Abstract
Background
Drug-induced QT interval prolongation, a risk factor for life-threatening ventricular arrhythmias, is a potential side effect of many marketed and withdrawn medications. The contribution of common genetic variants previously associated with baseline QT interval to drug-induced QT prolongation and arrhythmias is not known.
Methods
We tested the hypothesis that a weighted combination of common genetic variants contributing to QT interval at baseline, identified through genome-wide association studies, can predict individual response to multiple QT-prolonging drugs. Genetic analysis of 22 subjects was performed in a secondary analysis of a randomized, double-blind, placebo-controlled, cross-over trial of 3 QT-prolonging drugs with 15 time-matched QT and plasma drug concentration measurements. Subjects received single doses of dofetilide, quinidine, ranolazine and placebo. The outcome was the correlation between a genetic QT score comprising 61 common genetic variants and the slope of an individual subject’s drug-induced increase in heart rate corrected QT (QTc) vs. drug concentration.
Results
The genetic QT score was correlated with drug-induced QTc prolongation. Among white subjects, genetic QT score explained 30% of the variability in response to dofetilide (r = 0.55 [95% CI, 0.09–0.81], P = 0.02), 23% in response to quinidine (r = 0.48 [95% CI, −0.03 to 0.79], P = 0.06) and 27% in response to ranolazine (r = 0.52 [95% CI, 0.05 to 0.80], P = 0.03). Furthermore, the genetic QT score was a significant predictor of drug-induced torsade de pointes in an independent sample of 216 cases compared to 771 controls (r2 = 12%, P = 1×10−7).
Conclusion
We demonstrate that a genetic QT score comprising 61 common genetic variants explains a significant proportion of the variability in drug-induced QT prolongation and is a significant predictor of drug-induced torsade de pointes. These findings highlight an opportunity for recent genetic discoveries to improve individualized risk-benefit assessment for pharmacologic therapies. Replication of these findings in larger samples is needed to more precisely estimate variance explained and to establish the individual variants that drive these effects.
Trial Registration
http://clinicaltrials.gov/ Unique identifier: NCT01873950.
Keywords: antiarrhythmia agents, drug therapy, genetics, diagnostics, genetic testing, genomics, pharmacogenomics, pharmacology, torsade de pointes
Introduction
The U.S. government recently launched a Precision Medicine Initiative to move away from a “one-size-fits-all-approach” for medical therapies and instead take into account specific characteristics of individual patients.1 Outside of oncology, advances in pharmacogenomics have been limited, with the exception of the genetic basis of drug absorption, distribution, metabolism and excretion (pharmacokinetics), which are traits often controlled by one or a few genetic mechanisms rather than the many mechanisms responsible for most complex traits and diseases. Drug-induced QT prolongation (reflecting delayed ventricular repolarization), which is a risk factor for torsade de pointes, is a potential side effect of many marketed and withdrawn medications through their direct actions on the heart (pharmacodynamics).2
We previously performed genome-wide association studies (GWASs) of the electrocardiographic QT interval identifying many common genetic variants that contribute a modest increment in resting QT interval (e.g. ~1–3 ms/allele) when considered individually.3–5 We demonstrated that a genetic QT score is a strong predictor of baseline QT interval with individuals in the top quintile having a 15 ms higher QT interval compared to the bottom quintile,6 explaining up to 10% of QT variation (approximately 25% of its heritability).4 In the present study, we test the hypothesis that a weighted combination of common genetic variants contributing to QT at baseline will predict individual response to multiple QT-prolonging drugs and risk of torsade de pointes in a case-control study.
Methods
Clinical study design
The study was approved by the Food and Drug Administration Research Involving Human Subjects Committee and local institutional review boards. All subjects gave written informed consent. The study design and primary results (not including genetic analysis) have been previously published.7,8 The study was a randomized, double-blind, cross-over study of healthy subjects (Figure 1) at a phase 1 clinical research unit (Spaulding Clinical, West Bend, WI) to differentiate the effects of individual vs. multi-channel block on the electrocardiogram (ECG). The inclusion and exclusion criteria were similar to thorough QT studies. Subjects were 18–35 years old, 50–85 kg and without a family history of cardiovascular disease or unexplained sudden cardiac death. Subjects also had to have a baseline heart rate corrected QT (QTc) of <450 ms for men (470 ms for women) using Fridericia’s correction and fewer than 12 ventricular ectopic beats during a 3-hour continuous recording at screening.
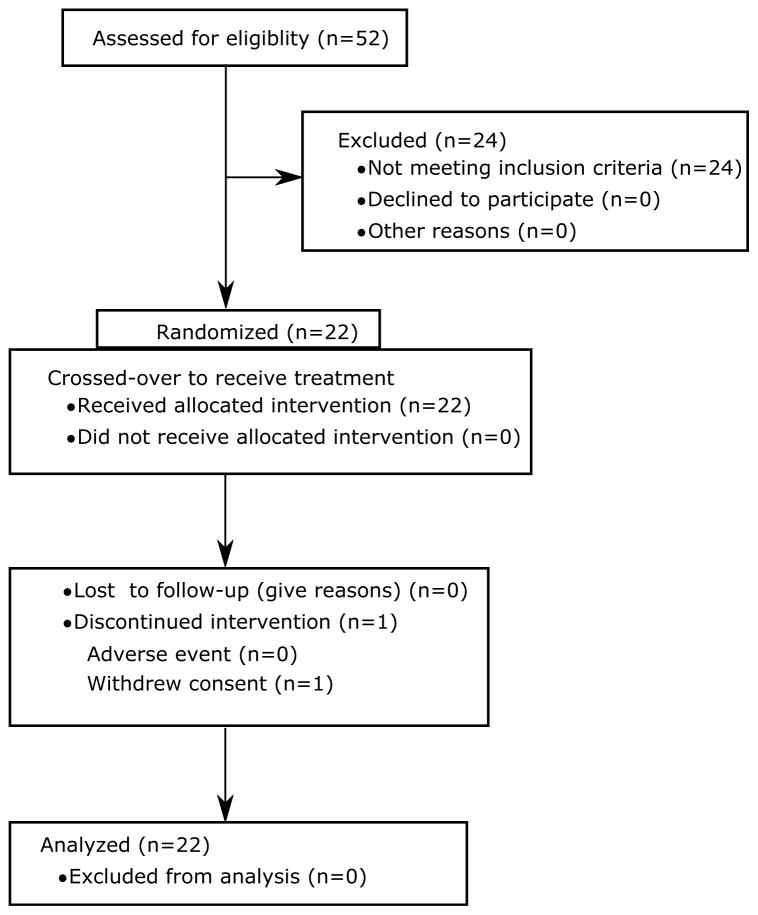
Figure 1.
