Abstract
Rationale
Macrophages reside in the healthy myocardium, participate in ischemic heart disease and modulate myocardial infarction (MI) healing. Their origin and roles in post-MI remodeling of non-ischemic remote myocardium, however, remain unclear.
Objective
This study investigated the number, origin, phenotype and function of remote cardiac macrophages residing in the non-ischemic myocardium in mice with chronic heart failure after coronary ligation.
Methods and Results
Eight weeks post-MI, fate mapping and flow cytometry revealed that a 2.9-fold increase in remote macrophages results from both increased local macrophage proliferation and monocyte recruitment. Heart failure produced by extensive MI, through activation of the sympathetic nervous system, expanded medullary and extramedullary hematopoiesis. Circulating Ly6Chigh monocytes rose from 64±5 to 108±9 /μl blood (p<0.05). Cardiac monocyte recruitment declined in Ccr2−/− mice, reducing macrophage numbers in the failing myocardium. Mechanical strain of primary murine and human macrophage cultures promoted cell cycle entry, suggesting that the increased wall tension in post-MI heart failure stimulates local macrophage proliferation. Strained cells activated the MAPK pathway, while specific inhibitors of this pathway reduced macrophage proliferation in strained cell cultures and in the failing myocardium (p<0.05). Steady-state cardiac macrophages, monocyte-derived and locally sourced macrophages isolated from failing myocardium expressed different genes in a pattern distinct from the M1/M2 macrophage polarization paradigm. In vivo silencing of endothelial cell adhesion molecules curbed post-MI monocyte recruitment to the remote myocardium and preserved ejection fraction (27.4±2.4 vs.19.1±2%, p<0.05).
Conclusions
Myocardial failure is influenced by an altered myeloid cell repertoire.
Keywords: macrophage, monocyte, heart failure
Subject codes: Inflammation, heart failure
Introduction
Heart failure commonly complicates ischemic heart disease. Incomplete reperfusion, large or multiple infarcts cause left ventricular remodeling, increased cardiac volumes, hypertrophy of remote myocardium and reduced ejection fraction. Loss of contractile units due to ischemic injury initiates chronic remodeling of the remote non-ischemic myocardium, promoting progressive pump failure. These patients have unacceptable mortality rates1–4, and ultimately can require left ventricular assist devices and heart transplantation to sustain life. The remodeling process of the remote myocardium involves myocyte hypertrophy, apoptosis and fibrosis, and leads to dilatation of the ventricle5, 6. In addition to the changes observed in myocytes, remodeling also alters non-myocyte parenchymal cells. Post-MI remodeling and heart failure associate with inflammation, documented by increased TNFα and circulating innate immune cells7–11.
Resident macrophages represent about 6–8% of the non-cardiomyocyte population in the healthy mouse myocardium12, 13, a number comparable to the frequency of resident macrophages in other tissues. Cardiac macrophage frequency is higher in newborn mice14 and increases orders of magnitude in ischemic myocardium15. The innate immune response after MI consists of an early inflammatory phase dominated by neutrophils and inflammatory monocytes. Around day 3, monocytes and macrophages with resolution phenotypes implement a transition to a reparative phase15. These two phases are essential for healing of the infarct in mice16, 17 and also occur in humans18. The inflammatory response receives reinforcement beyond resident cardiac macrophages; cells recruited to the infarct derive from the bone marrow and spleen, and increased sympathetic signaling to the bone marrow enhances hematopoiesis in the days after acute ischemia19, 20. The activation of monocyte production in the bone marrow and spleen begins with increased cell cycle entry and migration of Ccr2+ hematopoietic progenitor cells21, expanding the systemic pool of innate immune cells.
Macrophages have salutary functions in immune defense, wound healing and tissue homeostasis. For instance, macrophages may regulate angiogenesis, a process involved in adaptation to myocyte hypertrophy22, 23. Yet, macrophages also promote tissue destruction, for instance in atherosclerotic plaques24, 25 and, if inflammation resolution is delayed, in the acute infarct26. The roles of cardiac resident macrophages during chronic post-MI remodeling are incompletely understood. Preclinical data27, 28 indicate that macrophages increase in the remote zone after MI, but have not revealed their sources and functions. It was unclear whether changes in cardiac macrophages during left ventricular remodeling result from local proliferation (as in the steady-state12, 29), monocyte recruitment (as in acute ischemia12, 29), or both.
Here we test hypotheses regarding the origins of remote myocardial and systemic monocyte/macrophages and their progenitors during post-MI remodeling. We detail supply routes and mechanisms that trigger cell proliferation in the heart and hematopoietic organs. Fate mapping supports the hypothesis that both increased monocyte recruitment and local macrophage proliferation contribute to the expanded cell population in failing myocardium. Reinforcements to resident monocytes derive from increased production in the bone marrow and spleen. We report that mechanical strain, which rises in the left ventricular wall after MI, elicits macrophage proliferation. Expression profiling of steady-state, recruited, and locally sourced macrophages reveals that their phenotypes differ reflecting their complex phenotypic plasticity. Finally, inhibition of monocyte recruitment with nanoparticle-enabled RNAi of endothelial cell adhesion molecules reduces myocardial macrophage numbers and post-MI ventricular dilation.
Methods
Mice
Female C57BL/6J mice, female C57BL/6-Tg (UbcGFP) 30Scha/J) mice, male and female B6.129P2(Cg)-Cx3cr1tm2.1(cre/ERT2)Litt/WganJ mice expressing a Cre-ERT2 fusion protein and enhanced YFP, male and female B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J mice, and female B6.129S4-Ccr2tm1Ifc/J mice were purchased from Jackson. Male and female FVB/N-Adrb3tm1Lowl/J mice were donated by P. Frenette (Albert Einstein College of Medicine New York, NY, USA) and B. Lowell (Beth Israel Deaconess Medical Center, Boston, MA, USA). All procedures were approved by the Institutional Animal Care and Use Committee Subcommittee on Research Animal Care, Massachusetts General Hospital.
Myocardial infarction was induced by permanent ligation of the proximal left anterior descending coronary artery as described previously30. Mice were anesthetized with isoflurane, and received buprenophine (0.1 mg/kg i.p.) twice daily for three days, starting on the day of the surgery.
Parabiosis
Mice were joined in parabiosis as described perviously30. Mice were anesthetized with isoflurane and received buprenophine (0.1 mg/kg i.p.) twice daily for three days, starting on the day of the surgery. Experiments began 14 days after parabiosis surgery, as required to establish a shared circulation.
Mechanical cell strain
Mechanical deformation (distortion of 4% on surface area at 0.67Hz for 24h) was applied to cultured cells with a device that produces biaxial strain31–33. For the preparation of cells subjected to mechanical strain, autoclaved membranes were coated with 2 mg/mL of human serum fibronectin (Sigma-Aldrich) at 4°C.
Statistics
Statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc.). Results are mean ± standard error of mean unless stated otherwise. For two-group comparisons, an unpaired t-test was applied to normally distributed variables, and a Mann-Whitney test to non-normally distributed variables. For comparing more than two groups, an ANOVA test, followed by a Sidak’s test for multiple comparisons, was applied. P values of < 0.05 indicated statistical significance.
siRNA formulation into 7C1 nanoparticles was done as previously described34, 35.
Please see the supplemental material for siRNA dosing, flow cytometry, MRI, histology, ELISA and cell culture.
