A timely review of the structural basis of Ca2+-activated K+ channel modulation by regulator of conduction of K+ (RCK) domains
Abstract
Regulator of conduction of K+ (RCK) domains are ubiquitous regulators of channel and transporter activity in prokaryotes and eukaryotes. In humans, RCK domains form an integral component of large-conductance calcium-activated K channels (BK channels), key modulators of nerve, muscle, and endocrine cell function. In this review, we explore how the study of RCK domains in bacterial and human channels has contributed to our understanding of the structural basis of channel function. This knowledge will be critical in identifying mechanisms that underlie BK channelopathies that lead to epilepsy and other diseases, as well as regions of the channel that might be successfully targeted to treat such diseases.
Introduction
Cellular potassium homeostasis is governed by a combination of K+ transport proteins and ion channels, whose activity is linked to cellular metabolism and signal transduction (Hille, 2001; Kuo et al., 2005). In prokaryotes, constitutive K+ uptake is controlled in part by the protein complexes Trk or Ktr, which consist of “dual-pore” transmembrane and separate cytosolic regulatory domains (Albright et al., 2006, 2007; Cao et al., 2011, 2013; Vieira-Pires et al., 2013; Levin and Zhou, 2014). In contrast, K+ efflux is governed in part by so-called Kef proteins, which typically consist of channel-like transmembrane pores that are tethered to cytosolic regulatory domains (Jiang et al., 2002a; Roosild et al., 2004, 2009; Kuo et al., 2005; Parfenova et al., 2007; Kong et al., 2012). In each case, the cytosolic regulatory domain that controls transporter activity is a highly conserved modular domain known as the regulator of conduction of K+ (RCK) domain. RCK domains have also been identified in eukaryotic K+ channels and show a high degree of sequence conservation across phyla and kingdoms (Jiang et al., 2001, 2002a; Yuan et al., 2010, 2011; Leonetti et al., 2012; Hite et al., 2015).
In addition to Kef-like prokaryotic channels that include MthK and GsuK, RCK domains are found in eukaryotic channels of the Slo gene family, which includes the BK channel (Slo1, KCa1.1, and KCNMA1), Na+-activated K+ channels (Slo2.1-2.2, KCa4.1-4.2, and KCNT1-2), and a H+-inhibited K+ channel found in mammalian sperm cells (Slo3, KCa5.1, and KCNMC1; Albright et al., 2006; Ye et al., 2006; Wu et al., 2010; Yuan et al., 2011; Kong et al., 2012; Leonetti et al., 2012; Smith et al., 2012; Cao et al., 2013; Hite et al., 2015).
The molecular architecture common to prokaryotic and eukaryotic RCK-containing K+ channels is illustrated in Fig. 1. A Kef-like channel subunit consists of a transmembrane pore module that is tethered to an RCK domain; a second RCK domain is docked onto the tethered RCK domain to form an RCK dimer (Fig. 1 A). Four of these subunit assemblies constitute a channel, with the K+ permeation pathway formed at the confluence of the pore modules and with a modulatory unit, comprised of the RCK domains, assembled at the cytosolic side of the pore. Thus, this modulatory unit consists of a ring of eight RCK domains (shown in a bird’s-eye view in Fig. 1 B). Because of its role in gating of channel and transporter activity, this unit has been called the gating ring (Ye et al., 2006; Wu et al., 2010; Yuan et al., 2011; Smith et al., 2012). Eukaryotic (Slo) channels exhibit a similar overall architecture, except for the addition of transmembrane voltage-sensing domains on each subunit and two RCK domains tethered to each subunit in tandem (Fig. 1 C). Each of these features (voltage-sensing domains and tandem RCK domains) has been observed in some prokaryotic channels as well (Wu et al., 2010; Yuan et al., 2010, 2011; Kong et al., 2012; Leonetti et al., 2012). Importantly, however, these tandem-linked RCK domains form gating rings similar to those seen in Kef-like channels (Fig. 1 D).
Figure 1.
Molecular architecture of RCK domain–containing channels. (A) Schematic diagram of Kef-like channel subunits. Two of the four subunits are shown side by side to illustrate the K+ permeation pathway (arrow). The pore module (red) is tethered to an RCK domain (cyan). A second, identical RCK domain (yellow) associates tightly with the tethered domain. These paired RCK domains from the four subunits form a gating ring, as shown in B. (B) Crystal structure of the gating ring from the MthK channel (PDB accession no. 3RBZ), shown as a bird’s-eye view from above the membrane, with the transmembrane pore removed for clarity. Positions of Ca2+ ions are represented by green spheres. (C) Schematic diagram of a BK channel subunit. Each subunit contains a transmembrane voltage-sensing domain (S0–S4 helices, white), pore module (red), and two tandem RCK domains that make up the Ca2+ sensor (RCK1, magenta; RCK2, dark purple), with an architecture analogous to the one shown in A. (D) Crystal structure of the BK gating ring (PDB accession no. 3U6N) shown as a bird’s-eye view (as in B). Positions of Ca2+ ions in the Ca2+ bowl site are represented by green spheres.
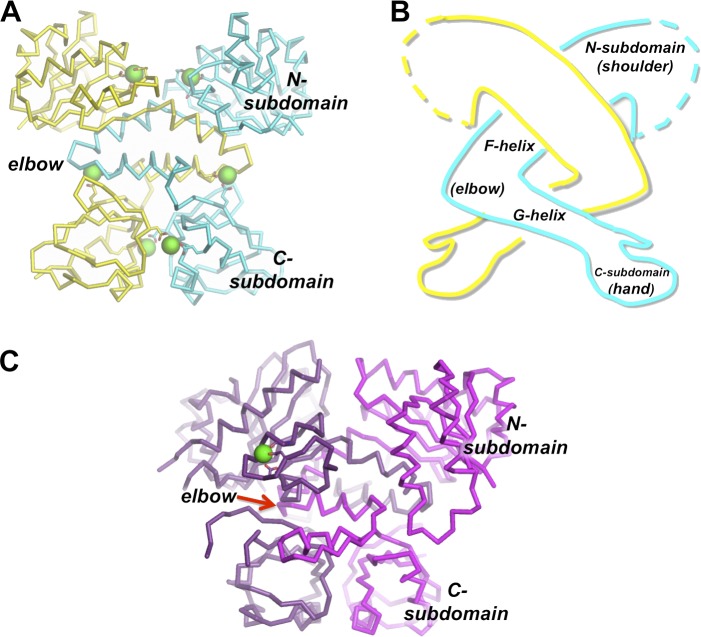
Each single RCK domain consists of a well-conserved N-terminal “Rossmann-fold” subdomain linked to a less well-conserved C-terminal subdomain via a helix-turn-helix segment (Fig. 2 A; Jiang et al., 2001, 2002a; Dong et al., 2005). In turn, RCK domains are paired to form a dimeric unit that is assembled in an “arm-in-arm” architecture (Fig. 2 B). In this architecture, the “turn” in each of the intertwined helix-turn-helix segments forms an “elbow,” although this has been observed to be a static elbow and not a flexing one among RCK domains observed in different conformations (Ye et al., 2006; Yuan et al., 2011; Smith et al., 2012, 2013). This arm-in-arm architecture is conserved among eukaryotic tandem RCK domains (Fig. 2 C).
Figure 2.
Structures of prokaryotic and eukaryotic RCK domains. (A) Crystal structure of an RCK domain homodimer from the MthK channel (PDB accession no. 4L73). Positions of Ca2+ ions are represented by enlarged green spheres. (B) Cartoon illustrating the arm-in-arm architecture of the RCK dimer with subdomain anatomy as indicated. (C) Crystal structure of the tandem RCK pseudodimer from the BK channel (PDB accession no. 3U6N). The position of the Ca2+ ion in the Ca2+ bowl site is represented by a green sphere.
