Abstract
Symmetry is one of three derived relations (along with transitivity and reflexivity) that indicate that explicitly trained conditional relations are equivalence relations and that the elements of those trained relations are members of a stimulus class. Although BA symmetry is typically observed after AB conditional discrimination training in humans, it has been an elusive phenomenon in other animals until just recently. This paper describes past unsuccessful attempts to observe symmetry in non-human animals and the likely reasons for that lack of success. I then describe how methodological changes made in response to the earlier findings have now yielded robust evidence for symmetry in pigeons, and what these changes indicate about the functional matching stimuli. Finally, I describe a theory of stimulus-class formation (Urcuioli, 2008) which specifies how and why symmetry and other derived relations arise from different sets of trained relations. These derived relations are noteworthy because they demonstrate an impressive repertoire of non-similarity-based categorization effects in animals and the generative effects of reinforcement and stimulus control processes on behavior.
Keywords: symmetry, transitivity, reflexivity, derived relations, stimulus-class formation, stimulus equivalence, functional stimuli, pigeons, key peck
The title of this paper is a deliberately modified version of the title of an influential paper by Sidman, Rauzin, Lazar, Cunningham, Tailby, and Carrigan (1982), “A search for symmetry in the conditional discriminations of rhesus monkeys, baboons, and children.” In that paper, Sidman et al. reported that the majority of the children in their study (ranging in age from 4 years - 8 months to 5 year - 9 months) showed symmetry, but that none of the five non-human primates did. Their findings helped to spur a wide-ranging effort throughout behavior analysis to understand the origins of equivalence relations (e.g., Barnes, 1994; Dube, McIlvane, Callahan, & Stoddard, 1993; Hayes, 1989; Horne & Lowe, 1996, 1997; Schusterman & Kastak, 1993; Sidman, 1994, 2000; Urcuioli, 2006; Vaughan, 1988; Zentall & Smeets, 1996). Naturally, the species differences Sidman et al. (1982) observed suggested that language or other human capabilities may be necessary for symmetry. However, Sidman et al. were careful to note the possibility that “experiential rather than…genetically related variables” (p. 42) may play a role and mentioned some possibly influential experiential variables – for example, multiple exemplar training of reinforced symmetrical relations. But they also said that “…symmetry’s very complexity should temper one’s optimism” (p. 43), noting that it requires “…the existence of the stimulus classes, sample and comparison” (p. 43) and that “Incorrect specification by the experimenter of the controlling stimuli…may be the most fundamental factor underlying the absence of symmetry.” (p. 43).
As I hope will become clear in this paper, their commentary was not only thorough but prescient. After describing some of the many failures to find symmetry, I show that the main culprit was incorrect specification of the functional matching stimuli (for pigeons, at least) and that symmetry was finally demonstrated once this was fully appreciated and properly taken into account. That recognition led to my theory of pigeons’ stimulus class formation (Urcuioli, 2008) which correctly predicts the conditions under which symmetry will emerge and also correctly predicts a variety of other derived relations indicative of stimulus class formation.
Early Unsuccessful Searches for Symmetry
The Sidman et al. (1982) paper reported 5 experiments in which Sidman and his colleagues looked for evidence of symmetry in different primate species after training them on arbitrary (AB) choice matching-to-sample. After high levels of baseline accuracy were achieved on the AB task, Sidman et al. then reversed the roles of the matching stimuli such that the former comparison stimuli now served as sample stimuli, and vice versa, to see if subjects would do the reverse of what they had been explicitly taught in training – i.e., whether they would accurately perform BA matching. Specifically, after having learned to match sample A1 to comparison B1 and sample A2 to comparison B2 in training, would subjects now consistently choose comparison A1 after sample B1 and consistently choose comparison A2 after sample B2? A positive test result would demonstrate that the explicitly trained conditional relations were symmetrical and would indicate that the trained relations were also equivalence relations (Sidman & Tailby, 1982; see also Sidman, 1990).
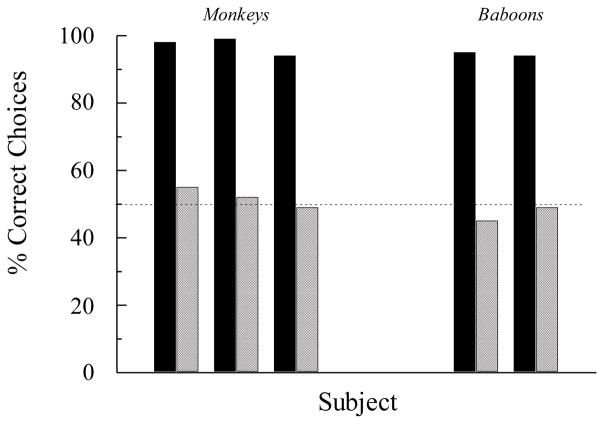
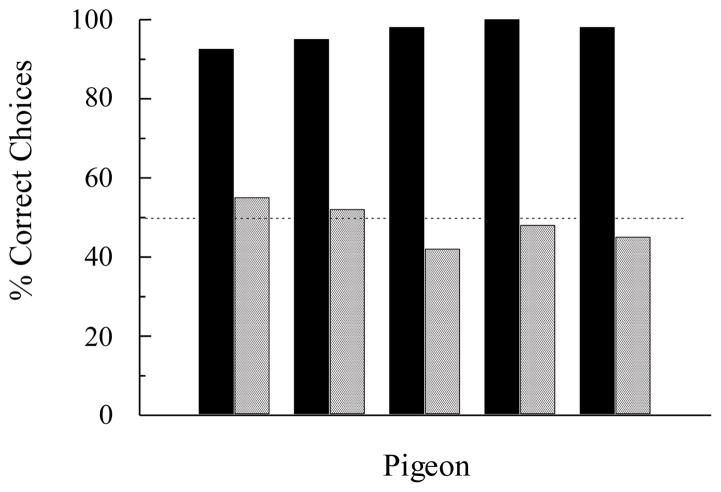
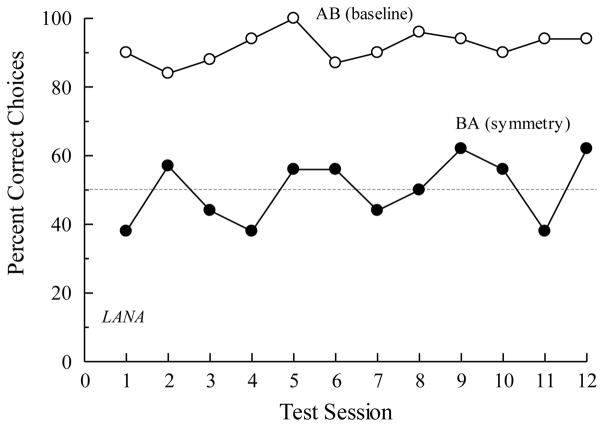
As I mentioned at the outset, Sidman et al. (1982) found that although four of the six children showed evidence for BA symmetry after AB conditional discrimination training (their Experiment 3), none of the rhesus monkeys (their Experiments 1 and 2) and neither of the baboons (their Experiment 5) did. During the critical tests, the non-human primates selected the symmetry-consistent comparisons with a frequency no different than that expected by chance. Figure 1 shows those results. Sidman et al. (1982) noted that their findings extended previous failures to find symmetry in pigeons (e.g., Hogan & Zentall, 1977; Rodewald, 1974). As the search for symmetry continued following their 1982 paper, the pattern of findings mostly confirmed the absence of symmetry in non-human animals when trained and tested in n-alternative choice matching-to-sample (D’Amato, Salmon, Loukas, & Tomie, 1985; Dugdale & Lowe, 2000; Gómez, García, & Pérez, 2014; Lionello-DeNolf & Urcuioli, 2002; Lipkens, Kop, & Matthijs, 1988; Meehan, 1999; Richards, 1988; Tomonaga, Matsuzawa, Fujita, & Yamamoto, 1991; Yamamoto & Asano, 1995; for a review, see Lionello-DeNolf, 2009). This is not to say that evidence for symmetry has been completely absent (e.g., Kastak & Schusterman, 1993; Tomonaga et al., 1991; Velasco, Huziwara, Machado, & Tomanari, 2010; see also García & Benjumea, 2006a, 2006b) but, rather, that it has been clearly the exception rather than the rule. Some additional examples of the “rule” are shown in Figures 2 and 3.
Figure 1.
The percentage of correct choices for individual rhesus monkeys and baboons on arbitrary (AB) matching-to-sample baseline trials (solid bars) and symmetry (BA) test trials (hatched bars). Adapted with permission from “A search for symmetry in the conditional discriminations of rhesus monkeys, baboons, and children”, by M. Sidman et al. 1982, Journal of the Experimental Analysis of Behavior, 37. ©1982 by the Society for the Experimental Analysis of Behavior.
Figure 2.
The percentage of correct choices for individual pigeons on arbitrary (AB) matching-to-sample baseline trials (solid bars) and symmetry (BA) test trials (hatched bars). From “A test of symmetry and transitivity in the conditional discrimination performances of pigeons”, by R. Lipkens, R. F. M. Kop, and W. Matthijs, 1988, Journal of the Experimental Analysis of Behavior, 49, p. 405. ©1988 by the Society for the Experimental Analysis of Behavior.
Figure 3.
The percentage of correct choices by a chimpanzee (Lana) on arbitrary (AB) matching-to-sample baseline trials and symmetry (BA) test trials over 12 consecutive test sessions. Adapted with permission from “Testing for symmetry in the conditional discriminations of language-trained chimpanzees”, by N. Dugdale and C. F. Lowe, 2000, Journal of the Experimental Analysis of Behavior, 73, p. 15. ©2000 by the Society for the Experimental Analysis of Behavior.