Results
Cardiac macrophages expand during the development of chronic heart failure post-MI
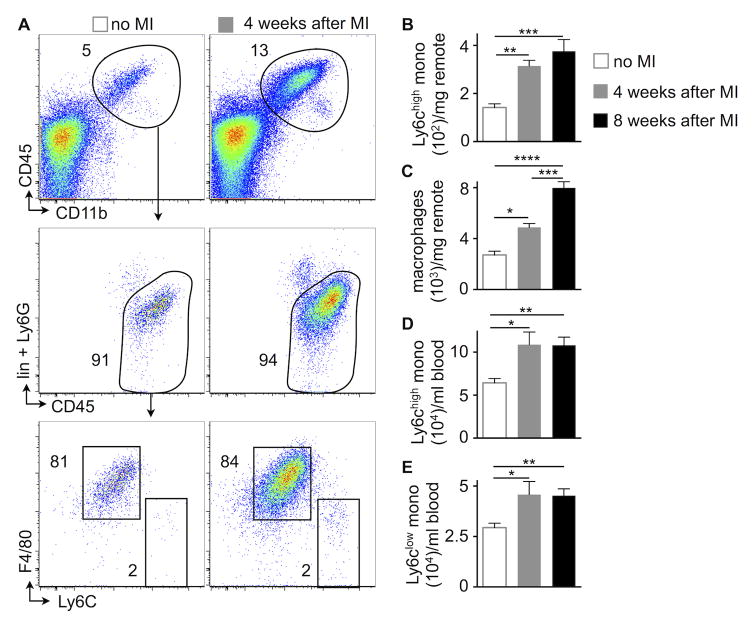
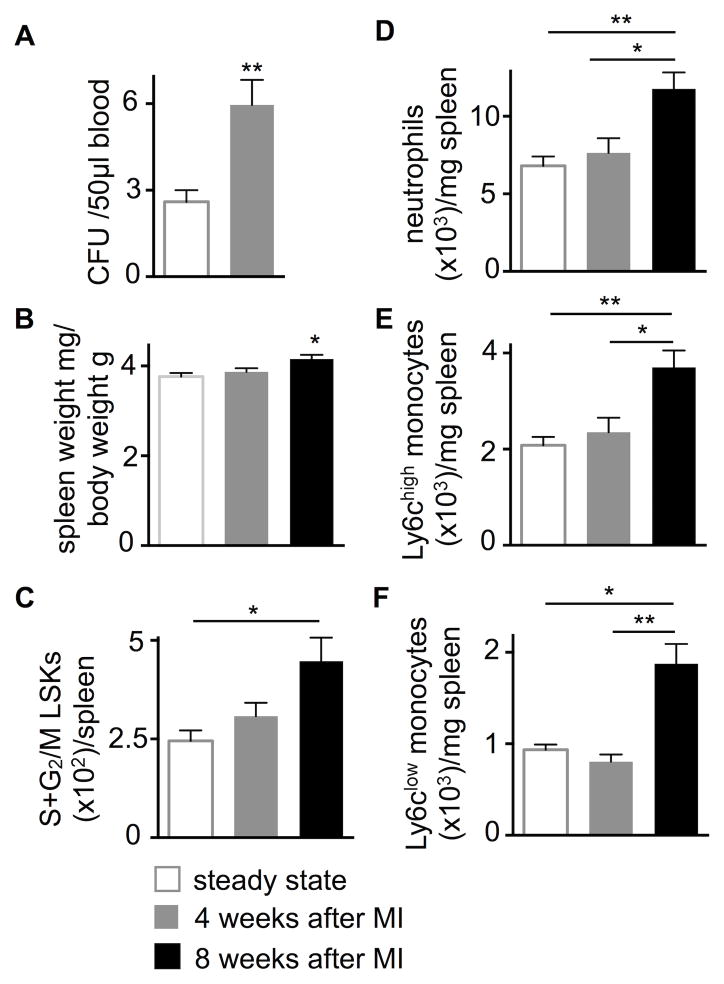
Macrophages populate the heart in the steady-state36, 37, die rapidly in acutely ischemic myocardium12 and are replaced by monocyte-derived macrophages12, 29. The behavior of macrophages that reside in the non-ischemic, remote myocardium after MI is less well understood, and it is unclear if and how they contribute to the development and progression of chronic heart failure. To examine this cell population, we ligated the left coronary artery proximally to induce large infarcts in mice. The heart to body weight ratio increased from 4.48 mg/g at steady-state to 6.65 mg/g at four (p<0.0001, n=15–20 per group) and 7.23 mg/g at eight weeks (p<0.0001, n=9–20 per group) after MI. In remote myocardium, which was isolated by microdissection and did not contain tissue from the infarct or the border zone, inflammatory monocyte and macrophage numbers per mg tissue increased progressively at 4 and 8 weeks after MI (Figure 1A–C). Macrophage numbers in the mature infarct scar were low in comparison to the non-ischemic myocardium and declined over time (Figure I in the Online Data Supplement). We detected blood monocytosis in HFrEF mice (Figure 1D and 1E), a finding that resembles findings in heart failure patients9, 10.
Figure 1. Expansion of cardiac macrophages in HFrEF.
A–C, Gating and quantification of myeloid cells in steady-state versus 4 and 8 weeks after MI in the remote area (i.e. the myocardium that was never ischemic), n=8–23 WT mice per group, mean±SEM, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
D and E, Blood monocytes in steady-state versus 4 and 8 weeks after MI, n=8–23 WT mice per group, mean±SEM, *p<0.05, **p<0.01.
Origins of macrophages in post-MI heart failure
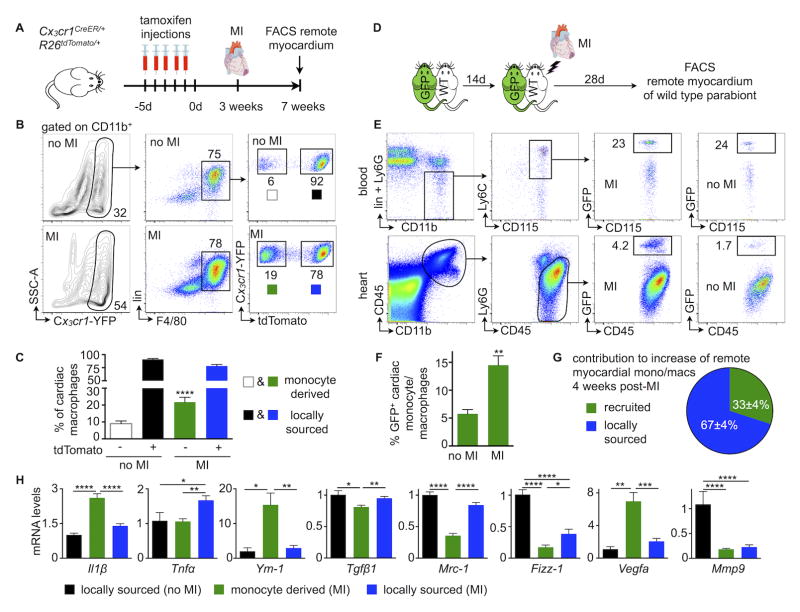
To determine if accumulation of remote myocardial macrophages relies on recruitment of monocytes from the blood, or alternatively from local proliferation, we pursued fate mapping experiments in Cx3cr1CreER/+ R26tdTomato/+ mice. In these mice, all fractalkine receptor (Cx3cr1) expressing cells, including circulating monocytes and cardiac resident macrophages, express yellow fluorescent protein (YFP). After injection of tamoxifen, all Cx3cr1pos cells also express the red fluorescent protein tdTomato. Thus, shortly after tamoxifen challenge, blood monocytes and resident macrophages exhibit red and yellow fluorescence (Figure II in the Online Data Supplement). Three weeks later, circulating monocytes are replaced by newly-made cells which derive from hematopoietic progenitors that do not express Cx3cr1. At this time point, blood monocytes and their progeny no longer express tdTomato (Figure II in the Online Data Supplement) while cells arising from local proliferation of Cx3cr1pos resident cardiac macrophages continue to express tdTomato. We infarcted mice three weeks after the last tamoxifen injection (Figure 2A) and assessed the myocardial frequencies of blood monocyte-derived YFPpos tdTomatoneg cells and locally sourced YFPpos tdTomatopos macrophages. A minor monocyte contribution to the cardiac macrophage pool in the steady state (9%) rose significantly in the remote myocardium of mice with HFrEF (21%, p<0.0001, Figure 2B and 2C).
