Abstract
Objective(s):
This study investigated the protective effect of tanshinone IIA sodium sulfonate (TSS) on ischemia-reperfusion (I/R) induced cardiac injury, and the underlying mechanism of action.
Materials and Methods:
Male Sprague-Dawley rats were subjected to a 30-min coronary arterial occlusion followed by 24 hours’ reperfusion. Half an hour before the left coronary artery ligation, rats were pretreated with TSS in three different dosages (15, 30, 70 mg/kg, IP). Twenty-four hours later, cardiac function was measured and the ratio of infarct size to area at risk (AAR) was calculated. Western blotting examined the expression of the inflammatory mediator high-mobility group box1 (HMGB-1), anti-apoptotic protein Bcl-2, pro-apoptotic mediators such as Bax and Caspase-3, markers of autophagy such as ratio of LC3B/LC3A and Beclin-1 expression.
Results:
Our results showed that TSS dose-dependently improves cardiac function, accompanied with decrease of HMGB1 level, increase of LC3B/LC3A ratio and increase of Beclin-1 expression. TSS treatment down-regulates Bax and Caspase-3 expression, while up-regulating Bcl-2 levels.
Conclusion:
TSS ameliorates I/R induced myocardial injury and improves cardiac function via reducing inflammation and apoptosis, while enhancing autophagy.
Keywords: Apoptosis, Autophagy, Ischemia/reperfusion (I/R), Tanshinone IIA sodium-sulfonate (TSS)
Introduction
Myocardial infarction (MI) is still a major public health problem worldwide with high mortality and morbidity (1, 2). While it is critical to re-establish blood flow as early as possible, reperfusion injury will be sustained. Various signaling pathways are responsible for inducing reperfusion injury, and exacerbating ischemic damage of the cardiac tissue (3). Therefore, exploring novel pharmacological agents to help salvage ischemia-reperfusion (I/R) damaged cardiac tissue may provide beneficial clinical outcomes for MI patients.
The mechanisms responsible for I/R injury have been widely investigated. Calcium overload, excessive production of reactive oxygen species (ROS), and the release of inflammatory factors are the major causative factors of cardiac I/R injury (4, 5). All of these factors finally contribute to the cardiomyocyte death, by necrosis and apoptosis, resulting in a decline of myocardial tissue function (6, 7). Inhibiting cardio-myocyte apoptosis is a proven strategy to protect against I/R injury. Moreover, up-regulation of the pro-inflammatory factors, chemokines, cytokines, and adhesive molecules also contribute to I/R induced tissue injury. It has been demonstrated that blockade of high-mobility group box 1 (HMGB-1), a pro-inflammatory mediator, can suppress inflammation, and attenuate myocardial apoptosis and I/R injury (8, 9).
Autophagy reportedly protects against cardiac injury (10). Moreover, autophagy was found to be impaired in cardiac I/R-induced injury (11, 12). We previously observed that autophagic markers, such as Beclin-1 and the ratio of LC3B/LC3A, were increased in response to cardiac I/R injury (13). Up-regulation of autophagy protects against myocardial I/R injury in the clinically relevant in vivo swine model of acute myocardial infarction (14). To date, data are still inconsistent about the regulation of autophagy signal pathway in response to I/R. Mild-to-moderate I/R may up-regulate autophagy level to play protective effects; however, severe I/R may impair autophagy levels thereafter play a detrimental role in the context of I/R.
Tanshinone IIA sodium sulfonate (TSS) is one of the major active constituents of Salvia miltiorrhiza bunge, which has been widely used for thousands of years in China for the treatment of various microcirculatory disturbance-related diseases (15). Previous studies have also shown that TSS is used for prevention and treatment of numerous diseases (8), (16). In the circulatory system, it has been demonstrated that TSS could protect against ischemia-reperfusion injury via inducing coronary artery vasodilatation (17). Recently, a number of studies have confirmed the protective effects of TSS in animal models such as improving cardiac function, limiting infarct size and exerting anti-apoptotic effect in response to I/R injury (18, 19). However, the effects and possible mechanisms of TSS on I/R induced cardiac injury require further investigation.
We hypothesized that cardioprotective effects of TSS involve not only the anti-apoptotic, but also its anti-inflammatory, as well as its pro-autophagic effects.
Materials and Methods
Experimental animals
Ten-week-old male Sprague-Dawley rats were purchased from Shanghai Laboratory Animal Center. Rats were housed under optimal conditions with standard hygiene, kept at a temperature of 25 °C with a 12/12 light/dark cycle, fed with standard rat chow and water ad libitum. The experiments were performed in accordance with the National Institutes of Health Guidelines for the Use of Laboratory Animals (NIH, publication number 85-23, revised 1996.), which were approved and performed according to guidelines for the care and use of animals established by Soochow University.