Not surprisingly, these sorts of results vis-à-vis the human findings raised the question of why it was so difficult to obtain evidence for symmetry in non-human animals. One entirely reasonable inference is that humans possess certain capabilities not found in our animal brethren (e.g., language), and these may be crucial for observing symmetry in particular and equivalence-class formation in general (Hayes, 1989; Horne & Lowe, 1996; 1997; Devany, Hayes, & Nelson, 1986). Alternatively, the problem may not be linguistically based but methodologically/conceptually based. To reiterate the point made by Sidman et al. (1982):
“Incorrect specification by the experimenter of the controlling stimuli in the conditional discriminations may be the most fundamental factor underlying the absence of symmetry.” (p. 43).
The Role of the Functional Matching Stimulus
Standard tests for symmetry in n-alternative matching involve the transposition of sample and comparison locations in the shift from training to testing. With pigeons, for instance, it is customary for the sample stimuli to appear on the center key of a three-key display and for the comparison stimuli to appear on the adjacent (left and right) side keys. When the roles of the baseline matching stimuli are reversed in testing, the stimuli formerly appearing as center-key samples now appear as side-key comparisons, and the stimuli formerly appearing as side-key comparisons now appear as center-key samples. If the controlling (i.e., functional) matching stimuli for the pigeon are simply the nominal stimuli, this is not problematic. However, if the functional matching stimuli for the pigeon includes where each matching stimulus appears (viz., its location), then the change is highly problematic (McIlvane, Serna, Dube, & Stromer, 2000; see also Iversen, 1997; Iversen, Sidman, & Carrigan, 1986; Sidman, 1992). Indeed, it invalidates the symmetry test because if pigeons learn to match “red-on-the-center-key” to “triangle-on-the-left/right-key” (as opposed to just “red” to “triangle”), a valid test is not to observe the accuracy of matching “triangle-on-the-center-key” to “red-on-the-left/right-key” because that relation involves novel stimuli rather than a reversal of the trained relation.
In fact, Lionello and Urcuioli (1998) provided independent evidence that the functional matching stimuli for pigeons in two-alternative matching-to-sample include the spatial location at which each matching stimulus appears. For example, in Experiment 1 of their study, pigeons learned to match vertical- and horizontal-line samples to vertical- and horizontal-line comparisons, respectively (AA matching), with each sample appearing on the center key and the two comparisons appearing on the adjacent side keys. Later, pigeons were tested for their ability to continue to accurately perform AA matching when the line samples now appeared on either the left or right side key and the comparisons appeared on the remaining two keys. Changing the locations at which the samples and comparisons appeared vis-à-vis training caused accuracies to drop to nearly chance levels (see also Urcuioli, 2007).
Given that the functional matching stimuli for pigeons consist of “what” and “where”, Lionello-DeNolf and Urcuioli (2000, Experiment 1) devised a procedure to reduce or eliminate control by “where” (location) by varying location – specifically, by presenting the sample stimulus at one location (viz., on the left key) on half of the matching trials and at another location (viz., on the right key) on the other half of the trials. (The comparisons always appeared on the two remaining keys.) After training this multiple-location task to high levels of accuracy, they presented the samples at a new (viz., center-key) location. Average accuracy on these novel sample location trials was approximately 80%, which compared favorably to the average baseline accuracy of 93%. Thus, multiple-location training had diminished control by location: Pigeons’ matching performances were more strongly controlled by the nominal line stimuli themselves (cf. Lionello & Urcuioli, 1998).
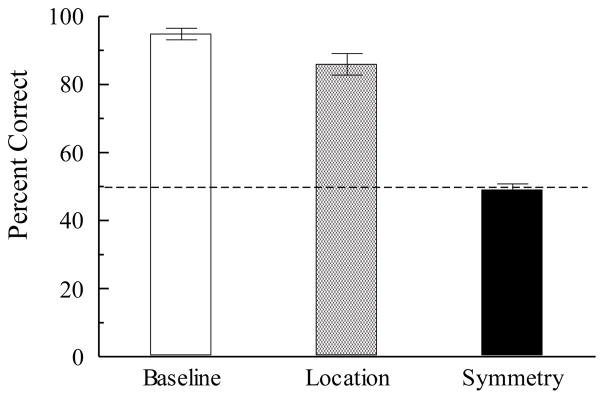
With an effective technique in hand for diminishing or eliminating control by stimulus location, Lionello-DeNolf and Urcuioli (2002) trained pigeons on AB (hue-line) matching in which each hue sample appeared half the time on the left key and half the time on the right key, and the line comparisons on the two remaining keys. After learning this arbitrary matching task to high levels of accuracy, pigeons were tested in sessions consisting of 48 baseline trials, 24 novel-location AB trials (i.e., hue samples appearing on the center key), and 24 symmetry (BA) trials with line samples appearing on the center key (and hue comparison on the adjacent side keys). Figure 4 shows the first-session test results. Despite accurate matching on the novel-location AB trials, the correct (symmetry-consistent) comparison was chosen, on average, only 49% of the time on the BA test trials. Apparently, even when pigeons learn to match the nominal samples to the nominal comparisons in two-alternative AB matching (i.e., by ignoring where these stimuli appear), they are unable to match the former comparisons to the former samples in a BA symmetry test (see also Urcuioli, 2008, Experiment 1A, 1B and 2). Follow-up experiments confirmed this result and showed that the absence of symmetry was not due to the absence of the prerequisite successive (sample) and simultaneous (comparison) discriminations (cf. Saunders & Green, 1999). These findings make it clear that changing sample and comparison locations in the shift from training to testing in standard n-alternative matching is not the sole reason for failures to observe symmetry.
Figure 4.
Average percentage of correct choices (± 1 standard error of the mean) across 12 pigeons on arbitrary (AB) matching-to-sample baseline trials with left- and right-key samples, arbitrary (AB) matching-to-sample trials with novel-location (center-key) samples, and symmetry (BA) test trials with center-key samples. Adapted with permission from “Stimulus control topographies and tests of symmetry in pigeons”, by K. M. Lionello-DeNolf and P. J. Urcuioli, 2002, Journal of the Experimental Analysis of Behavior, 78, pp. 472–473. ©2002 by the Society for the Experimental Analysis of Behavior.
Lionello-DeNolf and Urcuioli (2002; p. 493) speculated that another potential impediment to observing symmetry might be the temporal components associated with sample and comparison presentation (i.e., the sample always appears first, and comparisons always appear second, in a matching trial). If the functional stimuli also include a temporal/ordinal (“when”) component, this too would invalidate the symmetry test. In other words, if pigeons specifically learn to match “A1-in-ordinal-position-#1 to B1-in-position-#2”, they would not be expected to then match “B1-in-ordinal-position-#1 to A1-in-ordinal-position-#2” (see Sidman et al., 1982, p. 43 for a similar point). Other stimulus control topographies (McIlvane et al., 2000) that may also be important in two-alternative matching are differences (if any) in the required number of sample responses versus the required number of comparison responses (although see Urcuioli, 2008, Experiments 1A and 1B) and in the number of other stimuli appearing with the sample stimulus (viz., 0) versus with a particular comparison stimulus (viz., 1 or more).
Success in a Successive Matching Paradigm
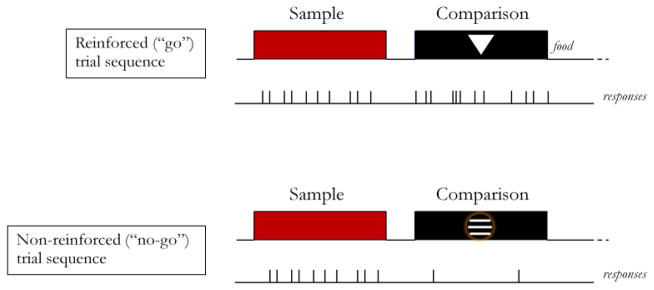
The turning point in the search for symmetry occurred when researchers used successive (go/no-go) matching rather than n-alternative matching as the training and testing paradigm (Frank & Wasserman, 2005; Urcuioli, 2008, Experiment 3). In successive matching, the single sample stimulus on each trial is followed by a single comparison stimulus presented in the same spatial location (e.g., Nelson & Wasserman, 1978; Wasserman, 1976). Typically, each stimulus is presented for an extended period of time (e.g., 5 or 10 s) with a short inter-stimulus interval separating the sample from the comparison (see Figure 5). Some sample-comparison sequences (e.g., red sample followed by triangle comparison) end with reinforcement for the first response to occur after the comparison-stimulus interval; other sequences (e.g., red sample followed by horizontal lines comparison) end without reinforcement (i.e., the comparison stimulus goes off response independently following the comparison-stimulus interval). Conditional discrimination performance is assessed by comparing the rate of comparison responding on reinforced trials with the rate of comparison responding on non-reinforced trials. If expressed as a ratio of the rate on reinforced trials divided by the rate over all (reinforced and non-reinforced) trials, “perfect” performance is indicated by a discrimination ratio (DR) = 1.00, and “chance” performance by a DR = 0.50.
Figure 5.
Depiction of a reinforced and a non-reinforced successive matching trial with sample and comparison responses.