Figure 2. Contribution of recruitment to cardiac macrophage expansion in HFrEF.
A, Experimental design.
B and C, Gating and quantification of resident versus bone marrow-derived cardiac macrophages in steady-state versus 4 weeks after MI, n=4–8 per group, mean±SEM, ****p<0.0001.
D, Experimental design.
E and F, Gating and quantification of chimerism for blood monocytes and cardiac monocytes and macrophages in steady-state versus 4 weeks after MI, n=4–10 pairs per group, mean±SEM, **p<0.01.
G, Relative contribution of monocyte-derived versus locally sourced macrophages to total remote monocyte/macrophage population 4 weeks after MI, n=4–10 pairs per group, mean±SEM.
H, Phenotyping of resident versus bone marrow-derived cardiac macrophages using fate mapping outlined in 2A (4 weeks after MI, n=4–8 per group, mean±SEM, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
In addition, we used parabiosis to follow HFrEF-induced changes in monocyte recruitment to failing myocardium. We surgically joined a UbcGFP mouse, in which all leukocytes express green fluorescent protein (GFP), with a wild type mouse (Figure 2D). Two weeks later, when the parabionts established a shared circulation, we induced a large infarct in the wild type parabiont (Figure 2D) and compared the chimerism of GFPpos monocytes and macrophages in the blood and heart to steady-state parabionts without MI. The contribution of recruited monocytes to the macrophage population in the remote myocardium rose 2.3±0.3-fold in infarcted parabionts (p<0.01, Figure 2E and 2F). Based on these data, we estimate that recruited monocytes contribute about one third to the expanded macrophage population in failing myocardium at 4 weeks after MI (Figure 2G, see the methods section for calculation).
To address the question whether macrophages in failing myocardium and those of different origins display distinct phenotypes, we isolated respective cell populations from the myocardium of Cx3cr1CreER/+ R26tdTomato/+ mice and compared their gene expression to steady-state by qPCR. Macrophages isolated from healthy and failing myocardium differed significantly in gene expression (Figure 2H). Monocyte-derived macrophages isolated from failing myocardium expressed more Il1β, Ym-1 and Vegfa, while locally sourced macrophages had higher mRNA for Tnfα, Tgfβ1 and Mrc-1. These differences diverge from the canonical M1/M2 macrophage polarization pattern. For instance, locally sourced macrophages in failing hearts expressed more Tnfα (a prototypical M1 gene) but also more Mrc-1 and Fizz-1 (both M2 genes) than monocyte-derived macrophages.
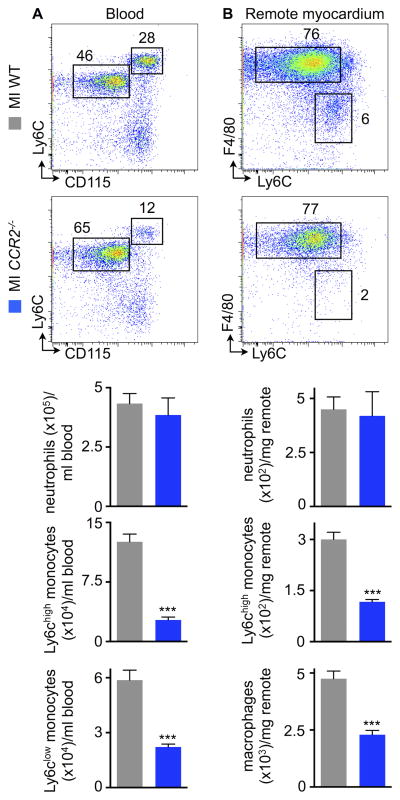
We next tested the role of the Ccl2/Ccr2 interaction in recruiting monocytes to the failing remote myocardium. Examination of the cellular source of Ccl2 in the remote myocardium revealed that capillary and arteriolar endothelial cells and to a lesser degree also macrophages produce Ccl2 (Figure III in the Online Data Supplement). Hence, we induced MIs in Ccr2−/− mice, which lack the Ccr2 chemokine receptor binding Ccl2. Monocyte release from the bone marrow into the blood and for the recruitment of monocytes to inflammatory sites requires Ccl2/Ccr2 interaction38–40. While neutrophil numbers did not change, monocyte counts fell in the blood of Ccr2−/− mice four weeks after MI (Figure 3A). Infarcted Ccr2−/− mice recruited significantly fewer Ly6Chigh monocytes to the remote myocardium. Thus, numbers of remote myocardial macrophages decreased (Figure 3B), which indicates that Ccr2-dependent monocyte bone marrow release and Ccr2-dependent monocyte recruitment to the heart contribute to the expanded macrophage pool in HFrEF.
Figure 3. Ccr2 dependent monocyte recruitment contributes to cardiac macrophage expansion.
A, Gating and quantification of blood myeloid cells in WT vs. Ccr2−/− mice, 4 weeks after MI, n=6–8 per group, mean±SEM, ***p<0.001.
B, Gating and quantification of cardiac myeloid cells in WT vs. Ccr2−/− mice, 4 weeks after MI, n=6–8 per group, mean±SEM, ***p<0.001.
Mechanical strain stimulates local macrophage proliferation
Steady-state cardiac macrophages self-renew through local proliferation12, 17, 29. Hence, we investigated the contribution of local proliferation to the increase in the remote myocardial macrophage population in mice with HFrEF. Remote macrophage proliferation rose significantly in HFrEF when compared to the steady-state (Figure 4A).
Figure 4. In situ macrophage proliferation and the role of biomechanical strain.
A, Gating and quantification of cardiac macrophage proliferation in steady-state versus 4 weeks after MI, n=6–9 per group, mean±SEM, **p<0.01.
B and C, Gating, quantification of cell numbers and proliferation with Ki67 (B) and BrdU (C) in stretched vs. non-stretched cultured murine peritoneal macrophages (n=6–8 dishes per group, mean±SEM, **p<0.01, ***p<0.001).
D, In-dish confocal microscopy, macrophage numbers and macrophage proliferation in stretched versus non-stretched murine cultured peritoneal macrophages (n=5 per group, mean±SEM, *p<0.01).
E, PhosphoErk1/2 (pT202/Y204) to total Erk1/2 ratio in stretched versus non-stretched cultured peritoneal murine macrophages by ELISA (n=6 per group, mean±SEM, *p<0.05).
F, Cell numbers and BrdU incorporation in stretched cultured peritoneal murine macrophages that were treated with Mek inhibitor (n=6 per group, mean±SEM, *p<0.05).
G and H, Gating and quantification of cardiac macrophage proliferation and numbers in mice with HFrEF, treated with a Mek inhibitor (4 weeks after MI, n=7–8 per group, mean±SEM, *p<0.05, ***p<0.001).
I, Blood monocytes in mice with HFrEF, treated with a Mek inhibitor (n=7–8 per group, mean±SEM).
