfected with non-targeting control siRNA or 3 different lamin A/C siRNAs (#9, 10, and 13: Qiagen), treated with 50 μM sodium arsenite for 3 hr, and 15 μg of whole cell extracts were loaded on 10% SDS-PAGE to measure expression levels of Nrf2, HMOX1, and NQO1 by Western blotting. Lamin A/C Western blot for the assessment of knockdown is also shown. B) HaCaT cells were transfected with non-targeting control siRNA or a mixture of lamin B1 and B2 siRNAs, treated with 50 μM sodium arsenite or 50 μM cadmium chloride for 4 hr, and 10 μg of cytoplasmic and nuclear extracts were loaded on 10% SDS-PAGE to measure expression levels of Nrf2 and HMOX1 by Western blotting. Western blots for lamins are shown for the assessment of lamin B1 and B2 knockdown. Quantification of Nrf2 and HMOX1 bands was analyzed with Image J software. Relative density after normalization to corresponding β-actin band is shown in parentheses. SiControl/arsenite(As) signal band was defined as 1.0. Representative Western blots from four experiments are shown.
Next we examined whether the deficiency of both lamins A/C and B may impair the activation of Nrf2. Similar experiments were performed except for transfection of all lamins A/C, B1 and B2 siRNAs together into HaCaT and SW480 cells as well. The results in Fig. 3A show that Nrf2 activation and Nrf2-regulated HMOX1 expression were normal in HaCaT cells even in lamins A/C plus B1/B2 knockdown cells in response to arsenite exposure. Similar results were observed in two other human cell lines SW480 (Fig. 3B) and K562 cells (not shown) after sodium arsenite or cadmium chloride treatment. Taken together, these results suggest that the core nuclear lamina components lamin A/C and lamin B1, B2 are dispensable for Nrf2 activation following arsenite and cadmium exposure.
Figure 3. The knockdown effects of all lamins A/C and B simultaneously on nuclear accumulation of Nrf2 and HMOX1 expression induced by arsenite or cadmium exposure.
A) HaCaT or B) SW480 cells were transfected with non-targeting control siRNA or a mixture of lamin A/C, B1 and B2 siRNAs, and treated with 50 μM sodium arsenite or 50 μM cadmium chloride for 4 hr (A) or 4hr and 8 hr (B). 15 μg of cytoplasmic and nuclear extracts in A) or only nuclear extracts in B) were loaded on 10% SDS-PAGE to measure expression levels of Nrf2 and HMOX1 by Western blotting. Western blots for lamins are shown for the assessment of each lamin knockdown. Quantification of Nrf2 and HMOX1 bands was analyzed with Image J software. Relative density after normalization to corresponding β-actin band is shown in parentheses. SiControl/arsenite(As) signal band was defined as 1.0. Representative Western blots from four experiments are shown.
Progerin-expressing primary HGPS fibroblasts retain the Nrf2 antioxidant mechanism
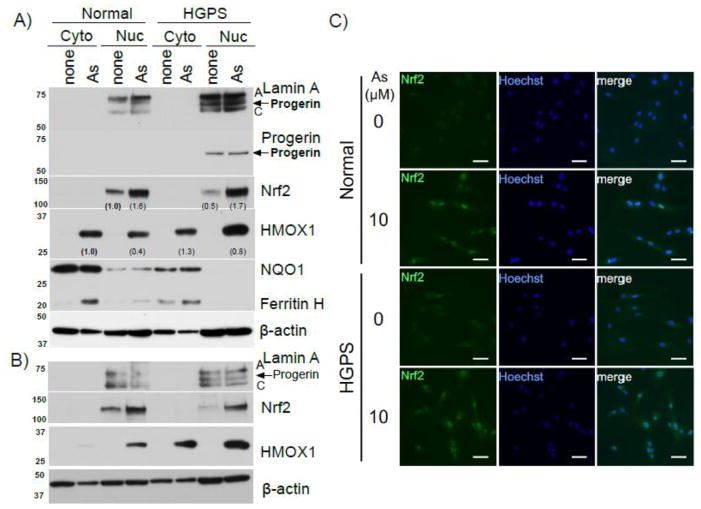
Progerin is a human lamin A dominant mutant responsible for the pathogenesis of HGPS [22]. Although the cellular mechanism of progerin-induced premature aging is obscure, increased oxidative stress in HGPS cells is understood as a plausible cause [24,29]. We hypothesized that progerin may interfere with the activation of Nrf2 and its downstream antioxidant gene expression through which oxidative stress may be elevated in HGPS cells. To test this hypothesis, Nrf2 nuclear accumulation and expression of Nrf2-regulated genes were investigated in normal and HGPS primary human fibroblasts after exposure to sodium arsenite. Progerin