Abstract
Objectives
Conversion rates from trial leads to permanent spinal cord stimulation (SCS) systems have important implications for healthcare resource utilization (HCRU) and pain management. We hypothesized conversion rates differ based on provider implant volume.
Materials and Methods
We designed a large, retrospective analysis using the Truven MarketScan database analyzing adult SCS patients with provider information available, with or without IPG implantation from the years 2007 to 2012. Patients were divided into three provider-based groups: high (>25), medium (9–24), and low (3–8) volume providers. Univariate and multivariate models identified factors associated with successful conversion.
Results
A total of 17,850 unique trial implants were performed by 3,028 providers. Of 13,879 patients with baseline data available, 8,981 (64.7%) progressed to permanent SCS. Higher volume providers were associated with slightly higher conversion rates (65.9% vs. 63.3% low volume, p=0.029), explant rates (9.2% vs. 7.7% medium volume, p=0.026), younger age (52.0 ± 13.4 years vs. 53.0 ± 13.4 years, p=0.0026), Medicare/Medicaid (47.8% vs. 35.0% low volume, p<0.0001), Southern region (53.5% vs. 38.9% low volume, p<0.0001), and higher Charlson comorbidity scores (1.0 (SD=1.4), p=0.0002). Multivariate regression results showed female gender (1.13 [95% CI: 1.05–1.22], p<0.001) and high volume providers associated with higher odds of successful trial conversion (1.12 [95% CI: 1.02–1.22], p=0.014).
Conclusions
In this nationwide analysis, high volume providers achieved higher trial-to-permanent SCS conversion rates than lower volume providers. The study has implications for both training requirements and referral patterns to delineate minimum implant experience necessary for provider proficiency. Future studies may be useful to understand HCRU differences.
Keywords: Spinal cord stimulator (SCS), trial conversion, chronic pain, outcomes, volume-outcome effect
Introduction
Chronic pain impacts 100 million adults in the United States every year, costing as much as $635 billion in increased medical costs and lost economic productivity1. Spinal cord stimulation (SCS) is an established modality for the treatment of intractable chronic pain, and has been shown to decrease refractory chronic pain and improve quality of life across a number of medical conditions2–6. Since the first volume-outcome relationships in surgical care were reported more than 30 years ago, there has been a continued debate about whether certain procedures should be restricted to high-volume centers7–10. Many studies have shown that higher provider volume is associated with lower postoperative complication rates after a variety of surgical procedures. Although the trend has most commonly been established with high-risk operations, it has also been shown in less complex procedures7,11–21.
Unfortunately, despite patient satisfaction, increased annual use, and sustained success of SCS22–25, a recent study reports the trial-to-permanent conversion rate at 42%26. These patients never receive a permanent spinal cord stimulator, as they do not improve sufficiently during the traditional trial period. Lasting approximately one week, the trial period helps to predict and scale expectations for full benefit from SCS27–33. The trial is considered successful if a 50% reduction in pain is achieved with the trial device in place, at which point the patient can proceed to permanent implantation. Trial-to-permanent conversion rates have been analyzed for their implication in overall device efficacy and have also been used as means of comparing provider proficiency in patient selection and procedure performance26,34. Beyond etiology of pain, other prognostic factors have been shown to predict trial conversion, including patient selection, geographic variation, insurance, age, trial-to-implant time, undergoing multiple trial phases, and previous back surgeries26,34–36.
Complications and failed trials result in high costs from continued attempts at pain management and repeated revisions26,35–38. While newer systems have reduced complications and improved trial conversion rates, there is a paucity of data regarding the impact of provider expertise and familiarity with SCS39,40. While differences may exist between practices, there may also exist intra-specialty and certainly individual provider technical differences that contribute to improved outcomes38,41,42. Although relations between volume and outcome have long been recognized, efforts to concentrate selective procedures or appropriate referral pathways to high-volume centers are only now beginning to gain momentum.
In this study, we hypothesized that patients with chronic pain who visit SCS implanters with high implant volume have higher trial-to-permanent conversion rates than patients who visit SCS implanters with low implant volume. Using the Truven Marketscan database, we examined SCS lead and implanted pulse generator (IPG) placement from 2007 to 2012 for the treatment of chronic pain. Our primary outcome was SCS trial-to-permanent conversion rate between those providers who frequently perform SCS implantation and those who perform such implantations less frequently.
Materials and Methods
Data Source
The present study is a large retrospective review utilizing the Truven Marketscan Database, a national database that includes information from Commercial Claims and Encounters, Medicare Supplemental and Coordination of Benefits, and Medicaid databases. Data within Marketscan includes United States inpatient admissions, inpatient services, outpatient services, and enrollment tables between 2007 and 2012, all of which are de-identified and collected from a third party, requiring no patient contact or consent. The study received IRB approval from the Institutional Review Board.
