Abstract
Background
Extracellular vesicles (EVs) represent a plausible molecular mechanism linking particulate matter (PM) inhalation to its systemic effects. Microvesicles (MVs) are released from many cell types in response to various stimuli. Increased body mass index (BMI) could modify the response to PM exposure due to enhanced PM uptake and/or an underlying pro-oxidative state.
We investigated the relationship between EV release and PM10/PM2.5 exposure in a cohort of 51 volunteers. Subjects were stratified based on their BMI to evaluate whether overweight BMI is a determinant of hypersusceptibility to PM effects.
Results
Exposure to PM10/PM2.5 was assessed with a personal sampler worn for 24 hours before plasma collection and confirmed with monitoring station data. Size and cellular origin of plasma EVs were characterized by Nanosight analysis and flow cytometry, respectively. Multivariate regression models were run after log-transformation, stratifying subjects based on BMI (≥ or <25 kg/m2).
PM exposure resulted in increased release of EVs, with the maximum observed effect for endothelial MVs. For PM10 and PM2.5, the adjusted geometric mean ratio and 95% confidence interval were 3.47 (1.30, 9.27) and 3.14 (1.23, 8.02), respectively. Compared to those in normal subjects, PM-induced EV alterations in overweight subjects were more pronounced, with visibly effect in all MV subtypes, particularly endothelial MVs.
Conclusions
Our findings emphasize the role of EV release after PM exposure and the susceptibility of overweight subjects. Larger studies with accurate exposure assessment and complete EVs characterization/content analysis, could further clarify the molecular mechanism responsible for PM effects and of hypersusceptibility of overweight subjects.
Abbreviations: EV, Extracellular Vesicles; PM, Particulate Matter; BMI, Body Mass Index; CVD, cardio vascular diseases; MV, Microvesicles; PCIS, personal cascade impactor sampler; ARPA, Agenzia Regionale Protezione Ambientale; NTA, nanoparticles tracking nalysis; CFSE, carboxyfluorescein diacetate N-succinimidyl ester; CI, confidence interval; IQR, Interquartile range; GMR, geometric mean ratio; EV-miRNA, Extracellular vesicles encapsulated microRNA
Keywords: PM exposure, Air pollution susceptibility, Overweight, Microvesicles, Flow cytometry
Highlights
-
•
We investigated the association between EV release and personal exposure to PM.
-
•
Increased PM exposure was associated with increased release of EVs.
-
•
The maximum effect was found for endothelial EVs.
-
•
PM-induced EV alterations were more pronounced in overweight subjects.
1. Introduction
Numerous epidemiological studies have shown that short-term exposure to particulate matter (PM) air pollution is associated with increased morbidity and mortality, primarily from cardiovascular disease (CVD) (Katsouyanni et al., 2001, Kinney and Ozkaynak, 1991, Schwartz and Dockery, 1992). Despite this consistently observed association between air pollution and sistemic effects, the underlying molecular mechanism responsible for this relationship is still poorly understood. It has been proposed that ambient particles trigger pulmonary oxidative stress and inflammatory responses that lead to the release of molecular signals into the circulatory system (Bollati et al., 2015). Determining the identity of these signals that link PM inhalation to systemic inflammation and endothelial response has been considered a high priority objective, in order to better understand possible pathway involved in determining the observed increase in CVD risk.
Recently, extracellular vesicles (EVs) have gained increasing consideration as a very plausible link. EVs represent a novel means for intercellular and between-tissue communication (Raposo and Stoorvogel, 2013), as they can travel from the tissue of origin to target cells and transfer their contents after being internalized (Hunter et al., 2008). These EVs are small (<1.0 µm) plasma membrane fragments that accumulate in biological fluids. Studies have shown that they play an important role in a variety of biological and pathological processes (Johnson et al., 2014), including atherosclerosis (Leroyer et al., 2008), thrombosis (Rautou et al., 2011), rheumatoid arthritis (Jungel et al., 2007), cancer (Jungel et al., 2007), and inflammation and sepsis (Prakash et al., 2012). Based on their size, they can be classified as exosomes (30–130 nm) and microvesicles (MVs, 130–700 nm). Exosomes are thought to be constitutively generated, stored, and released from the endosomal system, whereas MVs are shed from the plasma membrane in response to specific stimuli (Larson et al., 2014). MVs, in particular, are released from the external membrane of many, if not all, cell types. These MVs bear surface molecules of their parent cells that allow them to be targeted to recipient cells, thereby establishing a complex cross-talking network (Tkach and Thery, 2016). The presence of parent cell membrane proteins in MVs that are released to the plasma also permits characterization of their cellular origin (e.g., by flow cytometry). For clarity, in this paper, we will exclusively refer to EVs as the sum of MVs and exosomes, to MVs for those EVs that are shed from the plasma membrane and can be quantified by flow cytometry (usually with a size >130 nm) and to exosomes as EVs released from the endosomal system (usually with a size ≤130 nm).
Recent research findings suggest that increased body mass index (BMI) could be a susceptibility factor for the adverse effects of PM exposure. Increased susceptibility may be partially due to an increase in particle absorption: BMI was in fact associated with a graded increase in the estimated total lung dose of deposited fine particles in an inhalation study of healthy children aged 6–13 years (Bennett and Zeman, 2004). Moreover, overweight subjects are characterized by a low-grade systemic inflammatory state and adipose tissue dysfunction (Mraz and Haluzik, 2014). Increased weight is known to increase cell activation, favoring MV formation (Goichot et al., 2006). At the molecular level, obesity-related pathogenic factors causing adipocyte dysfunction include lipid and adipokine dysregulation, mitochondrial malfunction, and induction of cellular oxidative stress processes (Hotamisligil, 2010, Diaz-Ruiz et al., 2015). Taken together, these findings suggest that BMI may be an important effect modifier when investigating PM-mediated biological effects and related pathogenic mechanisms.