expression in HGPS fibroblasts was detected by Western blotting with anti-lamin A antibody, giving rise to a progerin band between lamin A and lamin C in the nuclear fraction of only HGPS samples (Fig. 4A). This was verified by Western blotting with an anti-progerin antibody (Fig. 4A). Contrary to our expectation, arsenite treatment induced nuclear accumulation of Nrf2 in both normal and HGPS fibroblasts (Fig. 4A). Furthermore, HMOX1 in the cytoplasm and nucleus after treatment with sodium arsenite was almost equivalent between normal and HGPS fibroblasts. Similar results were obtained when another normal human primary fibroblasts were compared with HGPS fibroblasts (Fig. 4B). We noted that the basal expression level of NQO1 was higher in normal fibroblasts compared with HGPS fibroblasts (Fig. 4A). Ferritin H, the major intracellular iron storage protein and regulated by Nrf2 through the ARE [38,39], was induced by arsenite in normal fibroblasts and to a lesser extent in HGPS fibroblasts (Fig. 4A). The nuclear accumulation of Nrf2 in both normal and HGPS fibroblasts in response to arsenite treatment was confirmed by immunofluorescent staining of Nrf2 (Fig. 4C).
Figure 4. Nrf2 antioxidant response to sodium arsenite in human normal and HGPS fibroblasts.
A, B) Human normal (GM08398 in A and GM07492 in B) and HGPS (AG11498) fibroblasts were treated with 10 μM sodium arsenite for 24 hr, and cytoplasmic and nuclear fractions were loaded on 10% SDS-PAGE to measure expression levels of Nrf2, progerin, lamin A/C, HMOX1, NQO1, ferritin H, and β-actin. In A), quantification of Nrf2 and HMOX1 bands was analyzed with Image J software. Relative density after normalization to corresponding β-actin band is shown in parentheses. Signal bands of untreated nuclear extract (for Nrf2) and sodium arsenite (As)-treated cytoplasmic extract (for HMOX1) in normal fibroblasts was defined as 1.0. Representative Western blots from five experiments are shown.
C) Human Normal (GM07492) and HGPS (AG11498) fibroblasts were treated with 10 μM sodium arsenite for 24 hr, and fixed. Cells were stained with anti-Nrf2 antibody, and nuclei were counterstained with Hoechst33342. Scale bar, 50 μm.
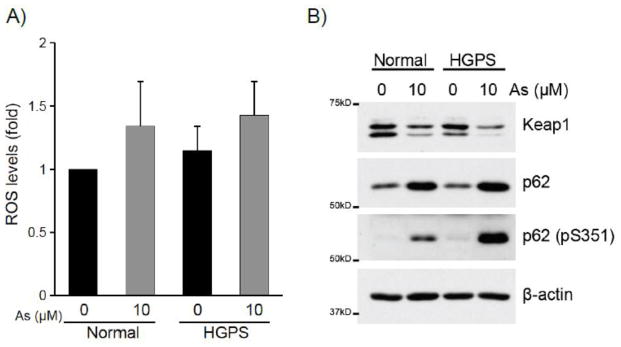
Arsenic is known to cause cellular toxicity in part through production of ROS, which may contribute to oxidation and inactivation of Keap1 leading to translocation and accumulation of Nrf2 in the nucleus. To elucidate the mechanism through which both normal and HGPS fibroblasts activate the Nrf2 antioxidant response to sodium arsenite, we measured intracellular ROS levels. We observed a similar trend of increased but not statistically significant ROS production in both normal and HGPS cells under arsenite stress (Fig. 5A). Recently it was shown that Keap1 is subject to autophagic degradation through interaction with an autophagic adaptor protein p62/SQSTM1 [14,15] upon phosphorylation at Ser351 [16,40], which in turn facilitates Nrf2 nuclear accumulation and activates cellular antioxidant response to oxidants and xenobiotics including arsenic [41]. We observed that sodium arsenite treatment increased the expression of p62/SQSTM1, significant induction of p62/SQSTM phosphorylation at Ser351, and concomitant downregulation of Keap1 in both normal and HGPS fibroblasts (Fig. 5B). Thus, the p62/SQSTM1-dependent activation mechanism of Nrf2 is fully functional in progerin-expressing HGPS cells in response to sodium arsenite.