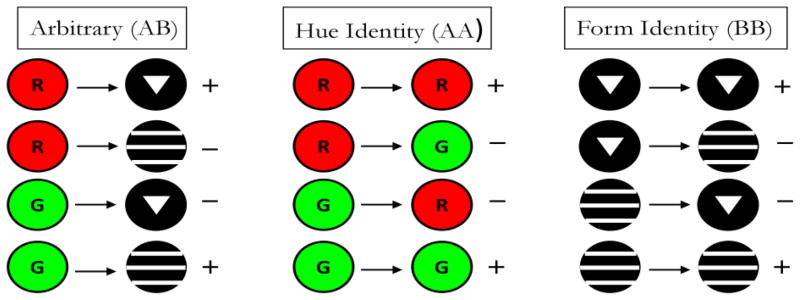
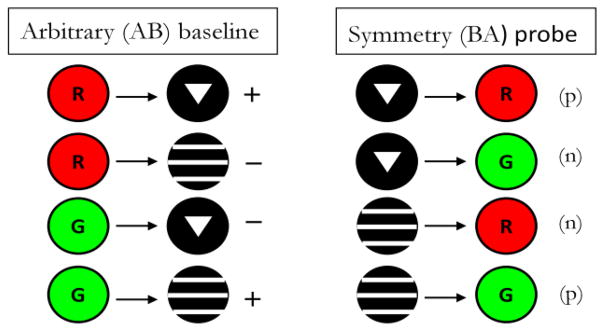
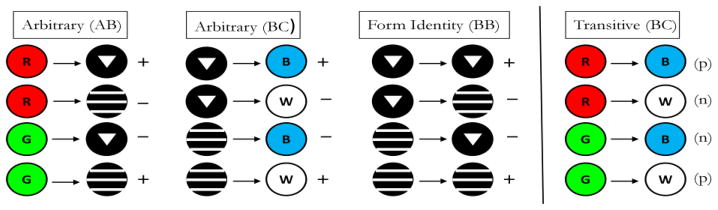
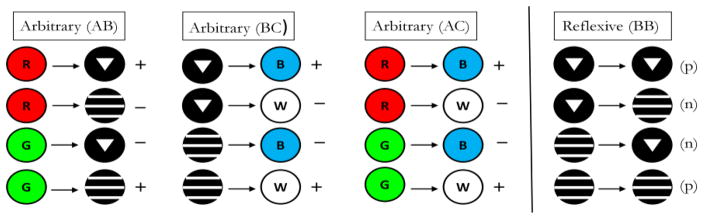
Pigeons in Frank and Wasserman (2005, Experiment 1) and Urcuioli (2008, Experiment 3) were concurrently trained to high levels of discriminative performance on 3 successive matching tasks – AB (arbitrary) matching, AA (identity) matching, and BB (identity) matching – prior to testing in which non-reinforced BA (symmetry) probe trials were infrequently presented among all of the baseline trials. Figure 6 shows the 3 baseline tasks used by Urcuioli (2008, Experiment 3). The singly presented sample stimuli are shown to the left of the arrows and the singly presented comparison stimuli are shown to the right of the arrows. “+” and “−” indicate reinforced and non-reinforced trials, respectively. Once a pigeon had acquired all three baseline tasks to criterion levels of performance (DRs ≥ .80 for five of six consecutive sessions, plus 10 subsequent overtraining sessions), testing commenced with non-reinforced BA (symmetry) probe trials intermixed among all of the various baseline trials. Figure 7 shows the four individual BA probe trial types next to their corresponding baseline AB trials. The “(p)” and “(n)” refer to probe trials that are the reverse of the reinforced (positive) baseline trials and the reverse of the negative (non-reinforced) baseline trials, respectively.
Figure 6.
The three successive matching tasks used during baseline training by Urcuioli (2008, Experiment 3). Sample stimuli are shown to the left of the arrows, and comparison stimuli are shown to the right of the arrows. “+” indicates trials ending in food reinforcement; “−” indicates trials ending without food reinforcement. Each row shows one of the four possible trial types for each concurrently trained task.
Figure 7.
The arbitrary (AB) successive matching baseline task and the symmetry (BA) probe trials appearing during testing in Urcuioli (2008, Experiment 3). (The two remaining baseline tasks are omitted for clarity). Sample stimuli are shown to the left of the arrows, and comparison stimuli are shown to the right of the arrows. “+” indicates trials ending in food reinforcement; “−” indicates trials ending without food reinforcement. “(p)” denotes test trials that are the reverse of the positive (reinforced) baseline trials; “(n)” denotes test trials that are the reverse of the negative (non-reinforced) baseline trials.
The original reason for including AA and BB training along with arbitrary (AB) training was to guarantee that pigeons saw each individual A (hue) and B (form) stimulus both as a sample and as a comparison prior to testing. In other words, including these identity matching tasks was meant to reduce or eliminate any generalization decrement that might occur if pigeons were to see each stimulus in a different temporal/ordinal position for the first time in testing.
It is also important to recognize some of the advantages that successive matching has over n-alternative matching in testing for symmetry. First, each sample and each comparison is presented singly, thus eliminating any concern about different numbers of other stimuli appearing along with the samples versus the comparisons. Second, samples and comparisons always appear in the same spatial location, making the shift from training to testing seamless in this regard. Third, all requisite discriminations (Saunders & Green, 1999) between samples and between comparisons are in place prior to testing, by virtue of the fact that all involve successive discriminations learned in training.
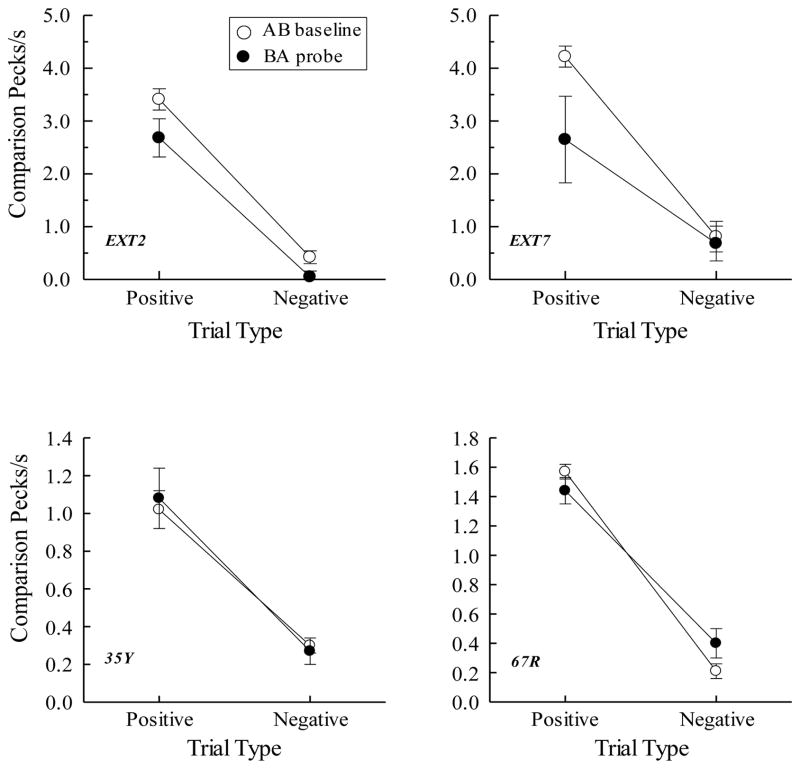
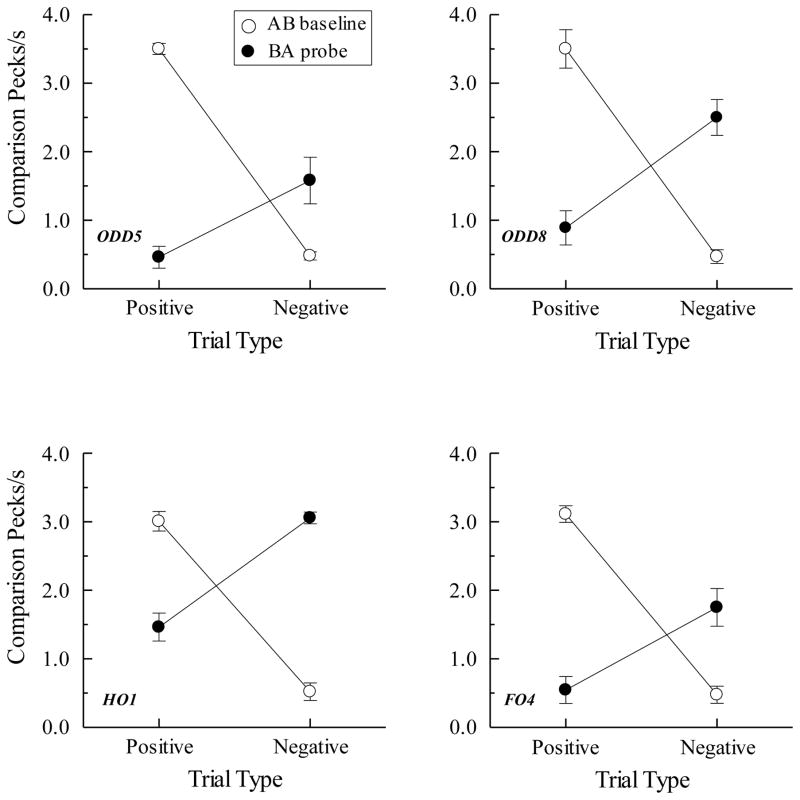
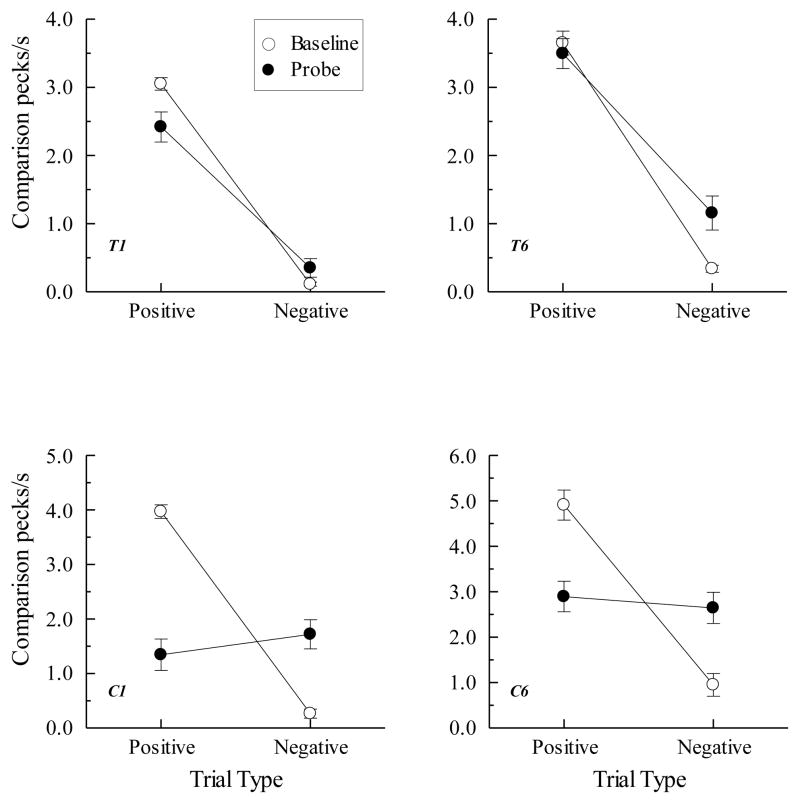
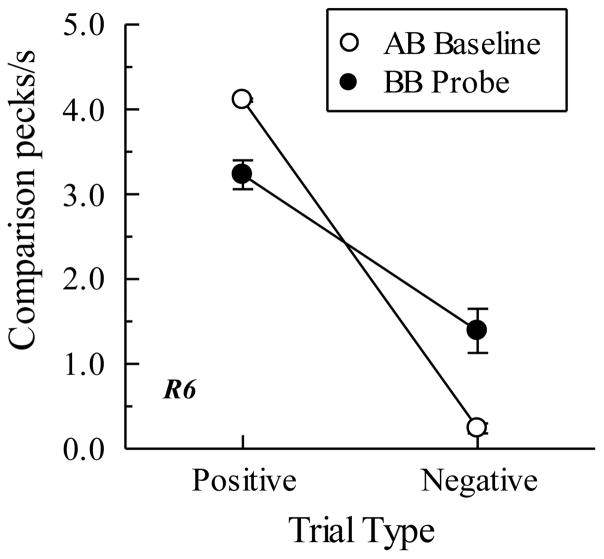
The top row of Figure 8 shows the symmetry (BA probe) results for two pigeons in Urcuioli (Experiment 3) over their first two test sessions along with their corresponding AB baseline performances. Plotted are comparison response rates (in pecks/s) on the reinforced (positive) and non-reinforced (negative) baseline trials (open circles) and the corresponding rates on the reverse of those trials (filled circles). Clearly, pigeons pecked more on symmetry (BA) probes that were the reverse of the positive baseline (AB) relations than on probes that were the reverse of the negative baseline (AB) relations. The bottom row shows the symmetry results averaged over all test sessions for the two pigeons in Frank and Wasserman (2005, Experiment 1) who used color clip-art stimuli as their matching stimuli. Their findings, too, demonstrate symmetry.