RCK domains are known to bind a diverse range of biological ligands, including nicotinamide adenine dinucleotide, ATP, and metal cations such as Na+ or Ca2+, and to modulate transporter or channel activity (Schlösser et al., 1993; Jiang et al., 2002a; Kröning et al., 2007; Yuan et al., 2010; Kong et al., 2012; Levin and Zhou, 2014). The efforts of several laboratories have contributed to an increasingly detailed understanding of the conformational changes that lead to ligand-dependent activation of RCK domains and their energetic contribution to K+ flux.
In this review, we focus on two exemplary ion channels, the prokaryotic MthK and eukaryotic BK channel, as excellent reviews of what RCK-regulated transporters are available (Levin and Zhou, 2014). We first describe the conformational repertoire of RCK gating rings in bacterial MthK channels. They have constituted a key model to understand the structural basis of BK channel gating by Ca2+ and other cations, which is presented in the second part of the review. Additionally, we discuss the relevance of this knowledge in the context of BK channels as potential therapeutic targets.
Insights from a channel found in sludge
Initial insights toward the physical structure of the RCK gating apparatus came from the crystal structure of a prokaryotic K+ channel, MthK, 15 years ago. The MthK channel was cloned from Methanobacterium thermoautotrophicum, an anaerobe that thrives in raw sewage at temperatures ranging from 40 to 70°C (Smith et al., 1997). Despite its humble origins, the MthK channel bears sequence similarity to the eukaryotic BK channel (Jiang et al., 2002a). Owing to this similarity, crystal structures from the MthK channel initially served as important guides for understanding the relation between RCK domain activation and gating of BK channels and continue to provide new insight toward mechanisms of channel activation (Jiang et al., 2002a,b; Bao et al., 2004; Yang et al., 2007, 2008; Hou et al., 2008; Pau et al., 2011; Smith et al., 2012, 2013; Liu et al., 2013).
Each of the MthK channel’s four primary subunits contains pore-lining helices that are tethered to a pair of RCK domains at the cytoplasmic side of the channel, as illustrated in Fig. 1 A (Jiang et al., 2002a). Using single-channel recordings, several laboratories contributed to development of an allosteric gating scheme that accounts for the major features of both Ca2+-dependent activation of the channel and inhibition of the channel by protonation (Zadek and Nimigean, 2006; Pau et al., 2010). Through this work and complementary structural studies by x-ray crystallography, it was deduced that the RCK domains bind at least eight Ca2+ ions to stabilize the open/conducting state of the channel’s transmembrane pore, whereas binding of at least eight protons contributes to inhibition of Ca2+-dependent gating, consistent with the idea that each of the eight RCK domains in a gating ring contributes one Ca2+ site and one H+ site that control gating (Dong et al., 2005; Ye et al., 2006; Pau et al., 2010; Smith et al., 2012, 2013).
Whereas initial structural studies identified a single Ca2+-binding site within the N-terminal subdomain, determined by acidic side chains of aspartate and glutamate residues (D184, E210, and E212), two experimental observations suggested that this initial view was too simple. First, MthK channels consistently display a very steep relation between open probability (Po) and [Ca2+], with Hill coefficients of nine or greater (Zadek and Nimigean, 2006; Pau et al., 2010); this relation is much steeper than one predicted from a model with only eight Ca2+-binding sites per channel. Second, mutation of the key Ca2+-binding residues D184 and E210 reduced, but did not eliminate, Ca2+-dependent activation of MthK, suggesting that there must be additional Ca2+-binding sites (Pau et al., 2011).
It was later discovered that each MthK RCK domain contributed two additional Ca2+-binding sites, for a total of three sites per RCK domain, each of which contributes energetically to activation of the channel (Pau et al., 2011; Smith et al., 2012, 2013). Each binding site was identified from a combination of electrophysiological and crystallographic data such that Ca2+-dependent activation of the channel can be eliminated only with combined mutations at all three binding sites.
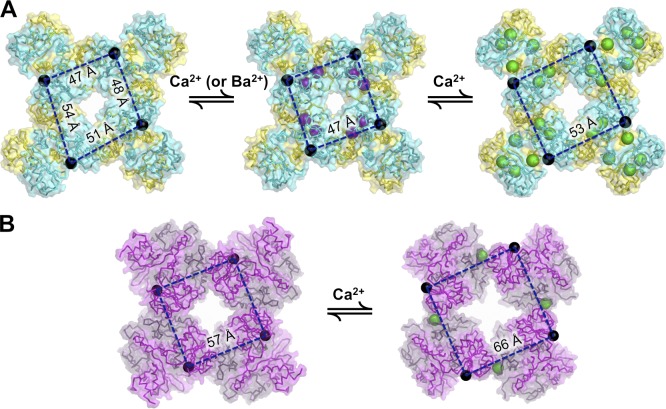
Further analysis in which the MthK RCK domain was crystallized with one, two, or all three of the Ca2+-binding sites occupied yielded further insight toward the contributions of individual sites to stabilization of domain conformations, as well as allosteric coupling between the sites (Smith et al., 2012, 2013). This work demonstrated that conformational changes at both N-terminal and C-terminal subdomains contribute to RCK domain activation and subsequent channel opening and that these different Ca2+-binding sites can interact energetically to affect channel gating. Interestingly, in the absence of ligand, the MthK gating ring can contain RCK dimers in multiple conformations, yielding gating rings that do not exhibit fourfold symmetry (Fig. 3 A; Ye et al., 2006). Binding of the divalent cations Ca2+ or Ba2+ within the N-terminal subdomain appears to affect interactions between neighboring RCK dimers in a gating ring to stabilize a fourfold symmetric conformation and facilitate channel opening (Smith et al., 2012). Binding of Ca2+ ions at additional sites in the C-terminal subdomain result in formation of intersubunit Ca2+ bridges between RCK domains within each dimer, resulting in a conformational change and further facilitation of channel opening (Dong et al., 2005; Ye et al., 2006; Pau et al., 2011; Smith et al., 2012, 2013).
Figure 3.
Ca2+-dependent conformations of MthK and BK gating rings. (A) Conformations of the MthK gating ring in (left to right) unliganded, singly liganded, and fully liganded states. N termini, which are tethered to pore-lining helices of the channel, are represented by black spheres; purple spheres represent Ba2+ ions in the singly liganded ring, and green spheres represent Ca2+ ions. RCK dimers comprising the unliganded gating ring can assume two different conformations, thus breaking fourfold symmetry. The singly liganded (Ba2+ bound) state stabilizes a single, symmetric conformation that facilitates channel opening, and opening is further facilitated by additional liganding. In the fully liganded state, distances between all N termini are uniformly greater than in the unliganded or singly liganded states, consistent with movements that could open the transmembrane pore. (B) Conformations of the BK gating ring in (left to right) the unliganded and Ca2+-bound states, in which Ca2+ (green spheres) is bound at the Ca2+ bowl. As with the MthK gating ring, binding of Ca2+ increases distances between the RCK domain N termini, which in turn underlies channel opening.
Together, these structural analyses are consistent with the idea that ligand binding can stabilize activated conformations of the gating ring that in turn can stabilize the open pore, whereas in the absence of ligand, these activated conformations are much less stable. Similar interactions among multiple ligands may be at play in other RCK domain–containing K+ channels, and it will be important to further understand their mechanisms in both functional and structural terms (Qian et al., 2006; Sweet and Cox, 2008; Kong et al., 2012).