Among many other adaptations during left ventricular remodeling, mechanical forces, which influence macrophage behavior32, 41–45, change in the left ventricular wall. During chronic post-MI remodeling and acutely after a large infarct, ventricular filling pressures can increase substantially from a normal left ventricular end-diastolic pressure of ~ 5mmHg to values that can exceed 30mmHg. This alteration profoundly increases wall tension and stress46, leading to myocyte slippage47, 48 and chamber dilation5, 6, 49, 50. Increased mechanical strain also acts on macrophages, which can sense tissue forces32, 51. We therefore hypothesized that increased strain accelerates macrophage proliferation. We isolated murine peritoneal macrophages, plated them on a membrane covered with a fibronectin layer and exposed them to biaxial mechanical strain (4% membrane deformation, 0.67 Hz) for 24 hours. Mechanical strain increased proliferation and consequently the numbers of macrophages (Figure 4B, 4C). In addition to flow cytometry, confocal microcopy of strained dishes documented increased proliferation and numbers of primary peritoneal murine macrophages (Figure 4D). To probe the pathways involved, we assayed mitogen-activated protein kinase (Mapk) signaling which relies on the protein kinases Fak and Src that both associate with cytoskeletal structures altered by deformation52–55. Further downstream, activated Mapk (Erk1/2, p38 Mapk) leads to expression of genes that regulate cell cycle entry56. Indeed, strain activated the Mapk pathway in cultured peritoneal macrophages, indicated by increased Erk phosphorylation (Figure 4E). Mek1/2 inhibition diminished strain-induced proliferation in vitro (Figure 4F). In vivo, Mek1/2 inhibition for three weeks, starting one week after coronary artery ligation, reduced cardiac macrophage proliferation (Figure 4G) and numbers (Figure 4H). Mek1/2 inhibition did not affect blood monocytes (Figure 4I). Using primary human macrophages, we likewise observed higher cell numbers after exposure to mechanical strain (Figure 5A). Human samples obtained from patients with ischemic cardiomyopathy undergoing left ventricular assist device implantation had more Ki67+ macrophages in regions remote to chronic infarcts when compared to control samples from unused donor hearts (Figure 5B).
Figure 5. Strain enhances proliferation of human macrophages.
A, Strain exposure of human macrophages. Gating and quantification of stretched versus non-stretched human primary macrophages (n=12 per group).
B, Histological evaluation of heart tissue obtained from patients with ischemic cardiomyopathy undergoing left ventricular assist device implantation. Controls are unused donor hearts (n=8–11 per group, mean±SEM, **p<0.01).
HFrEF stimulates hematopoiesis
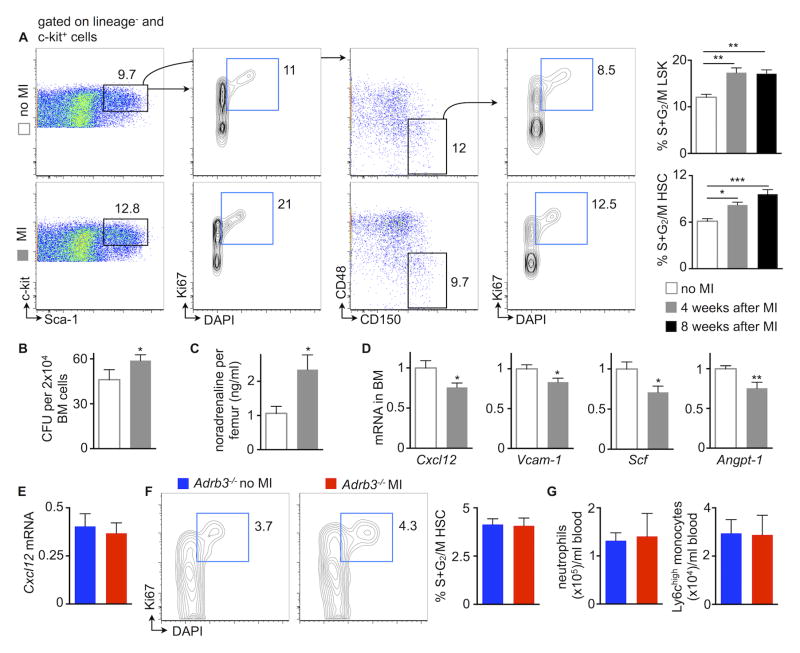
Because mice with HFrEF develop blood monocytosis (Figure 1), we investigated the contribution of the bone marrow and spleen to increased monocyte supply. In the bone marrow, hematopoietic stem and progenitor cells (HSPC) proliferated more vigorously during HFrEF than in steady-state (Figure 6A). In line with these data, the number of colony forming units increased in the bone marrow of mice with HFrEF (Figure 6B). To explore which mechanism increased bone marrow myelopoiesis, we investigated the sympathetic nervous system (SNS), which undergoes activation in patients with heart failure57. Mice with HFrEF had higher bone marrow noradrenaline levels (Figure 6C), a neurotransmitter that signals to hematopoietic niche cells through the β3 adrenergic receptor. As a result, mesenchymal stromal cells alter their supply of hematopoietic niche factors58. Indeed, Cxcl12 and Angiopoietin1, both promoters of HSC quiescence59, 60, fell in the bone marrow of mice with HFrEF (Figure 6D). In addition, HFrEF reduced Scf and Vcam-1 (Figure 6D), which retain HSC in the bone marrow niche61. When we neutralized bone marrow SNS signaling by generating HFrEF in Adrb3−/− mice, Cxcl12 expression was preserved (Figure 6E), resulting in unchanged HSC proliferation (Figure 6F). Blood neutrophil and monocyte levels did not increase in Adrb3−/− mice 4 weeks after MI (Figure 6G).
Figure 6. HFrEF activates bone marrow hematopoiesis.
A, Gating and quantification of bone marrow hematopoietic stem and progenitor cell proliferation in steady-state versus 4 and 8 weeks after MI, n=9–11 per group, mean±SEM, *p<0.05, **p<0.01, ***p<0.001.
B, Bone marrow colony forming unit (CFU) assay in steady-state versus HFrEF (n=5 per group, mean±SEM, *p<0.05).
C, Bone marrow noradrenaline in steady-state versus 4 weeks after MI, n=5–7 per group, mean±SEM, *p<0.05.
D, mRNA of bone marrow hematopoietic stem cell (HSC) retention factors (Cxcl12, chemokine (C-X-C motif) ligand 12; Vcam-1, vascular cell adhesion molecule 1; Scf, stem cell factor; Angpt1, angiopoietin-1) in bone marrow in steady state versus 4 weeks after MI, n=10 per group, mean±SEM, *p<0.05, **p<0.01.
E, mRNA of HSC retention factor Cxcl12 in steady-state Adrb3−/− versus Adrb3−/− mice 4 weeks after MI, n=5–6 per group, mean±SEM.
F, Gating and quantification of bone marrow hematopoietic stem and progenitor cell proliferation in steady-state Adrb3−/− versus Adrb3−/− mice 4 weeks after MI, n=5–6 per group, mean±SEM.
G, Quantification of blood neutrophils and monocytes in steady-state Adrb3−/− versus Adrb3−/− mice 4 weeks after MI, n=5–6 per group, mean±SEM.
In agreement with reduced bone marrow HSC retention factors in HFrEF (Figure 6D), HSPC release into blood increased (Figure 7A). These cells seeded the spleen and induced extramedullary hematopoiesis: eight weeks after MI, spleen weight (Figure 7B), splenic HSPC proliferation (Figure 7C) and splenic numbers of innate immune cells rose (Figure 7D–F). Taken together, these data indicate that HFrEF leads to increased extramedullary myelopoiesis, which contributes to the expanded systemic pool of innate immune cells.
Figure 7. HFrEF activates splenic myelopoiesis.
A, Blood colony forming unit (CFU) assay in steady-state versus 4 weeks after MI, n=5–12 per group, mean±SEM, **p<0.01.
B, Spleen weight in steady-state versus 4 and 8 weeks after MI, n=9–20 per group, mean±SEM, *p<0.05.
C, Splenic hematopoietic stem and progenitor cell proliferation in steady state versus 4 and 8 weeks after MI, n=7–16 per group, mean±SEM, *p<0.05.
D–F, Splenic myeloid cells in steady-state versus 4 and 8 weeks after MI, n=7–16 per group, mean±SEM, *p<0.05, **p<0.01.