Our hypothesis was that EVs produced by the respiratory system (Kesimer et al., 2009) can be disseminated through the bloodstream (Orozco and Lewis, 2010) and can play a role in the complex pathway leading to an increased f CVD risk (Bollati et al., 2015). Therefore, we evaluated EV release, both quantitatively (i.e., total number and size) and qualitatively (i.e., characterizing vesicles by their specific cellular origin), in volunteers with a measured exposure to size-fractionated urban PM assessed by both personal sampling with gravimetric analysis and background ambient monitoring stations, during a 24-h period before plasma collection. In addition, we stratified subjects according to their BMI to determine whether being overweight increases susceptibility to PM-induced adverse effects.
2. Methods
2.1. Study subjects
We recruited 51 healthy volunteers who answered an ad hoc developed announcement posted on the SPHERE Project website (http://users.unimi.it/sphere). After signing a detailed informed consent form, each subject was asked to wear a miniaturized personal sampling device to measure PM ≤10 µm and PM ≤2.5 µm (PM10 and PM2.5) during a 24-h period, beginning at 9 a.m. (day 1). On the following day, each subject underwent venous blood drawing at 9 a.m. (day 2) and returned the sampling device to the study team. All subjects also completed a questionnaire collecting detailed personal data, including anthropometric characteristics (e.g., height and weight), education, area of residence, job position and location, time spent commuting in traffic, alcohol consumption, smoking habits, drugs, and pre-existing medical conditions. Subjects were recruited from November 2014 to March 2015, a period of the year characterized by high levels of air pollution in Northern Italy due to mainly traffic exhaust emissions and residential heating combined with peculiar meteorological phenomena, such as thermal inversion (Carugno et al., 2016). During the 24-h personal sampling period, subjects were invited to perform their routine occupational and leisure activities and continue their voluntary habits.
2.2. Exposure assessment and validation
The subjects’ personal exposure to PM10 and PM2.5 was measured with a miniaturized personal cascade impactor sampler (PCIS; SKC Inc., Eighty Four, PA, USA). The sampler consisted of four impaction stages with cut-off diameters of 2.5, 1.0, 0.5, and 0.25 µm followed by a backup filter stage for particles <0.25 µm. Stretch polytetrafluoroethylene/polytetrafluoroethylene (S-PTFE/PTFE) filters (25 mm diameter and 0.8 µm porosity) were used as impaction substrates, and the backup filters were PTFE w/polymethylpentene ring membranes (37 mm diameter and 2 µm porosity). For this study, the original sampler was modified by excluding the 1.0, 0.5, and 0.25 impaction stages. A field intercomparison test was performed to verify the agreement between the original and the modified configuration. The resulting mean relative error was <1%.
PM concentrations were determined by gravimetric analysis. The net PM mass was obtained by weighing filters before and after sampling with a microbalance (Micro 1000; Gibertini, Novate, MI, Italy) inside a temperature- and relative humidity-controlled (20±1 °C, 50±5%) cabinet (Activa Climatic; Aquaria, Lacchiarella, MI, Italy). Quality of the weighing procedure was assessed using the American Society of Testing and Materials D 6552 method. The limit of detection was 9 µg and 5 µg for PM10 and PM2.5, respectively, which approximately corresponds to 0.7 µg/m3 and 0.4 µg/m3, respectively, for 24-h samplings at 9 L/min. The PM sampler was connected to a sampling pump (Leland Legacy; SKC Inc.) operated at a flow rate of 9 L/min and carried by subjects in the breathing zone. During night time, the instruments were placed in the living room. Ambient concentrations of PM10 and PM2.5 were also obtained from the regional air quality monitoring network (ARPA). Average PM10 and PM2.5 levels, calculated based on measurements from the three stations located in downtown Milan, were selected as suitable estimates of PM air concentrations in the study area, as the majority of study subjects resided and/or worked in Milan. Thus, these PM10 and PM2.5 estimates represented the contribution of outdoor contamination to personal PM exposure. PM levels measured by air monitoring stations were then compared with the results from PCIS.
2.3. Blood collection and EV isolation
Blood was collected in EDTA tubes and processed within 2 h of the phlebotomy. EDTA-treated blood was centrifuged at 1200×g for 15 min at room temperature to obtain platelet-free blood plasma. Plasma was further centrifuged at 1000, 2000, and 3000×g for 15 min at 4 °C, and the resulting pellet of cell debris was discarded. To prepare EV pellets for Nanosight analysis and flow cytometry, 1.5 mL of fresh plasma was transferred to an ultracentrifuge tube (Quick-Seal, Round-Top, polypropylene, 13.5 mL; Beckman Coulter, Inc., Indianapolis, IN, USA), which was then filled up with phosphate-buffered saline (PBS) previously passed 3× through a sterile membrane filter unit (Stericup-VP, 0.10 µm, polyethersulfone; EMD Millipore, Billerica, MA, USA) to minimize contribution of interfering background particles. Plasma was then spun in a benchtop ultracentrifuge (Optima MAX-XP; Beckman Coulter, Inc.) at 110,000×g for 75 min at 4 °C, to obtain an EV-rich pellet. The pellet was resuspended with 500 µL of triple membrane 0.1 µm filtered PBS for further analysis.
2.4. Nanosight analysis
The number and dimension of EVs were assessed by nanoparticle tracking analysis (NTA). This technique measures the Brownian motion of vesicles suspended in a fluid and displays them in real time through a charge-coupled device camera with high sensitivity. The Nanosight LM10-HS system (NanoSight Ltd., Amesbury, UK), was used to visualize the EVs by laser light scattering. Five 30-sec recordings were performed for each sample. Collected data were analyzed with NTA software, which provided high-resolution particle size distribution profiles and EV concentration measurements. EVs were expressed as 106 for 1 ml of plasma, and classified as total (size: 30–700 nm), exosomes (size: 30–130 nm), and MVs (size: 131–700 nm).
