Using in vivo experimentation and an in vitro microfluidic system, Wolf et al. demonstrate that monocytes require low levels of self-made TNF for their survival, both during monopoiesis and under specific immune challenges. They highlight the significance of this autonomous mechanism in a mouse multiple sclerosis model in which TNF-deficient monocytes survive less in the inflamed spinal cord, resulting in delayed disease onset.
Abstract
Monocytes are circulating mononuclear phagocytes, poised to extravasate to sites of inflammation and differentiate into monocyte-derived macrophages and dendritic cells. Tumor necrosis factor (TNF) and its receptors are up-regulated during monopoiesis and expressed by circulating monocytes, as well as effector monocytes infiltrating certain sites of inflammation, such as the spinal cord, during experimental autoimmune encephalomyelitis (EAE). In this study, using competitive in vitro and in vivo assays, we show that monocytes deficient for TNF or TNF receptors are outcompeted by their wild-type counterpart. Moreover, monocyte-autonomous TNF is critical for the function of these cells, as TNF ablation in monocytes/macrophages, but not in microglia, delayed the onset of EAE in challenged animals and was associated with reduced acute spinal cord infiltration of Ly6Chi effector monocytes. Collectively, our data reveal a previously unappreciated critical cell-autonomous role of TNF on monocytes for their survival, maintenance, and function.
Introduction
Monocytes are circulating mononuclear phagocytes and comprise two main subsets, which in mice have been defined as CCR2+ CX3CR1int CD43lo Ly6Chi and CCR2− CX3CR1hi CD43hi Ly6Clo cells (Geissmann et al., 2003; Ginhoux and Jung, 2014; Mildner et al., 2016). Monocytes develop in steady state in the BM from common monocyte precursors (cMOPs; Hettinger et al., 2013) that themselves derive from monocyte/macrophage DC precursors (MDP; Fogg et al., 2006; Varol et al., 2007). Ly6Chi monocytes are short lived (Yona et al., 2013) and poised to home to sites of inflammation to give rise to cells with DC or macrophage features (Mildner et al., 2013). Ly6Clo cells are progeny of Ly6Chi monocytes, display more extended half-lives (Yona et al., 2013), and are patrolling cells specialized in surveillance of vascular integrity (Auffray et al., 2007). As such, Ly6Clo cells can be considered a blood-resident macrophage population (Ginhoux and Jung, 2014). Infiltrates of Ly6Chi monocytes are prominent in neuroinflammatory disorders, such as the animal model of multiple sclerosis (MS), experimental autoimmune encephalomyelitis (EAE), which involves blood–brain barrier breakage (Mildner et al., 2009). Emerging evidence indicates distinct functional contributions of monocyte-derived cells and central nervous system (CNS)–resident microglia (Shechter et al., 2009; Ajami et al., 2011; Yamasaki et al., 2014).
Proinflammatory cytokines, such as IL-1β, IL-6, and TNF, are hallmarks of CNS inflammation (Murphy et al., 2010). TNF has pleiotropic actions and can induce both cell death and apoptosis resistance (Karin and Lin, 2002). In MS lesions, TNF is highly abundant and produced by many cells (Hofman et al., 1989; Bitsch et al., 2000). TNF is synthesized as a membrane-bound molecule but can be shed by the metalloprotease TNF-α–converting enzyme (TACE; Horiuchi et al., 2007). Both transmembrane and shed TNF isoforms have activity with partially distinct impact. Membrane-tethered TNF displays higher affinity for TNFR2 (p75) and was proposed to predominantly promote cell survival (Grell et al., 1995), whereas shed TNF binds preferentially to the death domain containing TNFR1 (p55), which may trigger apoptosis as shown in in vitro assays (Grell et al., 1998). Thus, TNF can act in diverse and even adverse ways (Caminero et al., 2011).
Using mice harboring conditional mutant TNF alleles, it was shown that TNF produced by myeloid cells mediates LPS-induced septic shock and protection from intracellular pathogens (Grivennikov et al., 2005). Moreover, when subjected to EAE, lyzMCre:tnffl/fl mice displayed delayed disease initiation, though no amelioration of the subsequent chronic pathology (Kruglov et al., 2011). This suggested a specific role of myeloid TNF in EAE onset. However, as the LysM promoter displays activity in both neutrophils and monocytes and low penetrance in microglia (Goldmann et al., 2013), it remained unclear which myeloid cells produce the critical TNF and what the underlying mechanisms are.
Here, we investigate the importance of TNF signaling in steady-state and inflammatory monocytes. We reveal a novel critical role of autonomous TNF signaling as a survival factor for monocytes, both in steady state and under neuroinflammatory conditions. Moreover, using the CX3CR1Cre/CreER system (Yona et al., 2013), we investigated the relative contribution of TNF derived from microglia or monocytes for EAE induction and show that microglial TNF is dispensable for EAE development. In contrast, absence of autonomous TNF from the monocyte infiltrate impaired survival of the cells and delayed EAE induction.
Results and discussion
Requirement of cell-intrinsic and -nonintrinsic TNF during monocyte development
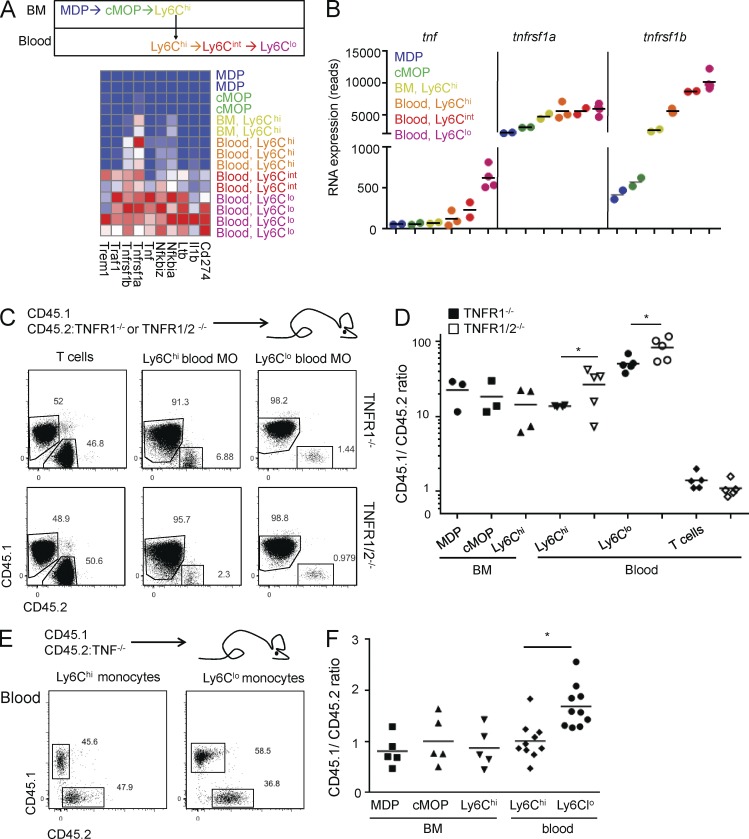
Transcriptome analysis of monocytes and their BM precursors revealed a consistent, though modest, expression of TNF, which increases with the steady-state differentiation of Ly6Chi monocytes into Ly6Clo cells (Fig. 1, A and B; unpublished data). Moreover, with progression from the Ly6Chi via the Ly6Cint to the Ly6Clo stage, monocytes gradually increased expression of many NF-κB signature genes, including tnf and its receptors tnfrsf1a (encoding TNFR1) and tnfrsf1b (encoding TNFR2), as part of their conversion program (Fig. 1, A and B). Tnfrsf1a levels are high already at the MDP stage and increase moderately (about twofold) with monocyte development, whereas tnfrsf1b displays low expression in BM precursors but is ∼20-fold induced during development, peaking in Ly6Clo blood monocytes. Interestingly, TNF expression levels are significantly correlated with expression of both TNFR1 (r = 0.597; P < 0.05) and TNFR2 (r = 0.809; P < 0.001).
Figure 1.
Absence of TNF signaling impairs steady-state monocytes. (A) Scheme illustrating monocyte development (top) and heat map of RNA sequencing expression data of NF-κB–driven genes in sorted MDP, cMOP, and Ly6Chi BM monocytes, as well as Ly6Chi, Ly6Cint, and Ly6Clo blood monocytes (bottom). (B) RNA sequencing reads for tnf, tnfrsf1a, and tnfrsf1b expression throughout sequential monocyte development stages in A. n = 2–3. (C) FACS analysis of T cells and Ly6Chi and Ly6Clo monocytes (MO) extracted from blood of either [TNFR1−/−/WT > WT] (top) or [TNFR1/2−/−/WT > WT] (bottom) mixed BM chimeras. (D) Quantification of myeloid cells and precursors. n = 3–5. Mann-Whitney U test was used. (E) FACS analysis of Ly6Chi and Ly6Clo monocytes extracted from blood of [TNF/WT > WT] mixed BM chimeras. (F) Quantification of myeloid cells and precursors in C and D. n = 5–10. Student’s t test was used. *, P < 0.05.