Monocyte-derived macrophages contribute to adverse remodeling after MI
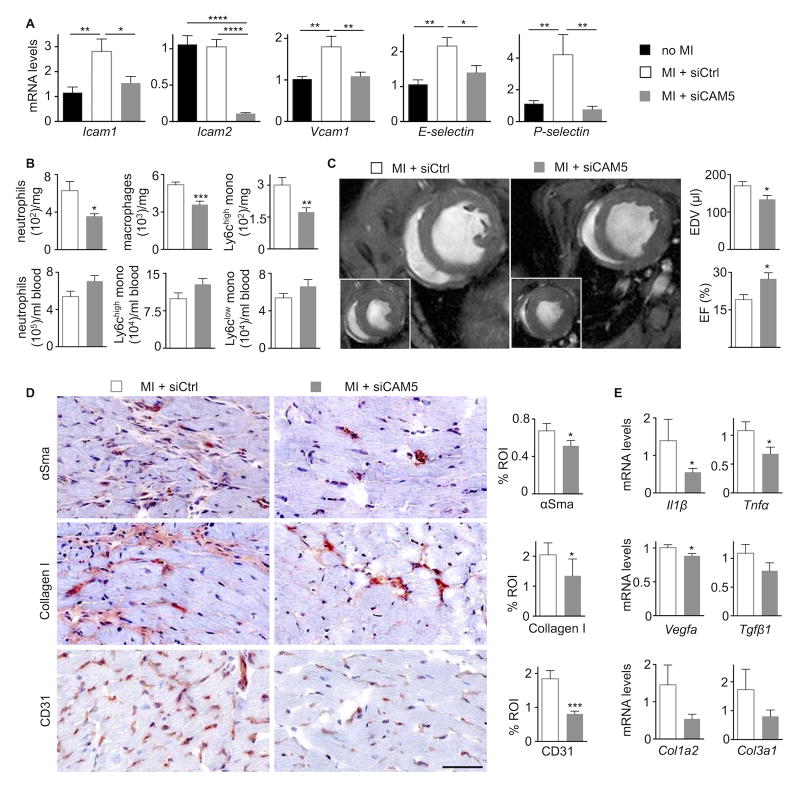
Adhesion molecules are necessary for extravasation of leukocytes and their recruitment into sites of inflammation62. Myocardial expression of Icam-1, Vcam-1, E- and P-selectin increased significantly 4 weeks post MI (Figure 8A). Employing a recently established in vivo RNAi approach34, 35, we silenced all 5 major adhesion molecules in cardiac endothelial cells (Figure 8A). This method relies on nanoparticle delivery of siRNA to endothelial cells and curbs leukocyte recruitment, as previously shown for atherosclerotic plaque and acute ischemia35. To examine whether monocyte-derived macrophages contribute causally to the development of HFrEF, we treated mice with endothelial-avid nanoparticles that contained either siRNA silencing all five cell adhesion molecules or an irrelevant control siRNA. The treatment began one week after coronary artery ligation to reduce interference with the acute inflammatory phase after MI. After three weeks of RNAi, neutrophil, monocyte and macrophage numbers decreased significantly in the remote myocardium while blood leukocyte levels did not change (Figure 8B). We then examined the consequences of reduced myeloid cell recruitment by cardiac MRI. RNAi treatment led to smaller end-diastolic volumes and less impaired left ventricular ejection fraction (Figure 8C), while it did not influence infarct volume (Figure IV in the Online Data Supplement). The treatment reduced myofibroblast content, collagen deposition and capillary frequency in the myocardium (Figure 8D). Myocardial mRNA levels of Il1β, Tnfα and Vegfa declined as well (Figure 8E).
Figure 8. Recruited macrophages contribute to HFrEF development.
A, Endothelial cell adhesion molecule mRNA levels in remote myocardium, values normalized to Gapdh (treatment with either siCtrl or siCAM5 for three weeks starting one week after MI, n=9–11 per group, mean±SEM, *p<0.05, ***p<0.001, ****p<0.0001).
B, Blood and cardiac myeloid cells in mice with HFrEF that received RNAi treatment with either siCtrl or siCAM5 for three weeks starting one week after MI (n=9–11 per group, mean±SEM, *p<0.05, **p<0.01, ***p<0.001).
C, Evaluation of post-MI remodeling by cardiac MRI. Each panel shows the mid-ventricular short axis view at end-diastole and end-systole (inset). End-diastolic volumes (EDV) and left ventricular ejection fraction (EF) were measured on day 28 after MI (n=9–11 per group, mean±SEM, *p<0.05).
D, Immunohistochemical evaluation of remote myocardium in mice with HFrEF for myofibroblasts (α-smooth muscle actin, αSMA), collagen (collagen-1), and vessels (CD31). Bar graphs show percentage of positive staining per region of interest (ROI) or number of vessels per high-power field (hpf). Scale bar, 50 μm (n=9–11 per group).
E, mRNA levels in remote myocardium, values normalized to Gapdh (treatment with either siCtrl or siCAM5 for three weeks starting one week after MI (n=9–11 per group, mean±SEM, *p<0.05).
Discussion
A large MI usually leads to left ventricular dilation, infarct expansion, hypertrophy of the remote myocardium and reduced cardiac output. Current standard of care measures do not completely interrupt this pathogenic sequence; hence the need for orthogonal strategies, based on newly identified pathways63. Here we describe that the expansion of remote myocardial macrophages in mice with MI depends on both local macrophage proliferation and recruitment of monocytes that derived from hematopoietic progenitors in the bone marrow and spleen. We identify increased sympathetic input through the β3 adrenergic receptor as a signal that fuels cell cycle entry of bone marrow hematopoietic progenitor cells, which leads to higher systemic numbers of monocytes. These monocytes enter the remote myocardium through canonical Ccl2/Ccr2 chemokine/chemokine receptor interaction, and through increased expression of endothelial cell adhesion molecules. We further identify mechanical strain as a putative cue leading to increased local proliferation of cardiac macrophages. Finally, we show that dampening monocyte recruitment, by means of adhesion molecule silencing started one week after coronary ligation, attenuates post-MI remodeling.
Data on the abundance, origin and function of macrophages are expanding rapidly64, 65. Within the last decade, we have learned that most organs harbor resident macrophages, including the healthy heart and arteries. These cells form a network interspersed between parenchymal cells. In cardiovascular disease, macrophages have received considerable attention in atherosclerotic plaque and in the acutely ischemic heart. Macrophage functions likely help maintain cardiovascular health; however, activation of their inflammatory actions can unleash functions that promote disease15, 37. Their oversupply leads to ischemic vascular complications, organ damage and maladaptive infarct repair. Thus, depending on location, number and phenotype, macrophages can protect or harm, rendering indiscriminate targeting of the immune system unlikely to succeed as a therapy. Hence, there is a need for a precise understanding of macrophage physiology and both their salutary and detrimental actions.
Prior work in humans and in mice indicates that, at least at chronic time points investigated by us, the infarct scar is relatively stable. In clinical delayed enhancement MRI, the tissue volume of bright infarct signal decreases over time66, 67. Fundamental studies in rodents after coronary ligation indicate that inflammatory processes subside in the ischemic zone, while active, inflammation-associated remodeling occurs in the remote myocardium that was initially not ischemic68, 69, 70. Hence, our study focused on macrophages residing in the myocardium remote from the ischemic zone.