2.5. Flow cytometry
EVs were characterized with the MACSQuant Analyzer flow cytometer (Miltenyi Biotec, Calderara di Reno, BO, Italy) according to a customer-provided protocol (https://goo.gl/8un69P). The Fluoresbrite Carboxylate Size Range Kit I (0.2, 0.5, 0.75, and 1 µm) was used to set the calibration gate on the MACSQuant Analyzer system. To evaluate EV integrity, 60-µL sample aliquots were stained with 0.02 µM 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE) at 37 °C for 20 min in the dark (Pospichalova et al., 2015). CFSE is a vital dye non fluorescent molecule, able to enter into MV, where intracellular esterases remove the acetate group and convert the molecule into the fluorescent ester form. Each aliquot of CFSE-stained sample was then incubated with a specific antibody: CD14-APC (Clone TÜK4) to distinguish MVs from macrophages and/or monocytes, CD105-APC (clone: 43A4E1) to distinguish MVs from endothelial cells, CD326 (EpCAM)-APC (clone: HEA-125) to distinguish MVs from epithelial cells, CD66abce-FITC (clone: TET2) to distinguish MVs from neutrophils, and CD61-APC (clone: Y2/51) to distinguish MVs from platelets. All antibodies were purchased from Miltenyi Biotec. Before use, each antibody was centrifuged at 17,000×g for 30 min at 4 °C to eliminate aggregates. A stained PBS (control) sample was used to detect autofluorescence of the antibody. Quantitative multiparameter analysis of flow cytometry data (expressed as 103 for 1 ml of plasma) was carried out using FlowJo software (Tree Star, Inc., Ashland, OR, USA).
2.6. Statistical analysis
Personal and lifestyle characteristics of enrolled subjects were expressed as mean±SD for continuous variables and percentages for categorical variables. Exposure data and EV counts exhibited a non-normal distribution and were therefore expressed as the median, range, and interquartile range (IQR). PM10 and PM2.5 exposure data measured by PCIS were cross-validated with the ARPA data (representing the average background PM levels in downtown Milan) by Wilcoxon signed-rank test and Spearman rank correlation (r) analysis.
Before applying linear regression models, exposure data were log (base 10) transformed to approximate linearity, and MV counts were log (base e) transformed to achieve a normal distribution. Effects were thus expressed as the geometric mean ratio (GMR) with 95% confidence interval (CI), which corresponds to the exponential of the β regression coefficient when the dependent variable is on the log-scale. GMR indicates the number of times the EV count changes for a 10 times (log10 unit) increase in PM concentration (as measured by PCIS). Regression models were adjusted for age (continuous data), gender, BMI (continuous data), and smoking habits (categories: never, former, and current smoker). To evaluate the possible effect modification of BMI, subjects were stratified according to the standard CDC definition of overweight subjects (BMI < or ≥25 kg/m2) and two separate linear regression models (adjusted for age, gender and smoking) were run. Interaction was tested by adding an interaction variable (categorical BMI:log10 PM exposure) to the multivariate regression model. A p-value ≤0.05 was considered as statistically significant (two-tailed). Data analysis was performed using SAS (version 9.3; SAS Institute Inc., Cary, NC, USA) and Stata (version 13; StataCorp, College Station, TX, USA).
3. Results
The main characteristics of the study subjects are reported in Table 1. Females comprised 58.8% of subjects, with a mean age of 48.7±8.4 years (range: 38–73). Mean BMI was 24.4±3.2 kg/m2 (range: 19–30), with 24 subjects classified as overweight. Most of the subjects were never smokers (54.9%), had a university degree (62.7%), and were currently employed (94.1%). Almost half of the study participants lived in Milan. Another 27% lived in the province of Milan, and almost all worked within the city.
Table 1.
Demographic and lifestyle characteristics of study participants (n = 51).
| Characteristic | N (%) |
|---|---|
| Age, years | |
| Mean, ±SD | 48.7, ±8.4 |
| <40 | 4 (7.8) |
| 40–44 | 18 (35.3) |
| 45–49 | 10 (19.6) |
| 50–54 | 7 (13.7) |
| 55+ | 12 (23.5) |
| Gender | |
| Male | 21 (41.2) |
| Female | 30 (58.8) |
| Body Mass Index, Kg/m2 | |
| Mean, ±SD | 24.4,±3.2 |
| <25 | 25 (49.0) |
| ≥25 | 24 (47.1) |
| Missing | 2 (3.9) |
| Smoking habit | |
| Never smoker | 28 (54.9) |
| Former smoker | 13 (25.5) |
| Current smoker | 10 (19.6) |
| Education | |
| University | 32 (62.7) |
| High school | 13 (25.5) |
| Other | 6 (11.8) |
| Occupation | |
| Currently employed | 48 (94.1) |
| Retired | 3 (5.9) |
| Living area | |
| City of Milan | 24 (47.1) |
| Province of Milan, outside city area | 14 (27.4) |
| Province of Monza-Brianza | 7 (13.7) |
| Other provinces in Lombardy | 6 (11.8) |
| Work area | |
| City of Milan | 43 (84.4) |
| Other | 4 (7.8) |
| Unknown | 4 (7.8) |
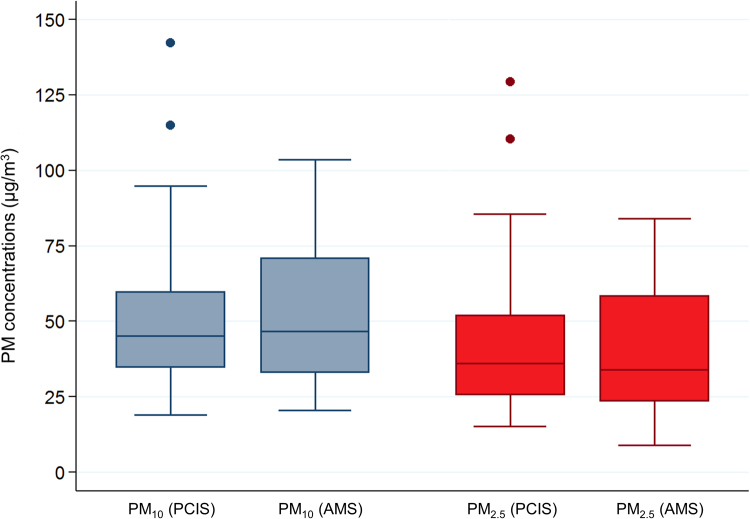
As shown in Fig. 1, median PM10 and PM2.5 exposure levels measured by ambient monitoring stations did not differ from those measured by PCIS (PM10: 46.8 µg/m3 vs. 45.1 µg/m3, respectively; PM2.5: 34.0 µg/m3 vs. 36.1 µg/m3, respectively; Wilcoxon test for equality of medians: p = 0.28 for PM10 and p = 0.48 for PM2.5), and they were significantly correlated (PM10: Spearman's r = 0.59; PM2.5: r = 0.68; both p<0.001). At the same time, PM10 and PM2.5 levels from PCIS resulted highly correlated (r = 0.97; p<0.001).