The eukaryotic BK channel
Similar to the MthK channel, an intracellular gating ring formed by eight RCK domains is present in the eukaryotic BK channel (Fig. 1 D). However, whereas in MthK (and other prokaryotes), the RCK domains forming the gating ring are identical to one another, each BK channel subunit contains two nonidentical RCK domains (namely RCK1 and RCK2) that are linked in tandem (Fig. 1 C; Yuan et al., 2010). Thus, in the BK channel, four RCK1–RCK2 tandems form an intracellular gating ring that is nonetheless remarkably similar in overall structure to the gating ring of MthK (Fig. 1). In addition, BK channel gating is regulated by voltage, which is sensed by charged amino acids in transmembrane segments S2, S3, and S4 (Stefani et al., 1997; Díaz et al., 1998; Horrigan and Aldrich, 1999; Horrigan et al., 1999; Ma et al., 2006; Lee and Cui, 2010; Pantazis et al., 2010). The mechanism by which BK functionally integrates these two stimuli has been described by allosteric models (McManus and Magleby, 1991; Cox et al., 1997; Rothberg and Magleby, 1998, 1999; Horrigan and Aldrich, 2002). The common feature of these models is that the individual structural modules sensing Ca2+ (gating ring) and voltage (transmembrane voltage-sensing domain) undergo structural rearrangements between resting and activated conformations, leading to channel opening. Either the Ca2+ or voltage sensors can open the channel independently as well as synergistically as a result of allosteric interactions between them (Horrigan and Aldrich, 2002; Latorre et al., 2017). The ability of BK channels to be regulated by Ca2+ and voltage turns these channels into essential physiological couplers of Ca2+ and membrane voltage signaling, providing a negative feedback mechanism controlling Ca2+ influx to the cell. Consequently, BK channels are key regulators of neuronal action potential firing, neurotransmitter release, or smooth muscle contractile tone. Inherited defects in BK channel function lead to disease, including high blood pressure, seizure and epilepsy, or urinary incontinence (Latorre et al., 2017). The gating ring constitutes an essential structure involved in the coupling between Ca2+ ligation and channel activation.
Structures of gating rings isolated from eukaryotic BK channels were first solved in unliganded and Ca2+-bound forms, corresponding to two gating ring conformations (Fig. 3 B; Wu et al., 2010; Yuan et al., 2011). Remarkably, Ca2+ binding to the BK channel underlies a similar “expansion” of the gating ring seen in activation of the MthK gating ring (Fig. 3), although this is achieved by ligand binding at completely different loci. Specifically, the BK channel gating ring contains a key Ca2+-binding site (the “Ca2+ bowl”) at the interface between adjacent RCK dimers (discussed below); thus, Ca2+ binding at this site has a fundamentally distinct impact on BK gating ring structure from that seen in the MthK gating ring. In addition, the BK gating ring has additional Ca2+-binding sites aside from the Ca2+ bowl (Schreiber and Salkoff, 1997; Bao et al., 2002; Xia et al., 2002; Sweet and Cox, 2008; Zhang et al., 2010). The structural impact of these sites has been recently revealed in the published structure of the full-length BK channel from Aplysia californica (Hite et al., 2017; Tao et al., 2017) as described below. This review compares RCK domains in three different organisms: mouse (m), A. californica (a), and human (h), which often have different residue numbers for the same functional amino acids or for amino acids in the same key structural locations, so the species will be indicated for every residue mentioned.
Role of the Ca2+ bowl
The high calcium sensitivity of BK channels has been attributed to a binding site known as the Ca2+ bowl, which contains a stretch of aspartate residues within RCK2 (Wei et al., 1994; Schreiber and Salkoff, 1997; Schreiber et al., 1999). Two of these aspartates (hD895 and hD897 and mD898 and mD900; Table 1) are each critical for both Ca2+-dependent activation through this site as well as direct coordination of Ca2+, whereas other aspartate residues in this segment appear to provide structural stability through interactions with surrounding side chains (Bao et al., 2004; Yuan et al., 2010). These interactions may depend on occupancy of the Ca2+ bowl; for example, the hD892 side chain (mD895) appears to form a hydrogen bond with the side chain of hQ907 when the bowl is unoccupied, whereas hD894 (mD897) appears to form salt bridges with hR1018 and hK1030 when Ca2+ is bound (Wu et al., 2010; Yuan et al., 2010). These localized Ca2+-dependent changes at the Ca2+ bowl may contribute to Ca2+-dependent structural rearrangements that underlie gating, as suggested by moderately reduced BK Ca2+ sensitivity in the mD895A and mD897A mutants (Bao et al., 2004). The structure of the complete Slo1 channel from A. californica, in which the Ca2+ ion is coordinated by residues aD905, aD907, aQ899, and aD902 of the RCK2 subunit, is consistent with previous work performed with channels from higher organisms (Hite et al., 2017; Tao et al., 2017).
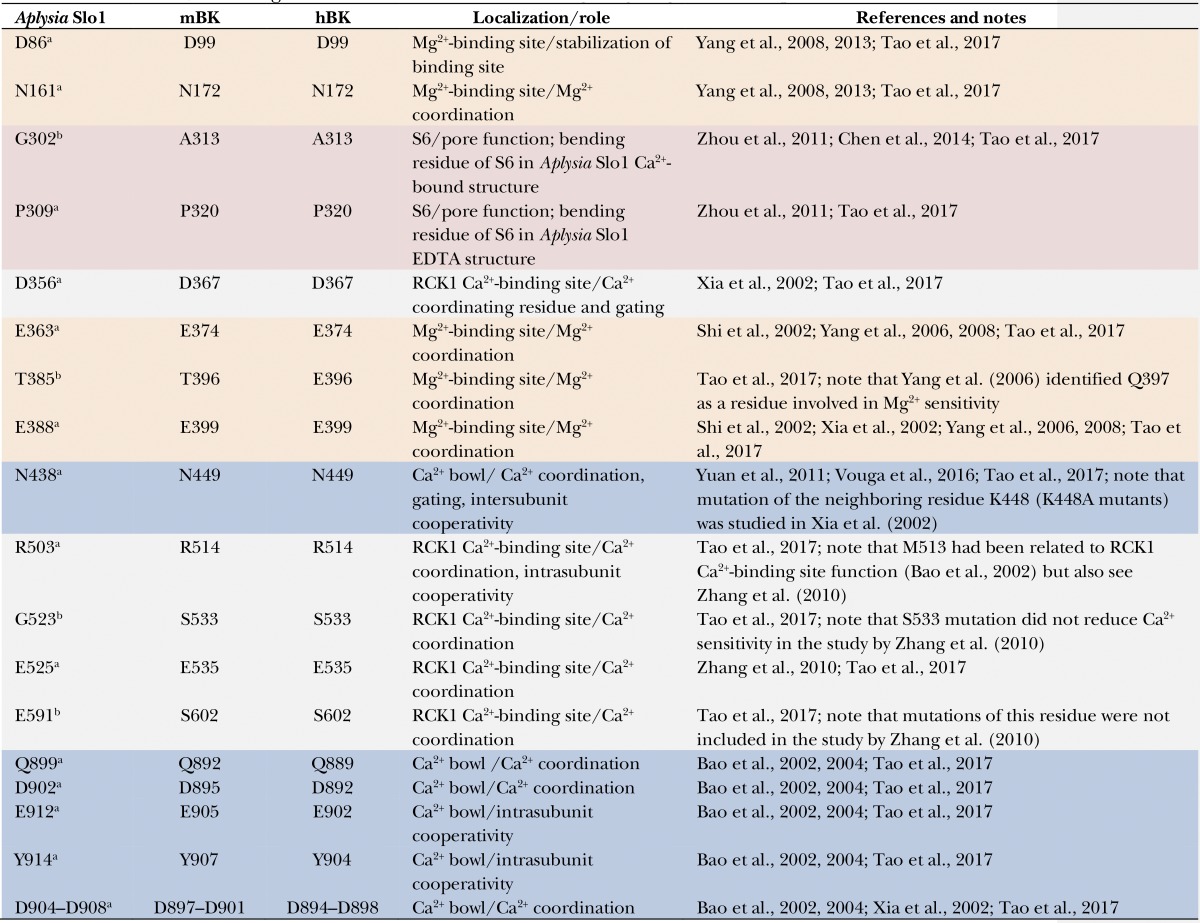
Table 1. Residues relevant to gating ring function reported in the literature.