Myocardial I/R model
The I/R model was performed as described previously (20). Briefly, rats were anesthetized with 10% chloral anesthesia (350 mg/kg, IP), cardiac I/R was performed by exposing the heart at the fifth intercostal space followed by a slipknot (6-0 silk) below the left descending coronary artery. Regional left ventricular ischemia was performed via occlusion of the coronary artery by clamping it together with the propylene tube. After 30 min of ischemia, the slipknot was released and followed by 24 hr of reperfusion. Half an hour before the left coronary artery ligation, rats were pretreated intraperitoneally with TSS (offered by Shanghai NO.1 Biochemical & Pharmaceutical Co, Ltd) in three different doses (15, 30, 70 mg/kg), which was according to previous observation with the TTS dose from 1-30 mg/kg and effectively inhibited the infarct from dose of 10 mg/kg (21). Rats were randomized into five groups: (1) sham group, rats received same procedure without occlusion of coronary artery, (n=10); (2) I/R group, rats received the same volume of saline alone, (n=10); (3) I/R+TSS-L group, I/R rats received TSS 15 mg/kg (n=10); (4) I/R+TSS-M group, I/R group received TSS 30 mg/kg (n=9); (5) I/R+TSS-H group, I/R rats received TSS 70 mg/kg group (n=9).
Cardiac function measurements
Twenty-four hours after reperfusion, rats were anesthetized with 10% chloral anesthesia (350 mg/kg, IP), and hemodynamic parameters were measured using a heart performance analysis system (ALCBIO, Shanghai Alcott Biotech CO., LTD.). The left femoral artery and right common carotid artery were isolated. A polystyrene PE-50 catheter was inserted into the left ventricle via right common carotid artery, with the other end connected to the analysis system. The major parameters of cardiac function were derived or calculated from the continuously obtained pressure signal and included systolic arterial pressure (SAP), the rate of maximum positive and negative left ventricular pressure development (±LVdp/dtmax), and the left ventricular end-diastolic pressure (LVEDP).
Measurement of ratio of myocardial infarct area to area at risk
After rat cardiac function was measured under anesthetized condition with 10% chloral anesthesia (350 mg/kg, IP), rat hearts were excised immediately after cardiac function measurements and perfused with Evans blue (1%, 4 ml) via the coronary artery under ligation of the left descending coronary artery with the remaining sutures. Hearts were traversely cut into 1-2 mm slices along the ligation point, placed in 1.25% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma, USA) solution in phosphate-buffered saline (PBS), incubate for 10 min at 37 °C. The ischemic regions (area at risk, AAR) and the infarct area (white area is not stained by TTC) were recorded by digital camera, and the blue area (stained by Evans blue; non-ischemic area) were analyzed with a digital imaging system (NIH image software). The ratio of myocardial infarct area to area-at-risk (AAR) was calculated.
Western blot analysis
Myocardial tissues (AAR tissue) were homogenized with radioimmunoprecipitation assay (RIPA) buffer (50 mm Tris, pH 7.0, 150 mM NaCl, 1% Triton-X-100) containing phenylmenthanesulfonyl fluoride (R&D Systems Inc., Minneapolis, US). Homogenates were centrifuged at 12,000 × g for 10 min at 4 °C. Cell protein were separated by SDS-PAGE and transferred to PVDF membranes (Hybond TM-ECL; Amersham Pharmacia Biotech, Inc.). The membranes were blocked in 5% nonfat milk in PBS and 0.1% Tween-20 at room temperature. The blots were then incubated with primary antibody: anti-Caspase-3 antibody (1:1000, abcam, Inc.), anti-Bcl-2 antibody (1:1000, Immunoway Biotech, Inc.), anti-Bax antibody (1:1000, abcam, Inc.), anti-Beclin-1 (1:1000, Santa Cruz Biotech, Inc.), anti-LC3 (1:1000, abcam, Inc.), anti-HMGB1 (1:1000, abcam, Inc.), or anti- glyceraldehyde 3-phosphate dehydro -genase (GAPDH) (Santa Cruz Biotech, Inc.). Then the membranes were incubated for 1 hour with a secondary antibody (HRP-conjugated anti-rabbit Ig-G, 1:2000). Excess antibody was washed off with TBS-T three times (15 min each) before incubation enhanced chemiluminescent reagent (ECL, R&D Systems Inc, Minneapolis, USA) for 1 min. Subsequently, the membrane was exposed to X-ray film. Immunoreactive bands were detected by the analysis of X-ray films using the software of Image J. The quantity of target proteins was normalized by GAPDH expression.