Fig. 1.
Box plots summarizing the exposure levels to PM10 (grey) and PM2.5 (red) measured by Personal Cascade Impactor Samplers (PCIS) and Ambient Monitoring Stations (AMS) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
EV counts quantified by Nanosight analysis and characterized by flow cytometry are shown in Table 2. Median count of total EVs was 1838.6×106/ml PL, which included a larger fraction of MVs (median =1558.8×106/ml PL) relative to that of the smaller-sized exosomes (median=281.4×106/ml PL). As for cellular origin by flow cytometry, the larger MV fraction originated from platelets (CD61+). Smaller proportions of MVs derived from neutrophils (CD66+), macrophages/monocytes (CD14+), and epithelial (EpCAM+) and endothelial (CD105+) cells were also identified.
Table 2.
Quantification of EV fractions and MV subtypes.
| Exosomes and MV characteristics | Median (Q1-Q3) | Range | |
|---|---|---|---|
| EV counta (106/ml PL) | Total EV (Exosomes + MV) | 1838.6 (1566.0–2321.6) | 944.4–3255.0 |
| Exosomes | 281.4 (164.7–367.8) | 55.2–818.1 | |
| MV | 1558.8 (1338.5–2030.4) | 889.2–2870.5 | |
| MV subtypesb (103/ml PL) | MVCD61+(platelets) | 264.1 (115.4–411.7) | 39.9–1427.7 |
| MVCD66+(neutrophils) | 7.8 (4.4–15.2) | 2.0–40.4 | |
| MVEpCAM+(epithelium) | 11.5 (7.0–15.7) | 3.0–41.9 | |
| MVCD105+(endothelium) | 10.9 (6.7–19.9) | 2.5–38.2 | |
| MVCD14+(macrophages/ monocytes) | 13.8 (8.0–27.8) | 2.9–113.5 | |
Q1–Q3, first quartile –third quartile.
Counts of EV fractions obtained by Nanosight analysis.
Counts of MV subtypes obtained by flow cytometry.
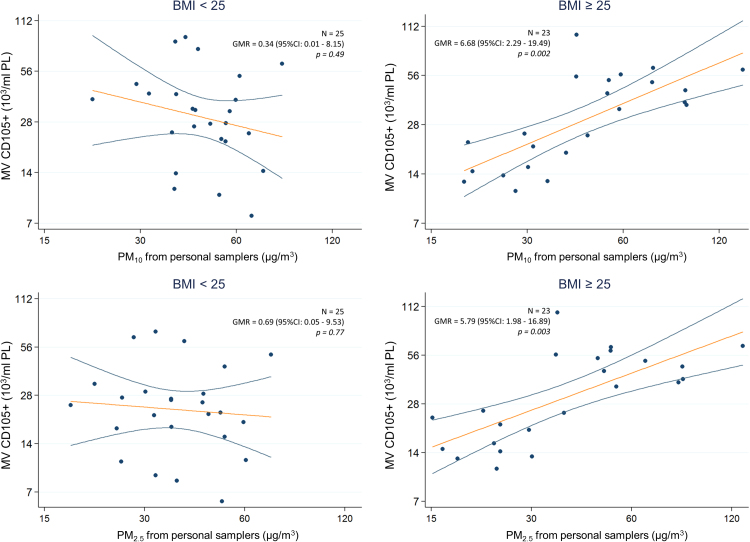
Neither univariate nor multivariate regression analysis in the whole population (Table 3) revealed a clear pattern of association between EV count and PM exposure. However, multivariate analysis revealed a PM-related increase of MVs derived from the endothelium (PM10: adjusted GMR = 3.47; 95% CI: 1.30, 9.27; p = 0.01; PM2.5: adjusted GMR = 3.14; 95% CI: 1.23, 8.02; p = 0.02) and from macrophages/monocytes (PM10: adjusted GMR = 3.45; 95% CI: 1.00, 11.94; p = 0.05; PM2.5: adjusted GMR = 3.70; 95% CI: 1.16, 11.86; p = 0.03). After stratifying by BMI (Table 4), no association between PM exposure and EV total count (in details total EV, exosomes and MV) was found in either normal or overweight subjects. Compared to that in normal subjects, the overweight subjects showed a much more pronounced PM-related effect among MVs derived from all cellular subtypes; however, this effect was particularly strong for MVs of endothelial origin, with approximately a 6× increase in MVs for each log10 unit increase in PM exposure (PM10: GMR = 6.68; 95% CI: 2.29, 19.49; p = 0.002; PM2.5: GMR = 5.79; 95% CI: 1.98, 16.89; p = 0.003) (Table 4 and Fig. 2). Interaction tests were formally performed to assess effect modification between BMI and PM exposure (see Table 4, last column to the right) and were statistically, or borderline statistically significant for all the considered cellular origins except for macrophages/monocytes, indicating a sensibly larger PM effect among overweight subjects.
