Abstract
Retroviral vectors were constructed containing a rat beta-glucuronidase cDNA driven by heterologous promoters. Vector-mediated gene transfer into human and canine beta-glucuronidase-deficient mucopolysaccharidosis type VII fibroblasts completely corrected the deficiency in beta-glucuronidase enzymatic activity. In primary cultures of canine mucopolysaccharidosis type VII retinal pigment epithelial cells, which contain large amounts of undegraded glycosaminoglycan substrates, vector correction restored normal processing of specific glycosaminoglycans in the lysosomal compartment. In canine mucopolysaccharidosis type VII bone marrow cells, beta-glucuronidase was expressed at high levels in transduced cells. Thus, the vector-encoded beta-glucuronidase was expressed at therapeutic levels in the appropriate organelle and corrected the metabolic defect in cells exhibiting the characteristic pathology of this lysosomal storage disorder.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier E. H., Davisson M. T., Beamer W. G., Ganschow R. E., Vogler C. A., Gwynn B., Lyford K. A., Maltais L. M., Wawrzyniak C. J. Murine mucopolysaccharidosis type VII. Characterization of a mouse with beta-glucuronidase deficiency. J Clin Invest. 1989 Apr;83(4):1258–1266. doi: 10.1172/JCI114010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. F., Desnick R. J. Affinity purification of alpha-galactosidase A from human spleen, placenta, and plasma with elimination of pyrogen contamination. Properties of the purified splenic enzyme compared to other forms. J Biol Chem. 1981 Feb 10;256(3):1307–1316. [PubMed] [Google Scholar]
- Choudary P. V., Barranger J. A., Tsuji S., Mayor J., LaMarca M. E., Cepko C. L., Mulligan R. C., Ginns E. I. Retrovirus-mediated transfer of the human glucocerebrosidase gene to Gaucher fibroblasts. Mol Biol Med. 1986 Jun;3(3):293–299. [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P. W., Jolly J. D., Jones J. B., Anderson W. F., Lothrop C. D., Jr Gene transfer into hematopoietic progenitor cells from normal and cyclic hematopoietic dogs using retroviral vectors. Blood. 1988 Mar;71(3):717–722. [PubMed] [Google Scholar]
- Faust P. L., Wall D. A., Perara E., Lingappa V. R., Kornfeld S. Expression of human cathepsin D in Xenopus oocytes: phosphorylation and intracellular targeting. J Cell Biol. 1987 Nov;105(5):1937–1945. doi: 10.1083/jcb.105.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman W. H., Goldman S. S., DeLellis R. Dual localization of beta-glucuronidase in endoplasmic reticulum and in lysosomes. Nature. 1967 Feb 4;213(5075):457–460. doi: 10.1038/213457a0. [DOI] [PubMed] [Google Scholar]
- Freeman C., Hopwood J. J. Human liver N-acetylglucosamine-6-sulphate sulphatase. Catalytic properties. Biochem J. 1987 Sep 1;246(2):355–365. doi: 10.1042/bj2460355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T. Progress toward human gene therapy. Science. 1989 Jun 16;244(4910):1275–1281. doi: 10.1126/science.2660259. [DOI] [PubMed] [Google Scholar]
- Gallagher P. M., D'Amore M. A., Lund S. D., Ganschow R. E. The complete nucleotide sequence of murine beta-glucuronidase mRNA and its deduced polypeptide. Genomics. 1988 Apr;2(3):215–219. doi: 10.1016/0888-7543(88)90005-5. [DOI] [PubMed] [Google Scholar]
- Hall C. W., Cantz M., Neufeld E. F. A -glucuronidase deficiency mucopolysaccharidosis: studies in cultured fibroblasts. Arch Biochem Biophys. 1973 Mar;155(1):32–38. doi: 10.1016/s0003-9861(73)80006-2. [DOI] [PubMed] [Google Scholar]
- Haskins M. E., Desnick R. J., DiFerrante N., Jezyk P. F., Patterson D. F. Beta-glucuronidase deficiency in a dog: a model of human mucopolysaccharidosis VII. Pediatr Res. 1984 Oct;18(10):980–984. doi: 10.1203/00006450-198410000-00014. [DOI] [PubMed] [Google Scholar]
- Kantoff P. W., Kohn D. B., Mitsuya H., Armentano D., Sieberg M., Zwiebel J. A., Eglitis M. A., McLachlin J. R., Wiginton D. A., Hutton J. J. Correction of adenosine deaminase deficiency in cultured human T and B cells by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6563–6567. doi: 10.1073/pnas.83.17.6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Kwok W. W., Schuening F., Stead R. B., Miller A. D. Retroviral transfer of genes into canine hemopoietic progenitor cells in culture: a model for human gene therapy. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4552–4555. doi: 10.1073/pnas.83.12.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunoff D., Nicol D. M., Pritzi P. Uptake of beta-glucuronidase by deficient human fibroblasts. Lab Invest. 1973 Oct;29(4):449–453. [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Jolly D. J., Friedmann T., Verma I. M. A transmissible retrovirus expressing human hypoxanthine phosphoribosyltransferase (HPRT): gene transfer into cells obtained from humans deficient in HPRT. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4709–4713. doi: 10.1073/pnas.80.15.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanohara A., Sharkey M. F., Witztum J. L., Steinberg D., Friedmann T. Efficient expression of retroviral vector-transduced human low density lipoprotein (LDL) receptor in LDL receptor-deficient rabbit fibroblasts in vitro. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6538–6542. doi: 10.1073/pnas.85.17.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C. D., Katz F. E., Joshi G., Millar J. L. A cell line secreting stimulating factors for CFU-GEMM culture. Blood. 1984 Jul;64(1):152–155. [PubMed] [Google Scholar]