Statistical analysis
The SPSS 18.0 software was used for statistical analysis. Data were presented as the mean±SEM. Grouped data were analyzed using a one-way analysis of variance followed by the Student-Newman-Keuls test. A P<0.05 was considered to be statistically significant.
Results
TSS improves cardiac function after I/R injury
To determine the effects of TSS on cardiac function in rats subject to I/R injury, cardiac function measure-ments were performed 24 hr after reperfusion. I/R significantly decreases cardiac function relative to the sham control group, by decreasing the SAP, Pmax, ± dp/dtmax, LVEDP and other parameters (Table 1). TSS dose-dependently improves the cardiac function parameters relative to I/R group.
Table 1.
Effects of tanshinone IIA sodium sulfonate (TSS) on cardiac function in response to I/R in rat
| Sham | I/R | I/R+TSS-L | I/R+TSS-M | I/R+TSS-H | |
|---|---|---|---|---|---|
| HR(bpm) | 332.5±20.4 | 369.5±16.6 | 377.8±33.1 | 354.4±25.4 | 374.6±34.3 |
| RRI(ms) | 205.5±16.5 | 177.6±11.2 | 195.4±37.5 | 197.3±20.0 | 236.2±71.5 |
| SAP (mmHg) | 83.8±2.9 | 61.7±8.8* | 68.9±8.4 | 89.6±3.3† | 77.1±5.7 |
| DAP (mmHg) | 61.1±3.0 | 47.2±6.4 | 50.8±7.3 | 66.2±3.8 | 58.7±4.1 |
| MAP (mmHg) | 70.3±2.3 | 53.4±7.3 | 57.9±7.6 | 75.8±3.1 | 66.4±4.5 |
| PP (mmHg) | 22.7±3.8 | 14.5±3.1 | 18.2±3.2 | 23.3±3.8 | 18.4±3.8 |
| Pmax (mmHg) | 98.2±2.5 | 68.9±3.9* | 84.8±4.8† | 93.1±1.2† | 93.3±1.8† |
| Pmin (mmHg) | 2.8±0.9 | 8.0±3.5 | 6.8±1.3 | 7.8±2.1 | 4.1±1.2 |
| Pmean (mmHg) | 42.9±1.8 | 30.9±7.2* | 42.4±3.0† | 49.6±1.6† | 44.5±2.0† |
| Lvedp (mmHg) | 15.4±2.3 | 43.8±5.9* | 35.4±5.6 | 22.8±3.8† | 22.2±4.9† |
| P@dp/dtmax (mmHg) | 69.3±2.2 | 43.8±9.5 | 65..6±4.9 | 76.4±2.6 | 68.7±3.6 |
| P@-dp/dtmax (mmHg) | 50.4±2.4 | 32.6±7.6 | 53.3±3.3 | 58.8±2.5 | 55.2±2.0 |
| RPP | 32449±1761 | 21823±4507 | 31632±2907 | 35224±2321 | 35558±3181 |
| dp/dtmax (mmHg/s) | 4943±142 | 2262±187* | 3265±120† | 4297±203† | 3901±133† |
| -dp/dtmax (mmHg/s) | 4479±155 | 2047±161* | 2787±257† | 4010±176† | 3859±189† |
| At(CFL)(CFU) | 102.2±15.4 | 25.1±2.6* | 59.6±13.2† | 92.0±16.6† | 90.1±4.1† |
| A1(CFL)(CFU) | 28.9±5.1 | 7.1±0.8* | 13.3±3.4† | 23.5±6.3† | 13.6±0.9† |
| A2(CFL)(CFU) | 18.5±0.8 | 5.3±0.6* | 10.1±1.2† | 17.1±3.1† | 13.3±0.7† |
| A3(CFL)(CFU) | 15.0±0.7 | 5.8±0.4* | 12.6±3.7† | 15.2±1.2† | 13.7±0.9† |
| A4(CFL)(CFU) | 35.6±9.1 | 6.3±1.5 | 23.6±7.4 | 36.2±9.2 | 49.5±22.5 |
| As(CFL)(CFU) | 47.5±5.2 | 11.8±1.3* | 23.5±4.4† | 40.5±8.4† | 26.8±1.3† |
| Ad(CFL)(CFU) | 50.8±9.8 | 10.9±2.3* | 36.2±9.5† | 51.5±10.1† | 63.2±22.6† |
| Smax (CRHL) | 313.7±15.4 | 151.8±11.4* | 201.5±13.9† | 297.5±35.3† | 241.0±12.5† |
| Smin (CRHL)(mmHg2/s2) | -408.3±18.6 | -157.1±14.5* | -253.3±22.3† | -359.2±27.6† | -271.3±21.9† |
| Dmax (CRHL)(mmHg2/s) | 321.3±15.2 | 178.7±23.2* | 197.3±31.2 | 312.6±22.2† | 247.6±23.3† |
| Dmin (CRHL)(mmHg2/s2) | -299.9±16.8 | -135.3±17.8* | -226.3±25.9† | -320.0±24.5† | -252.4±22.2† |