Figure 5. p62/SQSTM1 phosphorylation at Ser351 and Keap1 downregulation in sodium arsenite-treated human normal and HGPS fibroblasts.
A) Human normal (GM07492) and HGPS (AG11498) fibroblasts were treated with 10 μM sodium arsenite for 24 hr. Intracellular ROS were detected after incubation with CM-H2DCFDA and flow cytometry. The results are mean + sd. of three independent experiments. The ROS level in untreated normal fibroblasts was defined as 1.0. B) Human normal (GM07492) and HGPS (AG11498) fibroblasts were treated with 10 μM sodium arsenite for 24 hr. 5 μg of cytoplasmic extracts were loaded on 10% SDS-PAGE and expression of Keap1, p62/SQSTM1, phosphorylated p62/SQSTM1 at Ser351 were analyzed by Western blotting. β-actin is shown as a loading control of each sample.
Since the Nrf2-ARE antioxidant system regulates a battery of antioxidant detoxification genes at the transcriptional level, we next employed a pathway-focused gene q-PCR array for quantitative analysis of the mRNA expression profiles of oxidative stress-related genes in human primary normal and HGPS fibroblasts after exposure to sodium arsenite. This q-PCR array contains 84 genes responsive to oxidative stress, including major Nrf2-regulated genes such as HMOX1, NQO1, and also SQSTM1. When basal mRNA expression levels (without arsenite treatment) were compared between normal and HGPS fibroblasts, 40 and 4 genes were downregulated and upregulated, respectively, by more than 2-fold in HGPS fibroblasts (Supplemental Table I). When compared between with and without sodium arsenite treatment, 22 and 28 genes were upregulated in normal primary fibroblasts and HGPS fibroblasts, respectively (Table I). Among those 22 and 28 upregulated genes, 20 genes were commonly upregulated in both normal and HGPS fibroblasts after sodium arsenite treatment (Fig. 6 and Table I). 8 of highly induced Nrf2-regulated genes (HMOX1, SQSTM1, GCLM, GCLC, TXNRD1, TXN1, PRDX1, and NQO1) were further verified by RT-q-PCR. The results in Fig. 6 suggest that all these genes including HMOX1 were significantly and similarly upregulated by sodium arsenite treatment in both normal and HGPS fibroblasts. However, the basal mRNA levels of HMOX1, SQSTM1, NQO1, and GCLM had the tendency of lower expression in HGPS fibroblasts compared to normal fibroblasts (Fig. 6 and Supplemental Table I).
Table I.
Human normal (GM07492) and HGPS (AG11498) fibroblasts were treated with 10 μM sodium arsenite for 24 hr, and total RNA was used for human oxidative stress Finder RT2 profiler PCR array. More than 2-fold upregulated genes by sodium arsenite treatment are listed. The genes highlighted by bold letters are commonly induced in both normal and HGPS fibroblasts.