- Neufeld E. F., Fratantoni J. C. Inborn errors of mucopolysaccharide metabolism. Science. 1970 Jul 10;169(3941):141–146. doi: 10.1126/science.169.3941.141. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Rosenfeld M. G., Kreibich G., Gubler U., Sabatini D. D., Adesnik M., Andy R. Nucleotide sequence of rat preputial gland beta-glucuronidase cDNA and in vitro insertion of its encoded polypeptide into microsomal membranes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7292–7296. doi: 10.1073/pnas.83.19.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne W. R., Miller A. D. Design of vectors for efficient expression of human purine nucleoside phosphorylase in skin fibroblasts from enzyme-deficient humans. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6851–6855. doi: 10.1073/pnas.85.18.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima A., Kyle J. W., Miller R. D., Hoffmann J. W., Powell P. P., Grubb J. H., Sly W. S., Tropak M., Guise K. S., Gravel R. A. Cloning, sequencing, and expression of cDNA for human beta-glucuronidase. Proc Natl Acad Sci U S A. 1987 Feb;84(3):685–689. doi: 10.1073/pnas.84.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owerbach D., Luis A. J. Phenobarbital induction of egasyn: availability of egasyn in vivo determines glucuronidase binding to membrane. Biochem Biophys Res Commun. 1976 Apr 5;69(3):628–634. doi: 10.1016/0006-291x(76)90922-0. [DOI] [PubMed] [Google Scholar]
- Palmer T. D., Hock R. A., Osborne W. R., Miller A. D. Efficient retrovirus-mediated transfer and expression of a human adenosine deaminase gene in diploid skin fibroblasts from an adenosine deaminase-deficient human. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1055–1059. doi: 10.1073/pnas.84.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson D. F., Haskins M. E., Jezyk P. F., Giger U., Meyers-Wallen V. N., Aguirre G., Fyfe J. C., Wolfe J. H. Research on genetic diseases: reciprocal benefits to animals and man. J Am Vet Med Assoc. 1988 Nov 1;193(9):1131–1144. [PubMed] [Google Scholar]
- Powell P. P., Kyle J. W., Miller R. D., Pantano J., Grubb J. H., Sly W. S. Rat liver beta-glucuronidase. cDNA cloning, sequence comparisons and expression of a chimeric protein in COS cells. Biochem J. 1988 Mar 1;250(2):547–555. doi: 10.1042/bj2500547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchman E. H., Guzman N. A., Desnick R. J. Human alpha-L-iduronidase. I. Purification and properties of the high uptake (higher molecular weight) and the low uptake (processed) forms. J Biol Chem. 1984 Mar 10;259(5):3132–3140. [PubMed] [Google Scholar]
- Schuchman E. H., Toroyan T. K., Haskins M. E., Desnick R. J. Characterization of the defective beta-glucuronidase activity in canine mucopolysaccharidosis type VII. Enzyme. 1989;42(3):174–180. doi: 10.1159/000469027. [DOI] [PubMed] [Google Scholar]
- Sly W. S., Quinton B. A., McAlister W. H., Rimoin D. L. Beta glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr. 1973 Feb;82(2):249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- Sorge J., Kuhl W., West C., Beutler E. Complete correction of the enzymatic defect of type I Gaucher disease fibroblasts by retroviral-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Feb;84(4):906–909. doi: 10.1073/pnas.84.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead R. B., Kwok W. W., Storb R., Miller A. D. Canine model for gene therapy: inefficient gene expression in dogs reconstituted with autologous marrow infected with retroviral vectors. Blood. 1988 Mar;71(3):742–747. [PubMed] [Google Scholar]
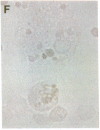
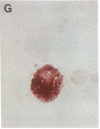
- Stramm L. E., Haskins M. E., Aguirre G. D. Retinal pigment epithelial glycosaminoglycan metabolism: intracellular versus extracellular pathways. In vitro studies in normal and diseased cells. Invest Ophthalmol Vis Sci. 1989 Oct;30(10):2118–2131. [PubMed] [Google Scholar]
- Stramm L. E., Haskins M. E., McGovern M. M., Aguirre G. D. Tissue culture of cat retinal pigment epithelium. Exp Eye Res. 1983 Jan;36(1):91–101. doi: 10.1016/0014-4835(83)90092-1. [DOI] [PubMed] [Google Scholar]
- Stramm L. E. Synthesis and secretion of glycosaminoglycans in cultured retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1987 Apr;28(4):618–627. [PubMed] [Google Scholar]
- Waheed A., Gottschalk S., Hille A., Krentler C., Pohlmann R., Braulke T., Hauser H., Geuze H., von Figura K. Human lysosomal acid phosphatase is transported as a transmembrane protein to lysosomes in transfected baby hamster kidney cells. EMBO J. 1988 Aug;7(8):2351–2358. doi: 10.1002/j.1460-2075.1988.tb03079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Johnston D. E., Jefferson D. M., Mulligan R. C. Correction of the genetic defect in hepatocytes from the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4421–4425. doi: 10.1073/pnas.85.12.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]