Equivalent amino acid numbers are shown for A. californica (Aplysia Slo1), mouse (mBK), and human BK (hBK). Table rows are color coded according to residue location: S6 (red), Mg2+-binding site (orange), RCK1 Ca2+-binding site (gray), and Ca2+ bowl (blue). Sequence alignment was performed using the Clustal Omega tool from EMBL-EBI and the following protein sequences: A. californica high conductance calcium-activated potassium channel (GenBank accession no. AAR27959.1), Mus musculus mbr5 mslo (GenBank accession no. AAA39746.1), and Homo sapiens calcium-activated potassium channel subunit α-1 isoform b (GenBank accession no. NP_002238.2).
Conserved residue.
Nonconserved residue.
The Ca2+ bowl is located very close to the intersubunit interface in the gating ring structure, in a different location than that of any of the Ca2+-binding sites of MthK. In the context of the gating ring, the Ca2+ ion within the Ca2+ bowl appears to be additionally coordinated by the side chain of hN449 in the RCK1 region of the adjacent subunit (aN438; Yuan et al., 2011; Hite et al., 2017; Tao et al., 2017). Based on the observation that the overall Ca2+-dependent conformational change in the gating ring involves relative movements of the subunits at the RCK1–Ca2+ bowl interface, the nexus formed by the hN449 side chain may be an important component of the gating machinery (Hite et al., 2017). Consistent with this idea, mutations at hN449 diminish Ca2+-dependent intersubunit interactions and Ca2+ sensitivity of the channel (Vouga et al., 2016). It will be important to determine the energetic contributions of other components of this interface.
Other binding sites outside the Ca2+ bowl
Two additional independent sites have been proposed to bind Ca2+ within the RCK1 domain. In the isolated gating ring structures obtained in high Ca2+ concentrations, no electron density attributable to Ca2+ was found at either of these sites, and a model was proposed to accommodate a high-affinity binding site at the RCK1 domain (Zhang et al., 2010). In the full channel A. californica Slo1 structure, strong density is detected consistent with a Ca2+ ion coordinated by side chains contributed by aD356 and aE525 and main-chain oxygens from aR503, aG523, and aE591 (Tao et al., 2017). Among these, residues aD356, aE525, and aR503 (mD367, mE535, and mR514) are highly conserved among BK channels and were previously identified from rigorous functional studies as being important for Ca2+ sensing (Xia et al., 2002; Zhang et al., 2010). Additionally, the A. californica Slo1 structure reveals that the conserved aR503 side chain forms key interactions with highly conserved residues in the Ca2+ bowl site (mE905 and mY907; Table 1), providing a structural basis for cooperative interactions between the two Ca2+ sites, as noted below (Rothberg and Magleby, 1998, 1999; Qian et al., 2006).
Another site at the RCK1 exhibits millimolar affinity for divalent cations and is thought to underlie activation of the channel by Mg2+ (Oberhauser et al., 1988). This site has been proposed to be formed at the interface between the gating ring and the transmembrane domains (mD99 and mN172 from the transmembrane region and mE374 and mE399 at the RCK1 region), constituting an interacting site between the voltage-sensing domain and the gating ring (Yang et al., 2007, 2008; Zhang et al., 2010). Consistent with the functional studies, the structure of the full-length Slo1 channel from A. californica shows a density peak at the interface between the gating ring and the transmembrane domain, most likely corresponding to a Mg2+ ion coordinated by residues aE363, aE388, aT385, and aN161 and a water molecule. Interestingly, the residue equivalent to mD99 (aD86 in A. californica Slo1) seems not to participate in direct coordination of the divalent cation, although it may be involved in maintaining the site stability (Tao et al., 2017). Interestingly, Mg2+ has been also proposed to interact with the gating ring through the Ca2+ bowl site (Javaherian et al., 2011; Miranda et al., 2016).
Ca2+-binding sites in the gating ring are not equivalent. In addition to different binding affinities for Ca2+, the structural differences among these sites are made evident by differential interactions with other chemically distinct divalent cations. For example, Ba2+ selectively interacts with the Ca2+ bowl (Zeng et al., 2005), whereas Cd2+ appears to act through the RCK1 domain–binding site (Zeng et al., 2005; but see Zhang et al., 2010). Other divalent cations of smaller ionic radius, such as Mn2+, Co2+, Mg2+, and Ni2+, bind uniquely to the low-affinity binding RCK1 site (Zhou et al., 2012). Although these cations may not have physiological relevance to BK channel function, they are useful tools to assess the properties of different cation binding sites as well as their independent role in the conformational rearrangements of the gating ring (see below).
RCK movements in functional BK channels
How is Ca2+ binding mechanically transduced into pore opening? Comparison of isolated gating ring x-ray structures obtained in the absence and presence of high Ca2+ shows little change in the layer formed by the RCK2 domains, whereas that formed by the four RCK1 domains seems to be expanded by >10 Å (Yuan et al., 2011). Large conformational changes induced by Ca2+ have also been measured in isolated tetrameric gating rings in solution (Javaherian et al., 2011). Because this region of the gating ring is directly linked to the channel’s pore-forming helices, this expansion could represent the direct link between Ca2+ binding and the opening of the pore in BK channels (Savalli et al., 2006; Yusifov et al., 2008, 2010; Lee and Cui, 2010). However, the physiological relevance of these crystal structures and biochemical studies was limited by the fact that only part of such a complex channel was studied. Cryo–electron microscopy structures of the full-length Slo1 channel from A. californica have been recently obtained both in an EDTA-treated, ligand-free conformation and in a Ca2+- and Mg2+-bound conformation, leading the authors to propose a structure-based mechanism of channel activation involving Ca2+ binding–induced tilting of the RCK1 N lobe regions (also known as the AC regions, βA-αC; Hite et al., 2017). Interestingly, this mechanism entails two pathways, one through direct pulling of the S6 helices via the RCK1-S6 polypeptide linkers and the other via noncovalent protein–protein interfaces between the gating ring and the transmembrane domains as well as the S4–S5 linker. This is an interesting finding because the existence of a covalent linkage between membrane domains and the RCK domains forming the gating ring had led to the idea that variations in the tension of such linkers may be the major mechanism transducing ligand-induced rearrangements of the gating ring into pore opening. Although some experimental work supported this notion (Niu et al., 2004), the lack of mechanical linkages between the gating ring and membrane domains in some bacterial transporters (i.e., KtrAB) suggested that other interactions between the RCK domains and the gating pore may be relevant for function (Lingle, 2007).
Conformational changes between subunits at the level of the gating ring during activation of functional BK channels have been studied experimentally using patch-clamp fluorometry on membrane patches containing fluorescently labeled BK channels (Giraldez et al., 2005; Miranda et al., 2013, 2016). Large changes in fluorescence resonance energy transfer (FRET) were observed upon Ca2+ binding and channel activation. Simultaneous FRET and electrophysiological recordings using two different locations of the fluorescent probes showed that the linkers between RCK1 and RCK2 domains (around hH667 sites) from adjacent subunits get closer upon Ca2+ binding, whereas the regions close to the Ca2+ bowl (hN860) are relatively moved apart. This movement is dependent on Ca2+ binding to the specific sites, as mutation of the Ca2+ bowl and/or the lower affinity Ca2+-binding sites gradually impairs state-dependent FRET changes. Surprisingly, the FRET changes detected by Miranda et al. (2013) do not directly track with open probability. The electrophysiological data were well described by the standard allosteric model (Sweet and Cox, 2008); however, this model could not describe the electrophysiological and fluorescence data simultaneously, even after adding a large number of modifications to it (Miranda et al., 2013). With the available data, the authors concluded that, to explain simultaneously the movement of the gating ring and the channel gating, the standard model would need to be extended, implying that the conformational change in the gating ring has a more complex relation to pore opening than previously thought (Miranda et al., 2013). These observations cannot be fully correlated with the recently available full-length structures, which have been obtained only in extreme conditions of low and high Ca2+ and Mg2+ (Hite et al., 2017; Tao et al., 2017). These structures provide the framework to design new experiments toward full understanding of the dynamic structural rearrangements occurring in the gating ring of intact BK channels.