Study Sample
Unique instances of percutaneous and paddle SCS trials were identified: individuals underwent implantation of a percutaneous SCS lead (CPT-4: 63650) or a paddle SCS lead (CPT-4: 63655) without a simultaneous implantation of a percutaneous IPG (CPT-4: 63685) (Supplementary Table 1). Additional inclusion criteria included age ≥18. The provider inclusion criteria included involvement in a minimum of three SCS trial implants and the provider type (i.e. anesthesiology, neurosurgery, orthopedic, and PM&R), which was represented by at least 100 unique instances to include those providers that typically perform such procedures. Cases that did not contain provider IDs or baseline data were excluded. Finally, cases that were coded ambiguously based on SCS lead and IPG placement were also excluded (Supplementary Figure 1).
Such criteria produced a final patient cohort of 13,879. Only the first trial and its associated provider was included to ensure each patient was only included once. Of the providers meeting the inclusion criteria, implanters were differentiated into high implant volume (>25 documented implants), medium implant volume (9–24 documented implants), and low implant volume (<3–8 documented implants). Such volume cutoffs were assigned based on rounding to the nearest whole number implant to approximate the cohort into thirds. The 13,879 patients were analyzed according to their provider volume based on these low, medium, and high groupings.
Main Outcome Measure
Six cohorts of chronic pain patients were identified, including successful trials from low/medium/high volume implanter and failed trial from low/medium/high volume implanter. The main outcome measure was the trial-to-permanent conversion rate with SCS trial implantation as the index event. Successful trial was defined by percutaneous or paddle lead implant followed by IPG implant. Due to the possibility of errors in billing date, IPG implants within one week of SCS lead implants were also included. Failed trials were defined as a percutaneous or paddle lead implant not accompanied by IPG implant.
Statistical Analysis
Descriptive statistics were reported and demarcated by low, medium, and high volume status. Counts and percentages were reported for categorical variables. Means, standard deviations, medians, and quartiles were provided for continuous variables. Chi-square test was used for the group difference for categorical variables, and Kruskal Wallis test was used for the group difference for the continuous variable. Univariate logistic regression model was used to test the significance of provider volume. Multivariate logistic regression model was used to quantify the likelihood of permanent conversion with age at first SCS lead implant, gender, provider volume, year of procedure, insurance source, employment status, and Charlson comorbidity score as the covariates. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were reported for trial-to-conversion rates. All analyses were conducted with SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient Cohort
A total of 13,879 unique patients undergoing SCS trials between 2007 and 2012 were identified in the Truven Marketscan database. These patients were 62.9% female with an average age of 52.5 ± 13.3 years. Approximately 44.1% of patients were located in the Southern geographic region, 23.8% were actively employed, and 41.1% of patients were insured through either Medicaid or Medicare (Table 1). 32.3% were classified as patients of high-volume (>25) providers, 31.6% were classified as patients of medium-volume (9–24) providers, and 36.0% were classified as patients of low-volume (3–8) providers.
Table 1.
Baseline Characteristics By Implant Volume
| Low (3–8) (N=5002) |
Medium (9–24) (N=4388) |