| > 2-fold upregulated genes by Arsenic | |||
|---|---|---|---|
| GM07492 | GM07492 | ||
| Gene symbol | Fold regulation | Gene symbol | Fold regulation |
| HMOX1 | 96.31 | HMOX1 | 340.49 |
| SQSTM1 | 7.49 | GCLM | 14.45 |
| UCP2 | 5.7 | SQSTM1 | 14.03 |
| NCF2 | 5.42 | NCF2 | 13.21 |
| GCLM | 4.51 | SRXN1 | 9.54 |
| SRXN1 | 4.42 | FTH1 | 9.09 |
| TXN | 4.15 | PREX1 | 8.88 |
| FTH1 | 3.86 | GSR | 7.76 |
| GSR | 3.66 | CCL5 | 6.04 |
| BNIP3 | 3.41 | HSPA1A | 5.12 |
| TXNRD1 | 3.4 | TXN | 5.09 |
| CYGB | 2.71 | PRNP | 4.64 |
| NQO1 | 2.66 | NQO1 | 4.24 |
| APOE | 2.51 | BNIP3 | 3.87 |
| SOD2 | 2.5 | PRDX1 | 3.72 |
| ALOX12 | 2.37 | GCLC | 3.53 |
| GCLC | 2.36 | SOD2 | 3.48 |
| SOD1 | 2.35 | SFTPD | 3.41 |
| GPX3 | 2.31 | SOD1 | 3.32 |
| HSPA1A | 2.28 | TTN | 3.07 |
| PRDX1 | 2.23 | ALOX12 | 2.62 |
| EPHX2 | 2.09 | ALB | 2.43 |
| TXNRD1 | 2.35 | ||
| NOX5 | 2.22 | ||
| UCP2 | 2.2 | ||
| MGST3 | 2.18 | ||
| MSRA | 2.06 | ||
| GTF2I | 2.04 | ||
Figure 6. Expression prolife of oxidative stress pathway genes in sodium arsenite-treated human normal and HGPS fibroblasts.
Human normal (GM07492) and HGPS (AG11498) fibroblasts were treated with 10 μM sodium arsenite for 24 hr, and total RNA was used for human oxidative stress Finder RT2 profiler PCR array. A white or gray circle indicates 2-fold induced or repressed genes in normal or HGPS fibroblasts by sodium arsenite treatment, respectively. The overlap (20 genes) indicates commonly regulated genes in both normal and HGPS fibroblasts. The RNA samples are further analyzed by RT-qPCR for mRNA levels of HMOX1, SQSTM1, GCLM, TXNRD1, TXN, GCLC, PRDX1, and NQO1. Data are mean + s.d. (*p<0.05, #p<0.01 by one-way ANOVA followed by post hoc Tukey test) of three independent experiments, normalized by mRNA expression level of β-actin relative to untreated normal fibroblasts.
DISCUSSION
The aim of this study was to characterize the roles of normal nuclear lamins and a mutant form of lamin A, progerin, in the Nrf2-ARE antioxidant pathway in response to Nrf2-activating xenobiotics, arsenite and cadmium. To characterize the role of normal lamins in this pathway, we knocked down lamin A/C, or lamins B1 and B2, or all together in human culture cell lines and asked whether the Nrf2-ARE system is affected under lamin-deficient conditions in response to arsenite or cadmium exposure. Given that appropriate expression levels of these lamins are important to maintain normal tissue function and cellular homeostasis both in vivo and in vitro cellular levels [20,26,27], this knockdown approach is straightforward to address our first question. In spite of the fact that lamins are critical components of nuclear lamina in the inner nuclear membrane to regulate chromatin architecture and nuclear integrity [19], the deficiency in lamins A/C or lamins B1 and B2, or all these lamins did not affect increased Nrf2 protein stabilization and subsequent translocation to the nucleus in response to arsenite or cadmium exposure (Fig. 1). Unexpectedly, HMOX1 protein induced by arsenite or cadmium was partly translocated to the nucleus equivalently in both control and lamin knockdown cells (Fig. 1). Unlikely but one possibility not excluded in this study is that our lamin knockdown with siRNAs did not sufficiently silence expression of lamin proteins enough to impact the Nrf2-ARE activation system. In particular, lamin A/C proteins were still detectable in our Western blotting after the knockdown (Fig. 2A). Although this possibility still cannot be ruled out, the facts that lamin A/C haploinsufficiency in mice and humans caused cardiomyopathy and muscular dystrophy [42,43] and that the lamin A deficiency in human dermal fibroblasts promoted cellular senescence [44], indicate that appropriate expression levels of lamin A/C are critical in their normal function to maintain the integrity of the nuclear lamina. Taken all together, these results suggest that not only Nrf2 but cytoplasmic-nuclear shuttling mechanisms of some other proteins (e.g. HMOX1) appear to be maintained in lamin-deficient cells.