Figure 8.
(Top row). Comparison response rates (in pecks/s) on AB successive matching baseline trials and BA probe trials for two pigeons averaged over their first two symmetry test sessions in Urcuioli (2008, Experiment 3). Positive = reinforced baseline trials and the symmetrical versions of them. Negative = non-reinforced baseline trials and the symmetrical versions of them. (Bottom row). Corresponding response rates for two pigeons averaged over eight symmetry test sessions in Frank and Wasserman (2005, Experiment 1). Adapted with permission from “Associative symmetry in the pigeon”, by A. J. Frank and E. A. Wasserman, Journal of the Experimental Analysis of Behavior, 84, p. 155. ©2005 by the Society for the Experimental Analysis of Behavior.
Campos, Urcuioli, and Swisher (2014) demonstrated that pigeon also show BA symmetry after AB successive matching training when combined with concurrent training on AA and BB successive oddity (as opposed to AA and BB identity). Oddity contingencies arrange reinforcement for pecking a comparison that does not physically match the preceding sample and non-reinforcement for pecking a comparison that does match the preceding sample. The Campos et al. (2014) findings support the original hypothesis (Frank and Wasserman, 2005) that the function of the two matching tasks concurrently trained with AB successive matching is to insure familiarity with each stimulus both as a sample and as a comparison prior to testing.
However, this familiarity hypothesis was earlier brought into question by Frank (2007) who showed that BA symmetry did not emerge after concurrent training on three arbitrary successive matching tasks - AB, CA, and BD. Here, too, baseline training insures that the B samples and the A comparisons appearing in the BA symmetry test were previously seen in these roles (viz., in the BD and CA baseline tasks, respectively). Clearly, then, some other factor appears to be responsible for the emergence of BA symmetry after AB, AA, and BB successive matching training (Frank & Wasserman, 2005; Urcuioli, 2008, Experiment 3).
One possibility is that identity matching training is crucial (Frank & Wasserman, 2005; Frank, 2007) but this can be readily dismissed given the Campos et al. (2014) data. Besides, Frank (2007, Experiment 2) showed that concurrently training CC and DD identity matching with AB arbitrary matching failed to yield BA symmetry.
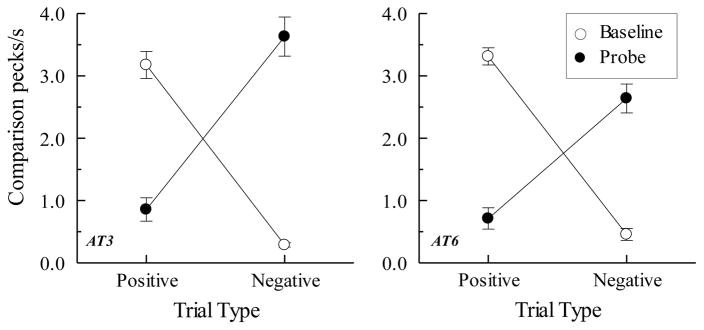
An important clue to the factors responsible for BA symmetry in successive matching was provided by Urcuioli (2008, Experiment 4). In his experiment (see also Urcuioli & Swisher, 2012a), pigeons were trained on AB successive matching accompanied by concurrent training on one oddity task (viz., AA oddity) and one identity task (viz., BB identity). When later tested for BA symmetry, pigeons showed a most unusual pattern of responding: They pecked the comparisons more frequently on BA probe trials that were the reverse of the negative (non-reinforced) AB baseline trials. In other words, their pattern of probe-trial responding was precisely the opposite of what would be expected by BA symmetry. Urcuioli (2008, Experiment 4) called this peculiar emergent effect “antisymmetry”. Figure 9 shows some selected examples of antisymmetry from that study and from Urcuioli and Swisher (2012a).
Figure 9.
(Top row). Comparison response rates (in pecks/s) on AB successive matching baseline trials and BA probe trials for two pigeons averaged over all symmetry test sessions in Urcuioli (2008, Experiment 4). Positive = reinforced baseline trials and the symmetrical versions of them. Negative = non-reinforced baseline trials and the symmetrical versions of them. Adapted with permission from “Associative symmetry, antisymmetry, and a theory of pigeons’ equivalence-class formation”, by P. J. Urcuioli, Journal of the Experimental Analysis of Behavior, 90, p. 275. Copyright 2008 by the Society for the Experimental Analysis of Behavior. (Bottom row). Corresponding comparison response rates for two pigeons averaged over all symmetry test sessions in Urcuioli and Swisher (2012). Adapted with permission from “A replication and extension of the antisymmetry effect in pigeons”, by P. J. Urcuioli and M. J. Swisher, Journal of the Experimental Analysis of Behavior, 98, p. 290. ©2012 by the Society for the Experimental Analysis of Behavior.
Revisiting the Functional Matching Stimulus
Frank’s (2007, Experiment 1) data and those obtained by Urcuioli (2008, Experiment 4) and Urcuioli and Swisher (2012a) make it abundantly clear that presenting each matching stimulus both as a sample and as a comparison during baseline successive matching training does not have the same impact on stimulus control as presenting each sample and each comparison stimulus in different spatial locations during choice matching-to-sample training (Lionello-DeNolf and Urcuioli, 2002). In other words, it does not cause pigeons to ignore ordinal position (“when”) unlike multiple-location training which does cause pigeons to ignore spatial location (“where”). Considering each situation in more detail reveals why this is so.
Table 1 depicts AB choice matching contingencies when each sample (Red or Green) sometimes appear at one spatial location (on the left key) and at other times at a different spatial location (on the right key). For simplicity, the correct (reinforced) comparison alternative on each trial is represented as C1+ and C2+ (i.e., neither the incorrect alternative nor the particular locations of the correct alternatives are shown). Note that where the nominal sample (R or G) appears on a matching trial is irrelevant to which comparison alternative is reinforced. The more valid or predictive cue for reinforced choice is the color of the sample, so it should acquire a substantial degree of control over choice. Location is a less valid (indeed, a non-predictive) cue, so its influence on choice performance should be effectively neutralized, in line with other examples of relative validity effects on incidental stimulus control in operant and Pavlovian conditioning (Wagner, Logan, Haberlandt, & Price, 1968; see also Couvillon, Klosterhalfen, & Bitterman, 1983; Shanks, 1991; Wasserman, 1974).
Table 1.
Arbitrary matching contingencies in choice matching-to-sample in which the sample appears on the left key on half of the trials and on the right key on the other half.
| R-left → C1+ |
| R-right → C1+ |
| G-left → C2+ |
| G-right → C2+ |
Note. R = red, G = green, C1= one comparison choice alternative, C2 = another comparison choice alternative, and “+” = reinforced choice. The sample stimulus is shown to the left of the arrows, and the reinforced comparison is shown to the right of the arrows. “left” and “right” indicate on which key the sample stimulus appears.