Table 3.
Association between PM exposure (data from personal sampler) and different classes of Extracellular Vesicles (EV). Linear regression models after log transformation of both PM exposure and outcomes.
|
PM10from personal samplers |
PM2.5from personal samplers |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Univariate regression |
Multivariate regressiona |
Univariate regression |
Multivariate regressiona |
||||||||||
| Exosomes and MV characteristics | GMR | (95% CI) | p | GMR | (95% CI) | p | GMR | (95% CI) | p | GMR | (95% CI) | p | |
| EV countb (106/ml PL) | Total EV | 1.13 | (0.76, 1.69) | 0.53 | 1.05 | (0.7, 1.57) | 0.83 | 1.17 | (0.81, 1.69) | 0.39 | 1.05 | (0.72, 1.55) | 0.78 |
| Exosomes | 0.89 | (0.38, 2.1) | 0.79 | 0.60 | (0.26, 1.39) | 0.23 | 0.97 | (0.44, 2.13) | 0.93 | 0.58 | (0.26, 1.29) | 0.18 | |
| MV | 1.21 | (0.83, 1.75) | 0.31 | 1.18 | (0.8, 1.75) | 0.40 | 1.24 | (0.88, 1.74) | 0.22 | 1.20 | (0.83, 1.74) | 0.33 | |
| MV subtypesc (103/ml PL) | MVCD61+(platelets) | 1.58 | (0.43, 5.78) | 0.48 | 1.49 | (0.35, 6.33) | 0.58 | 1.62 | (0.49, 5.34) | 0.42 | 1.52 | (0.39, 5.98) | 0.54 |
| MVCD66+(neutrophils) | 1.56 | (0.49, 5.00) | 0.45 | 1.89 | (0.54, 6.61) | 0.31 | 1.55 | (0.53, 4.53) | 0.42 | 1.91 | (0.58, 6.24) | 0.28 | |
| MVEpCAM+(epithelium) | 1.64 | (0.68, 3.95) | 0.26 | 1.86 | (0.71, 4.91) | 0.20 | 1.47 | (0.65, 3.32) | 0.34 | 1.67 | (0.66, 4.21) | 0.27 | |
| MVCD105+(endothelium) | 3.10 | (1.29, 7.46) | 0.01 | 3.47 | (1.3, 9.27) | 0.01 | 2.88 | (1.28, 6.45) | 0.01 | 3.14 | (1.23, 8.02) | 0.02 | |
| MVCD14+(macr./monoc.) | 2.54 | (0.82, 7.9) | 0.11 | 3.45 | (1, 11.94) | 0.05 | 2.49 | (0.88, 7.06) | 0.09 | 3.70 | (1.16, 11.86) | 0.03 | |
GMR, Geometric Mean Ratio.
Linear regression model adjusted for age, gender, BMI, and smoking habit (never, former, current smoker); PM10/PM2.5 values missing for 1 record.
Counts of EV fractions obtained by Nanosight analysis.
Counts of MV subtypes obtained by flow cytometry.
Table 4.
Association between PM exposure and different classes of EV, stratifying subjects by BMI (< vs ≥25 kg/m2). Results from a multivariate regression models adjusted for age, gender and smoking habit (PM10/PM2.5 values missing for 1 record, BMI values missing for 2 records).
|
BMI <25 (n=25) |
BMI ≥25 (n=23) |
|||||||
|---|---|---|---|---|---|---|---|---|
| GMR | (95% CI) | p | GMR | (95% CI) | p | p for interaction | ||
| PM10exposure | ||||||||
| EV counta (106/ml PL) | Total EV | 1.11 | (0.37, 3.37) | 0.85 | 1.25 | (0.68, 2.30) | 0.45 | 0.43 |
| Exosomes | 1.31 | (0.08, 22.19) | 0.85 | 0.81 | (0.28, 2.29) | 0.67 | 0.93 | |
| MV | 1.15 | (0.43, 3.10) | 0.77 | 1.36 | (0.74, 2.51) | 0.30 | 0.41 | |
| MV subtypesb (103/ml PL) | MVCD61+(platelets) | 0.04 | (0.00, 1.62) | 0.09 | 5.93 | (0.77, 45.63) | 0.08 | 0.01 |
| MVCD66+(neutrophils) | 0.19 | (0.01, 7.10) | 0.35 | 3.91 | (0.80, 19.08) | 0.09 | 0.04 | |
| MVEpCAM+(epithelium) | 0.16 | (0.01, 2.53) | 0.18 | 3.36 | (0.97, 0.97) | 0.06 | 0.02 | |
| MVCD105+(endothelium) | 0.34 | (0.01, 8.15) | 0.49 | 6.68 | (2.29, 19.49) | 0.002 | 0.03 | |
| MVCD14+(macr./monoc.) | 0.74 | (0.01, 51.76) | 0.88 | 3.23 | (0.93, 11.24) | 0.06 | 0.47 | |
| PM2.5exposure | ||||||||
| EV counta (106/ml PL) | Total EV | 1.10 | (0.44, 2.74) | 0.83 | 1.28 | (0.71, 2.29) | 0.39 | 0.44 |
| Exosomes | 1.15 | (0.11, 11.72) | 0.90 | 0.81 | (0.30, 2.23) | 0.67 | 0.99 | |
| MV | 1.17 | (0.52, 2.64) | 0.69 | 1.39 | (0.77, 2.51) | 0.25 | 0.42 | |
| MV subtypesb (103/ml PL) | MVCD61+(platelets) | 0.16 | (0.01, 3.79) | 0.24 | 5.62 | (0.78, 40.40) | 0.08 | 0.02 |
| MVCD66+(neutrophils) | 0.41 | (0.02, 8.24) | 0.54 | 3.66 | (0.79, 17.01) | 0.09 | 0.07 | |
| MVEpCAM+(epithelium) | 0.30 | (0.03, 3.09) | 0.30 | 2.77 | (0.80, 9.57) | 0.10 | 0.05 | |
| MVCD105+(endothelium) | 0.69 | (0.05, 9.53) | 0.77 | 5.79 | (1.98, 16.89) | 0.003 | 0.06 | |
| MVCD14+(macr./monoc.) | 1.50 | (0.05, 48.64) | 0.81 | 3.32 | (1.01, 10.88) | 0.05 | 0.56 | |
GMR, Geometric Mean Ratio.