TNFR1-deficient monocytes are present in similar numbers and subset distribution as monocytes in WT blood (Fig. S1 A). To probe for a potential role of TNF in monocyte generation and survival in a competitive setting, we generated mixed BM chimeras, in which lethally irradiated WT mice received equal amounts of WT BM (CD45.1) and BM deficient for either TNF receptor 1 (TNFR1−/−) or both TNF receptors (TNFR1/2−/−; CD45.2; Fig. 1, C and D; and Fig. S1 B). When analyzed after 4 wk, the blood T cell compartment of the chimeras displayed similar representation of both genotypes. However, TNFR1−/− Ly6Chi monocytes (CD45.1/2 ratio ∼15-fold) and, even more profoundly, TNFR1−/− Ly6Clo monocytes (CD45.1/2 ratio ∼50-fold) were outcompeted by their WT counterparts (Fig. 1 C). A similar effect was evident in [WT/TNFR1/2−/− > WT] BM chimeras, with an additive impact of the TNFR2 mutation, which was significant in Ly6Clo monocytes (CD45.1/2 ratio ∼75-fold), in line with their up-regulation of TNFR2 (Fig. 1, C and D). Of note, examination of monocytic BM precursors, including MDP, cMOP, and BM Ly6C+ monocytes, also revealed an overrepresentation of the WT genotype (Fig. 1 D, and Fig. S1, C and D). This indicates TNF requirement during monopoiesis. However, the further bias among the Ly6Clo blood monocytes of the [WT/TNFR1/2−/−] chimeras, together with the up-regulation of both TNF and TNFR2 in these cells, suggests an additional role of TNF in Ly6Chi to Ly6Clo monocyte conversion (Fig. 1 D).
These data raised the possibility that TNF has an autonomous effect on monocytes. To probe more rigorously such a scenario, we next generated mixed BM chimeras with TNF−/− (CD45.2) and TNF+/+ (CD45.1) BM. Representations of TNF-sufficient and -deficient Ly6Chi blood monocytes, as well as MDP, cMOP, and Ly6Chi BM monocytes, in these chimeras were equal, indicating no requirement of autonomous TNF throughout monocyte development up to this point (Fig. 1, E and F; and Fig. S1 E). In contrast, TNF-deficient Ly6Clo monocytes were significantly underrepresented as compared with TNF-sufficient monocytes (Fig. 1 F). Because these cells retain TNF receptor expression, these data imply an intrinsic requirement for cell-autonomous TNF by these monocytes.
Absence of TNF signaling leads to excessive monocyte cell death
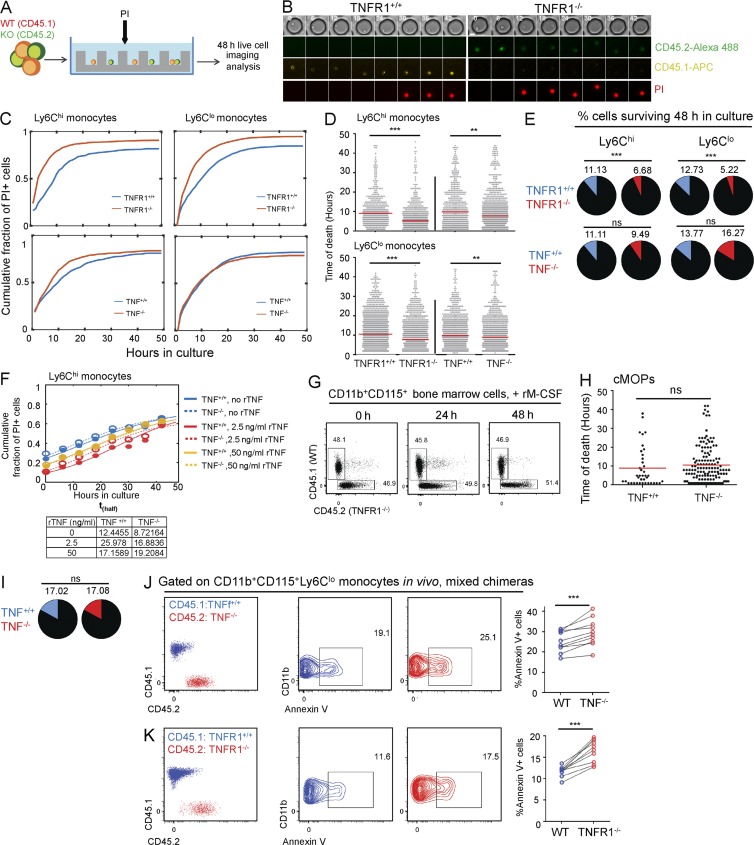
Cell-autonomous TNF could be required for either monocyte development or survival. To test the latter option, we resorted to an in vitro microfluidic system (Zaretsky et al., 2012). Specifically, we isolated circulating Ly6Chi and Ly6Clo monocytes from WT, TNF−/−, and TNFR1−/− mice and co-cultured them in microwells (Zaretsky et al., 2012) in the presence of the dead cell marker propidium iodide (PI) for 48 h (Fig. 2 A). Co-culture of WT and knockout monocytes in separate microwells in the same array allows for direct comparison of their death rate and survival, with single-cell resolution. In this assay, cells can initially respond only to TNF they secrete themselves; at later time points though, TNF can diffuse from TNF-secreting cells and affect cells in neighboring microwells with a typical diffusion time between wells of ∼24 h. The single-cell real-time analysis for cell death revealed that monocytes deprived of TNFR1 died significantly more rapidly than their WT counterpart, including both Ly6Chi and Ly6Clo cells. Moreover, the fraction of cells surviving 48 h in culture was significantly lower compared with TNFR1-sufficient monocytes (Fig. 2, B–E). A similar result was obtained with TNF-deficient Ly6Chi monocytes, which also exhibited faster death rates. However, the fraction of surviving cells at the end of the assay was similar to that of WT monocytes, probably because of TNF secreted by WT monocytes diffusing from neighboring microwells (Fig. 2, C–E). Earlier studies demonstrated that low doses of TNF can promote myeloid cell survival (Cowburn et al., 2004; Lehner et al., 2012). After administration of 2.5 ng/ml exogenous recombinant TNF, both TNF-sufficient and -deficient Ly6Chi monocytes were indeed partially rescued, as evident by a twofold increase of thalf, the time point at which the fraction of dying cells reached half of its maximal value, in both genotypes (Fig. 2 F; see Materials and methods for a description of thalf calculation). This effect was less substantial with a higher TNF dose (50 ng/ml; Fig. 2 F). Mixed cultures of MACS (magnetic-activated cell sorting)-sorted CD11b+CD115+ BM monocytes from TNFR−/− (CD45.2) and TNF−/− (CD45.1) mice, treated with the macrophage differentiation factor M-CSF, showed no disadvantage of the TNFR1-deficient cells, indicating that autonomous TNF is dispensable for monocyte-derived macrophage differentiation (Fig. 2 G). In addition, cMOPs isolated from TNF−/− (CD45.2) and TNF+/+ (CD45.1) mice displayed similar death rates and fractions of surviving cells, indicating that autonomous TNF is not required for monopoiesis (Fig. 2, H and I), corresponding to lack of TNF expression in cMOPs (Fig. 1, A and B). To relate these findings to the in vivo setting, we performed a staining for surface phosphatidylserine, as an indicator of cell death induction, on blood monocytes of the mixed chimeras. Interestingly, mutant Ly6Clo monocytes in [WT/TNF−/− > WT] and [WT/TNFR1−/− > WT], i.e., cells deficient for either the cytokine or its receptor, displayed increased annexin V staining for the apoptotic marker as compared with WT cells, indicating that autonomous TNF is required for monocyte survival in vivo (Fig. 2, J and K).
Figure 2.