In contrast, which comparison is reinforced in successive matching (or, for that matter, in choice matching tasks) always depends on what appeared before it (viz., the sample). Stated otherwise, the “before” aspect of a stimulus cannot be made irrelevant: It is part of what defines the “sample”. Likewise, part of what defines a “comparison” is that it appears “after” the sample. If we assume, then, that the functional matching stimuli in successive matching consist of “what” a pigeons sees (its nominal features) and “when” it sees it (first or second in a matching trial – i.e., as a sample and as a comparison), then the proper designation of a matching trial consisting of a red sample followed by a triangle comparison is not “R-T” but “R1-T2”, where 1 = appearing in the first ordinal position in a trial (as a sample) and 2 = appearing in the second ordinal position in a trial as a comparison). From this perspective, the symmetrical relation is “T2-R1”, although this is physically impossible because a stimulus appearing second cannot come before a stimulus appearing first.
Nevertheless, if the functional matching stimuli in successive matching are conceptualized in terms of “what” and “when” (ignoring the “where” component because all successive matching stimuli appear in the same spatial location), then it is possible with some additional assumptions to account for both the symmetry and antisymmetry findings, as I describe in the next section.
A Theory of Pigeons’ Stimulus Class Formation
Table 2 lists the four major assumptions of my theory of pigeons’ stimulus class formation (Urcuioli, 2008). The first has just been described – namely, that the functional matching stimuli consists of “what” and “when”. Thus, a red sample can be designated as R1, which is functionally different from a red comparison (viz., R2). Similarly, a triangle sample and a triangle comparison are designated as T1 and T2, respectively, and a horizontal-lines sample and a horizontal-lines comparison are designated as H1 and H2, respectively. These six matching stimuli are those used in AB, AA, and BB successive matching training depicted in Figure 6.
Table 2.
The assumptions of Urcuioli’s (2008) theory of pigeons’ stimulus class formation
|
The second assumption states that successive matching is particularly conducive to stimulus class formation because half of all matching trials end in non-reinforcement independently of the pigeons’ level of discriminative performance. In other words, the proportion of non-reinforced to reinforced matching trials in successive matching is exactly the same (50%) at the end of training when pigeons confine the majority of their comparison responses to the reinforced trials (viz., when they’ve learned the conditional relations) as it is at the beginning of training when pigeons are responding non-differentially (viz., prior to learning those relations). This is entirely different than in n-alternative matching-to-sample where the proportion of non-reinforced to reinforced trials drops precipitously from the beginning to the end of training; indeed pigeons may only rarely experience a non-reinforced sample-comparison relation after they’ve learned n-alternative matching.
Third, the stimulus classes resulting from successive matching training consist of the elements of the reinforced sample-comparison relations. Thus, if red sample – triangle comparison trials consistently end in reinforcement, a [R1, T2] class develops. Likewise, if green sample – horizontal comparison trials consistently end in reinforcement, a [G1, H2] class develops, and so forth. I used these two particular classes as an example because they represent the reinforced conditional relations for AB successive matching shown in Figure 6, and because the difference in their composition reflects the hypothesized effects of continual non-reinforcement of red sample – horizontal comparison and green sample – triangle comparison trials. Stated otherwise, the red sample (R1) and the horizontal comparison (H2) are in different classes, as are the green sample (G1) and the triangle comparison (T2), precisely because combinations of these elements are never reinforced.
Fourth, I assume that elements common to more than one class produce class merger. Descriptively, the [R1, T2] class from arbitrary (AB) matching and the [T1, T2] class from form identity (BB) matching should merge given their common T2 element. Likewise, the [R1, T2] class and the [R1, R2] class from hue identity (AA) matching should merge given their common R1 element.
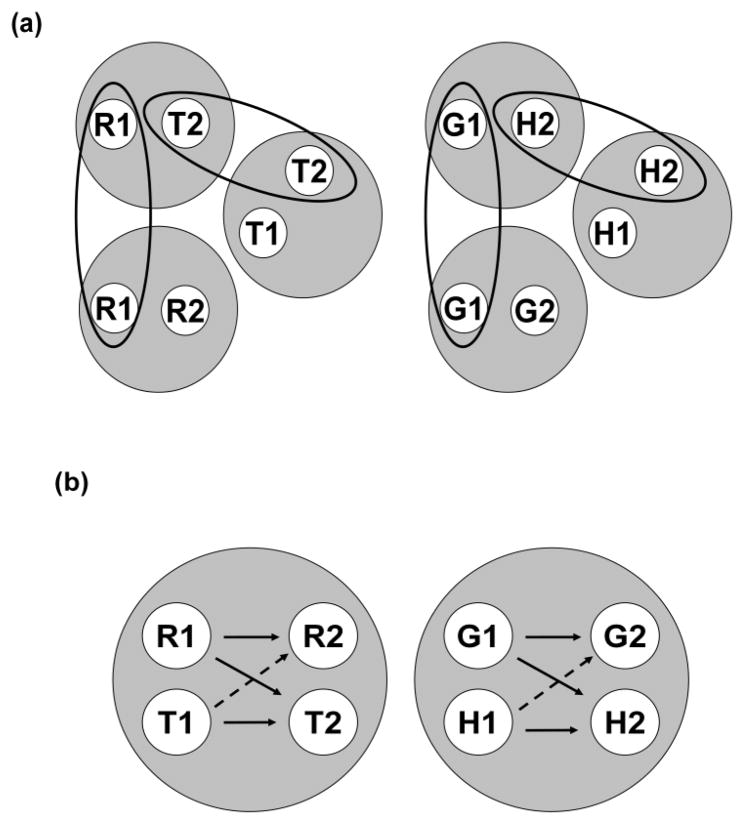
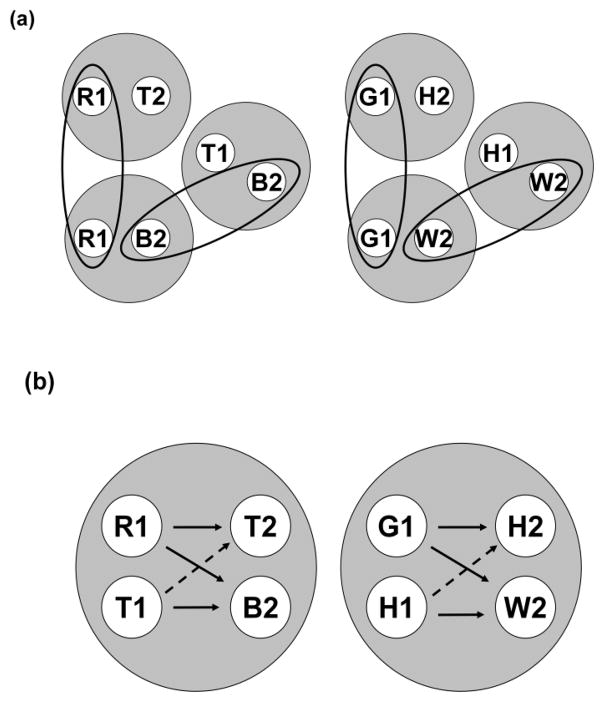
The top half (a) of Figure 10 shows a visual depiction of the six 2-member stimulus classes hypothesized to result from the AB, AA, and BB successive matching contingencies shown in Figure 6. The two AB classes ([R1, T2] and [G1, H2]) are shown at the top of Figure 10a, the two AA classes ([R1, R2] and [G1, G2]) are shown at the bottom, and the two BB classes ([T1, T2] and [H1, H2]) are shown in the middle and slightly to the right of the other classes. Ellipses encompass those elements common to more than one stimulus class (e.g., T2). The bottom half (b) of Figure 10 shows the two 4-member classes hypothesized to result from class merger via the common elements. Solid-line arrows connect the elements of the six explicitly reinforced sample-comparison relations (e.g., R1 (red sample) and T2 (triangle comparison)). Dashed-line arrows connect the elements comprising the symmetrical versions of the reinforced AB relations (viz., the “positive” BA probe trials – cf. Figure 7). If we assume that pigeons respond relatively more to a comparison in the same class as its preceding sample, this predicts higher comparison response rates on positive than on negative BA probe trials – precisely the pattern shown in Figure 8.
Figure 10.
(a) The six stimulus classes hypothesized to result from AB (hue-form) arbitrary, AA (hue identity), and BB (form identity) successive matching training. R = red, G = green, T = triangle, H = horizontal, 1 = first ordinal position (sample stimulus), 2 = second ordinal position (comparison stimulus). Ellipses highlight common elements across classes. (b) The two 4-member stimulus classes hypothesized to result from class merger via common elements. Solid and broken arrows denote explicitly trained relations and predicted symmetry relations, respectively. R = red, G = green, T = triangle, H = horizontal, 1 = first ordinal position (sample stimulus), 2 = second ordinal position (comparison stimulus).
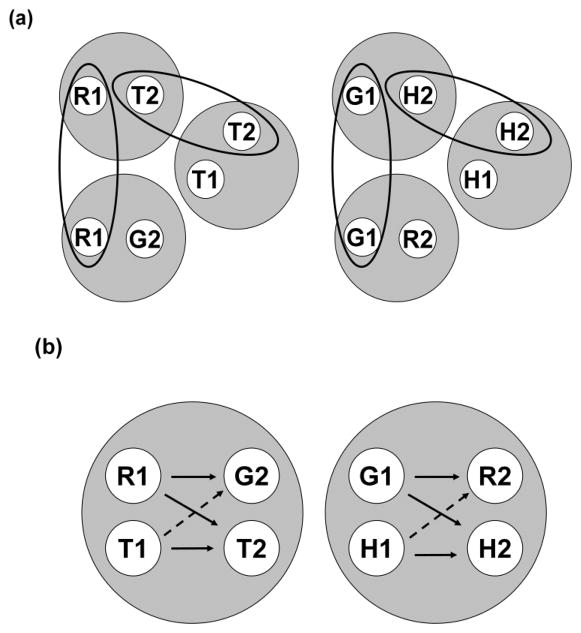
How does Urcuioli’s (2008) theory predict antisymmetry? In other words, if one of the concurrently trained tasks is, say, hue oddity rather than hue identity, why does the theory predict that pigeons will respond more to the comparisons on negative than on positive BA probe trials? Figure 11 shows the stimulus-class analysis for this situation. Figure 11a shows the six 2-member classes for AB arbitrary, AA oddity, and BB identity successive matching. The only difference between these classes and those shown in Figure 10a can be seen in the bottom row: AA oddity yields a [R1, G2] class and a [G1, R2] class because responding to the green (not the red) comparison is reinforced after a red sample and responding to the red (not the green) comparison is reinforced after a green sample. Ellipses again encompass elements common to more than one class, resulting via class merger in the two 4-member classes shown in Figure 11b. Solid-line arrows again connect the elements of the six reinforced sample-comparison relations, and the dashed-line arrows connect the elements of those BA probe trials which should yield relatively high rates of comparison responding.