Proteolytic cleavages of lamins were observed during apoptosis induced by low serum conditions [31], p53 [30,32], anti-Fas and staurosporine [34], giving rise to C-terminal fragments of 46–47 kDa lamin A [30,31], 37 kDa lamin C [30], and a little smaller than 50 kDa lamin B1 [32]. Caspase 6 was identified as the protease responsible for all these cleavages at a conserved V-E-I/V-D sequence (e.g. D(Asp)230 of lamin A, Fig. 1B)[30,33,34]. We did not detect these cleaved lamins probably because cells were not apoptotic in our experimental conditions. However, treatment of SW480 cells with cadmium chloride (50 μM) gave rise to new forms of cleaved lamins A, B1, and B2 (Fig. 1A). Of note, pre-lamin A (664 amino acids, 74 kDa) is subject to immediate processing by proteolytic cleavage at Tyr646 with a zinc metalloprotease ZMPSTE24 [45], therefore the lamin A in Fig. 1A should be the mature form of lamin A (646 amino acids). The slightly smaller lamin A therefore looked like progerin induced by cadmium chloride treatment (Fig. 1A, C) but it was not progerin translated from an accidentally spliced lamin A transcript (Fig. 1D). This cleavage was relatively specific to treatment with cadmium chloride and in SW480 cells throughout our experiments in this study. We did not observe these cleaved lamins in HaCaT cells or human primary fibroblasts even at 100 μM cadmium chloride. Cadmium chloride appears to induce or activate a protease in SW480 cells that cleaves lamin A between 572 and 646 amino acids, and the lamin B1 and B2 conserved sequence at the C-terminal ~ 400 amino acid position containing the amino acid sequences of LLDVKLALDME(S/N)AYRKLLEGEEERLKLSPSPSSRVTVSRA.
It has recently been reported by Kubben et al., that Nrf2 activity is impaired by progerin in human HGPS cells, in which Nrf2 preferentially interacts with progerin by competing with the mature wild type lamin A resulting in Nrf2 subnuclear mislocalization [29]. In their study, HGPS fibroblasts or tet-induced expression of GFP-progerin in normal human skin fibroblasts showed more than 50% reduction in ARE-luciferase activity compared to normal fibroblasts [29]. Consistently, they found that more than 200 Nrf2-ARE regulated genes were repressed in human HGPS fibroblasts compared to normal fibroblasts [29]. These experiments demonstrated the repressive effect of progerin on the basal activity of Nrf2. This may be consistent with our results of higher basal expression of some of Nrf2-regulated genes in untreated normal human fibroblasts compared to HGPS fibroblasts (Supplemental Table and Fig. 6). However, it should be noted that the HGPS fibroblasts AG11498 in our study became senescent much earlier than the normal fibroblasts GM08398 or GM07492, which may contribute to the lower basal expression levels of Nrf2-regulated genes. We also obtained two other HGPS fibroblasts (AG06917 and AG01972, Coriell Institute) and confirmed normal Nrf2 nuclear accumulation and HMOX1 induction following arsenite treatment but they became senescent after the pilot experiment. Kubben et al., also tested the effect of sulforaphane, an Nrf2-activating phytochemical, on Nrf2 nuclear accumulation in tet-inducible GFP-progerin fibroblasts and observed that tet-induced expression of progerin did not affect the stability of Nrf2 nor nuclear-cytoplasmic shuttling [29]. This is in agreement with our results of this study that the nuclear accumulation of Nrf2 following arsenic or cadmium treatment is fully functional in HGPS fibroblasts (Fig. 4). Furthermore, we observed that progerin-expressing HGPS fibroblasts still retain the p62/SQSTM-dependent mechanism (Fig. 5) and the ability of full response to arsenite exposure leading to induction of many Nrf2-regulated antioxidant detoxification genes (Fig. 6). Given the fact that the phenotypes of Nrf2 knockout mice are relatively normal [46] unless challenged with xenobiotics and oxidative stress [47–49], repression of the basal Nrf2-ARE antioxidant system by progerin in HGPS cells may be only a part of mechanisms behind the pathogenesis of HGPS.