Absence of TNF signaling increases apoptosis of circulating monocytes. (A) Scheme of in vitro co-culturing of TNF/TNFR1-deficient and WT monocytes in microwells. (B) Traces of co-cultured WT (TNFR1+/+) and TNFR1−/− monocytes monitored in culture for 48 h. WT/TNFR1−/− cells were distinguished by fluorescently labeled antibodies against CD45.1/CD45.2, respectively. Cell death time was monitored using PI. Bar, 10 μm. (C and D) Cumulative fraction of dead monocytes (PI+) during 48 h (C) and timing of PI signal (indicating time of cell death) for individual cells (D) in co-cultures of: Ly6Chi WT/TNFR1−/− monocytes (n = 567 and 960 cells, respectively), Ly6Chi WT/TNF−/− (n = 641 and 1,348), Ly6Clo WT/TNFR1−/− (n = 1,567 and 1,766), and Ly6Clo WT/TNF−/− (n = 1,121 and 1,162). Red lines in D show means. **, P < 0.01; ***, P < 0.001. Mann-Whitney U test was used. (E) Fraction of monocytes surviving after 48 h of co-culture. ***, P < 0.001. χ2 test was used. (F) Cumulative fraction of dead (PI+) Ly6Chi WT/TNF−/− monocytes co-cultured in microwell arrays during 48 h without TNF (n = 80 and 164) or with 2.5 ng/ml (n = 192 and 142) or 50 ng/ml (n = 112 and 181) recombinant TNF (rTNF). Closed and open circles show data (WT and TNF−/−, respectively), and continuous and dashed lines represent the best-fit logistic curve (see Materials and methods). The table shows thalf, the time by which the curves reach half of their maximum value. (G) FACS analysis of mixed cultures of MACS-sorted CD115+CD11b+ BM cells of CD45.1 and CD45.1/CD45.2 TNFR1−/− mice in the presence of 10 ng/ml M-CSF. Data are representative of three biological repeats. rM-CSF, recombinant M-CSF. (H and I) Timing of PI signal for individual cells (H) and fraction of cells surviving after 48 h (I) of cMOPs sorted from BM of WT and TNF−/− mice, co-cultured in microwell arrays. n = 47 and 170. (J and K) Fraction of annexin V+ Ly6Clo monocytes in BM chimeric [TNF/WT > WT] (F) and [TNFR1−/−/WT > WT] (G). n = 9–10. ***, P < 0.001. Paired Student’s t test was used.
In conclusion, the data obtained from the competitive in vivo experiments and the single-cell in vitro analysis established the requirement of autonomous TNF production of monocytes for their survival.
Ly6Chi effector monocytes up-regulate TNF and TNFR1 during EAE
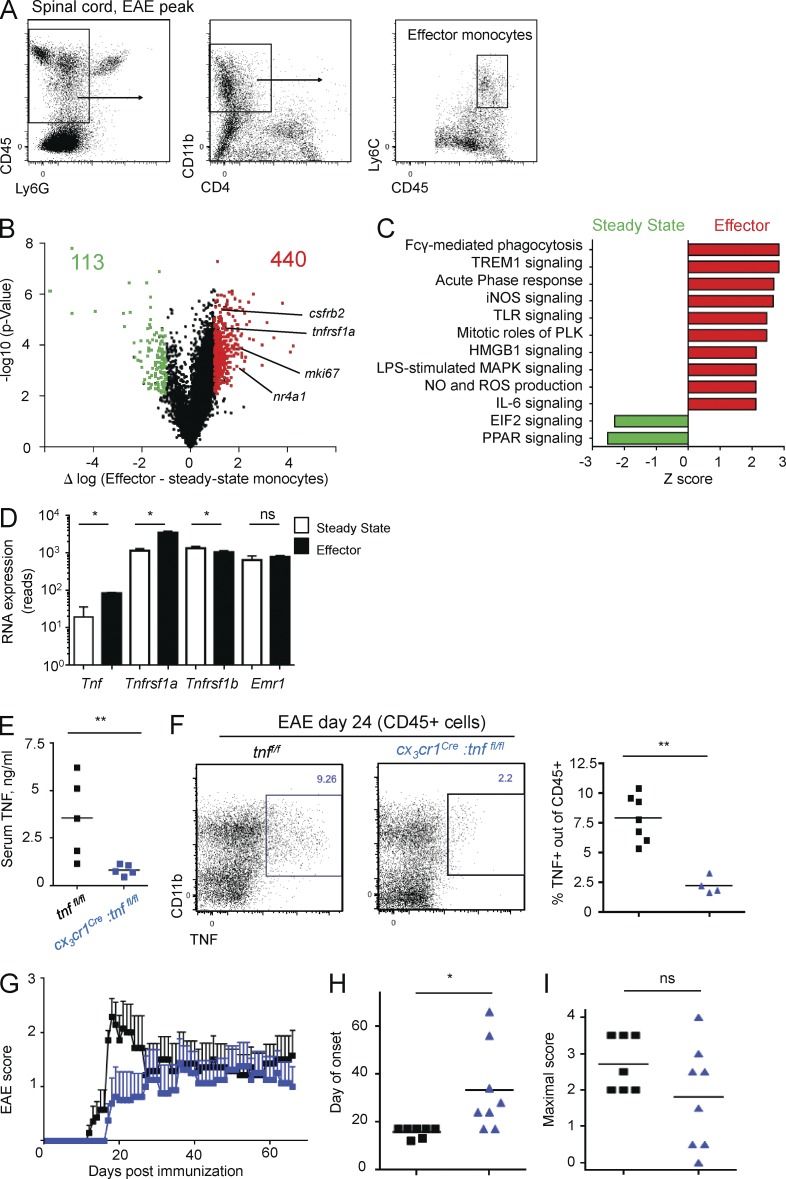
Monocyte infiltrates have been shown to play a critical role in the establishment of EAE that is distinct from microglia (Mildner et al., 2009; Ajami et al., 2011; Yamasaki et al., 2014). To investigate potential roles of monocyte- or macrophage-derived TNF in EAE development and probe for the physiological importance of the autonomous loop, we isolated the monocytic spinal cord infiltrate defined as CD45hi CD11b+ Ly6Chi Ly6G− cells, hereafter termed effector monocytes (Mildner et al., 2013), from EAE-induced mice at the pre–EAE onset stage (day 9 after immunization; score 0; Fig. 3 A). The time point before onset of EAE paralysis was chosen to minimize secondary effects induced by demyelination and cell death. Comparative transcriptome analysis with Ly6Chi steady-state monocytes (Lavin et al., 2014) revealed a list of 440 genes up-regulated in spinal cord effector monocytes compared with steady-state monocytes and 133 down-regulated genes, after filtering for genes displaying an absolute >1 log expression difference and p-value <0.01 (Fig. 3 B, and Tables S1 and S2). Canonical pathway analysis showed significant enrichment for inflammatory pathways, such as TLR, IL-6, and TREM1 signaling, as well as Fcγ receptor-mediated phagocytosis and nitric oxide and reactive oxygen species production (Fig. 3 C). Among the genes up-regulated was csfr2b, encoding the GM-CSF receptor and recently shown to drive proinflammatory monocyte activity in EAE (Croxford et al., 2015), as well as nr4a1, which negatively regulates monocyte pathology in EAE (Shaked et al., 2015); moreover, we noted high expression of tnfrsf1a (TNFR1) and the nuclear protein mki67, indicating proliferation, as previously reported (Fig. 3, B and C; Ajami et al., 2011). Although only modestly expressed, TNF also was significantly induced in effector monocytes, as compared with robust myeloid genes such as emr1 (encoding the F4/80 antigen), which was equally highly expressed in both populations (Fig. 3 D). Of note, TNFR2 levels were moderately reduced in effector monocytes (Fig. 3 D).
Figure 3.
Total monocyte/macrophage TNF deletion affects EAE onset. (A) Gating strategy for spinal cord–infiltrating effector monocytes, used for all cell sorting and analysis. (B) Volcano plot performed for comparison of sorted steady-state blood monocytes and effector monocyte infiltrates in the spinal cord at EAE day 9. Numbers indicate genes significantly up-regulated (red) or down-regulated (green) in effector monocytes compared with steady-state monocytes (log difference >1 or <−1; P < 0.05). (C) Canonical pathway analysis performed for genes significantly changed in D. Z-score >2 or <−2. iNOS, inducible nitric oxide synthase; NO, nitric oxide; PLK, polo-like kinase; PPAR, peroxisome proliferator-activated receptor; ROS, reactive oxygen species. (D) Normalized reads of tnf, tnfsr1a, tnfrsf1b, and emr1 expression in steady state and effector monocytes, as in Fig. 2 E. n = 3–4. Mann-Whitney U test was used. (E) ELISA of sera collected from tnffl/fl and cx3cr1Cre:tnffl/fl mice 4 h after intraperitoneal challenge with 200 µg LPS in vivo. n = 5. Mann-Whitney U test was used. (F) TNF production by myeloid cells in spinal cords of tnffl/fl and cx3cr1Cre:tnffl/fl mice at day 24 after MOG35–55 immunization, analyzed by FACS. n = 7–4. Mann-Whitney U test was used. (G–I) EAE disease course (G), day of onset (H), and individual maximal score (I) in tnffl/fl and cx3cr1Cre:tnffl/fl mice immunized with MOG35–55 peptide. n = 7–8. Student’s t test was used. Data are representative of three independent experiments. *, P < 0.05; **, P < 0.01. Results are expressed as means ± SEM.