Figure 11.
(a) The six stimulus classes hypothesized to result from AB (hue-form) arbitrary, AA (hue oddity), and BB (form identity) successive matching training. R = red, G = green, T = triangle, H = horizontal, 1 = first ordinal position (sample stimulus), 2 = second ordinal position (comparison stimulus). Ellipses highlight common elements across classes. (b) The two 4-member stimulus classes hypothesized to result from class merger via common elements. Solid and broken arrows denote explicitly trained relations and predicted symmetry relations, respectively. R = red, G = green, T = triangle, H = horizontal, 1 = first ordinal position (sample stimulus), 2 = second ordinal position (comparison stimulus).
Close inspection of these probes vis-à-vis the baseline AB relations reveals a rather interesting prediction. Specifically, although the red sample – triangle comparison (R1→T2) combination was reinforced in AB training, the theory predicts that pigeons should respond more in BA testing to a triangle sample – green comparison (T1→G2) combination! (Note that a green sample followed by a triangle comparison was non-reinforced (negative) in baseline AB training.) This probe-trial pattern of responding is just the opposite of symmetry – hence the term “antisymmetry”. This counterintuitive finding provides powerful support for my theory overall and for its assumption that the functional matching stimuli for pigeons include each stimulus’ ordinal position.
Other predicted derived relations
Symmetry is just one of three behavioral properties of stimulus equivalence (Sidman & Tailby, 1982). The other two are transitivity and reflexivity. Recent experiments in my lab have shown that pigeons exhibit transitivity and reflexivity after training under precisely those baseline conditions my theory says should yield them.
Transitivity requires baseline training on two sets of arbitrary conditional relations, AB and BC. Transitive AC relations are often then observed in humans (Fields, Adams, Verhave, & Newman, 1990; Lazar, Davis-Lang, & Sanchez, 1984; Pilgrim & Galizio, 1990, 1995; Sidman, Cresson, & Willson-Morris, 1974). The results obtained from non-human animals are less consistent (e.g., D’Amato et al., 1985; Lipkens et al., 1988; Sidman et al., 1982, Experiment 4). My theory of stimulus-class formation predicts that for pigeons at least, AB and BC baseline training is insufficient to yield transitive AC relations. Instead, the theory predicts that BB identity must be trained along with AB and BC arbitrary (see Figure 12) for transitivity to emerge. The reason is that BB identity provides the elements needed for the two-member classes arising from AB and BC training to merge into larger four-member classes containing the A samples and C comparisons comprising AC transitive relations. Without concurrent BB identity training, the two-member classes cannot merge because despite appearances, the common “B” in AB and BC successive matching are actually different stimuli for pigeons.
Figure 12.
The three successive matching tasks during baseline training (left three columns) and the transitivity probe trials (right column) in Urcuioli and Swisher (in press, Experiment 1). Sample stimuli are shown to the left of the arrows, and comparison stimuli are shown to the right of the arrows. “+” indicates baseline trials ending in food reinforcement; “−” indicates baseline trials ending without food reinforcement. “(p)” denotes test trials that are combinations of a reinforced AB and a reinforced BC baseline trial. “(n)” denotes test trials that are combinations of a reinforced AB and a non-reinforced BC baseline trial.
The top row of Figure 13 shows data from two pigeons (Urcuioli & Swisher, in press, Experiment 1) trained on the three baseline relations shown in the left-most columns of Figure 12, and then tested on AC transitivity probes (right column of Figure 12). Both showed transitivity in testing: They responded more frequently to the comparisons on probe trials consisting of samples and comparisons from two reinforced baseline relations sharing the same nominal B stimulus (positive probes) than on probe trials consisting of samples and comparisons from a reinforced and a non-reinforced baseline relation sharing the same nominal B stimulus (negative probes). The bottom row shows results obtained from two other pigeons trained only on AB and BC successive matching. As predicted, they did not show transitivity: They responded non-differentially to the comparisons on positive and negative probe trials (although see Strasser, Ehrlinger, & Bingman, 2004).
Figure 13.
(Top row). Comparison response rates (in pecks/s) on AB and BC baseline trials and AC probe trials for two pigeons trained on AB, BC, and BB successive matching averaged over all transitivity test sessions in Urcuioli and Swisher (in press, Experiment 1). Positive = reinforced baseline trials and probe trials resulting from combinations of reinforced AB and BC baseline trials. Negative = non-reinforced baseline trials and probe trials resulting from combinations of a reinforced AB and a non-reinforced BC baseline trial, or vice versa. (Bottom row) Corresponding comparison response rates for two pigeons trained only on AB and BC successive matching in Urcuioli and Swisher (in press, Experiment 2).
Given the findings from our symmetry studies (Urcuioli, 2008, Experiments 3 and 4; Urcuioli & Swisher, 2012a), what would happen if AB and BC successive matching training were accompanied by concurrent training on BB-oddity (rather than BB-identity)? Would this change the pattern of comparison responding observed on AC probe trials such that pigeons would now respond more frequently to the comparisons on negative than on positive AC probe trials? The answer is “Yes.” We called this finding “anti-transitivity” (Urcuioli & Swisher, in press, Experiment 2) – see Figure 14.
Figure 14.
Comparison response rates (in pecks/s) on AB and BC baseline trials and AC probe trials for two pigeons trained on AB, BC, and BB-oddity successive matching averaged over all transitivity test sessions in Urcuioli and Swisher (in press, Experiment 2).
The third of the three derived relations of equivalence is reflexivity, matching each stimulus to itself despite no explicit training to do so. Sweeney and Urcuioli (2010, Experiment 1) showed what appeared to be reflexivity in pigeons after training them on three sets of conditional go/no-go relations that Urcuioli’s (2008) theory predicts should yield this emergent effect. Specifically, six pigeons were concurrently trained on AB, BA, and BB successive matching after which they received periodic non-reinforced AA probe trials. In testing, five of the six pigeons responded significantly more frequently to the comparisons on positive (matching) AA probe trials than on negative (non-matching) AA probe trials – in short, showing emergent matching of each stimulus to itself.
However, subsequent experiments (e.g., Urcuioli, 2011) questioned whether this represented true reflexivity or generalized identity matching instead. Generalized identity refers to the finding that explicitly reinforcing identity matching with one set of stimuli transfers (generalizes) to a completely different set of stimuli (e.g., Barros, Galvão, & McIlvane, 2002; Blaisdell & Cook, 2005; Dube, Iennaco, & McIlvane, 1993; Lowenkron, 1988; Oden, Thompson, & Premack, 1988; Wright & Katz, 2006). Note that in Sweeney and Urcuioli (2010), one set of explicitly trained relations (BB) was identity matching and, in testing, identity relations appeared with a different set of stimuli (viz., AA). A generalized identity interpretation of the data suggests that the same pattern of AA test results should be observed if training consists of AB, BA, and CC successive matching (cf. Sweeney & Urcuioli, 2010). Here, CC identity training involves stimuli not appearing in the arbitrary AB and BA tasks. My theory of stimulus-class formation predicts that emergent AA matching should not be observed after such training. Contrary to prediction, two of four pigeons trained in this fashion showed significantly higher comparison-response rates on matching than on non-matching AA probe trials. In addition, two pigeons showed this result when later tested on BB probe trials.
Given that generalized identity may have contributed in whole or in part to the results reported by Sweeney and Urcuioli (2010), demonstrating reflexivity requires that baseline training not involve reinforced identity matching (nor relations that could yield emergent reflexive-like relations via transitivity - Urcuioli & Swisher, 2012b). Recently, Melissa Swisher and I have devised such a procedure (see Figure 15). Pigeons are concurrently trained on three arbitrary matching tasks –AB, BC, AC (left 3 columns), after which they received periodic non-reinforced BB (reflexivity) probe trials (right column). The theoretical prediction, shown visually in Figure 16, is that pigeons will respond more frequently to the comparisons on matching BB probe trials [“(p)” = positive probes] than on non-matching BB probe trials [“(n)” = negative probes]. Data from one pigeon in this on-going experiment are shown in Figure 17. A clear reflexivity effect is evident in this pigeon’s behavior. This is the first demonstration of true reflexivity in any animal, human or non-human.
Figure 15.
Three arbitrary successive matching tasks used for baseline training (left three columns) to test for emergent reflexivity (right column). Sample stimuli are shown to the left of the arrows, and comparison stimuli are shown to the right of the arrows. “+” indicates baseline trials ending in food reinforcement; “−” indicates baseline trials ending without food reinforcement. “(p)” denotes positive (matching) probe trials. “(n)” denotes negative (non-matching) probe trials.
Figure 16.