Table 1. Cardiac function parameters. HR (Heart rate), RRI (the R-R interval), SAP (Systolic arterial pressure), DAP (Diastolic arterial pressure), MAP (Mean arterial pressure), PP (Pulse pressure), Pmax (the maximum of left ventricular pressure development), Pmin (the minimum of left ventricular pressure development), Pmean (the mean of ventricular pressure development), LVEDP (left ventricular end-diastolic pressure), P@dp/dtmax (the left ventricular pressure corresponding to the rates of maximum positive left ventricular pressure development), P@-dp/dtmax (the left ventricular pressure corresponding to the rates of maximum negative left ventricular pressure development), RPP (rate pressure product), +dp/dtmax (rates of maximum positive left ventricular pressure development), -dp/dtmax (rates of maximum negative left ventricular pressure development), CFL (cardiac force loop), At(CFL) (total area of CFL), A1(CFL) (area of the first CFL), A2(CFL) (area of the second CFL), A3(CFL) (area of the third CFL), A4(CFL) (area of the forth CFL), As(CFL) (systolic area of CFL), Ad(CFL) (diastolic area of CFL), CRHL (contraction relaxation harmoniousness loop), Smax (CRHL) (the maximum of positive left ventricular systolic pressure of d2p/dt2), Smin (CRHL) (the maximum of negative left ventricular systolic pressure of d2p/dt2), Dmax (CRHL) (the maximum of positive left ventricular diastolic pressure of d2p/dt2), Dmin (CRHL) (the maximum of negative left ventricular diastolic pressure of d2p/dt2) were measured by a cardiac function analysis system. Values were expressed as mean ± SEM. Sham: Sham group (n=10); I/R: ischemic/reperfusion group (n=10). I/R+TSS-L: Low dose (15mg/kg) TSS pretreatment group (n=10). I/R+TSS-M: Mean dose (30 mg/kg) TSS pretreatment group (n=9). I/R+TSS-H: High dose (70 mg/kg) TSS pretreatment group (n=9).
P<0.05 compared with sham group.
P<0.05 compared with I/R group
TSS reduces myocardial infarct size after I/R injury
Cardiomyocyte injury is characterized by myocar-dial infarct size. To determine whether TSS attenuates I/R-induced cardiomyocyte injury, ratio of infarct size to AAR was calculated. Our data show that TSS significantly reduces the ratio of infarct size to AAR in a dose-dependent manner (Figure 1).
Figure 1.
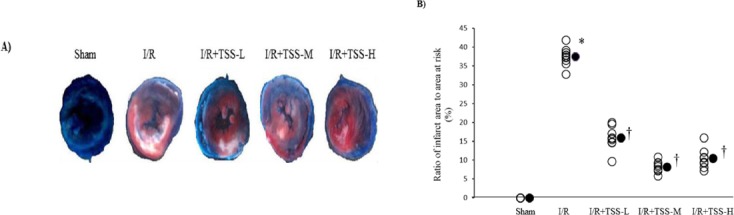
Effects of tanshinone IIA sodium sulfonate (TSS) on myocardial infarct size of I/R rats. A: Cross-section of rat left ventricle ring following myocardial I/R injury. Representative photos show the effect of TSS pretreatment reducing infarct size. B: The ratio of infarct size (white area is not stained by TTC) to area at risk (red area is stained by TTC) was measured, Sham: Sham group (n=10); I/R: ischemic/reperfusion group (n=10). I/R+TSS-L: Low dose (15 mg/kg) TSS pretreatment group (n=10). I/R+TSS-M: Mean dose (30 mg/kg) TSS pretreatment group (n=9). I/R+TSS-H: High dose (70 mg/kg) SS pretreatment group (n=9). All data were expressed as mean±SEM. *P<0.05 compared with sham group. † P<0.05 compared with I/R group
TSS suppresses inflammatory cytokine HMGB1 expression in rat I/R model
To investigate the effects of TSS on inflammatory factors, the expression of HMGB1 was determined by western blot analysis. The data demonstrated that cardiac I/R markedly increased the HMGB1 protein expression compared with the sham group (P<0.05, Figure 2). Pretreatment with TSS significantly suppresses HMGB1 expression compared with the I/R group (P<0.05, Figure 2). These results suggest that TTS may exert protective effect through inhibition of inflammatory cytokine expression.