Counts of EV fractions obtained by Nanosight analysis.
Counts of MV subtypes obtained by flow cytometry.
Fig. 2.
Association between MV from endothelium (CD105+) and PM exposure measured by personal samplers. Scatterplots of MV (103/ml PL) versus PM10 (above) or PM2.5 (below) levels (µg/m3), in subjects with BMI <25 (left) or ≥25 (right) kg/m2. Covariate-adjusted Geometric Mean Ratios and corresponding 95% Confidence Intervals [GMR (95%CI)] in MV estimated per log10-unit increase in PM are shown.
4. Discussion
In this study, we observed that exposure of healthy volunteers to PM10 and PM2.5 over a 24-h period can modulate release of EVs, particularly those of endothelial origin. The association resulted mainly attributable to overweight subjects. To our knowledge, this is the first study that has investigated the association between short-term PM exposure and EV release in humans.
The importance of PM-related EV increase has been recently addressed by Rodosthenous et al. (2016), who found an association between ambient PM2.5 levels and increased levels of EV-encapsulated microRNAs (EV-miRNAs) among 22 older individuals randomly selected from the Normative Aging Study in the greater Boston area. As this study was conducted on frozen samples, a precise counting of EVs and determination of their cellular origin was not feasible. Hence, the authors acknowledged that the observed overall increase in blood EV-miRNA levels may have originated either from an increase in numbers of EV released by blood cells or tissues sensitive to air pollution or from an increase in the amount of specific miRNAs loaded into EVs prior to their release into the circulation. With the ability to analyze fresh samples, we had the opportunity in the present study to analyze EV count and MV cellular origin by contemporary methods (i.e., Nanosight and flow cytometry, respectively). These methods allowed us to better describe a complex systemic phenomenon involving EV release in response to proinflammatory stimuli.
Concordant results were observed in our previous study of foundry workers exposed to metal-rich PM, which revealed alterations in EV-miRNA levels in response to short-term PM exposure (Bollati et al., 2015). Interestingly, previous studies have shown that elevated numbers of EVs in healthy subjects are linked to increased risk of developing CVD (Chironi et al., 2006, Ueba et al., 2010) and that EVs likely act as powerful intercellular messengers in the pathogenesis of CVD (Loyer et al., 2014). Although we did not present any cardiovascular or, more in general, clinical outcome, we believe that, together with the above mentioned studies, our study can support the hypothesis that EVs release (from endothelium, in particular) could represent a molecular mechanism involved in mediating short-term systemic response to PM exposures.
Considering the entire study population, we observed an effect of PM2.5 and PM10 exposure on monocyte/macrophage EV release. Alveolar macrophages represent one of the first target of PM action, as a direct uptake of PM by phagocytes, such as by macrophages, has been demonstrated (Hiraiwa and van Eeden, 2013).
Our findings are even more interesting in light of the fact that one of the most important gaps in our current knowledge regarding PM-related health effects is the identification of susceptible subjects (Chen et al., 2007). Although they are based on a relatively small study population, our results suggest that the PM effect is considerably more pronounced among individuals with a BMI >25 kg/m2 (i.e., the overweight group). As a potentially critical factor of susceptibility to PM effects, BMI should be carefully considered in all studies that investigate the biological role of EVs. Being overweight, as well as obesity, is a very common condition, the prevalence of which is increasing worldwide and which represents a major public health issue (Ahima, 2011). Adipose tissue appears to play a role in development of the proinflammatory state and in shifting the redox balance toward a more oxidative state. Therefore, it is reasonable to speculate that subjects with unbalanced or altered redox signaling could be particularly susceptible to PM-related health effects as a result of dysregulation of the pathways that link particle exposure to systemic manifestations.
It has been shown that PM can produce oxidative stress in both in vitro and in vivo systems, and we have recently observed a strong increase in oxidative stress markers associated with exposure to high metal-rich PM levels in affected workers (Bertazzi et al., 2014). It is worth noting that the role of MVs in redox signaling has been regarded as a very promising area of investigation, as recently reviewed by Larson and collaborators (Larson et al., 2014). According to our data, the most pronounced PM effect was observed in endothelial MVs. The endothelium is a well-known homeostatic organ that is essential for the regulation of vascular tone and structure (Versari et al., 2009). Under physiological conditions, stimulation of the endothelium leads to production and release of nitric oxide, which diffuses to surrounding tissue and cells and exerts its cardiovascular protective effects by relaxing media-smooth muscle cells and thus preventing leukocyte adhesion and migration into the arterial wall, muscle cell proliferation, platelet adhesion and aggregation, and adhesion molecule expression (Taddei et al., 2003). In the presence of cardiovascular risk factors during the earliest stages of the disease process, the endothelium undergoes functional and structural alterations (Taddei et al., 2003). Given this scenario, the observed increase of endothelial MVs following exposure to a cardiovascular risk factor (i.e., PM exposure) might be biologically relevant. Moreover, after deposition on alveolar epithelium, naïve PM or modified PM following macrophage phagocytosis can pass through the alveolar-capillary membrane and directly interact with pulmonary endothelium (Tamagawa et al., 2008), which may become a direct target for PM effects. As previously described in Northern Italian environmental studies, our subjects were exposed to quite a large range of PM levels, allowing us to investigate biological effects across a wider PM exposure range and at higher PM concentrations compared to those measured in previous investigations (Rodosthenous et al., 2016).