CONCLUSION
Knocking down lamins in three human cell lines or expression of progerin in HGPS primary human fibroblasts did not impair arsenite-induced activation of Nrf2 and downstream antioxidant gene expression. Thus, nuclear lamins and progerin play marginal roles in the antioxidant Nrf2 response.
Supplementary Material
Highlights.
Progerin, a truncated lamin A mutant, causes Hutchinson-Gilford Progeria Syndrome (HGPS), a premature aging syndrome.
Oxidative stress may be involved in the pathogenesis of HGPS.
This study tested whether lamins are important for the Nrf2 antioxidant system, and whether progerin compromises it.
Knockdown of lamins or expression of progerin in HGPS did not impair arsenic-induced Nrf2 activation and downstream antioxidant gene expression.
Nuclear lamins and progerin are dispensable for antioxidant Nrf2 response to arsenic and cadmium.
Acknowledgments
This work was supported by the NIH grant R01GM088392 from the National Institute of General Medical Sciences to Y. Tsuji and in part by P30ES025128 from the National Institute of Environmental Health Sciences to Center for Human Health and the Environment (CHHE). We are grateful to Dr. Kohta Ikegami for his helpful advice and discussion.
Abbreviations
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GCLC
Glutamate cysteine ligase catalytic subunit
- GCLM
Glutamate cysteine ligase modifier subunit
- HGPS
Hutchinson-Gilford Progeria Syndrome
- HMOX1
Heme oxygenase-1
- Keap1
Kelch-like ECH-associated protein 1
- NQO1
NAD(P)H quinone oxidoreductase 1
- Nrf2
Nuclear factor-E2-related factor 2
- PRDX1
Peroxiredoxin 1
- ROS
Reactive oxygen species
- SQSTM1
Sequestosome 1
- TXN
Thioredoxin
- TXNRD1
Thioredoxin reductase 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hei TK, Liu SX, Waldren C. Mutagenicity of arsenic in mammalian cells: role of reactive oxygen species. Proc Natl Acad Sci, USA. 1998;95:8103–8107. doi: 10.1073/pnas.95.14.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, Nawrot T, Vangronsveld J, Smeets K. Cadmium stress: an oxidative challenge. Biometals. 2010;23:927–940. doi: 10.1007/s10534-010-9329-x. [DOI] [PubMed] [Google Scholar]
- 4.Leonard SS, Harris GK, Shi X. Metal-induced oxidative stress and signal transduction. Free Radic Biol Med. 2004;37:1921–1942. doi: 10.1016/j.freeradbiomed.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Tapio S, Grosche B. Arsenic in the aetiology of cancer. Mutat Res. 2006;612:215–246. doi: 10.1016/j.mrrev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Douillet C, Currier J, Saunders J, Bodnar WM, Matousek T, Styblo M. Methylated trivalent arsenicals are potent inhibitors of glucose stimulated insulin secretion by murine pancreatic islets. Toxicol Appl Pharmacol. 2013;267:11–15. doi: 10.1016/j.taap.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nawrot TS, Staessen JA, Roels HA, Munters E, Cuypers A, Richart T, Ruttens A, Smeets K, Clijsters H, Vangronsveld J. Cadmium exposure in the population: from health risks to strategies of prevention. Biometals. 2010;23:769–782. doi: 10.1007/s10534-010-9343-z. [DOI] [PubMed] [Google Scholar]
- 8.Avery SV. Molecular targets of oxidative stress. Biochem J. 2011;434:201–210. doi: 10.1042/BJ20101695. [DOI] [PubMed] [Google Scholar]
- 9.Ghezzi P, Jaquet V, Marcucci F, Schmidt HH. The oxidative stress theory of disease: levels of evidence and epistemological aspects. Br J Pharmacol. 2016 doi: 10.1111/bph.13544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi A, Ohta T, Yamamoto M. Unique function of the Nrf2-Keap1 pathway in the inducible expression of antioxidant and detoxifying enzymes. Methods Enzymol. 2004;378:273–286. doi: 10.1016/S0076-6879(04)78021-0. [DOI] [PubMed] [Google Scholar]