Monocyte- but not microglia-derived TNF is critical for EAE onset
The up-regulation of TNF and TNFR1 in effector monocytes mirrored the induction paralleling the generation of Ly6Clo blood monocytes, suggesting a similar survival mechanism. To study the role of TNF expression for the function of mononuclear phagocytes in the context of EAE, we crossed cx3cr1Cre and cx3cr1CreER animals (Yona et al., 2013) to mice harboring floxed tnf alleles (Grivennikov et al., 2005). The cx3cr1Cre system broadly affects tissue macrophages, as well as monocyte-derived cells and a fraction of DCs (Yona et al., 2013; Aychek et al., 2015). However, the tamoxifen (TAM)-inducible cx3cr1CreER system targets long-lived cells that express the CX3CR1 chemokine receptor at the time of TAM treatment. The latter includes microglia and nonparenchymal CNS macrophages (Goldmann et al., 2016) but excludes monocytes (Goldmann et al., 2013) and hence allows discrimination of microglial and monocyte-derived TNF. First, we investigated the EAE disease course in cx3cr1Cre:tnffl/fl animals. To confirm the broad TNF deletion in tissue macrophages and monocytes, we challenged the animals with LPS, which is known to result in systemic TNF release (Grivennikov et al., 2005). Cx3cr1Cre:tnffl/fl mice displayed 4 h after intraperitoneal LPS challenge significantly lower serum TNF levels than littermate controls (Fig. 3 E). To probe for EAE development in cx3cr1Cre:tnffl/fl mice, we immunized the animals with MOG35–55 peptide and monitored disease emergence up to day 70. Total myeloid-derived TNF was significantly reduced in cx3cr1Cre:tnffl/fl mice in the spinal cord at EAE day 24 (Fig. 3 F), and TNF signal was abolished in both resident microglia and CD45hiLy6Chi and CD45hiLy6Clo monocytic infiltrates (Fig. S2 A). In accordance with earlier studies performed with TNF−/− animals (Kassiotis and Kollias, 2001; Kruglov et al., 2011), cx3cr1Cre:tnffl/fl mice exhibited a delayed and less sharp EAE onset (mean day 31), as compared with TNFfl/fl controls, which showed an earlier and synchronized start (mean 14 d; Fig. 3, G and H). However, eventually, the severity of disease symptoms was comparable between the mutants and littermate controls, both with respect to the mean maximal score and extent of demyelination (Fig. 3 I and Fig. S2 E). Of note, when animals from several EAE experiments were pooled together, statistically significant effects were seen both with respect to disease onset and maximal severity, which was slightly reduced in cx3cr1Cre:tnffl/fl animals (Fig. S2 B).
EAE development relies on generation of autoreactive CD4+ effector T cells, triggered by peripheral, and potentially also CNS-associated, DCs (Codarri et al., 2013). Delayed disease onset could indicate impaired T cell priming. However, analysis of lymph node T cells (day 6 after immunization) and spinal cord T cells of diseased animals (days 13 and 24) revealed similar numbers of CD4+ effector T cells, as well as T helper 1 cell (Th1 cell) and Th17 cell prevalence in cx3cr1Cre:tnffl/fl and tnffl/fl mice. In addition, histology revealed no significant difference in CD3+ cell numbers (Fig. S2, F and G). Finally, in vitro recall responses of lymph node CD4+ effector T cells to secondary exposure to MOG35–55 peptide, measured by the percentage of CD44+ cells, were unaffected in cx3cr1Cre:tnffl/fl mice (Fig. S2 H). Thus, the CD4+ T cell compartment of cx3cr1Cre:tnffl/fl mice is seemingly intact.
To investigate whether the EAE delay we observed in cx3cr1Cre:tnffl/fl mice was caused by a lack of TNF production by monocyte-derived phagocytes or tissue-resident microglia, we resorted to the cx3cr1CreER system (Yona et al., 2013). First, we isolated microglia from TAM-treated tnffl/fl and cx3cr1CreER:tnffl/fl animals and challenged them ex vivo with 10 µg/ml LPS for 4 h. Tnffl/fl microglia responded to stimulation with robust induction of TNF, whereas cx3cr1CreER:tnffl/fl microglia failed to do so, confirming their lack of the tnf gene (Fig. S3 A). To investigate the impact of the microglial TNF deficiency on EAE development, tnffl/fl and cx3cr1CreER:tnffl/fl animals were immunized 6 wk after TAM treatment with MOG35–55 peptide in CFA. Spinal cord microglia obtained from MOG35–55-immunized cx3cr1CreER:tnffl/fl mice lacked TNF production (Fig. S3, B and C). However, despite the prominent TNF expression in WT microglia, cx3cr1CreER:tnffl/fl mice displayed an EAE disease course similar to that of littermate controls, both with respect to severity and onset (Fig. S3, D–F). No statistically significant change in day of onset or maximal severity was seen even when animals from several experiments were pooled together, despite a nonsignificant trend of reduced mortality in cx3cr1CreER:tnffl/fl animals (Fig. S3, G and H). This establishes that microglial TNF is dispensable for EAE initiation in the MOG35–55 peptide/CFA-induced C57BL/6 model.
Monocytic infiltrates are reduced in the absence of autonomous TNF
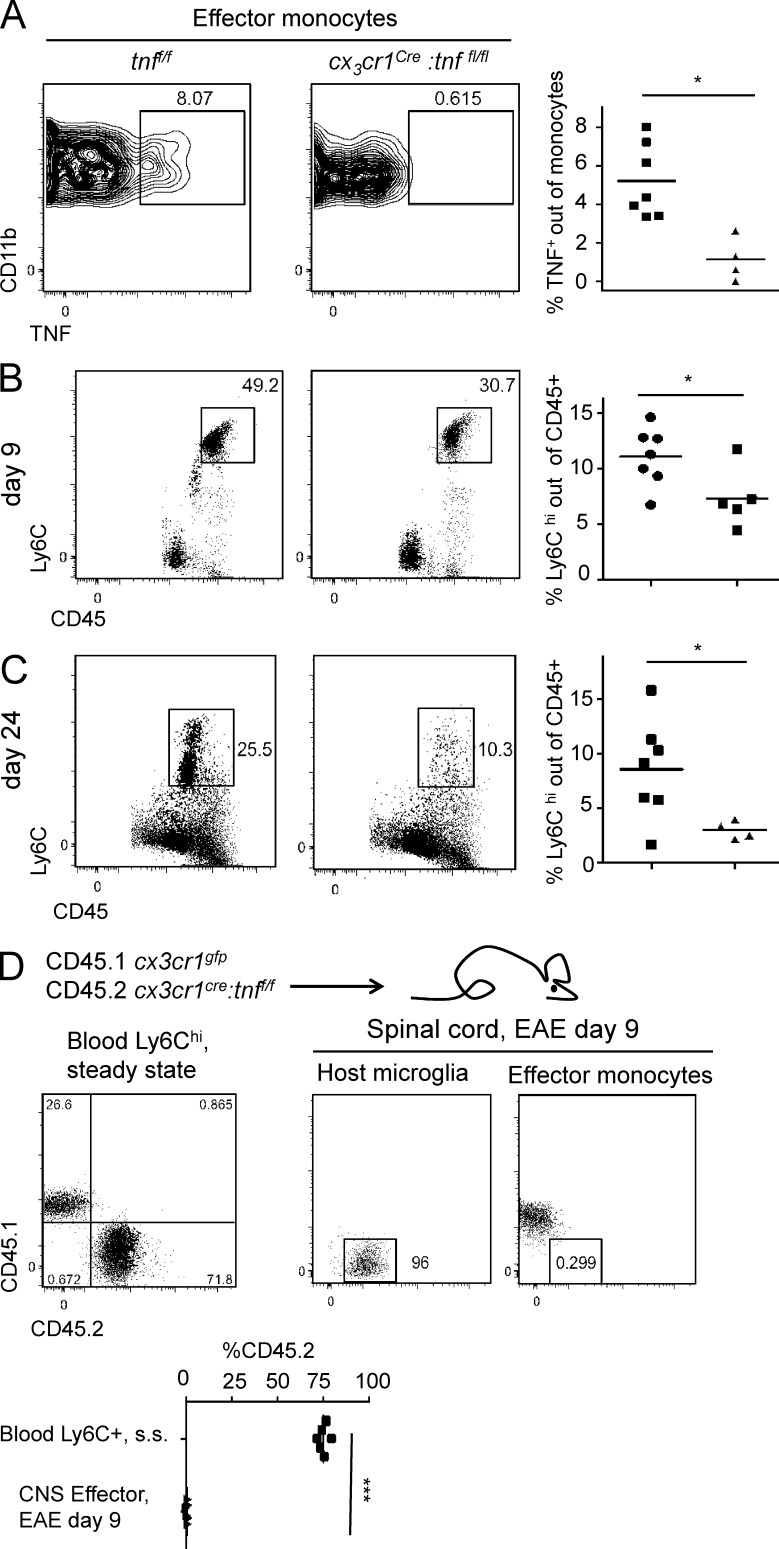
Neither peripheral T cell priming nor CNS infiltration of T cells seemed to depend on mononuclear phagocyte-derived TNF, and the microglia-restricted TNF deletion did not alter the disease course. Hence, we focused on the role of TNF production by CNS-infiltrating monocytes. In steady state, blood monocytes of cx3cr1Cre:tnffl/fl mice display normal numbers (Fig. S2 C). A fraction of the WT (tnffl/fl) effector monocyte infiltrate in the spinal cords of diseased animals displayed TNF expression; however, as expected, the latter was absent from effector monocytes of cx3cr1Cre:tnffl/fl mice (Fig. 4 A). Notably though, at day 9 after MOG immunization, i.e., before disease onset, and on day 24, effector monocyte numbers in spinal cords were found significantly reduced in cx3cr1Cre:tnffl/fl mice, as compared with controls (Fig. 4, B and C). However, effector monocytes of cx3cr1Cre:tnffl/fl mice expressed normal levels of MHCII, indicating similar activation of the cells in the absence of TNF (Fig. S2 D). Thus, we observed a positive correlation between numbers of Ly6Chi effector monocytes and their TNF expression and EAE onset.