High (25+) (N=4489) |
Total (N=13879) |
p-value | |
|---|---|---|---|---|---|
| Successful Trial, N(%) | 0.0292 | ||||
| No | 1834 (36.7%) | 1532 (34.9%) | 1532 (34.1%) | 4898 (35.3%) | |
| Yes | 3168 (63.3%) | 2856 (65.1%) | 2957 (65.9%) | 8981 (64.7%) | |
| Explant, N(%) | 0.0257 | ||||
| No | 4569 (91.3%) | 4052 (92.3%) | 4074 (90.8%) | 12695 (91.5%) | |
| Yes | 433 (8.7%) | 336 (7.7%) | 415 (9.2%) | 1184 (8.5%) | |
| Inpatient, N(%) | 0.0906 | ||||
| Inpatient | 92 (1.8%) | 101 (2.3%) | 111 (2.5%) | 304 (2.2%) | |
| Outpatient | 4910 (98.2%) | 4287 (97.7%) | 4378 (97.5%) | 13575 (97.8%) | |
| Charlson comorbidity score, N(%) | 0.0002 | ||||
| N | 4944 | 4333 | 4427 | 13704 | |
| Mean (SD) | 0.9 (1.3) | 0.9 (1.3) | 1.0 (1.4) | 0.9 (1.3) | |
| Median | 0.0 | 0.0 | 0.0 | 0.0 | |
| Employment Status, N(%) | <0.0001 | ||||
| Active Full Time | 1555 (31.1%) | 1114 (25.4%) | 635 (14.1%) | 3304 (23.8%) | |
| Retiree | 977 (19.6%) | 581 (13.2%) | 404 (8.9%) | 1962 (14.2%) | |
| Other | 2470 (49.4%) | 2693 (61.5%) | 3450 (76.9) | 8613 (62.1%) | |
| Gender, N(%) | 0.4712 | ||||
| Male | 1887 (37.7%) | 1611 (36.7%) | 1645 (36.6%) | 5143 (37.1%) | |
| Female | 3115 (62.3%) | 2777 (63.3%) | 2844 (63.4%) | 8736 (62.9%) | |
| Age at SCS, N(%) | 0.0026 | ||||
| Mean (SD) | 53.0 (13.4) | 52.3 (13.1) | 52.0 (13.4) | 52.5 (13.3) | |
| Median | 53.0 | 52.0 | 52.0 | 52.0 | |
| Region, N(%) | <0.0001 | ||||
| Northeast Region | 506 (10.1%) | 152 (3.5%) | 59 (1.3%) | 717 (5.2%) | |
| North Central Region | 856 (17.1%) | 632 (14.4%) | 273 (6.1%) | 1761 (12.7%) | |
| South Region | 1947 (38.9%) | 1776 (40.5%) | 2403 (53.5%) | 6126 (44.1%) | |
| West Region | 743 (14.9%) | 497 (11.3%) | 170 (3.8%) | 1410 (10.2%) | |
| Unknown Region | 7 (0.1%) | 6 (0.1%) | 11 (0.2%) | 24 (0.2%) | |
| Medicaid | 943 (18.9%) | 1325 (30.2%) | 1573 (35.0%) | 3841 (27.7%) | |
| Insurance source, N(%) | <0.0001 | ||||
| Commercial Insurance | 3255 (65.1%) | 2580 (58.8%) | 2343 (52.2%) | 8178 (58.9%) | |
| Medicaid | 943 (18.9%) | 1325 (30.2%) | 1573 (35.0%) | 3841 (27.7%) | |
| Medicare | 804 (16.1%) | 483 (11.0%) | 573 (12.8%) | 1860 (13.4%) | |
| Year, N(%) | <0.0001 | ||||
| 2007 | 670 (13.4%) | 682 (15.5%) | 628 (14.0%) | 1980 (14.3%) | |
| 2008 | 803 (16.1%) | 762 (17.4%) | 852 (19.0%) | 2417 (17.4%) | |
| 2009 | 1006 (20.1%) | 936 (21.3%) | 1017 (22.7%) | 2959 (21.3%) | |
| 2010 | 809 (16.2%) | 658 (15.0%) | 665 (14.8%) | 2132 (15.4%) | |
| 2011 | 851 (17.0%) | 712 (16.2%) | 683 (15.2%) | 2246 (16.2%) | |
| 2012 | 863 (17.3%) | 638 (14.5%) | 644 (14.3%) | 2145 (15.5%) |
Baseline Characteristics
At baseline, the average age of our patient cohort differed slightly between the implanters (p=0.0026), at 52.0 ± 13.4 years in high volume implanters and 53.0 ± 13.4 years in low volume implanters. Gender distribution was similar across all implant groups. A large majority of the patients in the cohort of high volume implanters was located in the Southern region (53.5% in high volume implanters vs. 38.9% in low volume implanters, p<0.0001). Amongst the high volume implanters, 47.8% of the patient cohort had either Medicare or Medicaid insurance type (vs. 35% in low volume implanters, p<0.0001) (Table 1).
Outcomes
Overall, 8,981 patients (64.7%) proceeded to have a permanent SCS system implanted after successful SCS trial, whereas 4,898 patients (35.3%) had a failed trial. In terms of trial-to-permanent conversion rate, high volume providers had a higher conversion rate at 65.9%, with medium volume providers at 65.1%, and low volume providers at 63.3% (p=0.0292) (Figure 1).
Figure 1. SCS Provider Volume by Conversion Rate.
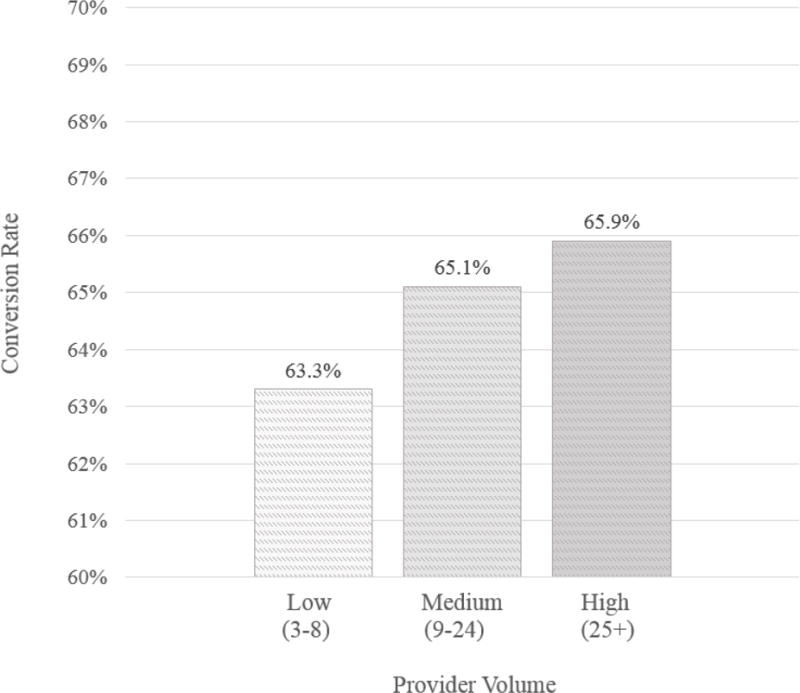
High volume providers display higher rates of trial-to-permanent spinal cord stimulator conversion.