Figure 2.
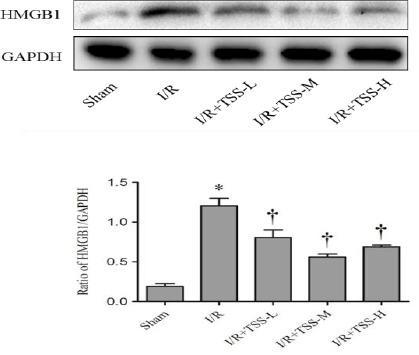
Effect of tanshinone IIA sodium sulfonate (TSS) on the expression of HMGB1 protein. Top: the representative Western blot of each group. Down: Densitometric analysis of HMGB1 expression normalized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. All data were expressed as mean±SEM. *P<0.05 compared with sham group. † P<0.05 compared with I/R group
TSS inhibits expression of pro-apoptotic proteins and increases expression of anti-apoptotic proteins after myocardial I/R
Bcl-2 and Bax genes are reported to play a crucial role in cell survival or death after apoptotic stimuli (22). Caspase-3 is also an important component of the apoptotic pathway. The effect of TSS on Bcl-2, Bax, and caspase-3 expression in myocardial tissue were analyzed by Western blot. Compared with those in sham group, I/R treatment significantly increases the levels of caspase-3 (P<0.05, Figure 3B) and Bax (P<0.05, Figure 3C), and decreases the levels of Bcl-2 (P<0.05, Figure 3D). Compared with those in I/R group, pretreatment with TSS significantly reduces levels of caspase-3 (P<0.05, Figure 3B) and Bax (P<0.05, Figure 3C), and enhances the levels of Bcl-2 (P<0.05, Figure 3D). These results indicate that TSS could reduce I/R induced cardiac apoptosis.
Figure 3.
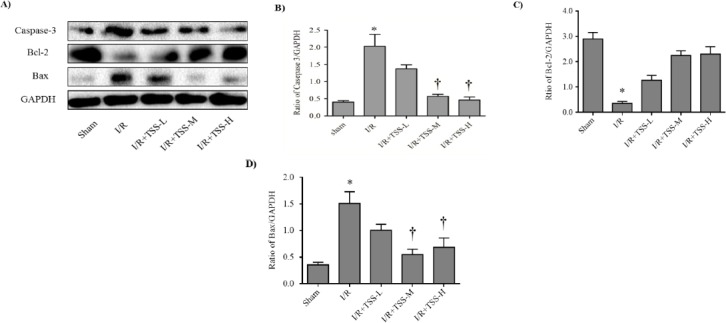
Effects of tanshinone IIA sodium sulfonate (TSS) on the expression of Bcl-2, Bax, Caspase-3. A: the representative Western blot for each group (n=9). B: Densitometric analysis of caspase-3 expression normalized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression, C: Densitometric analysis of Bcl-2 expression normalized by GAPDH expression, D: Densitometric analysis of Bax expression normalized by GAPDH expression. All data were expressed as mean±SEM. *P<0.05 compared with sham group. † P<0.05 compared with I/R group
TSS up-regulates the expression of Beclin-1 and increases the ratio of LC3B to LC3A in I/R model rat
To investigate the effects of TSS on autophagy levels, the expression of Beclin-1 and the ratio of LC3B to LC3A were determined by western blot analysis. Figure 4B) and the ratio of LC3B to LC3A (P<0.05, Figure 4C). Pretreatment with TSS increases the expression of Beclin-1 (P<0.05, Figure 4B) and ratio of LC3B to LC3A (P<0.05, Figure 4C) in a dose-dependent manner compared with the I/R group. These results suggest that TSS may protect cardiac I/R induced injury via up-regulation of autophagy levels.
Figure 4.
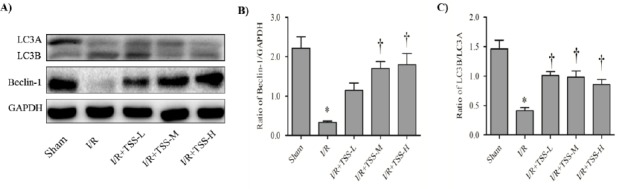
Effects of tanshinone IIA sodium sulfonate (TSS) on the expression of Beclin-1 and the ratio of LC3B/LC3A. A: the representative Western blot of each group. B: Densitometric analysis of Beclin-1 expression normalized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression, C: ratio of LC3B/LC3A. All data were expression as mean±SEM. *P<0.05 compared with sham group. † P<0.05 compared with I/R group
Discussion
Although the rapid restoration of blood flow through the occluded left coronary artery is the most effective therapy to reduce infarct area and enhance the clinical outcome after acute myocardial infarction. Reperfusion itself causes additional cardiomyocyte apoptosis and triggers inflammation in a process termed ‘myocardial I/R injury’ (23). The major finding of present study is that TSS could improve cardiac function and reduce myocardial injury by inhibiting cell apoptosis cascades, reducing the release of inflammatory factors, and activating autophagic pathways.