- 12.Mitsuishi Y, Motohashi H, Yamamoto M. The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Front Oncol. 2012;2:200. doi: 10.3389/fonc.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 15.Lau A, Wang XJ, Zhao F, Villeneuve NF, Wu T, Jiang T, Sun Z, White E, Zhang DD. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, Hoshii T, Hirao A, Takagi K, Mizushima T, Motohashi H, Lee MS, Yoshimori T, Tanaka K, Yamamoto M, Komatsu M. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Guo Y, Yu S, Zhang C, Kong AN. Epigenetic regulation of Keap1-Nrf2 signaling. Free Radic Biol Med. 2015;88:337–349. doi: 10.1016/j.freeradbiomed.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raices M, D’Angelo MA. Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions. Nat Rev Mol Cell Biol. 2012;13:687–699. doi: 10.1038/nrm3461. [DOI] [PubMed] [Google Scholar]
- 19.Ho CY, Lammerding J. Lamins at a glance. J Cell Sci. 2012;125:2087–2093. doi: 10.1242/jcs.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke B, Stewart CL. The nuclear lamins: flexibility in function. Nat Rev Mol Cell Biol. 2013;14:13–24. doi: 10.1038/nrm3488. [DOI] [PubMed] [Google Scholar]
- 21.Capell BC, Collins FS. Human laminopathies: nuclei gone genetically awry. Nat Rev Genet. 2006;7:940–952. doi: 10.1038/nrg1906. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raharjo WH, Enarson P, Sullivan T, Stewart CL, Burke B. Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery-Dreifuss muscular dystrophy. J Cell Sci. 2001;114:4447–4457. doi: 10.1242/jcs.114.24.4447. [DOI] [PubMed] [Google Scholar]
- 24.Richards SA, Muter J, Ritchie P, Lattanzi G, Hutchison CJ. The accumulation of un-repairable DNA damage in laminopathy progeria fibroblasts is caused by ROS generation and is prevented by treatment with N-acetyl cysteine. Hum Mol Genet. 2011;20:3997–4004. doi: 10.1093/hmg/ddr327. [DOI] [PubMed] [Google Scholar]
- 25.Barascu A, Le Chalony C, Pennarun G, Genet D, Imam N, Lopez B, Bertrand P. Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation. EMBO J. 2012;31:1080–1094. doi: 10.1038/emboj.2011.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malhas AN, Lee CF, Vaux DJ. Lamin B1 controls oxidative stress responses via Oct-1. J Cell Biol. 2009;184:45–55. doi: 10.1083/jcb.200804155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimi T, Butin-Israeli V, Adam SA, Hamanaka RB, Goldman AE, Lucas CA, Shumaker DK, Kosak ST, Chandel NS, Goldman RD. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 2011;25:2579–2593. doi: 10.1101/gad.179515.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scaffidi P, Misteli T. Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome. Nat Med. 2005;11:440–445. doi: 10.1038/nm1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubben N, Zhang W, Wang L, Voss TC, Yang J, Qu J, Liu GH, Misteli T. Repression of the Antioxidant NRF2 Pathway in Premature Aging. Cell. 2016;165:1361–1374. doi: 10.1016/j.cell.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao L, Perez D, White E. Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol. 1996;135:1441–1455. doi: 10.1083/jcb.135.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oberhammer FA, Hochegger K, Froschl G, Tiefenbacher R, Pavelka M. Chromatin condensation during apoptosis is accompanied by degradation of lamin A+B, without enhanced activation of cdc2 kinase. J Cell Biol. 1994;126:827–837. doi: 10.1083/jcb.126.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandler JM, Alnemri ES, Cohen GM, MacFarlane M. Activation of CPP32 and Mch3 alpha in wild-type p53-induced apoptosis. Biochem J. 1997;322(Pt 1):19–23. doi: 10.1042/bj3220019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi A, Alnemri ES, Lazebnik YA, Fernandes-Alnemri T, Litwack G, Moir RD, Goldman RD, Poirier GG, Kaufmann SH, Earnshaw WC. Cleavage of lamin A by Mch2 alpha but not CPP32: multiple interleukin 1 beta-converting enzyme-related proteases with distinct substrate recognition properties are active in apoptosis. Proc Natl Acad Sci U S A. 1996;93:8395–8400. doi: 10.1073/pnas.93.16.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orth K, Chinnaiyan AM, Garg M, Froelich CJ, Dixit VM. The CED-3/ICE-like protease Mch2 is activated during apoptosis and cleaves the death substrate lamin A. J Biol Chem. 1996;271:16443–16446. [PubMed] [Google Scholar]