Using low-volume as a reference, the results for the univariate and adjusted multivariate model were similar. After adjusting for the previously mentioned demographic factors, multivariate analysis showed a 12% increased likelihood of trial-to-permanent conversion in the high-volume group (OR: 1.12; p=0.014; 95% CI: 1.02–1.22). As shown in the multivariate model, the medium-volume group was 8% more likely to convert to permanent SCS based on the adjusted model (OR: 1.08; p=0.080; 95% CI: 0.99–1.18) (Table 2).
Table 2.
Multivariate Logistic Regression for Successful Conversion
| OR (95% CI)a | p-value | |
|---|---|---|
| Age | ||
| 0.99 (0.99, 1.00) | <.001 | |
| Charlson comorbidity score | ||
| 0.99 (0.96, 1.02) | 0.524 | |
| Gender of Patient | ||
| Female | 1.13 (1.05, 1.22) | <.001 |
| Male | reference | . |
| Implant volume | ||
| High (>25) | 1.12 (1.02, 1.22) | 0.014 |
| Medium (9–24) | 1.08 (0.99, 1.18) | 0.080 |
| Low (3–8) | reference | . |
| Year | ||
| 2012 | 0.97 (0.85, 1.10) | 0.610 |
| 2011 | 1.18 (1.04, 1.35) | 0.011 |
| 2010 | 1.13 (0.99, 1.29) | 0.068 |
| 2009 | 1.03 (0.91, 1.16) | 0.684 |
| 2008 | 0.99 (0.87, 1.12) | 0.867 |
| 2007 | reference | . |
| Insurance source | ||
| Medicare | 0.99 (0.86, 1.14) | 0.854 |
| Medicaid | 0.95 (0.86, 1.05) | 0.330 |
| Commercial Insurance | reference | . |
| Employment status | ||
| Other | 1.02 (0.92, 1.12) | 0.709 |
| Retiree/Medicare | 0.94 (0.82, 1.07) | 0.323 |
| Eligible/Disabled | ||
| FT/PT | reference | . |
OR, odds ratio; CI, confidence interval
The mean duration to IPG implant following SCS lead implant was prolonged in the low volume cohort, at 50.3 days (median 29.0 days) versus medium or high volume implanters (mean[median]: 45.6[26.0] and 48.5[28.0], respectively).
Discussion
In this study, we attempted to quantify the impact of provider’s procedural volume on trial-to-permanent SCS conversion. Our expectation was that high implant volume providers would have higher trial-to-permanent conversion rates. This was indeed corroborated by our results, albeit to a lesser degree than expected. On average, high-volume providers have 2.6% higher trial-to-conversion rates with at least a 12% increased likelihood of successful trial compared to low-volume providers. Our study suggests consideration of a minimum numeric threshold for trial implantation necessary for a clinician to be considered a ‘standard of care’ provider for SCS. Although standardized training requirements have been previously explored42, annual institutional and practice performance requirements may also be required and pursued for continued excellence in patient care and appropriate administration of SCS technology.
Patient Charlson comorbidity scores were slightly higher in the high volume providers, suggesting high volume providers are possibly treating more medically challenging patients. Whether this is a function of referral patterns, self-selection, or other providers “passing the buck” to their more experienced colleagues is worth exploring. Interestingly, high volume providers were also associated with higher rates of explant, which could be due to a variety of reasons such as lower threshold for defining treatment failure, more complicated case mix, or patient expectations when seeking treatment at high vs. low volume centers. At least one previous study has shown loss of clinical effect as a primary factor in explantation43. It will be useful to further explore this trend, given the association between treatment outcomes and healthcare resource utilization. While insurance type, employment status, and geographic region did not predict higher trial-to-permanent conversion rates, the independent predictor of implant year, showing 18% increased odds of conversion in 2011, may suggest technological advances have contributed to the overall improved success of SCS and thus increased conversion rates over time.