Figure 4.
Effector monocytes are reduced because of absence of autonomous TNF. (A) FACS analysis of effector monocytes gated as in Fig. 3 A from MOG35–55 immunized in tnffl/fl and cx3cr1Cre:tnffl/fl mice at day 24. n = 4–7. Mann-Whitney U test was used. (B and C) FACS analysis of spinal cord CD45hiLy6Chi infiltrate in MOG35–55 immunized in tnffl/fl and cx3cr1Cre:tnffl/fl mice at day 9 (n = 5–7; B) or 24 (n = 4–7; C) after immunization. Mann-Whitney U test was used. (D) FACS analysis circulating steady-state (s.s.) Ly6Chi monocytes (gated as in Fig. S1 B) compared with spinal cord effector monocytes (gated as in Fig. 3 A) at day 9 after MOG35–55 immunization in [CD45.1 cx3cr1gfp:tnf+/+/CD45.2 cx3cr1Cre:tnffl/fl > CD45.2 WT] chimeric mice. Resident CD45.2+ microglia (gated as in Fig. S3 B) are presented as reference cells for CD45.2 signal. n = 6. Student’s t test was used. *, P < 0.05; ***, P < 0.001.
The reduced number of infiltrating monocytes in the spinal cords of cx3cr1Cre:tnffl/fl mice could stem from environmental impairment, such as reduced activation of endothelial cells, which are known to depend on TNF (Madge and Pober, 2001) and recruit monocytes via the CCL2/CCR2 axis (Paul et al., 2014). However, CD31+ CD45− endothelial cells isolated from spinal cord of MOG-challenged cx3cr1Cre:tnffl/fl mice and controls up-regulated expression of VCAM1, suggesting an unaltered endothelial response (Fig. S2 I). Alternatively, the reduction of effector monocytes in cx3cr1Cre:tnffl/fl mice could result from autonomous TNF-mediated survival, similar to that observed for steady-state Ly6Clo monocytes. To probe this option, we generated mixed BM chimeras with cx3cr1Cre:tnffl/fl (CD45.2) and cx3cr1gfp:tnf+/+ (CD45.1) BM. [Cx3cr1Cre:tnffl/fl/cx3cr1gfp:tnf+/+ > WT] mice were immunized with MOG peptide in CFA. Analysis of effector monocytes in the spinal cord 9 d after MOG immunization revealed an extensive out-competition of the TNF-deficient by the TNF-proficient (CD45.1) effector monocytes (Fig. 4 D). Interestingly, in dextran sulfate sodium (DSS)–induced colitis, an inflammatory condition in which monocytes do not up-regulate TNF (Zigmond et al., 2012), colonic Ly6Chi CD45.2 tnf−/− monocytes were represented in the same percentages as steady-state Ly6Chi blood monocytes, indicating that not all inflammatory conditions require autonomous TNF survival signal (Fig. S1 F). Collectively, these data show that absence of the autonomous TNF signal can have a critical impact on the performance of effector monocytes in both steady-state and inflammatory settings.
Here, we report the critical role of cell-intrinsic TNF for the survival and function of blood and effector monocytes in steady state and in a model of autoimmune neuroinflammation. The concept of cell-autonomous/autocrine TNF signals has been discussed before in different cellular contexts. In cultured human monocyte-derived DCs, autocrine TNF was shown to inhibit apoptosis mediated by LPS and polyinosinic:polycytidylic acid, by regulating the balance between pro- and antiapoptotic proteins (Cowburn et al., 2004; Lehner et al., 2012). CpG-induced autocrine TNF of BM-derived mouse macrophages was suggested to amplify NF-κB activation (Caldwell et al., 2014). Moreover, LPS stimulation of these cells was recently shown to induce cell-intrinsic TNF-mediated necroptosis, if not inhibited by CYLD (Legarda et al., 2016). These experiments used primary cell cultures to dissect paracrine from autocrine TNF secretion. However, experimental evidence supporting the in vivo relevance and physiological implications of self-TNF and its impairment has been missing.
Specifically, here, we demonstrate that prosurvival signaling triggered by autonomous TNF is important for monocyte differentiation and monocyte maintenance. Of note, in the EAE model, inflammatory signals, which activate Ly6Chi monocytes and recruit them to the site of inflammation, also initiate autocrine TNF as well as TNFR1 expression. Moreover, TNF is found to be up-regulated after the conversion of Ly6Chi monocytes into Ly6Clo cells in the steady state, alongside the induction of its receptors, especially TNFR2. The TNF-triggered signal seems also important for the survival of effector Ly6Chi monocytes infiltrating the spinal cord, as, in its absence, CNS monocyte numbers are reduced, and EAE disease development is delayed. Interestingly, BM monocyte precursors also require TNF, as indicated by the disadvantage of TNFR1-deficient cells compared with WT, but can acquire TNF from noncell-intrinsic origins and do not seem to express autonomous TNF.
Notably, we find TNF to be produced at levels that are detectable by flow cytometry only in a small fraction of effector monocytes; likewise, Ly6Clo monocytes also express low amounts of TNF. However, interestingly, it has been reported that low concentrations of TNF, which could escape our readout, can promote survival, whereas high concentrations can confer apoptosis and collateral damage (van den Berg et al., 2001). This paradoxical effect of TNF on monocytes, i.e., promotion of survival or apoptosis at low and high levels, respectively, is reminiscent of an IL-2 activity on T cells that we recently revealed as a mechanism that contributes to cell number homeostasis (Hart et al., 2014).
Although the deletion of myeloid TNF results in reduced monocyte numbers in the tissue, it does not significantly alter the extent of tissue damage caused by EAE; thus, monocyte-derived TNF is not required for demyelination itself. Likewise, microglial TNF is not required for the induction of EAE, probably because the massive generation of monocyte-derived macrophages can compensate for the loss of microglial TNF.
TNF is known primarily as a proinflammatory cytokine and a mediator of apoptosis (Wajant et al., 2003). However, TNF signaling activates the NF-κB signaling pathway, which also includes many genes promoting cell survival and inhibiting cell death by apoptosis (Karin and Lin, 2002). In human neutrophils, TNF can induce survival in an NF-κB–dependent manner (Cowburn et al., 2004). Moreover, human DCs were proposed to be protected by autocrine TNF from apoptosis, although in the performed in vitro assays, TNF provision by neighbor DCs could not be ruled out (Lehner et al., 2012).
Importantly, our study focuses on EAE disease onset. As our work is confined to C57BL/6 mice, we were bound to MOG35–55 peptide vaccination and can draw no conclusions on a relapsing/remitting EAE course, which might arguably better reflect the disease progression in humans (Berard et al., 2010). Future work using SJL mice and other peptides, such as proteolipid protein and myelin basic protein, will be required to elucidate the roles of myeloid TNF in the remyelination process and of monocytic or microglial TNF in EAE reoccurrence.
An even more important question is whether the intrinsic TNF survival mechanism we report exists in human monocytes. TNF antagonist so far proved to be inefficient or even detrimental in EAE (Caminero et al., 2011). However, our data suggest that neutralization of monocytic TNF, either by a specific antagonist or by blockade of the corresponding receptor, could be tested clinically for its potential to ameliorate MS in humans. Of particular interest in this respect are newly developed bispecific antibodies designed to specifically neutralize monocyte/macrophage TNF (Efimov et al., 2016). TNFR1−/− mice are protected from EAE, and selective neutralization of TNFR1 with antibody reduces disease severity (Williams et al., 2014). An alternative promising approach could be the monocyte-specific knockdown of TNF or its receptors by nanoparticle-encapsulated siRNA, as recently demonstrated for CCR2 (Majmudar et al., 2013).
To conclude, here, we describe a novel role for cell-intrinsic TNF in the survival of monocytes under both steady-state and pathological conditions. We show that this autocrine signal can alter significantly the disease course in autoimmune demyelination and potentially other conditions that involve acute recruitment of effector monocytes.