Interaction of the gating ring with the voltage sensors
Fluorescence-based studies indicate that the structural rearrangements of the gating ring show voltage dependence, but not in all regions (Miranda et al., 2013, 2015, 2016). Specifically, Ca2+-dependent FRET signals from the RCK1–RCK2 linkers region (hH667) have voltage dependence, whereas those of hN860 sites at the RCK2 do not. These differences in voltage dependence may reflect distinct interactions of different regions of the gating ring with the voltage-sensing domain during channel activation (Yang et al., 2007, 2008; Sweet and Cox, 2008; Savalli et al., 2012; Miranda et al., 2015). Consistently, with this hypothesis, the structure from A. californica Slo1 shows a large specific protein–protein interface between the gating ring and the transmembrane region containing the voltage sensors and S4–S5 linkers connecting the sensors to the pore. Together with other structural observations, this has lead Hite et al. (2017) to highlight the relevance of this interface in the channel activation mechanism by Ca2+. The structures suggest that Ca2+-induced rearrangements of the RCK1 N lobe directly produce a displacement of the voltage sensors away from the pore axis, which in turn induces an equivalent displacement of the S5 helices near the plasma surface, favoring pore opening (Hite et al., 2017).
Cooperativity between Ca2+-binding sites
Functional studies have demonstrated the existence of cooperative interactions between the Ca2+-binding sites (Rothberg and Magleby, 1999; Qian et al., 2006; Sweet and Cox, 2008; Savalli et al., 2012). The cryo–electron microscopy structure of the open A. californica Slo1 channel shows that the Ca2+ ion within the Ca2+ bowl is additionally coordinated by the side chain of aN438 (m449; Table 1) from the RCK1 N lobe region of the adjacent subunit. At the RCK1 site, the Ca2+ ion coordination is made complete by interaction with the aD356 from the RCK1 N lobe. Thus, the tilting on the RCK1 N lobe would simultaneously complete coordination of the cations at both the RCK1 site and the Ca2+ bowl, providing a structural basis for Ca2+-binding cooperativity. Additionally, intrasubunit cooperativity may also arise from the observed interaction of the aR503 residue from the RCK1 site with amino acids aE912 and aY914 from the Ca2+ bowl within the same subunit (Tao et al., 2017).
By taking advantage of the specific effects of different divalent cations, fluorescence studies have recently shown that activation of single high-affinity binding sites (either the Ca2+ bowl or the RCK1 site) by cations other than Ca2+ (i.e., Mg2+, Cd2+, and Ba2+) evoked significantly smaller conformational changes than those observed when both sites are occupied with Ca2+ (Miranda et al., 2016). This result indicates that both RCK domains can move independently when their specific binding sites are occupied by cations. Simultaneous occupation of both RCK1 and RCK2 sites by distinct cations is additive, emulating the effect of fully occupied Ca2+-binding sites (Miranda et al., 2016). It is tempting to speculate that binding of cations different from Ca2+ may not attain a complete coordination in the specific sites, thus yielding different levels of cooperativity between sites or no cooperativity at all.
Other players controlling the gating ring
The tissue-specific functional diversity of BK channels arises in part from the association of the pore-forming α subunits with any of four β and four γ regulatory subunits (Brenner et al., 2000a,b, 2005; Rothberg, 2012; Yan and Aldrich, 2012; Gonzalez-Perez et al., 2015), as well as the potential for multiple RCK domain structures and gating phenotypes through alternative splicing (Tseng-Crank et al., 1994; Glauser et al., 2011; Johnson et al., 2011; Shelley et al., 2013) or posttranslational modifications, such as palmytoylation (Shipston and Tian, 2016). Additionally, another regulatory mechanism may occur in macrocomplexes formed by BK and other ion channels, such as voltage-gated Ca2+ channels (Berkefeld and Fakler, 2013; Singh et al., 2016; Latorre et al., 2017). Much remains to be known about the molecular mechanisms underlying the effect of regulatory subunits on BK channel gating, some of which may include a direct interaction with the gating ring (Qian et al., 2002; Lee et al., 2010).
Conclusions and future perspectives
Our understanding of the structural basis of gating by RCK domains is rapidly evolving to the point where we can begin to tease apart the roles of chemical interactions between amino acids within the domains with great precision. We now have access to gating ring structures in the context of full-length channels, bringing the research field to a new knowledge level and setting the stage for new functional studies, having supported or confirmed much of the electrophysiological work performed over the past 30 years.
Untangling gating ring function may provide a starting point for computational drug design studies or genetic approaches to understand and treat BK channelopathies including epilepsy, ischemic heart disease, pulmonary disease, erectile dysfunction, and bladder instability. The search for drugs that activate BK channels has constituted an important goal for many years, although with only moderate clinical success. This could be attributed to many potential factors, such as low selectivity of compounds in vivo, because of the ubiquitous nature and functional diversity of BK channels (Bentzen et al., 2014). It will thus become important to study gating ring function in conjunction with regulatory subunits and other important players to better understand the impacts of these components on channel function and pharmacology.
Acknowledgments
The authors’ work is supported by grants from the National Science Foundation (MCB-1243803) and American Heart Association (16GRNT27460001) to B.S. Rothberg, the Spanish Ministry of Economy and Competitivity (SAF2013-50085-EXP to T. Giraldez), and the European Research Council under the European Union’s Horizon 2020 Research and Innovation Program (grant agreement 648936 to T. Giraldez).
The authors declare no competing financial interests.
Lesley C. Anson served as editor.