Variations in patient population and protocol across provider groups may partly explain our results. Trial-to-permanent conversion rates are impacted by disease type and the duration of pain experienced by the patient cohort43. Number of leads, lead placement, spinal level placement, and dermatomal targets can impact effective pain coverage after SCS implantation. SCS outcomes are also closely associated with patient follow-up, how providers choose to tailor stimulator characteristics, and the selectivity and threshold for interpreting a ‘trial failure’. Thus, factors other than technical proficiency and overall device efficacy may affect trial-to-conversion rates across providers in this study. Whether high volume providers have higher conversion rates due to more experience vis-à-vis larger caseloads, or if these providers receive greater referrals as a function of sub-specialization, cannot be inferred here.
There is a growing body of literature examining the variability of SCS outcomes and how they are dependent on not only patient factors, but also on physician expertise, surgical technique, and postoperative care31,33,44,45. An estimated 27,484 SCS systems were implanted in 2007 by medical and surgical specialties with widely varying training backgrounds, a number that most likely has escalated given increased awareness of SCS as a viable treatment alternative over recent years22. With no formal certification prerequisites as to number of procedures performed or additional fellowship training requirements across different specialties, it is clear that there is growing need for standardization of physician training in SCS42. In 2009, the North American Neuromodulation Society (NANS) released training and competency guidelines to address this need, highlighting three SCS training programs with requirements depending on the services offered by specific specialties. These required at minimum 25 follow-up visits with SCS patients in the capacity of primary operator and evaluator, and maintenance of competency with at least 10 device implants/revisions annually42. In particular, NANS defined an “SCS service” as a physician or group of physicians with a caseload of at least 30 implants or trials per year. A 2014 survey of pain medicine programs across the US reported that although there has been marked changes in pain practices over the past decade, there continues to be a high degree of variability with a total of 50 programs reporting an annual range of 5 to 200 trials and 0 to 150 permanent implantations performed41. Moreover, although additional fellowship training was cited to be the most educational experience for SCS competency, there exists a general lack of uniformity in experience across institutions.
While there have been several studies in the literature that have examined factors associated with spinal cord stimulation success26,31,33,44,45, the focus has been largely patient-based rather than identifying provider variables. Our study is the first to consider the effect of provider implant volume using a large national cohort. There are, however, limitations to our analysis. First and foremost, we recognize that trial-to-conversion rates cannot be assumed to be a metric for long-term SCS success. Second, this study is a US-based, retrospective and non-randomized, analysis of a patient cohort primarily weighted towards recent years and commercial insurance. We attempted to address this through a multivariate analysis and adjustment of our data for patient and hospital-related factors. Studies conducted in the UK have demonstrated lower rates of back surgery and utilization of SCS, but purportedly higher trial-to-conversion and lower explantation rates, which underscore the regional applicability of this study46,47 (personal communication). Thirdly, this database lacked information on factors that may affect optimal pain coverage in SCS patients, including number of leads, lead location, spinal level placement, and dermatomal targets. Both paddle and percutaneous trials were included, as the decision to use one or the other is largely based on provider and patient anatomy, and the study does not speak to how the conversion rates would differ if delineating lead type. A separate analysis of such factors may help evaluate for provider implant volume and customization of such characteristics. Similarly, other factors that may add to the breadth of discussion, such as changes to numerical pain scores, pain intensity, or quality and impact on quality of life, could not be analyzed in this patient cohort.
Despite the aforementioned limitations, our study does highlight the importance of considering provider and practice variables that directly impact patient outcomes. It would be interesting to identify if providers that experience high implant volume are associated with a particular specialty, or located at large group practices or academic institutions with access to ancillary resources. Future studies are required to understand the practice variations that make this possible, and to consider the effect of a successful conversion rate and concentration of implantation at higher volume centers on healthcare utilization.
Conclusion
Data is accumulating that provider volume is a plausible predictor of outcome in multiple interventional and surgical disciplines. The skillset and training of the individual surgeon is important, though it is also crucial that a multidisciplinary treatment team develops substantial experience in the management of these patients. In this large, nationwide analysis of 13,879 patients, our results indicate that providers performing a higher volume of SCS implants achieve significantly higher trial-to-permanent conversion rates, compared to providers performing a lower volume of SCS implants. We also identified demographic variables associated with high conversion rates that suggest trends that may impact patient selection in SCS. The demonstrated findings have implications for provider qualification and certification, as well as for the patient and referring provider. Patients, providers, and the field of neuromodulation should acknowledge volume-outcome results, as advanced health care systems will continue to face specialization and regionalization of procedures.
Supplementary Material
Acknowledgments
Funding Statement: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: Dr. Lad has received fees for serving as a speaker and consultant for Medtronic Inc., Boston Scientific, and St. Jude Medical. The remaining authors report no conflicts of interest or financial disclosures.