In our study, individual PM exposure was assessed using the PCIS that allowed us to take into better consideration the personal factors that may produce individual variability, such as indoor exposures, traffic exposure, etc. However, the PCIS was worn for only 24 h. We focused on short-term PM exposure because it has a greater biological effect on EV release. Effects from short-term exposure are quick and rapidly changing, and, unlike those from chronic exposure, they are not responsive to inherent adaptive mechanisms. We also based our analyses on data from the PCIS rather than from the ambient monitoring stations in order to minimize exposure misclassifications. It is remarkable that we observed a strong association between PM levels measured by PCIS and PM level estimates based on average measurements from ambient monitoring stations located in downtown Milan. This finding suggests the possibility that PCIS data could be reasonably integrated with exposure estimates based on air monitoring stations in order to investigate larger populations or to evaluate PM exposure over a more extended period of time.
We obtained our results from only 51 healthy subjects, which consequently yielded low statistical power. The size of the observed effects makes us confident that these changes in EV release are not due to chance, but one must be very cautious in generalizing our results to the larger Italian population with different characteristics and/or exposure conditions. We observed an enhanced effect among overweight subjects, which supports the biological plausibility of an increased susceptibility to PM in subjects with higher BMIs. However, a limitation of this study was that our investigation was limited to overweight subjects, as we did not investigate obese or severely obese subjects. Finally, it may be possible that other conditions strictly correlated to elevated BMI, such as unbalanced diet or sedentary habits, may explain the observed increase in EV release in response to PM exposure.
5. Conclusions
Our findings show that EV release is associated with short-term PM exposure and that overweight subjects are potentially more susceptible to EV release in response to this exposure. Larger studies that include accurate exposure assessment and complete EV characterization, as well as a detailed analysis of EV content, could better clarify the molecular mechanism responsible for the PM effects. Such studies could also further elucidate the underlying mechanism responsible for increased susceptibility to PM effects that appears to be evident in overweight and/or obese subjects, which likely represent a more susceptible population in terms of PM-induced EV release and air pollution-related health effects. If confirmed, these findings could help elucidate the subclinical biological changes that precede the onset of air pollution-associated diseases. Moreover, they could help us to identify susceptible subjects and develop new preventive policies for the promotion of public health.
Ethics approval and consent to participate
Each participant signed a written informed consent, approved by the Ethic Committee of the Fondazione Ca’Granda – Ospedale Maggiore Policlinico (approval number 1425), in accordance with the Helsinki Declaration principles.
Consent for publication
Not applicable.
Availability of data and material
Please contact corresponding author for data request.
Competing interests
The authors declare that they have no competing interests.
Funding
This project received support from the EU Programme “Ideas” (ERC-2011-StG 282413), principal investigator Prof. Valentina Bollati.
Acknowledgements
We thank the Occupational Medicine Medical Residents for their help in examining and recruiting the study subjects. We are grateful to the nurses of the “Medicina del Lavoro” Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Enrico Radice for database development, Elisabetta Angiolino from Regional Environmental Protection Agency (ARPA), as well as the volunteers who participated in the study.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.envres.2017.02.014.
Appendix A. Supplementary material
Supplementary material
.
References
- Ahima R.S. Digging deeper into obesity. J. Clin. Investig. 2011;121(6):2076–2079. doi: 10.1172/JCI58719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett W.D., Zeman K.L. Effect of body size on breathing pattern and fine-particle deposition in children. J. Appl. Physiol. 2004;97(3):821–826. doi: 10.1152/japplphysiol.01403.2003. [DOI] [PubMed] [Google Scholar]
- Bertazzi P.A., Cantone L., Pignatelli P., Angelici L., Bollati V., Bonzini M. Does enhancement of oxidative stress markers mediate health effects of ambient air particles? Antioxid. Redox Signal. 2014;21(1):46–51. doi: 10.1089/ars.2013.5694. [DOI] [PubMed] [Google Scholar]
- Bollati V., Angelici L., Rizzo G., Pergoli L., Rota F., Hoxha M. Microvesicle-associated microRNA expression is altered upon particulate matter exposure in healthy workers and in A549 cells. J. Appl. Toxicol. 2015;35(1):59–67. doi: 10.1002/jat.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carugno M., Consonni D., Randi G., Catelan D., Grisotto L., Bertazzi P.A. Air pollution exposure, cause-specific deaths and hospitalizations in a highly polluted Italian region. Environ. Res. 2016;147:415–424. doi: 10.1016/j.envres.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Chen J.C., Cavallari J.M., Stone P.H., Christiani D.C. Obesity is a modifier of autonomic cardiac responses to fine metal particulates. Environ. Health Perspect. 2007;115(7):1002–1006. doi: 10.1289/ehp.9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chironi G., Simon A., Hugel B., Del Pino M., Gariepy J., Freyssinet J.M. Circulating leukocyte-derived microparticles predict subclinical atherosclerosis burden in asymptomatic subjects. Arterioscler. Thromb. Vasc. Biol. 2006;26(12):2775–2780. doi: 10.1161/01.ATV.0000249639.36915.04. [DOI] [PubMed] [Google Scholar]