The most important therapy in response to cardiac I/R injury is to restore cardiac function to prevent organ failure. The preservation of cardiac function is the critical benchmark to measure when evaluating the clinical efficacy of various treatments for infarct patients (24). Previous reports have demonstrated that TSS could prevent I/R-induced cardiac injury (18, 21, 25). However, these observations do not provide data of the effect of TSS on cardiac function in response to I/R. In our study, we clearly demonstrated that TSS improves cardiac function after I/R injury. Our data also shows that TSS ameliorates both the constriction and relaxation function of the heart, which can be observed from SAP, Pmax, ± dp/dtmax and LVEDP data. Our results strongly support the protective effects of TSS on I/R induced cardiac injury. It should be noted that previous studies have demonstrated that TTS exerts protective effects even with lower dose of 10 mg/kg (21) or 20 mg/kg (25) than our effective dose of 30 mg/kg, this discrepancy may be due to the different models applied. Since, in the present study we could not find any more protective effects of TTS at dose of 70 mg/kg compared with dose of 30 mg/kg, we assume that the most effective dose of TTS is around 30 mg/kg.
Traditionally, it has been well recognized that cellular responses to I/R is highly related to the activation of apoptotic pathways, and various strategies were explored to suppress the activation of apoptosis (23, 26-28). As reported previously, TSS protects cardiomyocytes against oxidative stress-mediated apoptosis through inhibiting JNK activation (21). Findings have also demonstrated that TSS inhibits apoptosis of H9c2 cardiomyocytes via Akt and ERK1/2 phosphorylation (18). TSS protects rat myocardium against ischemia-reperfusion injury via activation of PI3K/Akt/FOXO3A/Bim pathway (25). We demonstrated that TSS dose-dependently suppresses the expression of pro-apoptotic proteins Bax and caspase-3, and up-regulates anti-apoptotic protein Bcl-2. These data suggest that TSS regulates the balance of pro-apoptotic and anti-apoptotic signaling pathways to exert protective effects, which are concordance with previous studies (18, 21, 25). Also, it should be noted that although our present observation does not focus on the effects of TTS on apoptotic signal pathways, we just speculated that TTS exerts anti-apoptotic effects via above-mentioned signal pathways such as Akt and ERK1/2 phosphorylation (18) and PI3K/Akt/FOXO3A/Bim pathway (25). More investigation should be performed to explore if there is other anti-apoptotic signal pathway involved in cardiac protective effects of TTS on I/R injury.
Under stress conditions, autophagy is activated either to meet the increased requirements for repair and detoxification, as a result of exposure to various damaging factors, or to produce energy and deliver building blocks for anabolic processes under starvation. Reportedly, autophagy has protective role in cardiac I/R injury (29). Up-regulation of autophagy increases resistance to myocardial I/R injury in the clinically relevant in vivo swine model of acute MI (14). Recently, it was also demonstrated that autophagy is impaired in cardiac I/R injury (11). We previously demonstrated that autophagic pathways are activated under cardiac I/R (13). Although several studies have demonstrated that TSS could protect I/R induced cardiac injury through various mechanisms, its effects on autophagy are unclear. This study shows that TSS up-regulates autophagic makers, Beclin-1 and the ratio of LC3B/LC3A. This suggests a novel mechanism for the protective effects of TSS in I/R induced cardiac injury model, which may occur via the activation of self-repair mechanisms to rescue damaged cells. Whether TSS up-regulates autophagic levels via two pathways responsible for I/R-induced autophagy involving either BNIP3 (30) or AMPK (31) still needs further investigation.
The activation of inflammatory cytokines and their contribution to I/R-induced cardiac injury have been widely investigated. HMGB-1, as a pro-inflammatory mediator, which is a non-chromosomal nuclear protein that maintains the nucleosome and regulates gene transcription, is released by necrotic cells, and activates innate macrophages, monocytes and apoptotic cells (32). HMGB-1 can significantly promote the apoptosis of neonatal myocytes and decrease the cell viability (32, 33). The HMGB1-TLR4 axis contributes to myocardial I/R injury via the up-regulation of cardiomyocyte apoptosis (9). Furthermore, it has been reported that HMGB-1 is related to tissue autophagy (34), which may also contribute the cell repair or damage. Several studies have demonstrated that TSS regulates the expression of inflammatory factors (35, 36). However, the effect of TSS on HMGB-1 remains unexplored. We demonstrated in this study that TSS suppresses HMGB-1 levels in cardiac tissue in response to I/R injury. This suggests that the protective effects of TSS may also occur via the regulation of pro-inflammatory factor HMGB-1.