Authorship Statement: Siyun Yang, Jichun Xie, Kelly Ryan Murphy, Beth Parente, Jing L. Han and Shivanand P. Lad designed and conducted the study, data collection, and data analysis. Kelly Ryan Murphy prepared the manuscript draft with important intellectual input from Jing L. Han and Syed Mohammed Qasim Hussaini. All authors approved the final manuscript. Jichun Xie and Siyun Yang had complete access to the study data.
References
- 1.Gaskin DJ, Richard P. The economic costs of pain in the United States. The Journal of Pain. 2012;13(8):715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Simpson EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J. Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation. Health technology assessment (Winchester, England) 2009 Mar;13(17):iii, ix–x, 1–154. doi: 10.3310/hta13170. [DOI] [PubMed] [Google Scholar]
- 3.North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98–106. doi: 10.1227/01.neu.0000144839.65524.e0. discussion 106–107. [DOI] [PubMed] [Google Scholar]
- 4.Kemler MA, de Vet HC, Barendse GA, van den Wildenberg FA, van Kleef M. Effect of spinal cord stimulation for chronic complex regional pain syndrome Type I: five-year final follow-up of patients in a randomized controlled trial. Journal of neurosurgery. 2008 Feb;108(2):292–298. doi: 10.3171/JNS/2008/108/2/0292. [DOI] [PubMed] [Google Scholar]
- 5.Lad SP, Babu R, Bagley JH, et al. Utilization of spinal cord stimulation in patients with failed back surgery syndrome. Spine. 2014 May 20;39(12):E719–727. doi: 10.1097/BRS.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 6.Yakovlev AE, Resch BE. Treatment of multifocal pain with spinal cord stimulation. Neuromodulation : journal of the International Neuromodulation Society. 2012 May-Jun;15(3):210–213. doi: 10.1111/j.1525-1403.2012.00435.x. discussion 213. [DOI] [PubMed] [Google Scholar]
- 7.Davies JM, Ozpinar A, Lawton MT. Volume-Outcome Relationships in Neurosurgery. Neurosurgery clinics of North America. 2015;26(2):207–218. doi: 10.1016/j.nec.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. New England Journal of Medicine. 1979;301(25):1364–1369. doi: 10.1056/NEJM197912203012503. [DOI] [PubMed] [Google Scholar]
- 9.Luft HS, Hunt SS, Maerki SC. The volume-outcome relationship: practice-makes-perfect or selective-referral patterns? Health services research. 1987;22(2):157. [PMC free article] [PubMed] [Google Scholar]
- 10.Merrill AL, Jha AK, Dimick JB. Clinical Effect of Surgical Volume. New England Journal of Medicine. 2016;374(14):1380–1382. doi: 10.1056/NEJMclde1513948. [DOI] [PubMed] [Google Scholar]
- 11.Cowan JA, Jr, Dimick JB, Leveque JC, Thompson BG, Upchurch GR, Jr, Hoff JT. The impact of provider volume on mortality after intracranial tumor resection. Neurosurgery. 2003 Jan;52(1):48–53. doi: 10.1097/00006123-200301000-00005. discussion 53–44. [DOI] [PubMed] [Google Scholar]
- 12.Cowan JA, Jr, Dimick JB, Thompson BG, Stanley JC, Upchurch GR., Jr Surgeon volume as an indicator of outcomes after carotid endarterectomy: an effect independent of specialty practice and hospital volume. Journal of the American College of Surgeons. 2002 Dec;195(6):814–821. doi: 10.1016/s1072-7515(02)01345-5. [DOI] [PubMed] [Google Scholar]
- 13.Jain N, Pietrobon R, Guller U, Shankar A, Ahluwalia AS, Higgins LD. Effect of provider volume on resource utilization for surgical procedures of the knee. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2005 May;13(4):302–312. doi: 10.1007/s00167-004-0516-6. [DOI] [PubMed] [Google Scholar]
- 14.Jain NB, Pietrobon R, Guller U, Ahluwalia AS, Higgins LD. Influence of provider volume on length of stay, operating room time, and discharge status for rotator cuff repair. Journal of shoulder and elbow surgery / American Shoulder and Elbow Surgeons … [et al] 2005 Jul-Aug;14(4):407–413. doi: 10.1016/j.jse.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Lawton MT, Du R. Effect of the neurosurgeon’s surgical experience on outcomes from intraoperative aneurysmal rupture. Neurosurgery. 2005 Jul;57(1):9–15. doi: 10.1227/01.neu.0000163082.20941.ef. discussion 19–15. [DOI] [PubMed] [Google Scholar]