The question of macrophage origin had previously been addressed for cardiovascular tissues in the steady-state29, 71, acute myocardial injury12 and atherosclerotic plaque72. In early atherogenesis, plaque macrophages derive from blood monocytes, but in established lesions the population expands due to local proliferation of these monocyte-derived cells72. In the healthy adult myocardium, blood monocytes do not contribute to the cardiac resident population to a major extent12, 29, a finding that our current fate mapping studies confirm. Comparable to the diseased vessel wall, monocyte recruitment increases in the chronically failing myocardium. Yet, local proliferation contributes the majority of cells at 4 weeks after MI. Whether the ratio of locally sourced versus blood monocyte derived macrophages changes dynamically while heart failure evolves remains to be investigated. Decreasing blood monocyte recruitment by in vivo RNAi improved remodeling and preserved left ventricular ejection fraction. These data support that blood monocyte levels could serve a prognostic marker.
The relevance of macrophage origins rests on whether or not recruited and locally sourced macrophages differ in function, which the difference in gene expression detected in this study implies. Monocyte-derived macrophages arise in the bone marrow and spleen. In mice with acute ischemia19 or exposure to chronic stress73, sympathetic nervous signaling induces higher myeloid cell output. A similar mechanism results in chronically elevated HSC activity in post-MI heart failure, as mice with a genetic deficiency of the β3 adrenergic receptor were protected from bone marrow microenvironmental signals that push hematopoietic progenitors into active cell cycle phases. Sympathetic nervous signaling and the resulting reduction of Cxcl12 also induces bone marrow release of myeloid cells and their progenitors19, 58. These progenitors take up residence in the spleen to establish extramedullary hematopoiesis in HFrEF. Prior splenectomy experiments revealed that about half of all infarct macrophages derive from the organ20, 74. Previous work has also implicated the spleen in heart failure, and splenectomy reduced chronic heart failure in mice28. Astonishingly, injection of splenocytes from a mouse with MI led to heart failure in an otherwise healthy recipient28, perhaps suggestive of autoimmunity. While it remains unclear which antigen induces autoimmunity after MI, these data highlight the complexity of the spleen as an immunological organ that harbors a panoply of immune cell types. Some of these splenocytes may have roles in heart failure, perhaps regulating myocyte health or macrophage activity. Eight weeks after a large MI, the spleen produces Ly6Chigh monocytes, a function that the organ does not exhibit in the steady-state. Clinical data on the importance of the spleen for cardiovascular health are sparse, with one study reporting increased cardiovascular mortality in patients that lost their spleen in World War II75. Confounding due to the predisposition to infections with encapsulated microorganisms in splenectomized humans complicates the interpretation of this observation. Imaging of spleen size, metabolism and proliferation might circumvent the paucity of human spleen tissue available for analysis. 18F-FDG PET data indicate that splenic glucose uptake increases in patients with acute coronary syndromes76, a finding that may relate to increased myelopoiesis78.
Wall stress increases in the failing heart, exposing all cells, including macrophages, to increased biomechanical strain. Macrophages respond to strain by inflammatory activation51 and increased expression of scavenger receptors32. These phenomena could be of importance in hypertension, which exposes arterial macrophages to increased mechanical forces32. Our in vitro data imply that strain increases macrophage proliferation. While the precise nature of mechanosensing remains uncertain, straining of cells in culture activated Mapk and Mek1/2 inhibition suppressed macrophage proliferation both in vitro and in vivo. Given that macrophage functions may differ substantially when cultured, the interpretation of these data requires caution. The MAPK pathway is activated in human heart failure78, although this effect may also relate to growth factor signaling and oxidative stress79. In addition to direct mechanical strain effects on macrophage proliferation, para- and autocrine pathways may contribute to the observed phenomenon. Our data motivate further study of the link between macrophage proliferation and strain, especially in the context of evidence for strain-induced proliferation in other cell types.
The origin of cells that promote disease may instruct the design of therapeutic interventions: if harmful cells accumulate due to recruitment, then inhibition of recruitment or dampening hematopoietic supply may prove beneficial. The result of the RNAi experiment (Figure 8), which used recently established technology to silence endothelial adhesion molecules34, 35, suggests that dampening myeloid cell recruitment might mitigate myocardial ischemic injury. These data also provide evidence for causal involvement of monocyte-derived macrophages in the evolution of heart failure. Several clinical trials indicate that in vivo RNAi is a clinically viable strategy80–82. The used nanomaterial directs uptake of siRNA to endothelial cells and leads to sufficient silencing with low toxicity34. The therapy began one week after coronary artery ligation to avoid interference with recruitment during the acute inflammatory phase after MI. Late effects of treatment on the infarct healing process are probably minor, but cannot be excluded entirely. A similar siRNA delivery strategy reduced the recruitment of monocytes to atherosclerotic plaque35, lesions found in patients with ischemic cardiomyopathy. Thus, this treatment approach may not only attenuate left ventricular remodeling but also decrease the risk of re-infarction. Safety studies will have to reveal whether such treatment compromises host defense against infection. In addition, we detected a small but significant reduction of Vegfa mRNA and reduced capillary density, in line with the high Vegfa mRNA levels in monocyte-derived cardiac macrophages. This observation sounds a cautionary note, as a mismatch of capillaries to hypertrophying myocytes might aggravate the balance between left ventricular oxygen supply and demand23. Reparative monocytes and macrophages can elaborate Vegfa after MI, and thus support regeneration and healing14, 16, 83. While the RNAi treatment that targeted monocyte recruitment proved overall beneficial, these considerations should prompt careful monitoring and dose selection to avoid undue decreases in myocardial Vegfa concentrations. Further, we found a non-significant trend towards higher blood leukocyte numbers in the siCAM5 treatment group (Figure 8B). While retention of leukocytes in circulation might partially explain this observation, we do not know how the treatment interferes with leukocyte homing to recycling organs such as bone marrow and spleen. These organs remove aging cells based on their elasticity, potentially independent of CAMs. Additionally, siCAM5 treatment also reduced remote myocardial neutrophil numbers. Thus, lower neutrophil counts might also contribute to the beneficial effects of RNAi on remodeling.
Our data raise a number of interesting questions. For instance, they suggest prioritization of genome-wide expression profiling of cardiac macrophage subsets isolated from failing myocardium. Such an undertaking could reveal unexpected functions and therapeutic options, if cell-specific deletion of identified genes improves post MI remodeling. Further, our observations suggest that monitoring of macrophage numbers and functions should inform novel therapeutic approaches to the treatment of heart failure, and may furnish mechanistic insights into their mode of action. The production of monocytes depends partially on β-adrenergic receptors19, and angiotensin II regulates the release of splenic monocytes75, suggesting that beta blockers and ACE inhibitors may act in part by altering leukocyte dynamics. Moreover, these data highlight the need to explore further the direct communication of macrophages with myocytes, fibroblasts and endothelial cells in cardiac pathophysiology.
Supplementary Material
Novelty and Significance.
What is known?
After large myocardial infarction, the myocardium undergoes a remodeling process that leads to hypertrophy, chamber dilation, heart failure and death.
In patients with heart failure, the white cell blood count correlates with mortality.
Macrophages participate in acute and chronic tissue remodeling via phagocytosis and cross talk with stromal cells.
What new information does this article contribute?
Higher sympathetic tone in mice with heart failure fuels systemic myeloid cell production in hematopoietic tissues.
Myocardial macrophages proliferate locally, potentially due to higher mechanical strain in the failing heart.
Inhibition of monocyte recruitment to the post-MI heart reduces heart failure, indicating that inflammatory bone marrow derived cardiac macrophages enhance left ventricular remodeling.
Macrophages reside in the healthy heart, and are recruited in large numbers into acutely ischemic tissues. While it is known that the cells regulate tissue health, their kinetics, sources and functions are not well understood in the setting of chronic heart failure. Here we describe that in the months after cardiac ischemia, macrophage numbers increase in the remote myocardium due to i) local macrophage proliferation and ii) due to monocyte recruitment. Increased local proliferation results in part from mechanic stress in failing hearts. Recruited monocytes, which are made in the bone marrow and spleen, contribute about one third to the increase in cardiac macrophages. If monocyte recruitment is dampened, left ventricular remodeling is attenuated. These data provide causal evidence that monocyte recruitment contributes to post-MI heart failure.