Footnotes
Abbreviations used:
- FRET
- fluorescence resonance energy transfer
- RCK
- regulator of conduction of K+
References
- Albright R.A., Ibar J.L., Kim C.U., Gruner S.M., and Morais-Cabral J.H.. 2006. The RCK domain of the KtrAB K+ transporter: multiple conformations of an octameric ring. Cell. 126:1147–1159. 10.1016/j.cell.2006.08.028 [DOI] [PubMed] [Google Scholar]
- Albright R.A., Joh K., and Morais-Cabral J.H.. 2007. Probing the structure of the dimeric KtrB membrane protein. J. Biol. Chem. 282:35046–35055. 10.1074/jbc.M704260200 [DOI] [PubMed] [Google Scholar]
- Bao L., Rapin A.M., Holmstrand E.C., and Cox D.H.. 2002. Elimination of the BKCa channel’s high-affinity Ca2+ sensitivity. J. Gen. Physiol. 120:173–189. 10.1085/jgp.20028627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Kaldany C., Holmstrand E.C., and Cox D.H.. 2004. Mapping the BKCa channel’s “Ca2+ bowl”. J. Gen. Physiol. 123:475–489. 10.1085/jgp.200409052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzen B.H., Olesen S.P., Rønn L.C., and Grunnet M.. 2014. BK channel activators and their therapeutic perspectives. Front. Physiol. 5:389 10.3389/fphys.2014.00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkefeld H., and Fakler B.. 2013. Ligand-gating by Ca2+ is rate limiting for physiological operation of BKCa channels. J. Neurosci. 33:7358–7367. 10.1523/JNEUROSCI.5443-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R., Jegla T.J., Wickenden A., Liu Y., and Aldrich R.W.. 2000a Cloning and functional characterization of novel large conductance calcium-activated potassium channel β subunits, hKCNMB3 and hKCNMB4. J. Biol. Chem. 275:6453–6461. 10.1074/jbc.275.9.6453 [DOI] [PubMed] [Google Scholar]
- Brenner R., Peréz G.J., Bonev A.D., Eckman D.M., Kosek J.C., Wiler S.W., Patterson A.J., Nelson M.T., and Aldrich R.W.. 2000b Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 407:870–876. 10.1038/35038011 [DOI] [PubMed] [Google Scholar]
- Brenner R., Chen Q.H., Vilaythong A., Toney G.M., Noebels J.L., and Aldrich R.W.. 2005. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat. Neurosci. 8:1752–1759. 10.1038/nn1573 [DOI] [PubMed] [Google Scholar]
- Cao Y., Jin X., Huang H., Derebe M.G., Levin E.J., Kabaleeswaran V., Pan Y., Punta M., Love J., Weng J., et al. 2011. Crystal structure of a potassium ion transporter, TrkH. Nature. 471:336–340. 10.1038/nature09731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Pan Y., Huang H., Jin X., Levin E.J., Kloss B., and Zhou M.. 2013. Gating of the TrkH ion channel by its associated RCK protein TrkA. Nature. 496:317–322. 10.1038/nature12056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Yan J., and Aldrich R.W.. 2014. BK channel opening involves side-chain reorientation of multiple deep-pore residues. Proc. Natl. Acad. Sci. USA. 111:E79–E88. 10.1073/pnas.1321697111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D.H., Cui J., and Aldrich R.W.. 1997. Allosteric gating of a large conductance Ca-activated K+ channel. J. Gen. Physiol. 110:257–281. 10.1085/jgp.110.3.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz L., Meera P., Amigo J., Stefani E., Alvarez O., Toro L., and Latorre R.. 1998. Role of the S4 segment in a voltage-dependent calcium-sensitive potassium (hSlo) channel. J. Biol. Chem. 273:32430–32436. 10.1074/jbc.273.49.32430 [DOI] [PubMed] [Google Scholar]
- Dong J., Shi N., Berke I., Chen L., and Jiang Y.. 2005. Structures of the MthK RCK domain and the effect of Ca2+ on gating ring stability. J. Biol. Chem. 280:41716–41724. 10.1074/jbc.M508144200 [DOI] [PubMed] [Google Scholar]
- Giraldez T., Hughes T.E., and Sigworth F.J.. 2005. Generation of functional fluorescent BK channels by random insertion of GFP variants. J. Gen. Physiol. 126:429–438. 10.1085/jgp.200509368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser D.A., Johnson B.E., Aldrich R.W., and Goodman M.B.. 2011. Intragenic alternative splicing coordination is essential for Caenorhabditis elegans slo-1 gene function. Proc. Natl. Acad. Sci. USA. 108:20790–20795. 10.1073/pnas.1116712108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez V., Xia X.M., and Lingle C.J.. 2015. Two classes of regulatory subunits coassemble in the same BK channel and independently regulate gating. Nat. Commun. 6:8341 10.1038/ncomms9341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. 2001. Ion Channels of Excitable Membranes. Third edition. Sinauer Associates, Inc., Sunderland, MA. 814 pp. [Google Scholar]
- Hite R.K., Yuan P., Li Z., Hsuing Y., Walz T., and MacKinnon R.. 2015. Cryo-electron microscopy structure of the Slo2.2 Na+-activated K+ channel. Nature. 527:198–203. 10.1038/nature14958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hite R.K., Tao X., and MacKinnon R.. 2017. Structural basis for gating the high-conductance Ca2+-activated K+ channel. Nature. 541:52–57. 10.1038/nature20775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan F.T., and Aldrich R.W.. 1999. Allosteric voltage gating of potassium channels II. Mslo channel gating charge movement in the absence of Ca2+. J. Gen. Physiol. 114:305–336. 10.1085/jgp.114.2.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan F.T., and Aldrich R.W.. 2002. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J. Gen. Physiol. 120:267–305. (published erratum appears in J. Gen. Physiol. 2002. 120:599) 10.1085/jgp.20028605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan F.T., Cui J., and Aldrich R.W.. 1999. Allosteric voltage gating of potassium channels I. Mslo ionic currents in the absence of Ca2+. J. Gen. Physiol. 114:277–304. 10.1085/jgp.114.2.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S., Xu R., Heinemann S.H., and Hoshi T.. 2008. Reciprocal regulation of the Ca2+ and H+ sensitivity in the SLO1 BK channel conferred by the RCK1 domain. Nat. Struct. Mol. Biol. 15:403–410. 10.1038/nsmb.1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaherian A.D., Yusifov T., Pantazis A., Franklin S., Gandhi C.S., and Olcese R.. 2011. Metal-driven operation of the human large-conductance voltage- and Ca2+-dependent potassium channel (BK) gating ring apparatus. J. Biol. Chem. 286:20701–20709. 10.1074/jbc.M111.235234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Pico A., Cadene M., Chait B.T., and MacKinnon R.. 2001. Structure of the RCK domain from the E. coli K+ channel and demonstration of its presence in the human BK channel. Neuron. 29:593–601. 10.1016/S0896-6273(01)00236-7 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Lee A., Chen J., Cadene M., Chait B.T., and MacKinnon R.. 2002a Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 417:515–522. 10.1038/417515a [DOI] [PubMed] [Google Scholar]
- Jiang Y., Lee A., Chen J., Cadene M., Chait B.T., and MacKinnon R.. 2002b The open pore conformation of potassium channels. Nature. 417:523–526. 10.1038/417523a [DOI] [PubMed] [Google Scholar]
- Johnson B.E., Glauser D.A., Dan-Glauser E.S., Halling D.B., Aldrich R.W., and Goodman M.B.. 2011. Alternatively spliced domains interact to regulate BK potassium channel gating. Proc. Natl. Acad. Sci. USA. 108:20784–20789. 10.1073/pnas.1116795108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong C., Zeng W., Ye S., Chen L., Sauer D.B., Lam Y., Derebe M.G., and Jiang Y.. 2012. Distinct gating mechanisms revealed by the structures of a multi-ligand gated K+ channel. eLife. 1:e00184 10.7554/eLife.00184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröning N., Willenborg M., Tholema N., Hänelt I., Schmid R., and Bakker E.P.. 2007. ATP binding to the KTN/RCK subunit KtrA from the K+-uptake system KtrAB of Vibrio alginolyticus: its role in the formation of the KtrAB complex and its requirement in vivo. J. Biol. Chem. 282:14018–14027. 10.1074/jbc.M609084200 [DOI] [PubMed] [Google Scholar]