(a) The six stimulus classes hypothesized to result from AB, BC, and AC successive matching training. R = red, G = green, B = blue, W = white, T = triangle, H = horizontal, 1 = first ordinal position (sample stimulus), 2 = second ordinal position (comparison stimulus). Ellipses highlight common elements across classes. (b) The two 4-member stimulus classes hypothesized to result from class merger via common elements. Solid and broken arrows denote explicitly trained relations and predicted reflexivity relations, respectively. R = red, G = green, B = blue, W = white, T = triangle, H = horizontal, 1 = first ordinal position (sample stimulus), 2 = second ordinal position (comparison stimulus).
Figure 17.
Comparison response rates (in pecks/s) on AB baseline trials and BB (reflexivity) probe trials for a pigeon trained on AB, BC, and AC successive matching averaged over all test sessions.
Summary and Conclusions
Pigeons and other non-human animals have long been known to demonstrate what Wasserman, DeVolder, and Coppage (1992; see also Wasserman & DeVolder, 1993) called “non-similarity-based” categorization or concepts. Unlike similarity-based concepts in which perceptually similar but nonidentical stimuli or objects are treated as belonging together (viz., to the same stimulus class – e.g., Wasserman, Kiedinger, & Bhatt, 1988), non-similarity-based concepts involve treating physically dissimilar stimuli or objects as belonging together. Acquired equivalence effects like those demonstrated in pigeons (Urcuioli, Zentall, Jackson-Smith, & Steirn, 1989), rats (e.g., Honey & Hall, 1989), sea lions (Schusterman, Reichmuth, & Kastak, 2000), dolphins (Von Fersen & Delius, 2000) and non-human primates (e.g., Bovet & Vauclair, 1998) are all examples of this. More broadly, these effects are instances of what Zentall, Wasserman, and Urcuioli (2014) called “associative concepts” in which arbitrary stimuli become interchangeable with one another via their learned association with another stimulus, a particular outcome, or a particular response.
Symmetry is, of course, another example, as are transitivity and reflexivity. A long-standing concern in regard to these three derived relations of equivalence, however, was their relative absence in non-human animals despite clear evidence of other forms of non-similarity-based categorization (e.g., Saunders, Williams, & Spradlin, 1996; see also Hayes, 1989). This dichotomy of derived relations led some to surmise that “…some species may be limited to unidirectional transfer that essentially involves the recombination of chains.” (Saunders et al., 1996, p. 105), that “…verbal behavior, particularly naming, may be critical for the establishment of arbitrary stimulus classes.” (Lowe, Horne, Harris, & Randle, 2002, p. 528), and that “…it may be the case that equivalence is a given only for the human species…” (Horne, Hughes, & Lowe, 2006, p. 271).
But things have changed dramatically since the time these comments were published. Armed with knowledge about the functional matching, my lab has successfully replicated the symmetry findings first reported by Frank and Wasserman (2005), has shown the other behavioral properties associated with equivalence (transitivity and reflexivity) and has demonstrated some never-before-seen emergent effects (antisymmetry and anti-transitivity). All have appeared in the non-verbal pigeon and all are, from what I can see, not amenable to explanation in terms of “recombination of chains” (i.e., mediated transfer). Instead, they provide support for the claims that “…equivalence relations arise directly from…reinforcement contingencies” (Sidman, 2008, p. 329) and that stimulus class formation “…may be a product of any procedure that serves to partition a set of stimuli into subsets of stimuli that are substitutable for one another in certain contexts.” (Saunders & Green, 1992, p. 239, italics added). In sum, they demonstrate the powerful generative effects of reinforcement and stimulus control processes on behavior across many species.
Footnotes
Reference for this article in Web is: http://conductual.com/content/successful-search-symmetry
Preparation of this manuscript was supported by NICHD Grant R01 HD061322 to Peter J. Urcuioli
References
- Barnes D. Stimulus equivalence and relational frame theory. The Psychological Record. 1994;44:91–124. [Google Scholar]
- Barros R, Galvão O, McIlvane WJ. Generalized identity matching-to-sample in Cebus apella. The Psychological Record. 2002;52:441–460. doi: 10.1007/s40732-014-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaisdell AP, Cook RG. Two-item same-different concept learning in pigeons. Learning & Behavior. 2005;33:67–77. doi: 10.3758/bf03196051. [DOI] [PubMed] [Google Scholar]
- Bovet D, Vauclair J. Functional categorization of objects and of their pictures in baboons (Papio anubis) Learning and Motivation. 1998;29:309–322. [Google Scholar]
- Campos HC, Urcuioli PJ, Swisher M. Concurrent identity training is not necessary for associative symmetry in successive matching. Journal of the Experimental Analysis of Behavior. 2014;101:10–25. doi: 10.1002/jeab.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvillon PA, Klosterhalfen S, Bitterman ME. Analysis of overshadowing in honeybees. Journal of Comparative Psychology. 1983;97:154–166. [Google Scholar]
- D’Amato MR, Salmon DP, Loukas E, Tomie A. Symmetry and transitivity in the conditional relations in monkeys (Cebus apella) and pigeons (Columba livia) Journal of the Experimental Analysis of Behavior. 1985;44:35–47. doi: 10.1901/jeab.1985.44-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devany JM, Hayes SC, Nelson RO. Equivalence class formation in language-able and language-disabled children. Journal of the Experimental Analysis of Behavior. 1986;46:243–257. doi: 10.1901/jeab.1986.46-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube WV, Iennaco FM, McIlvane WJ. Generalized identity matching to sample of two-dimensional forms in individuals with intellectual disabilities. Research in Developmental Disabilities. 1993;14:457–477. doi: 10.1016/0891-4222(93)90038-l. [DOI] [PubMed] [Google Scholar]
- Dube WV, McIlvane WJ, Callahan TD, Stoddard LT. The search for stimulus equivalence in non-verbal organisms. The Psychological Record. 1993;43:761–778. [Google Scholar]
- Dugdale N, Lowe CF. Testing for symmetry in the conditional discriminations of language-trained chimpanzees. Journal of the Experimental Analysis of Behavior. 2000;73:5–22. doi: 10.1901/jeab.2000.73-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields L, Adams BJ, Verhave T, Newman S. The effects of nodality on the formation of equivalence classes. Journal of the Experimental Analysis of Behavior. 1990;53:345–358. doi: 10.1901/jeab.1990.53-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank AJ. Unpublished doctoral dissertation. University of Iowa; 2007. An examination of the temporal and spatial stimulus control in Emergent symmetry in pigeons. [Google Scholar]
- Frank A, Wasserman E. Associative symmetry in the pigeon after successive matching-to-sample training. Journal of the Experimental Analysis of Behavior. 2005;84:147–165. doi: 10.1901/jeab.2005.115-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García A, Benjumea S. The emergence of symmetry in a conditional discrimination task using different responses as proprioceptive samples in pigeons. Journal of the Experimental Analysis of Behavior. 2006a;86:65–80. doi: 10.1901/jeab.2006.67-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García A, Benjumea S. Conditional discrimination of one’s own behavior in pigeons: The role of the length of the behavior–sample. International Journal of Psychology and Psychological Therapy. 2006b;6:331–342. [Google Scholar]
- Gómez J, García A, Pérez V. Failure to find symmetry after multiple exemplar training. Psicothema. 2014;26:434–441. doi: 10.7334/psicothema2013.352. [DOI] [PubMed] [Google Scholar]
- Hayes SC. Nonhumans have not yet shown stimulus equivalence. Journal of the Experimental Analysis of Behavior. 1989;51:385–392. doi: 10.1901/jeab.1989.51-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan DE, Zentall TR. Backward associations in the pigeon. American Journal of Psychology. 1977;90:3–15. [Google Scholar]
- Honey RC, Hall G. The acquired equivalence and distinctiveness of cues. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:338–346. [PubMed] [Google Scholar]
- Horne PJ, Hughes JC, Lowe CF. Naming and categorization in Young children: IV: Listener behavior training and transfer of function. Journal of the Experimental Analysis of Behavior. 2006;85:247–273. doi: 10.1901/jeab.2006.125-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne PJ, Lowe CF. On the origins of naming and other symbolic behavior. Journal of the Experimental Analysis of Behavior. 1996;65:185–241. doi: 10.1901/jeab.1996.65-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne PJ, Lowe CF. Toward a theory of verbal behavior. Journal of the Experimental Analysis of Behavior. 1997;68:271–296. doi: 10.1901/jeab.1997.68-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen IH. Matching-to-sample performance in rats: A case of mistaken identity? Journal of the Experimental Analysis of Behavior. 1997;68:27–45. doi: 10.1901/jeab.1997.68-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen IH, Sidman M, Carrigan P. Stimulus definition in conditional discrimination. Journal of the Experimental Analysis of Behavior. 1986;45:297–304. doi: 10.1901/jeab.1986.45-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar RM, Davis-Lang D, Sanchez L. The formation of visual stimulus equivalences in children. Journal of the Experimental Analysis of Behavior. 1984;41:251–266. doi: 10.1901/jeab.1984.41-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-DeNolf KM. The search for symmetry: 25 years in review. Learning & Behavior. 2009;37:188–203. doi: 10.3758/LB.37.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello KM, Urcuioli PJ. Control by sample location in pigeons’ matching to sample. Journal of the Experimental Analysis of Behavior. 1998;70:235–251. doi: 10.1901/jeab.1998.70-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-DeNolf KM, Urcuioli PJ. Transfer of pigeons' matching-to-sample to novel sample locations. Journal of the Experimental Analysis of Behavior. 2000;73:141–161. doi: 10.1901/jeab.2000.73-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-DeNolf KM, Urcuioli PJ. Stimulus control topographies and tests of symmetry in pigeons. Journal of the Experimental Analysis of Behavior. 2002;78:467–495. doi: 10.1901/jeab.2002.78-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenkron B. Generalization of delayed identity matching in retarded children. Journal of the Experimental Analysis of Behavior. 1988;50:163–172. doi: 10.1901/jeab.1988.50-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkens R, Kop PFM, Matthijs W. A test of symmetry and transitivity in the conditional discrimination performances of pigeons. Journal of the Experimental Analysis of Behavior. 1988;49:395–409. doi: 10.1901/jeab.1988.49-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe CF, Horne PJ, Harris FDA, Randle VRL. Naming and categorization in young children: Vocal tact training. Journal of the Experimental Analysis of Behavior. 2002;78:527–549. doi: 10.1901/jeab.2002.78-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvane WJ, Serna RW, Dube WV, Stromer R. Stimulus control topography coherence and stimulus equivalence: Reconciling test outcomes with theory. In: Leslie J, Blackman DE, editors. Experimental and applied analysis of human behavior. Reno, NV: Context Press; 2000. pp. 85–110. [Google Scholar]