It should be noted that the present study only focused on the short-term cardioprotective effect of TSS against I/R injury in rats. Further studies are needed to clarify whether TSS provides long-term functional cardioprotection, such as anti-cardiac remodeling. In addition, further investigations are required to investigate whether drug combination therapy will provide more beneficial strategies in the clinical application of TSS. However, adverse reactions of TSS administration have been observed clinically (37), which necessitates further studies on the optimal treatment conditions to optimize infract patient outcomes.
Conclusion
We demonstrate that TSS protects against I/R injury via reducing inflammatory factors, inhibiting apoptosis, and inducing autophagy.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (81270316, 81470563), the Research Program of Soochow University (Q413400111), and the Suzhou science and technology development projects (SYS201364, SYS201573).
Conflict of interest
The authors declare that there is no conflict of interest in this study.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez AD, Murray CC. The global burden of disease 1990-2020. Nat Med. 1998;4:1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res. 1999;43:860–878. doi: 10.1016/s0008-6363(99)00187-x. [DOI] [PubMed] [Google Scholar]
- 6.Kwak W, Ha YS, Soni N, Lee W, Park S-I, Ahn H, et al. Apoptosis imaging studies in various animal models using radio-iodinated peptide. Apoptosis. 2015;20:110–121. doi: 10.1007/s10495-014-1059-z. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 8.Zhai C-l, Zhang M-q, Zhang Y, Xu H-x, Wang J-m, An G-p, et al. Glycyrrhizin protects rat heart against ischemia-reperfusion injury through blockade of HMGB1-dependent phospho-JNK/Bax pathway. Acta Pharmacol Sin. 2012;33:1477–1487. doi: 10.1038/aps.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding H-S, Yang J, Chen P, Yang J, Bo S-Q, Ding J-W, et al. The HMGB1-TLR4 axis contributes to myocardial ischemia/reperfusion injury via regulation of cardiomyocyte apoptosis. Gene. 2013;527:389–393. doi: 10.1016/j.gene.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 10.Depre C, Vatner SF. Cardioprotection in stunned and hibernating myocardium. Heart Fail Rev. 2007;12:307–317. doi: 10.1007/s10741-007-9040-3. [DOI] [PubMed] [Google Scholar]
- 11.Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Diwan A. Autophagy is impaired in cardiac ischemia-reperfusion injury. Autophagy. 2012;8:1394–1396. doi: 10.4161/auto.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Hill JA, et al. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation. 2012;125:3170–3181. doi: 10.1161/CIRCULATIONAHA.111.041814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C, Hu L-X, Dong T, Wang G-Q, Wang L-H, Zhou X-P, et al. Apoptosis and autophagy contribute to gender difference in cardiac ischemia–reperfusion induced injury in rats. Life Sci. 2013;93:265–270. doi: 10.1016/j.lfs.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Przyklenk K, Undyala VV, Wider J, Sala-Mercado JA, Gottlieb RA, Mentzer RM., Jr Acute induction of autophagy as a novel strategy for cardioprotection: getting to the heart of the matter. Autophagy. 2011;7:432–433. doi: 10.4161/auto.7.4.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kai G, Xu H, Zhou C, Liao P, Xiao J, Luo X, et al. Metabolic engineering tanshinone biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. Metab Eng. 2011;13:319–327. doi: 10.1016/j.ymben.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Pan L-L, Liu X-H, Jia Y-L, Wu D, Xiong Q-H, Gong Q-H, et al. A novel compound derived from danshensu inhibits apoptosis via upregulation of heme oxygenase-1 expression in SH-SY5Y cells. Biochim Biophys Acta. 2013;1830:2861–2871. doi: 10.1016/j.bbagen.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Wu G-b, Zhou E-x, Qing D-x. Tanshinone II (A) elicited vasodilation in rat coronary arteriole: Roles of nitric oxide and potassium channels. Eur J Pharmacol. 2009;617:102–107. doi: 10.1016/j.ejphar.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 18.Yin Y, Guan Y, Duan J, Wei G, Zhu Y, Quan W, et al. Cardioprotective effect of Danshensu against myocardial ischemia/reperfusion injury and inhibits apoptosis of H9c2 cardiomyocytes via Akt and ERK1/2 phosphorylation. Eur J Pharmacol. 2013;699:219–226. doi: 10.1016/j.ejphar.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Wei B, Li WW, Ji J, Hu QH, Ji H. The cardioprotective effect of sodium tanshinone IIA sulfonate and the optimizing of therapeutic time window in myocardial ischemia/reperfusion injury in rats. Atherosclerosis. 2014;235:318–327. doi: 10.1016/j.atherosclerosis.2014.05.924. [DOI] [PubMed] [Google Scholar]