Acknowledgments
The authors thank Greg Wojtkiewicz, MS for assistance with imaging and the RNAi trial.
Funding Sources
This work was funded in part by grants from the from the National Heart, Lung, and Blood Institute (HL096576, HL117829, HL128264) and the MGH Research Scholar Award to MN. KL is supported by grants from the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (CHII2015-462), Foundation of Barnes-Jewish Hospital (8038-88), Burroughs Foundation Welcome Fund, and the NHLBI K08 HL123519.
Non-standard abbreviations and acronyms
- ADRB3
β3 adrenergic receptor
- Angpt-1
angiopoetin-1
- BrdU
5-bromo-2′-deoxyuridine
- Ccr2
Chemokine (C-C Motif) Receptor 2
- Cxcl12
chemokine (C-X-C motif) ligand 12
- DAPI
4,6-diamidino-2-phenylindole
- Erk
extracellular signal-regulated kinase
- GFP
green fluorescent protein
- HFrEF
heart failure with reduced ejection fraction
- HSC
hematopoietic stem cells
- HSPC
hematopoietic stem and progenitor cells
- LVAD
left ventricular assist device
- Mapk
mitogen-activated protein kinase
- Mek
mitogen-activated protein kinase kinase
- MI
myocardial infarction
- MRI
magnetic resonance imaging
- NA
noradrenaline
- Scf
stem cell factor
- SNS
sympathetic nervous system
- Vcam-1
vascular cell adhesion molecule 1
- WT
wild type
Footnotes
Disclosures
J.E.D., and D.G.A. have filed intellectual property protection related to 7C1 nanoparticles. The authors declare that they have no further competing interests.
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 2.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 3.Landmesser U, Drexler H. Chronic heart failure: an overview of conventional treatment versus novel approaches. Nat Clin Pract Cardiovasc Med. 2005;2:628–638. doi: 10.1038/ncpcardio0371. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJ. Clinical practice. Systolic heart failure. N Engl J Med. 2010;362:228–238. doi: 10.1056/NEJMcp0909392. [DOI] [PubMed] [Google Scholar]
- 5.Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128:388–400. doi: 10.1161/CIRCULATIONAHA.113.001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4:98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 8.Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44:1945–1956. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 9.Engstrom G, Melander O, Hedblad B. Leukocyte count and incidence of hospitalizations due to heart failure. Circ Heart Fail. 2009;2:217–222. doi: 10.1161/CIRCHEARTFAILURE.108.827071. [DOI] [PubMed] [Google Scholar]
- 10.Dixon DL, Griggs KM, Bersten AD, De Pasquale CG. Systemic inflammation and cell activation reflects morbidity in chronic heart failure. Cytokine. 2011;56:593–599. doi: 10.1016/j.cyto.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Francis GS. TNF-alpha and heart failure. The difference between proof of principle and hypothesis testing. Circulation. 1999;99:3213–3214. doi: 10.1161/01.cir.99.25.3213. [DOI] [PubMed] [Google Scholar]
- 12.Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, Swirski FK, Weissleder R, Nahrendorf M. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res. 2014;115:284–295. doi: 10.1161/CIRCRESAHA.115.303567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. Revisiting Cardiac Cellular Composition. Circ Res. 2016;118:400–409. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–1392. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilgendorf I, Gerhardt LM, Tan TC, et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114:1611–1622. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Laan AM, Ter Horst EN, Delewi R, Begieneman MP, Krijnen PA, Hirsch A, Lavaei M, Nahrendorf M, Horrevoets AJ, Niessen HW, Piek JJ. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur Heart J. 2014;35:376–385. doi: 10.1093/eurheartj/eht331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leuschner F, Rauch PJ, Ueno T, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutta P, Sager HB, Stengel KR, et al. Myocardial Infarction Activates CCR2(+) Hematopoietic Stem and Progenitor Cells. Cell Stem Cell. 2015;16:477–487. doi: 10.1016/j.stem.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014;114:565–571. doi: 10.1161/CIRCRESAHA.114.300507. [DOI] [PubMed] [Google Scholar]
- 23.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 25.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee WW, Marinelli B, van der Laan AM, et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol. 2012;59:153–163. doi: 10.1016/j.jacc.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res. 2014;114:266–282. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sager HB, Heidt T, Hulsmans M, Dutta P, Courties G, Sebas M, Wojtkiewicz GR, Tricot B, Iwamoto Y, Sun Y, Weissleder R, Libby P, Swirski FK, Nahrendorf M. Targeting Interleukin-1beta Reduces Leukocyte Production After Acute Myocardial Infarction. Circulation. 2015;132(20):1880–90. doi: 10.1161/CIRCULATIONAHA.115.016160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng GC, Briggs WH, Gerson DS, Libby P, Grodzinsky AJ, Gray ML, Lee RT. Mechanical strain tightly controls fibroblast growth factor-2 release from cultured human vascular smooth muscle cells. Circ Res. 1997;80:28–36. doi: 10.1161/01.res.80.1.28. [DOI] [PubMed] [Google Scholar]
- 32.Sakamoto H, Aikawa M, Hill CC, Weiss D, Taylor WR, Libby P, Lee RT. Biomechanical strain induces class a scavenger receptor expression in human monocyte/macrophages and THP-1 cells: a potential mechanism of increased atherosclerosis in hypertension. Circulation. 2001;104:109–114. doi: 10.1161/hc2701.091070. [DOI] [PubMed] [Google Scholar]
- 33.Schaffer JL, Rizen M, L’Italien GJ, Benbrahim A, Megerman J, Gerstenfeld LC, Gray ML. Device for the application of a dynamic biaxially uniform and isotropic strain to a flexible cell culture membrane. J Orthop Res. 1994;12:709–719. doi: 10.1002/jor.1100120514. [DOI] [PubMed] [Google Scholar]
- 34.Dahlman JE, Barnes C, Khan O, et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol. 2014 doi: 10.1038/nnano.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sager HB, Dutta P, Dahlman JE, et al. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci Transl Med. 2016;8:342ra80. doi: 10.1126/scitranslmed.aaf1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, Bilbao-Cortes D, Godwin JW, Rosenthal NA. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One. 2012;7:e36814. doi: 10.1371/journal.pone.0036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15:117–129. doi: 10.1038/nri3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, Kraemer D, Taffet G, Rollins BJ, Entman ML. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115:584–592. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- 39.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto K, Ikeda U, Shimada K. Role of mechanical stress in monocytes/macrophages: implications for atherosclerosis. Curr Vasc Pharmacol. 2003;1:315–319. doi: 10.2174/1570161033476565. [DOI] [PubMed] [Google Scholar]
- 42.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 43.Patel NR, Bole M, Chen C, Hardin CC, Kho AT, Mih J, Deng L, Butler J, Tschumperlin D, Fredberg JJ, Krishnan R, Koziel H. Cell elasticity determines macrophage function. PLoS One. 2012;7:e41024. doi: 10.1371/journal.pone.0041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trepat X, Deng L, An SS, Navajas D, Tschumperlin DJ, Gerthoffer WT, Butler JP, Fredberg JJ. Universal physical responses to stretch in the living cell. Nature. 2007;447:592–595. doi: 10.1038/nature05824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fereol S, Fodil R, Labat B, Galiacy S, Laurent VM, Louis B, Isabey D, Planus E. Sensitivity of alveolar macrophages to substrate mechanical and adhesive properties. Cell Motil Cytoskeleton. 2006;63:321–340. doi: 10.1002/cm.20130. [DOI] [PubMed] [Google Scholar]