Materials and methods
Mice
The following 4–24-wk-old mice were used: C57BL/6 (CD45.2 and CD45.1; B6.SJL-Ptprca Pep3b/Boyj; Jackson ImmunoResearch Laboratories, Inc.), cx3cr1gfp/+ (JAX stock 005582 B6.129P-Cx3cr1tm1Litt/J; Jung et al., 2000), cx3cr1Cre (JAX stock 025524 B6J.B6N[Cg]-Cx3cr1tm1.1[cre]Jung/J), cx3cr1CreER (JAX stock 020940 B6.129P2[C]-Cx3cr1tm2.1[cre/ERT2]Jung/J; Yona et al., 2013), tnff/f (Grivennikov et al., 2005), tnf−/− (Pasparakis et al., 1996), tnfrsf1a−/− (TNFR1−/−; Rothe et al., 1993), and tnfrsa1b−/−/tnfrsf1b (TNFR1/2 DKO) mice (Probert et al., 2000). All animals were on a C57BL/6 background, maintained in specific pathogen–free conditions, and handled according to protocols approved by the Weizmann Institute Animal Care Committee as per international guidelines.
In vitro monocyte assays
Blood monocytes and BM cMOPs were sorted using a FACSAria cell sorter (BD). Microwell arrays were designed using autoCAD (Autodesk Inc.) as an array of several hundreds of hexagonally spaced microwells, each being 25 µm in diameter and 60 µm in depth. Polydimethylsiloxane microwell arrays were fabricated using soft lithography as described previously (Zaretsky et al., 2012). The arrays were placed in glass-bottom 96-well plates. Monocytes were sorted and loaded into the microwell array: First, medium was removed and replaced with 25 × 103 monocytes in 100 µl RPMI medium. The plate was centrifuged at 300 g for 1 min to allow cells to settle in the microwells. Residual cells were aspirated, and wells were supplemented with 200 µl RPMI containing 0.5 ng anti-CD45.2–Alexa Fluor 488 and 0.5 ng anti-CD45.1–APC (BioLegend) and 2.5 µg/ml PI (Sigma-Aldrich) for a final dilution of 1:2 × 105 for the antibodies and 1:2 × 103 for PI (Zaretsky et al., 2012; Polonsky et al., 2016). For time-lapse experiments, a Ti-Eclipse microscope (Nikon) was used, equipped with an automated stage, incubator, and closed chamber that allows for CO2 flow over the 96-well plate. Cells were imaged using a 20× objective and monitored using brightfield illumination and three fluorescence channels: FITC, Cy3, and Cy5. Time-lapse videos were collected using iQ software (Andor Technology). Image analysis was performed using a custom built MATLAB tool. Only microwells that included a single cell were included in the analysis. Analysis was performed in several steps: (a) identification of microwells using edge detection in the brightfield images, (b) background subtraction and illumination correction of fluorescent images, (c) identification of microwells, in which mean fluorescent intensities (MFIs) corresponded to that of a single cell (MFI values of single cells were evaluated manually for several hundreds of microwells in three different fields of view), and (d) death times were determined for each microwell as the time in which MFI in the Cy3 channel (PI signal) was ≥500. thalf, the time in which the cumulative death curve reaches half of its maximum value, was calculated by fitting the logistic equation
in which M is the maximum value, and r is the steepness of the slope. Fitting was done using MATLAB. For the generation of monocyte-derived cells, BM of CD45.1 and CD45.2/tnf−/− mice was extracted and flushed with PBS. The BM suspension was incubated with biotinylated CD115 antibody (BioLegend) and was MACS sorted using streptavidin beads (Miltenyi Biotec). The sorted cells were grown in RPMI medium containing 10% FCS, 1% l-glutamine, 1% penicillin/streptomycin, and 10 ng/ml M-CSF (PeproTech).
Generation of BM chimeras
7–10-wk-old recipient animals were lethally irradiated (950 rad) and reconstituted with donor BM by i.v. injection of a minimum of 106 BM cells. Donor BM was isolated from femurs and tibiae of donor mice, filtered through a 70-µm mesh, and resuspended in PBS for i.v. injection.
Reagent administration
TAM (Sigma-Aldrich) was dissolved in 10% ethanol/90% corn oil (Sigma-Aldrich) to a 100 mg/ml final solution. To induce Cre-mediated gene recombination in 5–7-wk-old cx3cr1creER:tnff/f mice, 5 mg TAM was administered orally for five consecutive days (25 mg total) by gavage. For EAE induction, mice were injected into each flank with 50 µl emulsion containing 1 mg/ml MOG35–55 peptide (GeneScript), 1:4 PBS, and 1:2 Freund’s incomplete adjuvant (Sigma-Aldrich) enriched with killed Mycobacterium tuberculosis (BD), supplemented with 250 ng pertussis toxin (Sigma-Aldrich) administered i.p. on days 0 and 2. For DSS-induced colitis, mice were administrated 2% DSS (MP Biomedicals) in drinking water for 7 d (Zigmond et al., 2012).
EAE assessment
EAE was assessed according to an accepted assessment index (Stromnes and Goverman, 2006). The mice were monitored daily by being held at the base of the tail. The index is as follows: 0, no symptoms; 0.5, partial tail limp; 1, complete tail limp; 1.5, impaired gait; 2, loss of pinch reflex in hindlimbs; 2.5, one hindlimb paralysis; 3, complete hindlimb paralysis; 3.5, one forelimb paralysis; 4, complete forelimb paralysis; and 5, death.
Tissue extraction and flow cytometry
For peripheral analysis, blood was collected by tail bleeds; mononuclear cells were enriched by Ficoll density gradient centrifugation at 1,000 g for 15 min at 20°C with low acceleration and no brake. For lymph node analysis, tissues were collected and digested for 1 h with 1 mg/ml collagenase D (Roche) in PBS containing magnesium and calcium (PBS+/+; Beit Ha’emek) and then macerated mechanically and filtered through a 70-µm mesh. For CNS analysis, mice were perfused with 10 ml PBS via the left ventricle. Brain and spinal cord samples were harvested from individual mice, and tissues were homogenized and incubated with an HBSS solution containing 2% BSA (Sigma-Aldrich), 1 mg/ml collagenase D (Roche), and 0.15 mg/ml DNase1, filtered through a 70-µm mesh. Homogenized sections were resuspended in 40% percoll before density centrifugation (1,000 g for 15 min at 20°C with low acceleration and no brake). For spinal cord endothelium extraction, spinal cords were removed as for microglia extraction and digested with 0.1 mg/ml collagenase 11, 2 mg/ml collagenase 2, 0.2 mg/ml Dnase1, and 0.1 mg/ml hylurodinase 1 (Sigma-Aldrich). After cell suspension, cells were incubated in FACS buffer (PBS with 1% BSA, 2 mM EDTA, and 0.05% sodium azide) in the presence of staining antibody. For colon extraction after DSS, fecal content was flushed out with PBS, and the tissue was first incubated with HBSS containing 10% FCS, 2.5 mM EDTA, and 1 mM dithiothreitol. Next, the tissue was incubated with PBS containing 5% FCS, 1 mg/ml collagenase VIII (Sigma-Aldrich), and 0.1 mg/ml DNase I (Roche). For intracellular staining, the CytoFix/Cytoperm kit (BD) was used according to the instructions of the manufacturer. Cells were acquired on FACSCanto, LSRII, and LSRFortessa systems (BD) and analyzed with FlowJo software (Tree Star). For cell sorting, the FACSAria cell sorter (BD) was used. Antibodies used throughout the research are: CD11b (clone M1/70), CD45.1 (clone A20), CD45.2 (clone 104), B220 (clone RA3-6B2), CD115 (clone AFS98), Gr1 (clone RB6-8C5), Ly6C (clone AL21), Ly6G (clone H129.19), CD4 (clone H129.19), CD45 (clone 30F11), IL-17A (clone TC11-18H10.1), IFN-γ (clone XMG1.2), TNF (clone MP6-XT2), CD31 (clone 390), CD3 (clone 145-2C11), CD19 (clone 1D3/CD19), NK1.1 (clone PK136), VCAM1 (clone 429 MVCAM.A; all BioLegend), and F4/80 (clone BM8; Serotech).
RNA isolation, library construction, and analysis
RNA sequencing was performed as described previously (Jaitin et al., 2014). In brief, 103–104 cells from each population were sorted into 100–200 µl of lysis/binding buffer (Thermo Fisher Scientific). mRNA was captured with 12 µl of Dynabeads oligo(dT) (Thermo Fisher Scientific), washed, and eluted at 70°C with 10 µl of 10 mM Tris-Cl, pH 7.5. A mean of four million reads per library was sequenced and aligned to the mouse reference genome (NCBI 37; mm9) using TopHat (v2.0.10) with default parameters. Expression levels were calculated and normalized using ESAT software. RNA sequencing analysis focused on genes in the 25th percentile of expression with a twofold differential between at least two populations and a p-value <0.01. Canonical pathway analysis was performed using the Ingenuity program.