- 35.Pi J, Qu W, Reece JM, Kumagai Y, Waalkes MP. Transcription factor Nrf2 activation by inorganic arsenic in cultured keratinocytes: involvement of hydrogen peroxide. Exp Cell Res. 2003;290:234–245. doi: 10.1016/s0014-4827(03)00341-0. [DOI] [PubMed] [Google Scholar]
- 36.Ray PD, Huang BW, Tsuji Y. Coordinated regulation of Nrf2 and histone H3 serine 10 phosphorylation in arsenite-activated transcription of the human heme oxygenase-1 gene. Biochim Biophys Acta. 2015;1849:1277–1288. doi: 10.1016/j.bbagrm.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang BW, Ray PD, Iwasaki K, Tsuji Y. Transcriptional regulation of the human ferritin gene by coordinated regulation of Nrf2 and protein arginine methyltransferases PRMT1 and PRMT4. FASEB J. 2013;27:3763–3774. doi: 10.1096/fj.12-226043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuji Y. JunD activates transcription of the human ferritin H gene through an antioxidant response element during oxidative stress. Oncogene. 2005;24:7567–7578. doi: 10.1038/sj.onc.1208901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwasaki K, Mackenzie EL, Hailemariam K, Sakamoto K, Tsuji Y. Hemin-mediated regulation of an antioxidant-responsive element of the human ferritin H gene and role of Ref-1 during erythroid differentiation of K562 cells. Mol Cell Biol. 2006;26:2845–2856. doi: 10.1128/MCB.26.7.2845-2856.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashimoto K, Simmons AN, Kajino-Sakamoto R, Tsuji Y, Ninomiya-Tsuji J. TAK1 Regulates the Nrf2 Antioxidant System Through Modulating p62/SQSTM1. Antioxid Redox Signal. 2016;25:953–964. doi: 10.1089/ars.2016.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau A, Zheng Y, Tao S, Wang H, Whitman SA, White E, Zhang DD. Arsenic inhibits autophagic flux, activating the Nrf2-Keap1 pathway in a p62-dependent manner. Mol Cell Biol. 2013;33:2436–2446. doi: 10.1128/MCB.01748-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf CM, Wang L, Alcalai R, Pizard A, Burgon PG, Ahmad F, Sherwood M, Branco DM, Wakimoto H, Fishman GI, See V, Stewart CL, Conner DA, Berul CI, Seidman CE, Seidman JG. Lamin A/C haploinsufficiency causes dilated cardiomyopathy and apoptosis-triggered cardiac conduction system disease. J Mol Cell Cardiol. 2008;44:293–303. doi: 10.1016/j.yjmcc.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea JA, Duboc D, Fardeau M, Toniolo D, Schwartz K. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet. 1999;21:285–288. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- 44.Pekovic V, Gibbs-Seymour I, Markiewicz E, Alzoghaibi F, Benham AM, Edwards R, Wenhert M, von Zglinicki T, Hutchison CJ. Conserved cysteine residues in the mammalian lamin A tail are essential for cellular responses to ROS generation. Aging Cell. 2011;10:1067–1079. doi: 10.1111/j.1474-9726.2011.00750.x. [DOI] [PubMed] [Google Scholar]
- 45.Pendas AM, Zhou Z, Cadinanos J, Freije JM, Wang J, Hultenby K, Astudillo A, Wernerson A, Rodriguez F, Tryggvason K, Lopez-Otin C. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat Genet. 2002;31:94–99. doi: 10.1038/ng871. [DOI] [PubMed] [Google Scholar]
- 46.Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci U S A. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci U S A. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O’Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- 49.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.