- 16.Cowan JA, Jr, Dimick JB, Leveque J-C, Thompson BG, Upchurch GR, Jr, Hoff JT. The impact of provider volume on mortality after intracranial tumor resection. Neurosurgery. 2003;52(1):48–54. doi: 10.1097/00006123-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 17.De la Garza-Ramos R, Abt NB, Kerezoudis P, Krauss W, Bydon M. Provider volume and short-term outcomes following surgery for spinal metastases. Journal of Clinical Neuroscience. 2016;24:43–46. doi: 10.1016/j.jocn.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. New England Journal of Medicine. 2011;364(22):2128–2137. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu C-E, Lin T-K, Lee M-H, et al. The Impact of Surgical Experience on Major Intraoperative Aneurysm Rupture and Their Consequences on Outcome: A Multivariate Analysis of 538 Microsurgical Clipping Cases. PloS one. 2016;11(3):e0151805. doi: 10.1371/journal.pone.0151805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez AA, Dimick JB, Birkmeyer JD, Ghaferi AA. Understanding the volume-outcome effect in cardiovascular surgery: the role of failure to rescue. JAMA surgery. 2014 Feb;149(2):119–123. doi: 10.1001/jamasurg.2013.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jollis JG, Romano PS. Volume-outcome relationship in acute myocardial infarction: the balloon and the needle. Jama. 2000 Dec 27;284(24):3169–3171. doi: 10.1001/jama.284.24.3169. [DOI] [PubMed] [Google Scholar]
- 22.LETTER T. Estimates of annual spinal cord stimulator implant rises in the United States. 2009. [DOI] [PubMed] [Google Scholar]
- 23.Kumar K, Taylor RS, Jacques L, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008 Oct;63(4):762–770. doi: 10.1227/01.NEU.0000325731.46702.D9. discussion 770. [DOI] [PubMed] [Google Scholar]
- 24.Ohnmeiss DD, Rashbaum RF. Patient satisfaction with spinal cord stimulation for predominant complaints of chronic, intractable low back pain. The spine journal : official journal of the North American Spine Society. 2001 Sep-Oct;1(5):358–363. doi: 10.1016/s1529-9430(01)00083-3. [DOI] [PubMed] [Google Scholar]
- 25.Alo KM, Redko V, Charnov J. Four Year Follow-up of Dual Electrode Spinal Cord Stimulation for Chronic Pain. Neuromodulation : journal of the International Neuromodulation Society. 2002 Apr;5(2):79–88. doi: 10.1046/j.1525-1403.2002.02017.x. [DOI] [PubMed] [Google Scholar]
- 26.Huang KT, Martin J, Marky A, et al. A national survey of spinal cord stimulation trial-to-permanent conversion rates. Neuromodulation : journal of the International Neuromodulation Society. 2015 Feb;18(2):133–139. doi: 10.1111/ner.12199. discussion 139–140. [DOI] [PubMed] [Google Scholar]
- 27.Mathew L, Winfree C, Miller-Saultz D, Sonty N. Transcutaneous electrical nerve stimulator trial may be used as a screening tool prior to spinal cord stimulator implantation. Pain. 2010 Aug;150(2):327–331. doi: 10.1016/j.pain.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Oakley JC, Krames ES, Stamatos J, Foster AM. Successful long-term outcomes of spinal cord stimulation despite limited pain relief during temporary trialing. Neuromodulation : journal of the International Neuromodulation Society. 2008 Jan;11(1):66–73. doi: 10.1111/j.1525-1403.2007.00145.x. [DOI] [PubMed] [Google Scholar]
- 29.Olson KA, Bedder MD, Anderson VC, Burchiel KJ, Villanueva MR. Psychological variables associated with outcome of spinal cord stimulation trials. Neuromodulation : journal of the International Neuromodulation Society. 1998 Jan;1(1):6–13. doi: 10.1111/j.1525-1403.1998.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 30.Rainov NG, Heidecke V, Burkert W. Short test-period spinal cord stimulation for failed back surgery syndrome. Minimally invasive neurosurgery : MIN. 1996 Jun;39(2):41–44. doi: 10.1055/s-2008-1052214. [DOI] [PubMed] [Google Scholar]
- 31.Son BC, Kim DR, Lee SW, Chough CK. Factors associated with the success of trial spinal cord stimulation in patients with chronic pain from failed back surgery syndrome. Journal of Korean Neurosurgical Society. 2013 Dec;54(6):501–506. doi: 10.3340/jkns.2013.54.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinand ME, Madhusudan H, Davis B, Melgar M. Acute vs. Prolonged Screening for Spinal Cord Stimulation in Chronic Pain. Neuromodulation : journal of the International Neuromodulation Society. 2003 Jan;6(1):15–19. doi: 10.1046/j.1525-1403.2003.03002.x. [DOI] [PubMed] [Google Scholar]
- 33.Williams KA, Gonzalez-Fernandez M, Hamzehzadeh S, et al. A multi-center analysis evaluating factors associated with spinal cord stimulation outcome in chronic pain patients. Pain medicine (Malden, Mass) 2011 Aug;12(8):1142–1153. doi: 10.1111/j.1526-4637.2011.01184.x. [DOI] [PubMed] [Google Scholar]