- Meehan EF. Class-consistent differential reinforcement and stimulus class formation in pigeons. Journal of the Experimental Analysis of Behavior. 1999;72:97–115. doi: 10.1901/jeab.1999.72-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KR, Wasserman EA. Temporal factors influencing the pigeon’s successive matching-to-sample performance: Sample duration, intertrial interval, and retention interval. Journal of the Experimental Analysis of Behavior. 1978;30:153–162. doi: 10.1901/jeab.1978.30-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oden DL, Thompson RKR, Premack D. Spontaneous transfer of matching by infant chimpanzees (Pan troglodytes) Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:140–145. [PubMed] [Google Scholar]
- Pilgrim C, Galizio M. Relations between baseline contingencies and equivalence probe performances. Journal of the Experimental Analysis of Behavior. 1990;54:213–224. doi: 10.1901/jeab.1990.54-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgrim C, Galizio M. Reversal of baseline relations and stimulus equivalence. I. Adults. Journal of the Experimental Analysis of Behavior. 1995;63:225–238. doi: 10.1901/jeab.1995.63-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RW. The question of bidirectional associations in pigeons’ learning of conditional discrimination tasks. Bulletin of the Psychonomic Society. 1988;26:577–579. [Google Scholar]
- Rodewald HK. Symbolic matching-to-sample by pigeons. Psychological Reports. 1974;34:987–990. [Google Scholar]
- Saunders KJ, Williams DC, Spradlin JE. Derived stimulus control: Are there differences among procedures and processes? In: Zentall TR, Smeets PM, editors. Stimulus class formation in humans and animals. NY: Elsevier; 1996. pp. 93–109. [Google Scholar]
- Saunders RR, Green G. The nonequivalence of behavioral and mathematical equivalence. Journal of the Experimental Analysis of Behavior. 1992;57:227–241. doi: 10.1901/jeab.1992.57-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders RR, Green G. A discrimination analysis of training-structure effects on stimulus equivalence outcomes. Journal of the Experimental Analysis of Behavior. 1999;72:117–137. doi: 10.1901/jeab.1999.72-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schusterman RJ, Kastak D. A California sea lion (Zalophus Californianus) is capable of forming equivalence relations. The Psychological Record. 1993;43:823–839. [Google Scholar]
- Schusterman RJ, Reichmuth CJ, Kastak D. How animals classify friends and foe. Current Directions in Psychological Science. 2000;9:1–6. [Google Scholar]
- Shanks D. Categorization by a connectionist network. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1991;17:433–443. [Google Scholar]
- Sidman M. Equivalence relations: Where do they come from? In: Blackman DE, Lejeune H, editors. Behaviour analysis in theory and practice: Contributions and controversies. Hillsdale, NJ: Erlbaum; 1990. pp. 93–114. [Google Scholar]
- Sidman M. Adventitious control by the location of comparison stimuli in conditional discriminations. Journal of the Experimental Analysis of Behavior. 1992;58:176–182. doi: 10.1901/jeab.1992.58-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M. Equivalence relations and behavior: A research story. Boston: Authors Cooperative; 1994. [Google Scholar]
- Sidman M. Equivalence relations and the reinforcement contingency. Journal of the Experimental Analysis of Behavior. 2000;74:127–146. doi: 10.1901/jeab.2000.74-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M. Symmetry and equivalence relations in behavior. Cognitive Studies. 2008;15:322–332. [Google Scholar]
- Sidman M, Cresson O, Jr, Willson-Morris M. Acquisition of matching to sample via mediated transfer. Journal of the Experimental Analysis of Behavior. 1974;22:261–273. doi: 10.1901/jeab.1974.22-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Rauzin R, Lazar R, Cunningham S, Tailby W, Carrigan P. A search for symmetry in the conditional discriminations of rhesus monkeys, baboons, and children. Journal of the Experimental Analysis of Behavior. 1982;37:23–44. doi: 10.1901/jeab.1982.37-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Tailby W. Conditional discrimination vs. matching to sample: An expansion of testing paradigm. Journal of the Experimental Analysis of Behavior. 1982;37:5–22. doi: 10.1901/jeab.1982.37-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R, Ehrlinger JM, Bingman VP. Transitive behavior in hippocampallesioned pigeons. Brain, Behavior, and Evolution. 2004;63:181–188. doi: 10.1159/000076442. [DOI] [PubMed] [Google Scholar]
- Sweeney MM, Urcuioli PJ. A reflexivity effect in pigeons. Journal of the Experimental Analysis of Behavior. 2010;94:267–282. doi: 10.1901/jeab.2010.94-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonaga M, Matsuzawa T, Fujita K, Yamamoto J. Emergence of symmetry in a visual discrimination by chimpanzees (Pan troglodytes) Psychological Reports. 1991;68:51–60. doi: 10.2466/pr0.1991.68.1.51. [DOI] [PubMed] [Google Scholar]
- Urcuioli PJ. Responses and acquired equivalence classes. In: Wasserman EA, Zentall TR, editors. Comparative cognition: Experimental explorations of animal intelligence. NY: Oxford University Press; 2006. pp. 405–421. [Google Scholar]
- Urcuioli PJ. Sample and comparison location as factors in matching acquisition, transfer, and acquired equivalence. Animal Learning & Behavior. 2007;35:252–261. doi: 10.3758/bf03206431. [DOI] [PubMed] [Google Scholar]
- Urcuioli PJ. Associative symmetry, antisymmetry, and a theory of pigeons’ equivalence-class formation. Journal of the Experimental Analysis of Behavior. 2008;90:257–282. doi: 10.1901/jeab.2008.90-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ. Emergent identity matching after successive matching training. Reflexivity or generalized identity? Journal of the Experimental Analysis of Behavior. 2011;96:329–341. doi: 10.1901/jeab.2011.96-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ, Swisher MJ. A replication and extension of the antisymmetry effect in pigeons. Journal of the Experimental Analysis of Behavior. 2012a;98:283–293. doi: 10.1901/jeab.2012.98-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ, Swisher MJ. Emergent identity matching after successive matching training. II. Reflexivity or transitivity? Journal of the Experimental Analysis of Behavior. 2012b;97:5–27. doi: 10.1901/jeab.2012.97-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ, Swisher MJ. Transitive and anti-transitive emergent relations in pigeons: Support for a theory of stimulus-class formation. Behavioural Processes. doi: 10.1016/j.beproc.2014.07.006. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ, Zentall TR, Jackson-Smith P, Steirn JN. Evidence for common coding in many-to-one matching: Retention, intertrial interference, and transfer. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:264–273. [Google Scholar]
- Vaughan W., Jr Formation of equivalence sets in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:36–42. [Google Scholar]
- Velasco SM, Huziwara EM, Machado A, Tomonari GY. Associative symmetry by pigeons after few-exemplar training. Journal of the Experimental Analysis of Behavior. 2010;94:283–295. doi: 10.1901/jeab.2010.94-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Fersen L, Delius JD. Acquired equivalences between auditory stimuli in dolphins (Tursiops truncates) Animal Cognition. 2000;3:79–83. [Google Scholar]
- Wagner AR, Logan FA, Haberlandt K, Price T. Stimulus selection in animal discrimination learning. Journal of Experimental Psychology. 1968;76:177–186. doi: 10.1037/h0025414. [DOI] [PubMed] [Google Scholar]
- Wasserman EA. Stimulus-reinforcer predictiveness and selective discrimination learning in pigeons. Journal of Experimental Psychology. 1974;103:284–297. [Google Scholar]
- Wasserman EA. Successive matching-to-sample in the pigeon: Variations on the theme by Konorski. Behavior Research Methods & Instrumentation. 1976;8:278–282. [Google Scholar]
- Wasserman EA, DeVolder CL. Similarity- and nonsimilarity-based conceptualization in children and pigeons. The Psychological Record. 1993;43:779–793. [Google Scholar]
- Wasserman EA, DeVolder CL, Coppage DJ. Non-similarity based conceptualization in pigeons via secondary or mediated generalization. Psychological Science. 1992;6:374–379. [Google Scholar]
- Wasserman EA, Kiedinger RE, Bhatt RS. Conceptual behavior in pigeons: Categories, subcategories, and pseudocategories. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:235–246. [Google Scholar]
- Wright AA, Katz JS. Mechanisms of same/different concept learning in primates and avians. Behavioural Processes. 2006;72:234–254. doi: 10.1016/j.beproc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Yamamoto J, Asano T. Stimulus equivalence in a chimpanzee (Pan troglodytes) The Psychological Record. 1995;45:3–21. [Google Scholar]
- Zentall TR, Smeets PM. Stimulus class formation in humans and animals. NY: Elsevier; 1996. [Google Scholar]
- Zentall TR, Wasserman EA, Urcuioli PJ. Associative concept learning in animals. Journal of the Experimental Analysis of Behavior. 2014;101:130–151. doi: 10.1002/jeab.55. [DOI] [PMC free article] [PubMed] [Google Scholar]