Ex vivo stimulation
For Th1/Th17 cell stimulation, spinal cord or lymph node samples were prepared as mentioned in the Tissue extraction and flow cytometry section and were incubated for 3 h in RPMI medium containing 10% FCS, 1:100 penicillin streptomycin antibiotic, 1:100 l-glutamine, 1:100 MEM, and 1:100 sodium pyruvate (Beit Ha’emek) supplemented with 20 µg/ml MOG35–55 peptide (GeneScript). Then, the cell suspensions were further incubated with the same media, supplemented with 1 µg/ml brefeldin A (Sigma-Aldrich), for three additional hours before antibody staining. For ex vivo LPS stimulation, brain cell suspensions were incubated with the above RPMI medium supplemented with 10 µg/ml LPS for 2 h, followed by incubation in the presence of brefeldin A for an additional 2 h. Recombinant mouse TNF was purchased from PeproTech.
Histology
Histology was performed as described recently (Goldmann et al., 2013). In brief, mice were killed on day 24 after immunization and subsequently perfused with PBS. Subsequently, the spinal cords were removed, fixed in 4% buffered formalin, and embedded in paraffin. Then, tissue samples were sectioned and stained with Luxol fast blue (LFB-PAS) to assess the degree of demyelination and CD3 (1:100; MCA1477; AbD Serotec) to assess the degree of infiltration of T cells. Tissue sections were evaluated on a microscope (BX-61; Olympus) using cell-P software (Olympus).
ELISA
For ELISA, sera were extracted by tail bleed and centrifugation of blood at full speed for 7 min. TNF was measured using the DueSet kit (R&D Systems). ELISA was performed according to the manufacturer’s instructions.
Statistical analysis
Results are expressed as means ± SEM. Statistical analysis was performed using Student’s t test, Mann-Whitney U test, ANOVA, or repeated-measurements two-way ANOVA with posthoc Student’s t test or paired Student’s t test, as appropriate, using Prism (GraphPad Software).
Online supplemental material
Fig. S1 shows flow cytometric analysis of monocytes and monocyte precursors in the BM. Fig. S2 shows additional characterization of cx3cr1Cre:tnff/f mice. Fig. S3 shows microglia-restricted TNF deletion does not affect EAE onset. Tables S1 and S2 are included as Excel files. Table S1 lists genes up-regulated in spinal cord effector monocytes at EAE day 9 compared with Ly6Chi monocytes. Table S2 lists genes down-regulated in spinal cord effector monocytes at EAE day 9 compared with Ly6Chi monocytes.
Supplementary Material
Acknowledgments
We would like to thank all members of the Jung laboratory for helpful discussion and the staff of the Weizmann Institute animal facility for excellent animal care.
Work in the Jung laboratory was supported by the Israel Science Foundation (grant 887/11), the Minerva Foundation (grant 711747), and the European Research Council (AdvERC grant 340345).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- cMOP
- common monocyte precursor
- CNS
- central nervous system
- DSS
- dextran sulfate sodium
- EAE
- experimental autoimmune encephalomyelitis
- MACS
- magnetic-activated cell sorting
- MDP
- monocyte/macrophage DC precursor
- MS
- multiple sclerosis
- PI
- propidium iodide
- TAM
- tamoxifen
References
- Ajami B., Bennett J.L., Krieger C., McNagny K.M., and Rossi F.M.V.. 2011. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 14:1142–1149. 10.1038/nn.2887 [DOI] [PubMed] [Google Scholar]
- Auffray C., Fogg D., Garfa M., Elain G., Join-Lambert O., Kayal S., Sarnacki S., Cumano A., Lauvau G., and Geissmann F.. 2007. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 317:666–670. 10.1126/science.1142883 [DOI] [PubMed] [Google Scholar]
- Aychek T., Mildner A., Yona S., Kim K.W., Lampl N., Reich-Zeliger S., Boon L., Yogev N., Waisman A., Cua D.J., and Jung S.. 2015. IL-23-mediated mononuclear phagocyte crosstalk protects mice from Citrobacter rodentium-induced colon immunopathology. Nat. Commun. 6:6525 10.1038/ncomms7525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berard J.L., Wolak K., Fournier S., and David S.. 2010. Characterization of relapsing-remitting and chronic forms of experimental autoimmune encephalomyelitis in C57BL/6 mice. Glia. 58:434–445. 10.1002/glia.20935 [DOI] [PubMed] [Google Scholar]
- Bitsch A., Kuhlmann T., Da Costa C., Bunkowski S., Polak T., and Brück W.. 2000. Tumour necrosis factor alpha mRNA expression in early multiple sclerosis lesions: correlation with demyelinating activity and oligodendrocyte pathology. Glia. 29:366–375. [DOI] [PubMed] [Google Scholar]
- Caldwell A.B., Cheng Z., Vargas J.D., Birnbaum H.A., and Hoffmann A.. 2014. Network dynamics determine the autocrine and paracrine signaling functions of TNF. Genes Dev. 28:2120–2133. 10.1101/gad.244749.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminero A., Comabella M., and Montalban X.. 2011. Tumor necrosis factor alpha (TNF-α), anti-TNF-α and demyelination revisited: an ongoing story. J. Neuroimmunol. 234:1–6. 10.1016/j.jneuroim.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Codarri L., Greter M., and Becher B.. 2013. Communication between pathogenic T cells and myeloid cells in neuroinflammatory disease. Trends Immunol. 34:114–119. 10.1016/j.it.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Cowburn A.S., Deighton J., Walmsley S.R., and Chilvers E.R.. 2004. The survival effect of TNF-α in human neutrophils is mediated via NF-κB-dependent IL-8 release. Eur. J. Immunol. 34:1733–1743. 10.1002/eji.200425091 [DOI] [PubMed] [Google Scholar]
- Croxford A.L., Lanzinger M., Hartmann F.J., Schreiner B., Mair F., Pelczar P., Clausen B.E., Jung S., Greter M., and Becher B.. 2015. The Cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity. 43:502–514. 10.1016/j.immuni.2015.08.010 [DOI] [PubMed] [Google Scholar]
- Efimov G.A., Kruglov A.A., Khlopchatnikova Z.V., Rozov F.N., Mokhonov V.V., Rose-John S., Scheller J., Gordon S., Stacey M., Drutskaya M.S., et al. 2016. Cell-type-restricted anti-cytokine therapy: TNF inhibition from one pathogenic source. Proc. Natl. Acad. Sci. USA. 113:3006–3011. 10.1073/pnas.1520175113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogg D.K., Sibon C., Miled C., Jung S., Aucouturier P., Littman D.R., Cumano A., and Geissmann F.. 2006. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 311:83–87. 10.1126/science.1117729 [DOI] [PubMed] [Google Scholar]
- Geissmann F., Jung S., and Littman D.R.. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 19:71–82. 10.1016/S1074-7613(03)00174-2 [DOI] [PubMed] [Google Scholar]
- Ginhoux F., and Jung S.. 2014. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 14:392–404. 10.1038/nri3671 [DOI] [PubMed] [Google Scholar]
- Goldmann T., Wieghofer P., Müller P.F., Wolf Y., Varol D., Yona S., Brendecke S.M., Kierdorf K., Staszewski O., Datta M., et al. 2013. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16:1618–1626. 10.1038/nn.3531 [DOI] [PubMed] [Google Scholar]
- Goldmann T., Wieghofer P., Jordão M.J., Prutek F., Hagemeyer N., Frenzel K., Amann L., Staszewski O., Kierdorf K., Krueger M., et al. 2016. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 17:797–805. 10.1038/ni.3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell M., Douni E., Wajant H., Löhden M., Clauss M., Maxeiner B., Georgopoulos S., Lesslauer W., Kollias G., Pfizenmaier K., and Scheurich P.. 1995. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 83:793–802. 10.1016/0092-8674(95)90192-2 [DOI] [PubMed] [Google Scholar]
- Grell M., Wajant H., Zimmermann G., and Scheurich P.. 1998. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc. Natl. Acad. Sci. USA. 95:570–575. 10.1073/pnas.95.2.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S.I., Tumanov A.V., Liepinsh D.J., Kruglov A.A., Marakusha B.I., Shakhov A.N., Murakami T., Drutskaya L.N., Förster I., Clausen B.E., et al. 2005. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity. 22:93–104. 10.1016/j.immuni.2004.11.016 [DOI] [PubMed] [Google Scholar]
- Hart Y., Reich-Zeliger S., Antebi Y.E., Zaretsky I., Mayo A.E., Alon U., and Friedman N.. 2014. Paradoxical signaling by a secreted molecule leads to homeostasis of cell levels. Cell. 158:1022–1032. 10.1016/j.cell.2014.07.033 [DOI] [PubMed] [Google Scholar]