- 34.Kumar K, Wilson J. Factors affecting spinal cord stimulation outcome in chronic benign pain with suggestions to improve success rate. Springer; 2007. [DOI] [PubMed] [Google Scholar]
- 35.Huang KT, Hazzard MA, Babu R, et al. Insurance disparities in the outcomes of spinal cord stimulation surgery. Neuromodulation : journal of the International Neuromodulation Society. 2013 Sep-Oct;16(5):428–434. doi: 10.1111/ner.12059. discussion 434–425. [DOI] [PubMed] [Google Scholar]
- 36.Kumar K, Toth C, Nath RK, Laing P. Epidural spinal cord stimulation for treatment of chronic pain–some predictors of success. A 15-year experience. Surgical neurology. 1998 Aug;50(2):110–120. doi: 10.1016/s0090-3019(98)00012-3. discussion 120–111. [DOI] [PubMed] [Google Scholar]
- 37.Babu R, Hazzard MA, Huang KT, et al. Outcomes of percutaneous and paddle lead implantation for spinal cord stimulation: a comparative analysis of complications, reoperation rates, and health-care costs. Neuromodulation : journal of the International Neuromodulation Society. 2013 Sep-Oct;16(5):418–426. doi: 10.1111/ner.12065. discussion 426–417. [DOI] [PubMed] [Google Scholar]
- 38.Jang HD, Kim MS, Chang CH, Kim SW, Kim OL, Kim SH. Analysis of failed spinal cord stimulation trials in the treatment of intractable chronic pain. Journal of Korean Neurosurgical Society. 2008 Feb;43(2):85–89. doi: 10.3340/jkns.2008.43.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haddadan K, Krames ES. The effect of spinal cord stimulation, overall, and the effect of differing spinal cord stimulation technologies on pain, reduction in pain medication, sleep, and function. Neuromodulation : journal of the International Neuromodulation Society. 2007 Apr;10(2):156–163. doi: 10.1111/j.1525-1403.2007.00104.x. [DOI] [PubMed] [Google Scholar]
- 40.Kinfe TM, Schu S, Quack FJ, Wille C, Vesper J. Percutaneous implanted paddle lead for spinal cord stimulation: technical considerations and long-term follow-up. Neuromodulation : journal of the International Neuromodulation Society. 2012 Jul;15(4):402–407. doi: 10.1111/j.1525-1403.2012.00473.x. [DOI] [PubMed] [Google Scholar]
- 41.Gharibo C, Laux G, Forzani BR, Sellars C, Kim E, Zou S. State of the Field Survey: Spinal Cord Stimulator Use by Academic Pain Medicine Practices. Pain Medicine. 2014;15(2):188–195. doi: 10.1111/pme.12264. [DOI] [PubMed] [Google Scholar]
- 42.Henderson JM, Levy RM, Bedder MD, et al. NANS Training Requirements for Spinal Cord Stimulation Devices: Selection, Implantation, and Follow-up. Neuromodulation: Technology at the Neural Interface. 2009;12(3):171–174. doi: 10.1111/j.1525-1403.2009.00211.x. [DOI] [PubMed] [Google Scholar]
- 43.Hayek SM, Veizi E, Hanes M. Treatment-Limiting Complications of Percutaneous Spinal Cord Stimulator Implants: A Review of Eight Years of Experience From an Academic Center Database. Neuromodulation: Technology at the Neural Interface. 2015;18(7):603–609. doi: 10.1111/ner.12312. [DOI] [PubMed] [Google Scholar]
- 44.Sears NC, Machado AG, Nagel SJ, et al. Long-Term Outcomes of Spinal Cord Stimulation With Paddle Leads in the Treatment of Complex Regional Pain Syndrome and Failed Back Surgery Syndrome. Neuromodulation: Technology at the Neural Interface. 2011;14(4):312–318. doi: 10.1111/j.1525-1403.2011.00372.x. [DOI] [PubMed] [Google Scholar]
- 45.Taylor RS, Buyten JP, Buchser E. Spinal cord stimulation for complex regional pain syndrome: A systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. European Journal of Pain. 2006;10(2):91–91. doi: 10.1016/j.ejpain.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Tharmanathan P, Adamson J, Ashby R, Eldabe S. Diagnosis and treatment of failed back surgery syndrome in the UK: mapping of practice using a cross-sectional survey. British journal of pain. 2012 doi: 10.1177/2049463712466321. 2049463712466321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vyawahare B, Hallas N, Brookes M, Taylor RS, Eldabe S. Impact of the National Institute for Health and Care Excellence (NICE) guidance on medical technology uptake: analysis of the uptake of spinal cord stimulation in England 2008–2012. BMJ open. 2014;4(1):e004182. doi: 10.1136/bmjopen-2013-004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