- Diaz-Ruiz A., Guzman-Ruiz R., Moreno N.R., Garcia-Rios A., Delgado-Casado N., Membrives A. Proteasome dysfunction associated to oxidative stress and proteotoxicity in adipocytes compromises insulin sensitivity in human obesity. Antioxid. Redox Signal. 2015;23(7):597–612. doi: 10.1089/ars.2014.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goichot B., Grunebaum L., Desprez D., Vinzio S., Meyer L., Schlienger J.L. Circulating procoagulant microparticles in obesity. Diabetes Metab. 2006;32(1):82–85. doi: 10.1016/s1262-3636(07)70251-3. [DOI] [PubMed] [Google Scholar]
- Hiraiwa K., van Eeden S.F. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediat. Inflamm. 2013;2013:619523. doi: 10.1155/2013/619523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter M.P., Ismail N., Zhang X., Aguda B.D., Lee E.J., Yu L. Detection of microRNA expression in human peripheral blood microvesicles. PloS One. 2008;3(11):e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B.L., Iii, Kuethe J.W., Caldwell C.C. Neutrophil derived microvesicles: emerging role of a key mediator to the immune response. Endocr. Metab. Immune Disord. Drug Targets. 2014;14(3):210–217. doi: 10.2174/1871530314666140722083717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungel A., Distler O., Schulze-Horsel U., Huber L.C., Ha H.R., Simmen B. Microparticles stimulate the synthesis of prostaglandin E(2) via induction of cyclooxygenase 2 and microsomal prostaglandin E synthase 1. Arthritis Rheum. 2007;56(11):3564–3574. doi: 10.1002/art.22980. [DOI] [PubMed] [Google Scholar]
- Katsouyanni K., Touloumi G., Samoli E., Gryparis A., Le Tertre A., Monopolis Y. Confounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2 project. Epidemiology. 2001;12(5):521–531. doi: 10.1097/00001648-200109000-00011. [DOI] [PubMed] [Google Scholar]
- Kesimer M., Scull M., Brighton B., DeMaria G., Burns K., O'Neal W. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: a possible role in innate defense. FASEB J. 2009;23(6):1858–1868. doi: 10.1096/fj.08-119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney P.L., Ozkaynak H. Associations of daily mortality and air pollution in Los Angeles County. Environ. Res. 1991;54(2):99–120. doi: 10.1016/s0013-9351(05)80094-5. [DOI] [PubMed] [Google Scholar]
- Larson M.C., Hillery C.A., Hogg N. Circulating membrane-derived microvesicles in redox biology. Free Radic. Biol. Med. 2014;73:214–228. doi: 10.1016/j.freeradbiomed.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroyer A.S., Tedgui A., Boulanger C.M. Role of microparticles in atherothrombosis. J. Intern. Med. 2008;263(5):528–537. doi: 10.1111/j.1365-2796.2008.01957.x. [DOI] [PubMed] [Google Scholar]
- Loyer X., Vion A.C., Tedgui A., Boulanger C.M. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ. Res. 2014;114(2):345–353. doi: 10.1161/CIRCRESAHA.113.300858. [DOI] [PubMed] [Google Scholar]
- Mraz M., Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 2014;222(3):R113–R127. doi: 10.1530/JOE-14-0283. [DOI] [PubMed] [Google Scholar]
- Orozco A.F., Lewis D.E. Flow cytometric analysis of circulating microparticles in plasma. Cytom. Part A: J. Int. Soc. Anal. Cytol. 2010;77(6):502–514. doi: 10.1002/cyto.a.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospichalova V., Svoboda J., Dave Z., Kotrbova A., Kaiser K. Simplified protocol for flow cytometry analysis of fluorescently labeled exosomes and microvesicles using dedicated flow cytometer. J. Extracell Vesicles. 2015;31:25530. doi: 10.3402/jev.v4.25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash P.S., Caldwell C.C., Lentsch A.B., Pritts T.A., Robinson B.R. Human microparticles generated during sepsis in patients with critical illness are neutrophil-derived and modulate the immune response. J. Trauma Acute Care Surg. 2012;73(2):401–406. doi: 10.1097/TA.0b013e31825a776d. (discussion 406-407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautou P.E., Vion A.C., Amabile N., Chironi G., Simon A., Tedgui A. Microparticles, vascular function, and atherothrombosis. Circ. Res. 2011;109(5):593–606. doi: 10.1161/CIRCRESAHA.110.233163. [DOI] [PubMed] [Google Scholar]
- Rodosthenous R.S., Coull B.A., Lu Q., Vokonas P.S., Schwartz J.D., Baccarelli A.A. Ambient particulate matter and microRNAs in extracellular vesicles: a pilot study of older individuals. Part. Fibre Toxicol. 2016;13:13. doi: 10.1186/s12989-016-0121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., Dockery D.W. Increased mortality in Philadelphia associated with daily air pollution concentrations. Am. Rev. Respir. Dis. 1992;145(3):600–604. doi: 10.1164/ajrccm/145.3.600. [DOI] [PubMed] [Google Scholar]
- Taddei S., Ghiadoni L., Virdis A., Versari D., Salvetti A. Mechanisms of endothelial dysfunction: clinical significance and preventive non-pharmacological therapeutic strategies. Curr. Pharm. Des. 2003;9(29):2385–2402. doi: 10.2174/1381612033453866. [DOI] [PubMed] [Google Scholar]
- Tamagawa E., Bai N., Morimoto K., Gray C., Mui T., Yatera K. Particulate matter exposure induces persistent lung inflammation and endothelial dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;295(1):L79–L85. doi: 10.1152/ajplung.00048.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkach M., Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- Ueba T., Nomura S., Inami N., Nishikawa T., Kajiwara M., Iwata R. Plasma level of platelet-derived microparticles is associated with coronary heart disease risk score in healthy men. J. Atheroscler. Thromb. 2010;17(4):342–349. doi: 10.5551/jat.2964. [DOI] [PubMed] [Google Scholar]
- Versari D., Daghini E., Virdis A., Ghiadoni L., Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care. 2009;32(Suppl. 2):S314–S321. doi: 10.2337/dc09-S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material