- Kuo M.M.C., Haynes W.J., Loukin S.H., Kung C., and Saimi Y.. 2005. Prokaryotic K+ channels: from crystal structures to diversity. FEMS Microbiol. Rev. 29:961–985. 10.1016/j.femsre.2005.03.003 [DOI] [PubMed] [Google Scholar]
- Latorre R., Castillo K., Carrasquel-Ursulaez W., Sepulveda R.V., Gonzalez-Nilo F., Gonzalez C., and Alvarez O.. 2017. Molecular determinants of BK channel functional diversity and functioning. Physiol. Rev. 97:39–87. 10.1152/physrev.00001.2016 [DOI] [PubMed] [Google Scholar]
- Lee U.S., and Cui J.. 2010. BK channel activation: structural and functional insights. Trends Neurosci. 33:415–423. 10.1016/j.tins.2010.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee U.S., Shi J., and Cui J.. 2010. Modulation of BK channel gating by the ß2 subunit involves both membrane-spanning and cytoplasmic domains of Slo1. J. Neurosci. 30:16170–16179. 10.1523/JNEUROSCI.2323-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti M.D., Yuan P., Hsiung Y., and Mackinnon R.. 2012. Functional and structural analysis of the human SLO3 pH- and voltage-gated K+ channel. Proc. Natl. Acad. Sci. USA. 109:19274–19279. 10.1073/pnas.1215078109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E.J., and Zhou M.. 2014. Recent progress on the structure and function of the TrkH/KtrB ion channel. Curr. Opin. Struct. Biol. 27:95–101. 10.1016/j.sbi.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle C.J. 2007. Gating rings formed by RCK domains: keys to gate opening. J. Gen. Physiol. 129:101–107. 10.1085/jgp.200709739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Bukiya A.N., Kuntamallappanavar G., Singh A.K., and Dopico A.M.. 2013. Distinct sensitivity of slo1 channel proteins to ethanol. Mol. Pharmacol. 83:235–244. 10.1124/mol.112.081240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Lou X.J., and Horrigan F.T.. 2006. Role of charged residues in the S1-S4 voltage sensor of BK channels. J. Gen. Physiol. 127:309–328. 10.1085/jgp.200509421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O.B., and Magleby K.L.. 1991. Accounting for the Ca2+-dependent kinetics of single large-conductance Ca2+-activated K+ channels in rat skeletal muscle. J. Physiol. 443:739–777. 10.1113/jphysiol.1991.sp018861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda P., Contreras J.E., Plested A.J., Sigworth F.J., Holmgren M., and Giraldez T.. 2013. State-dependent FRET reports calcium- and voltage-dependent gating-ring motions in BK channels. Proc. Natl. Acad. Sci. USA. 110:5217–5222. 10.1073/pnas.1219611110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda P., Giraldez T., and Holmgren M.. 2015. Voltage dependence of BK channels gating ring motion studied by state dependent FRET. Biophys. J. 108:119a 10.1016/j.bpj.2014.11.666 [DOI] [Google Scholar]
- Miranda P., Giraldez T., and Holmgren M.. 2016. Interactions of divalent cations with calcium binding sites of BK channels reveal independent motions within the gating ring. Proc. Natl. Acad. Sci. USA. 113:14055–14060. 10.1073/pnas.1611415113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X., Qian X., and Magleby K.L.. 2004. Linker-gating ring complex as passive spring and Ca2+-dependent machine for a voltage- and Ca2+-activated potassium channel. Neuron. 42:745–756. 10.1016/j.neuron.2004.05.001 [DOI] [PubMed] [Google Scholar]
- Oberhauser A., Alvarez O., and Latorre R.. 1988. Activation by divalent cations of a Ca2+-activated K+ channel from skeletal muscle membrane. J. Gen. Physiol. 92:67–86. 10.1085/jgp.92.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis A., Gudzenko V., Savalli N., Sigg D., and Olcese R.. 2010. Operation of the voltage sensor of a human voltage- and Ca2+-activated K+ channel. Proc. Natl. Acad. Sci. USA. 107:4459–4464. 10.1073/pnas.0911959107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfenova L.V., Abarca-Heidemann K., Crane B.M., and Rothberg B.S.. 2007. Molecular architecture and divalent cation activation of TvoK, a prokaryotic potassium channel. J. Biol. Chem. 282:24302–24309. 10.1074/jbc.M703650200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau V.P., Abarca-Heidemann K., and Rothberg B.S.. 2010. Allosteric mechanism of Ca2+ activation and H+-inhibited gating of the MthK K+ channel. J. Gen. Physiol. 135:509–526. 10.1085/jgp.200910387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau V.P., Smith F.J., Taylor A.B., Parfenova L.V., Samakai E., Callaghan M.M., Abarca-Heidemann K., Hart P.J., and Rothberg B.S.. 2011. Structure and function of multiple Ca2+-binding sites in a K+ channel regulator of K+ conductance (RCK) domain. Proc. Natl. Acad. Sci. USA. 108:17684–17689. 10.1073/pnas.1107229108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Nimigean C.M., Niu X., Moss B.L., and Magleby K.L.. 2002. Slo1 tail domains, but not the Ca2+ bowl, are required for the β1 subunit to increase the apparent Ca2+ sensitivity of BK channels. J. Gen. Physiol. 120:829–843. 10.1085/jgp.20028692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Niu X., and Magleby K.L.. 2006. Intra- and intersubunit cooperativity in activation of BK channels by Ca2+. J. Gen. Physiol. 128:389–404. (published erratum appears in J. Gen. Physiol. 2006. 128:629) 10.1085/jgp.200609486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosild T.P., Lê K.T., and Choe S.. 2004. Cytoplasmic gatekeepers of K+-channel flux: a structural perspective. Trends Biochem. Sci. 29:39–45. 10.1016/j.tibs.2003.11.008 [DOI] [PubMed] [Google Scholar]
- Roosild T.P., Castronovo S., Miller S., Li C., Rasmussen T., Bartlett W., Gunasekera B., Choe S., and Booth I.R.. 2009. KTN (RCK) domains regulate K+ channels and transporters by controlling the dimer-hinge conformation. Structure. 17:893–903. 10.1016/j.str.2009.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg B.S. 2012. The BK channel: a vital link between cellular calcium and electrical signaling. Protein Cell. 3:883–892. 10.1007/s13238-012-2076-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg B.S., and Magleby K.L.. 1998. Kinetic structure of large-conductance Ca2+-activated K+ channels suggests that the gating includes transitions through intermediate or secondary states. A mechanism for flickers. J. Gen. Physiol. 111:751–780. 10.1085/jgp.111.6.751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg B.S., and Magleby K.L.. 1999. Gating kinetics of single large-conductance Ca2+-activated K+ channels in high Ca2+ suggest a two-tiered allosteric gating mechanism. J. Gen. Physiol. 114:93–124. (published erratum appears in J. Gen. Physiol. 1999. 114:337) 10.1085/jgp.114.1.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savalli N., Kondratiev A., Toro L., and Olcese R.. 2006. Voltage-dependent conformational changes in human Ca2+- and voltage-activated K+ channel, revealed by voltage-clamp fluorometry. Proc. Natl. Acad. Sci. USA. 103:12619–12624. 10.1073/pnas.0601176103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savalli N., Pantazis A., Yusifov T., Sigg D., and Olcese R.. 2012. The contribution of RCK domains to human BK channel allosteric activation. J. Biol. Chem. 287:21741–21750. 10.1074/jbc.M112.346171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlösser A., Hamann A., Bossemeyer D., Schneider E., and Bakker E.P.. 1993. NAD+ binding to the Escherichia coli K+-uptake protein TrkA and sequence similarity between TrkA and domains of a family of dehydrogenases suggest a role for NAD+ in bacterial transport. Mol. Microbiol. 9:533–543. 10.1111/j.1365-2958.1993.tb01714.x [DOI] [PubMed] [Google Scholar]