- 20.Zhang G-X, Kimura S, Murao K, Obata K, Matsuyoshi H, Takaki M. Inhibition of cytochrome c release by 10-N-nonyl acridine orange, a cardiolipin-specific dye, during myocardial ischemia-reperfusion in the rat. Am J Physiol Heart Circ Physiol. 2010;298:H433–H439. doi: 10.1152/ajpheart.00938.2009. [DOI] [PubMed] [Google Scholar]
- 21.Yang R, Liu A, Ma X, Li L, Su D, Liu J. Sodium tanshinone IIA sulfonate protects cardiomyocytes against oxidative stress-mediated apoptosis through inhibiting JNK activation. J Cardiovasc Pharmacol. 2008;51:396–401. doi: 10.1097/FJC.0b013e3181671439. [DOI] [PubMed] [Google Scholar]
- 22.Scarfò L, Ghia P. Reprogramming cell death: BCL2 family inhibition in hematological malignancies. Immunol Lett. 2013;155:36–39. doi: 10.1016/j.imlet.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 24.Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter RJ. Cardioprotection and pharmacological therapies in acute myocardial infarction: Challenges in the current era. World J Cardiol. 2014;6:100–106. doi: 10.4330/wjc.v6.i3.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang M-q, Zheng Y-l, Chen H, Tu J-f, Shen Y, Guo J-p, et al. Sodium tanshinone IIA sulfonate protects rat myocardium against ischemia-reperfusion injury via activation of PI3K/Akt/FOXO3A/Bim pathway. Acta Pharmacol Sin. 2013;34:1386–1396. doi: 10.1038/aps.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagai A, Dangas GD, Stone GW, Granger CB. Reperfusion strategies in acute coronary syndromes. Circ Res. 2014;114:1918–1928. doi: 10.1161/CIRCRESAHA.114.302744. [DOI] [PubMed] [Google Scholar]
- 27.Xu T, Wu X, Chen Q, Zhu S, Liu Y, Pan D, et al. The Anti-Apoptotic and Cardioprotective Effects of Salvianolic Acid A on Rat Cardiomyocytes following Ischemia/Reperfusion by DUSP-Mediated Regulation of the ERK1/2/JNK Pathway. PLoS One. 2014;9:e102292. doi: 10.1371/journal.pone.0102292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umansky SR, Cuenco GM, Khutzian SS, Barr PJ, Tomei LD. Post-ischemic apoptotic death of rat neonatal cardiomyocytes. Cell Death Differ. 1995;2:235–241. [PubMed] [Google Scholar]
- 29.Decker RS, Wildenthal K. Lysosomal alterations in hypoxic and reoxygenated hearts. I. Ultrastructural and cytochemical changes. Am J Pathol. 1980;98:425–444. [PMC free article] [PubMed] [Google Scholar]
- 30.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 31.Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol. 2013;93:865–873. doi: 10.1189/jlb.1212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu X, Zhou X, He B, Xu C, Wu L, Cui B, et al. Minocycline protects against myocardial ischemia and reperfusion injury by inhibiting high mobility group box 1 protein in rats. Eur J Pharmacol. 2010;638:84–89. doi: 10.1016/j.ejphar.2010.03.059. [DOI] [PubMed] [Google Scholar]
- 34.Sun X, Tang D. HMGB1-dependent and-independent autophagy. Autophagy. 2014;10:1873–1976. doi: 10.4161/auto.32184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Q, Chen H, Sheng L, Liang Y, Li Q. Sodium tanshinone IIA sulfonate prolongs the survival of skin allografts by inhibiting inflammatory cell infiltration and T cell proliferation. Int Immunopharmacol. 2014;22:277–284. doi: 10.1016/j.intimp.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Sun N, Li E, Wang Z, Zhao J, Wang S, He J, et al. Sodium tanshinone IIA sulfonate inhibits porcine reproductive and respiratory syndrome virus via suppressing N gene expression and blocking virus-induced apoptosis. Antivir Ther. 2014;19:89–95. doi: 10.3851/IMP2694. [DOI] [PubMed] [Google Scholar]
- 37.Liu HC, Liu HH. [Adverse reactions of tanshinone II(A) sodium sulfonate injection in treating 18 cases: an analysis of clinical features] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013;33:1287–1289. [PubMed] [Google Scholar]