- Hettinger J., Richards D.M., Hansson J., Barra M.M., Joschko A.C., Krijgsveld J., and Feuerer M.. 2013. Origin of monocytes and macrophages in a committed progenitor. Nat. Immunol. 14:821–830. 10.1038/ni.2638 [DOI] [PubMed] [Google Scholar]
- Hofman F.M., Hinton D.R., Johnson K., and Merrill J.E.. 1989. Tumor necrosis factor identified in multiple sclerosis brain. J. Exp. Med. 170:607–612. 10.1084/jem.170.2.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K., Kimura T., Miyamoto T., Takaishi H., Okada Y., Toyama Y., and Blobel C.P.. 2007. Cutting edge: TNF-α-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J. Immunol. 179:2686–2689. 10.4049/jimmunol.179.5.2686 [DOI] [PubMed] [Google Scholar]
- Jaitin D.A., Kenigsberg E., Keren-Shaul H., Elefant N., Paul F., Zaretsky I., Mildner A., Cohen N., Jung S., Tanay A., and Amit I.. 2014. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science. 343:776–779. 10.1126/science.1247651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S., Aliberti J., Graemmel P., Sunshine M.J., Kreutzberg G.W., Sher A., and Littman D.R.. 2000. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20:4106–4114. 10.1128/MCB.20.11.4106-4114.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., and Lin A.. 2002. NF-κB at the crossroads of life and death. Nat. Immunol. 3:221–227. 10.1038/ni0302-221 [DOI] [PubMed] [Google Scholar]
- Kassiotis G., and Kollias G.. 2001. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J. Exp. Med. 193:427–434. 10.1084/jem.193.4.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglov A.A., Lampropoulou V., Fillatreau S., and Nedospasov S.A.. 2011. Pathogenic and protective functions of TNF in neuroinflammation are defined by its expression in T lymphocytes and myeloid cells. J. Immunol. 187:5660–5670. 10.4049/jimmunol.1100663 [DOI] [PubMed] [Google Scholar]
- Lavin Y., Winter D., Blecher-Gonen R., David E., Keren-Shaul H., Merad M., Jung S., and Amit I.. 2014. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 159:1312–1326. 10.1016/j.cell.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legarda D., Justus S.J., Ang R.L., Rikhi N., Li W., Moran T.M., Zhang J., Mizoguchi E., Zelic M., Kelliher M.A., et al. 2016. CYLD proteolysis protects macrophages from TNF-mediated auto-necroptosis induced by LPS and licensed by type I IFN. Cell Reports. 15:2449–2461. 10.1016/j.celrep.2016.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner M., Kellert B., Proff J., Schmid M.A., Diessenbacher P., Ensser A., Dörrie J., Schaft N., Leverkus M., Kämpgen E., and Holter W.. 2012. Autocrine TNF is critical for the survival of human dendritic cells by regulating BAK, BCL-2, and FLIPL. J. Immunol. 188:4810–4818. 10.4049/jimmunol.1101610 [DOI] [PubMed] [Google Scholar]
- Madge L.A., and Pober J.S.. 2001. TNF signaling in vascular endothelial cells. Exp. Mol. Pathol. 70:317–325. 10.1006/exmp.2001.2368 [DOI] [PubMed] [Google Scholar]
- Majmudar M.D., Keliher E.J., Heidt T., Leuschner F., Truelove J., Sena B.F., Gorbatov R., Iwamoto Y., Dutta P., Wojtkiewicz G., et al. 2013. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 127:2038–2046. 10.1161/CIRCULATIONAHA.112.000116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A., Mack M., Schmidt H., Brück W., Djukic M., Zabel M.D., Hille A., Priller J., and Prinz M.. 2009. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. 132:2487–2500. 10.1093/brain/awp144 [DOI] [PubMed] [Google Scholar]
- Mildner A., Yona S., and Jung S.. 2013. A close encounter of the third kind: monocyte-derived cells. Adv. Immunol. 120:69–103. 10.1016/B978-0-12-417028-5.00003-X [DOI] [PubMed] [Google Scholar]
- Mildner A., Marinkovic G., and Jung S.. 2016. Murine monocytes: Origins, subsets, fates, and functions. Microbiol. Spectr. 4. [DOI] [PubMed] [Google Scholar]
- Murphy Á.C., Lalor S.J., Lynch M.A., and Mills K.H.G.. 2010. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav. Immun. 24:641–651. 10.1016/j.bbi.2010.01.014 [DOI] [PubMed] [Google Scholar]
- Pasparakis M., Alexopoulou L., Episkopou V., and Kollias G.. 1996. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184:1397–1411. 10.1084/jem.184.4.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D., Ge S., Lemire Y., Jellison E.R., Serwanski D.R., Ruddle N.H., and Pachter J.S.. 2014. Cell-selective knockout and 3D confocal image analysis reveals separate roles for astrocyte-and endothelial-derived CCL2 in neuroinflammation. J. Neuroinflammation. 11:10 10.1186/1742-2094-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky M., Chain B., and Friedman N.. 2016. Clonal expansion under the microscope: studying lymphocyte activation and differentiation using live-cell imaging. Immunol. Cell Biol. 94:242–249. 10.1038/icb.2015.104 [DOI] [PubMed] [Google Scholar]
- Probert L., Eugster H.P., Akassoglou K., Bauer J., Frei K., Lassmann H., and Fontana A.. 2000. TNFR1 signalling is critical for the development of demyelination and the limitation of T-cell responses during immune-mediated CNS disease. Brain. 123:2005–2019. 10.1093/brain/123.10.2005 [DOI] [PubMed] [Google Scholar]
- Rothe J., Lesslauer W., Lötscher H., Lang Y., Koebel P., Köntgen F., Althage A., Zinkernagel R., Steinmetz M., and Bluethmann H.. 1993. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 364:798–802. 10.1038/364798a0 [DOI] [PubMed] [Google Scholar]
- Shaked I., Hanna R.N., Shaked H., Chodaczek G., Nowyhed H.N., Tweet G., Tacke R., Basat A.B., Mikulski Z., Togher S., et al. 2015. Transcription factor Nr4a1 couples sympathetic and inflammatory cues in CNS-recruited macrophages to limit neuroinflammation. Nat. Immunol. 16:1228–1234. 10.1038/ni.3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R., London A., Varol C., Raposo C., Cusimano M., Yovel G., Rolls A., Mack M., Pluchino S., Martino G., et al. 2009. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 6:e1000113 10.1371/journal.pmed.1000113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromnes I.M., and Goverman J.M.. 2006. Passive induction of experimental allergic encephalomyelitis. Nat. Protoc. 1:1952–1960. 10.1038/nprot.2006.284 [DOI] [PubMed] [Google Scholar]
- van den Berg J.M., Weyer S., Weening J.J., Roos D., and Kuijpers T.W.. 2001. Divergent effects of tumor necrosis factor α on apoptosis of human neutrophils. J. Leukoc. Biol. 69:467–473. [PubMed] [Google Scholar]
- Varol C., Landsman L., Fogg D.K., Greenshtein L., Gildor B., Margalit R., Kalchenko V., Geissmann F., and Jung S.. 2007. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J. Exp. Med. 204:171–180. 10.1084/jem.20061011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajant H., Pfizenmaier K., and Scheurich P.. 2003. Tumor necrosis factor signaling. Cell Death Differ. 10:45–65. 10.1038/sj.cdd.4401189 [DOI] [PubMed] [Google Scholar]
- Williams S.K., Maier O., Fischer R., Fairless R., Hochmeister S., Stojic A., Pick L., Haar D., Musiol S., Storch M.K., et al. 2014. Antibody-mediated inhibition of TNFR1 attenuates disease in a mouse model of multiple sclerosis. PLoS One. 9:e90117 10.1371/journal.pone.0090117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R., Lu H., Butovsky O., Ohno N., Rietsch A.M., Cialic R., Wu P.M., Doykan C.E., Lin J., Cotleur A.C., et al. 2014. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 211:1533–1549. 10.1084/jem.20132477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S., Kim K.W., Wolf Y., Mildner A., Varol D., Breker M., Strauss-Ayali D., Viukov S., Guilliams M., Misharin A., et al. 2013. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 38:79–91. 10.1016/j.immuni.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky I., Polonsky M., Shifrut E., Reich-Zeliger S., Antebi Y., Aidelberg G., Waysbort N., and Friedman N.. 2012. Monitoring the dynamics of primary T cell activation and differentiation using long term live cell imaging in microwell arrays. Lab Chip. 12:5007–5015. 10.1039/c2lc40808b [DOI] [PubMed] [Google Scholar]
- Zigmond E., Varol C., Farache J., Elmaliah E., Satpathy A.T., Friedlander G., Mack M., Shpigel N., Boneca I.G., Murphy K.M., et al. 2012. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 37:1076–1090. 10.1016/j.immuni.2012.08.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.