- 46.Grossman W, Paulus WJ. Myocardial stress and hypertrophy: a complex interface between biophysics and cardiac remodeling. J Clin Invest. 2013;123:3701–3703. doi: 10.1172/JCI69830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 48.Nadal-Ginard B, Kajstura J, Anversa P, Leri A. A matter of life and death: cardiac myocyte apoptosis and regeneration. J Clin Invest. 2003;111:1457–1459. doi: 10.1172/JCI18611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383:1933–1943. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 51.Pugin J, Dunn I, Jolliet P, Tassaux D, Magnenat JL, Nicod LP, Chevrolet JC. Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol. 1998;275:L1040–50. doi: 10.1152/ajplung.1998.275.6.L1040. [DOI] [PubMed] [Google Scholar]
- 52.Naruse K, Yamada T, Sai XR, Hamaguchi M, Sokabe M. Pp125FAK is required for stretch dependent morphological response of endothelial cells. Oncogene. 1998;17:455–463. doi: 10.1038/sj.onc.1201950. [DOI] [PubMed] [Google Scholar]
- 53.Sai X, Naruse K, Sokabe M. Activation of pp60(src) is critical for stretch-induced orienting response in fibroblasts. J Cell Sci. 1999;112:1365–1373. doi: 10.1242/jcs.112.9.1365. [DOI] [PubMed] [Google Scholar]
- 54.Wang JG, Miyazu M, Matsushita E, Sokabe M, Naruse K. Uniaxial cyclic stretch induces focal adhesion kinase (FAK) tyrosine phosphorylation followed by mitogen-activated protein kinase (MAPK) activation. Biochem Biophys Res Commun. 2001;288:356–361. doi: 10.1006/bbrc.2001.5775. [DOI] [PubMed] [Google Scholar]
- 55.Wang JG, Miyazu M, Xiang P, Li SN, Sokabe M, Naruse K. Stretch-induced cell proliferation is mediated by FAK-MAPK pathway. Life Sci. 2005;76:2817–2825. doi: 10.1016/j.lfs.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 56.Whitmarsh AJ. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim Biophys Acta. 2007;1773:1285–1298. doi: 10.1016/j.bbamcr.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 57.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res. 2014;114:1815–1826. doi: 10.1161/CIRCRESAHA.114.302589. [DOI] [PubMed] [Google Scholar]
- 58.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 59.Kfoury Y, Mercier F, Scadden DT. SnapShot: The hematopoietic stem cell niche. Cell. 2014;158:228–228. e1. doi: 10.1016/j.cell.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 60.Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2012;12:49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 63.Libby P, Nahrendorf M, Swirski FK. Leukocytes Link Local and Systemic Inflammation in Ischemic Cardiovascular Disease: An Expanded “Cardiovascular Continuum”. J Am Coll Cardiol. 2016;67:1091–1103. doi: 10.1016/j.jacc.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 66.Thompson RC, Liu P, Brady TJ, Okada RD, Johnston DL. Serial magnetic resonance imaging in patients following acute myocardial infarction. Magn Reson Imaging. 1991;9(2):155–8. doi: 10.1016/0730-725x(91)90004-6. [DOI] [PubMed] [Google Scholar]
- 67.Ibrahim T, Hackl T, Nekolla SG, Breuer M, Feldmair M, Schömig A, Schwaiger M. Acute myocardial infarction: serial cardiac MR imaging shows a decrease in delayed enhancement of the myocardium during the 1st week after reperfusion. Radiology. 2010 Jan;254(1):88–97. doi: 10.1148/radiol.09090660. [DOI] [PubMed] [Google Scholar]
- 68.Berr SS, Xu Y, Roy RJ, Kundu B, Williams MB, French BA. Images in cardiovascular medicine. Serial multimodality assessment of myocardial infarction in mice using magnetic resonance imaging and micro-positron emission tomography provides complementary information on the progression of scar formation. Circulation. 2007 May 1;115(17):e428–9. doi: 10.1161/CIRCULATIONAHA.106.673749. [DOI] [PubMed] [Google Scholar]
- 69.Ross AJ, Yang Z, Berr SS, Gilson WD, Petersen WC, Oshinski JN, French BA. Serial MRI evaluation of cardiac structure and function in mice after reperfused myocardial infarction. Magn Reson Med. 2002 Jun;47(6):1158–68. doi: 10.1002/mrm.10166. [DOI] [PubMed] [Google Scholar]
- 70.Ramirez TA, Iyer RP, Ghasemi O, Lopez EF, Levin DB, Zhang J, Zamilpa R, Chou YM, Jin YF, Lindsey ML. Aliskiren and valsartan mediate left ventricular remodeling post-myocardial infarction in mice through MMP-9 effects. J Mol Cell Cardiol. 2014 Jul;72:326–35. doi: 10.1016/j.yjmcc.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ensan S, Li A, Besla R, et al. Self-renewing resident arterial macrophages arise from embryonic CX3CR1(+) precursors and circulating monocytes immediately after birth. Nat Immunol. 2016;17:159–168. doi: 10.1038/ni.3343. [DOI] [PubMed] [Google Scholar]
- 72.Robbins CS, Hilgendorf I, Weber GF, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heidt T, Sager HB, Courties G, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robinette CD, Fraumeni JFJ. Splenectomy and subsequent mortality in veterans of the 1939–45 war. Lancet. 1977;2:127–129. doi: 10.1016/s0140-6736(77)90132-5. [DOI] [PubMed] [Google Scholar]
- 76.Emami H, Singh P, MacNabb M, et al. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc Imaging. 2015;8:121–130. doi: 10.1016/j.jcmg.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarrazy V, Viaud M, Westerterp M, Ivanov S, Giorgetti-Peraldi S, Guinamard R, Gautier EL, Thorp EB, De Vivo DC, Yvan-Charvet L. Disruption of Glut1 in Hematopoietic Stem Cells Prevents Myelopoiesis and Enhanced Glucose Flux in Atheromatous Plaques of ApoE−/− Mice. Circ Res. 2016;118:1062–1077. doi: 10.1161/CIRCRESAHA.115.307599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cook SA, Sugden PH, Clerk A. Activation of c-Jun N-terminal kinases and p38-mitogen-activated protein kinases in human heart failure secondary to ischaemic heart disease. J Mol Cell Cardiol. 1999;31:1429–1434. doi: 10.1006/jmcc.1999.0979. [DOI] [PubMed] [Google Scholar]
- 79.O’Donoghue ML, Glaser R, Cavender MA, et al. Effect of Losmapimod on Cardiovascular Outcomes in Patients Hospitalized With Acute Myocardial Infarction: A Randomized Clinical Trial. JAMA. 2016;315:1591–1599. doi: 10.1001/jama.2016.3609. [DOI] [PubMed] [Google Scholar]
- 80.Zuckerman JE, Davis ME. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat Rev Drug Discov. 2015;14:843–856. doi: 10.1038/nrd4685. [DOI] [PubMed] [Google Scholar]
- 81.Coelho T, Adams D, Silva A, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369:819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 82.Tabernero J, Shapiro GI, LoRusso PM, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–417. doi: 10.1158/2159-8290.CD-12-0429. [DOI] [PubMed] [Google Scholar]
- 83.Howangyin KY, Zlatanova I, Pinto C, Ngkelo A, Cochain C, Rouanet M, Vilar J, Lemitre M, Stockmann C, Fleischmann BK, Mallat Z, Silvestre JS. Myeloid-Epithelial-Reproductive Receptor Tyrosine Kinase and Milk Fat Globule Epidermal Growth Factor 8 Coordinately Improve Remodeling After Myocardial Infarction via Local Delivery of Vascular Endothelial Growth Factor. Circulation. 2016;133:826–839. doi: 10.1161/CIRCULATIONAHA.115.020857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.