- Schreiber M., and Salkoff L.. 1997. A novel calcium-sensing domain in the BK channel. Biophys. J. 73:1355–1363. 10.1016/S0006-3495(97)78168-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber M., Yuan A., and Salkoff L.. 1999. Transplantable sites confer calcium sensitivity to BK channels. Nat. Neurosci. 2:416–421. 10.1038/8077 [DOI] [PubMed] [Google Scholar]
- Shelley C., Whitt J.P., Montgomery J.R., and Meredith A.L.. 2013. Phosphorylation of a constitutive serine inhibits BK channel variants containing the alternate exon “SRKR”. J. Gen. Physiol. 142:585–598. 10.1085/jgp.201311072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Krishnamoorthy G., Yang Y., Hu L., Chaturvedi N., Harilal D., Qin J., and Cui J.. 2002. Mechanism of magnesium activation of calcium-activated potassium channels. Nature. 418:876–880. 10.1038/nature00941 [DOI] [PubMed] [Google Scholar]
- Shipston M.J., and Tian L.. 2016. Posttranscriptional and posttranslational regulation of BK channels. Int. Rev. Neurobiol. 128:91–126. 10.1016/bs.irn.2016.02.012 [DOI] [PubMed] [Google Scholar]
- Singh H., Li M., Hall L., Chen S., Sukur S., Lu R., Caputo A., Meredith A.L., Stefani E., and Toro L.. 2016. MaxiK channel interactome reveals its interaction with GABA transporter 3 and heat shock protein 60 in the mammalian brain. Neuroscience. 317:76–107. 10.1016/j.neuroscience.2015.12.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.R., Doucette-Stamm L.A., Deloughery C., Lee H., Dubois J., Aldredge T., Bashirzadeh R., Blakely D., Cook R., Gilbert K., et al. 1997. Complete genome sequence of Methanobacterium thermoautotrophicum deltaH: functional analysis and comparative genomics. J. Bacteriol. 179:7135–7155. 10.1128/jb.179.22.7135-7155.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F.J., Pau V.P., Cingolani G., and Rothberg B.S.. 2012. Crystal structure of a Ba2+-bound gating ring reveals elementary steps in RCK domain activation. Structure. 20:2038–2047. 10.1016/j.str.2012.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F.J., Pau V.P., Cingolani G., and Rothberg B.S.. 2013. Structural basis of allosteric interactions among Ca2+-binding sites in a K+ channel RCK domain. Nat. Commun. 4:2621 10.1038/ncomms3621 [DOI] [PubMed] [Google Scholar]
- Stefani E., Ottolia M., Noceti F., Olcese R., Wallner M., Latorre R., and Toro L.. 1997. Voltage-controlled gating in a large conductance Ca2+-sensitive K+ channel (hslo). Proc. Natl. Acad. Sci. USA. 94:5427–5431. 10.1073/pnas.94.10.5427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet T.B., and Cox D.H.. 2008. Measurements of the BKCa channel’s high-affinity Ca2+ binding constants: effects of membrane voltage. J. Gen. Physiol. 132:491–505. 10.1085/jgp.200810094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X., Hite R.K., and MacKinnon R.. 2017. Cryo-EM structure of the open high-conductance Ca2+-activated K+ channel. Nature. 541:46–51. 10.1038/nature20608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng-Crank J., Foster C.D., Krause J.D., Mertz R., Godinot N., DiChiara T.J., and Reinhart P.H.. 1994. Cloning, expression, and distribution of functionally distinct Ca2+-activated K+ channel isoforms from human brain. Neuron. 13:1315–1330. 10.1016/0896-6273(94)90418-9 [DOI] [PubMed] [Google Scholar]
- Vieira-Pires R.S., Szollosi A., and Morais-Cabral J.H.. 2013. The structure of the KtrAB potassium transporter. Nature. 496:323–328. 10.1038/nature12055 [DOI] [PubMed] [Google Scholar]
- Vouga A.G., Hendron E., and Rothberg B.S.. 2016. Role of an intersubunit Ca2+ bridge in structure and function of BK channels. Biophys. J. 110:187a 10.1016/j.bpj.2015.11.1042 [DOI] [Google Scholar]
- Wei A., Solaro C., Lingle C., and Salkoff L.. 1994. Calcium sensitivity of BK-type KCa channels determined by a separable domain. Neuron. 13:671–681. 10.1016/0896-6273(94)90034-5 [DOI] [PubMed] [Google Scholar]
- Wu Y., Yang Y., Ye S., and Jiang Y.. 2010. Structure of the gating ring from the human large-conductance Ca2+-gated K+ channel. Nature. 466:393–397. 10.1038/nature09252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X.M., Zeng X., and Lingle C.J.. 2002. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 418:880–884. 10.1038/nature00956 [DOI] [PubMed] [Google Scholar]
- Yan J., and Aldrich R.W.. 2012. BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc. Natl. Acad. Sci. USA. 109:7917–7922. 10.1073/pnas.1205435109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Hu L., Shi J., and Cui J.. 2006. Tuning magnesium sensitivity of BK channels by mutations. Biophys. J. 91:2892–2900. 10.1529/biophysj.106.090159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Hu L., Shi J., Delaloye K., Horrigan F.T., and Cui J.. 2007. Mg2+ mediates interaction between the voltage sensor and cytosolic domain to activate BK channels. Proc. Natl. Acad. Sci. USA. 104:18270–18275. 10.1073/pnas.0705873104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Shi J., Zhang G., Yang J., Delaloye K., and Cui J.. 2008. Activation of Slo1 BK channels by Mg2+ coordinated between the voltage sensor and RCK1 domains. Nat. Struct. Mol. Biol. 15:1152–1159. 10.1038/nsmb.1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Yang H., Sun X., Delaloye K., Yang X., Moller A., Shi J., and Cui J.. 2013. Interaction between residues in the Mg2+-binding site regulates BK channel activation. J. Gen. Physiol. 141:217–228. 10.1085/jgp.201210794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S., Li Y., Chen L., and Jiang Y.. 2006. Crystal structures of a ligand-free MthK gating ring: insights into the ligand gating mechanism of K+ channels. Cell. 126:1161–1173. 10.1016/j.cell.2006.08.029 [DOI] [PubMed] [Google Scholar]
- Yuan P., Leonetti M.D., Pico A.R., Hsiung Y., and MacKinnon R.. 2010. Structure of the human BK channel Ca2+-activation apparatus at 3.0 Å resolution. Science. 329:182–186. 10.1126/science.1190414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P., Leonetti M.D., Hsiung Y., and MacKinnon R.. 2011. Open structure of the Ca2+ gating ring in the high-conductance Ca2+-activated K+ channel. Nature. 481:94–97. 10.1038/nature10670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusifov T., Savalli N., Gandhi C.S., Ottolia M., and Olcese R.. 2008. The RCK2 domain of the human BKCa channel is a calcium sensor. Proc. Natl. Acad. Sci. USA. 105:376–381. 10.1073/pnas.0705261105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusifov T., Javaherian A.D., Pantazis A., Gandhi C.S., and Olcese R.. 2010. The RCK1 domain of the human BKCa channel transduces Ca2+ binding into structural rearrangements. J. Gen. Physiol. 136:189–202. 10.1085/jgp.200910374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadek B., and Nimigean C.M.. 2006. Calcium-dependent gating of MthK, a prokaryotic potassium channel. J. Gen. Physiol. 127:673–685. 10.1085/jgp.200609534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X.H., Xia X.M., and Lingle C.J.. 2005. Divalent cation sensitivity of BK channel activation supports the existence of three distinct binding sites. J. Gen. Physiol. 125:273–286. 10.1085/jgp.200409239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Huang S.Y., Yang J., Shi J., Yang X., Moller A., Zou X., and Cui J.. 2010. Ion sensing in the RCK1 domain of BK channels. Proc. Natl. Acad. Sci. USA. 107:18700–18705. 10.1073/pnas.1010124107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Xia X.M., and Lingle C.J.. 2011. Cysteine scanning and modification reveal major differences between BK channels and Kv channels in the inner pore region. Proc. Natl. Acad. Sci. USA. 108:12161–12166. 10.1073/pnas.1104150108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zeng X.H., and Lingle C.J.. 2012. Barium ions selectively activate BK channels via the Ca2+-bowl site. Proc. Natl. Acad. Sci. USA. 109:11413–11418. 10.1073/pnas.1204444109 [DOI] [PMC free article] [PubMed] [Google Scholar]