CONSORT diagram for the study as reported in Vicente et al.8 Twenty four of the 52 screened subjects did not meet the inclusion criteria. Twenty two of the 28 subjects who met the inclusion criteria were randomized. All subjects completed the study, except one who withdrew prior to the last treatment period.
There was a 7-day washout period between each 24-hour treatment period. In the morning of each period, subjects received a single dose of 500 μg dofetilide (Tikosyn, Pfizer, New York, NY), 400 mg quinidine sulfate (Watson Pharma, Corona, CA), 1,500 mg ranolazine (Ranexa, Gilead, Foster City, CA), 120 mg verapamil hydrochloride (Heritage Pharmaceuticals, Edison, NJ) or placebo. As previously reported,7 verapamil did not prolong QTc at the dose administered and is not included in this analysis of the association of genetic variants with QTc prolongation.
Continuous ECGs were recorded at 500 Hz with an amplitude resolution of 2.5 μV. From the continuous recording, triplicate 10-second ECGs were extracted at pre-dose and 15 predefined time-points over 24 hours post-dose during which the subjects were resting in a supine position for 10 minutes. ECGs were extracted with stable heart rates and maximum signal quality using Antares software (AMPS-LLC, New York City, NY) at each of the 16 time-points.9 All post-dose time points were time-matched with blood samples for pharmacokinetic analysis. Plasma drug concentration was measured using a validated liquid chromatography with tandem mass spectroscopy method by Frontage Laboratories (Exton, Philadelphia, PA).7
Semi-automatic adjudication of the ECG intervals of the up-sampled ECGs was carried out blinded to treatment and time as previously described.7 For identification of the peak of the T-wave (Tpeak) and end of the T-wave (Tend), two ECG readers identified the global peak and end of the T-wave in the vector magnitude lead derived from the Guldenring transformation matrix.10 Tpeak was located by fitting a parabola through the T-wave peak. In the presence of a notch, the Tpeak was defined as the first discernible peak. Tend was determined using the tangent method, which involves locating the intersection between the line through the terminal descending part of the T-wave and isoelectric line. This approach of using the global vector magnitude lead to identify Tpeak and tangent method for Tend is not the same as Tpeak–Tend measured in a precordial lead, but produces more consistent measurements. In cases of low amplitude, flat T-waves, this does result in longer QT intervals. Disagreements on a T-wave being measureable, presence of a notch, or a difference of more than 5 ms in either T-peak or T-end were re-reviewed and adjudicated by an expert ECG reader. This was the case for only ~1.4% of ECGs.7 QT was corrected for heart rate with Fridericia’s formula (QTc) and J-Tpeak was corrected with Johannesen’s formula (J-Tpeakc = J–Tpeak/RR0.58 with RR in seconds), while Tpeak-Tend was not corrected for heart rate as it has minimal heart rate relationship at rest as previously described.11 The annotated ECG median beats are available on Physionet at https://physionet.org/physiobank/database/ecgrdvq/.12 A fully automated algorithm for Tpeak and Tend is also now available at https://github.com/FDA/ecglib.13
DNA extraction
Blood samples for isolation of DNA and genetic testing were collected and spotted onto Whatman FTA blood spot cards (Whatman Inc., Clifton, NJ, USA) by a research team member at check-in of the first period. DNA was extracted from Whatman FTA blood spot cards using Promega Tissue and Hair Extraction (Promega, Inc., Madison, WI, USA) kits. For samples with comparatively low yield, whole genome amplification was performed using the Qiagen REPLI-g Midi Kit (Qiagen, Inc., Venlo, Limburg, Netherlands). Samples were plated in duplicate from both raw extracted DNA and amplified DNA.
Primer selection and design
68 SNPs with established independent effects on QT interval from a large GWAS in 76,061 individuals of European descent, all meeting P < 5×10−8 threshold for statistical significance,4 were targeted for design in 3 multiplex assays using Sequenom custom software. Where assays for specific SNPs could not be designed, alternate SNPs that were highly correlated (r2 > 0.90 to the index SNP) and known to be equally associated with QT interval were attempted. In total, 63 SNPs were designed into three multiplexed pools; 5 SNPs could not be designed due to multiplexing limitations.
Genotyping and quality control
63 SNPs were attempted on the Sequenom MALDI-TOF platform. DNA with and without whole-genome amplification was tested in duplicate (88 wells for 22 individuals) on 384-well plates with DNA from an additional 200 individuals genotyped for a separate study. For a given individual in whom two samples were genotyped, the sample with the highest genotyping call rate was selected for analysis. 61 SNPs with call rate > 90% and Hardy-Weinberg equilibrium P > 0.001 across all plated samples (22+200) were retained for further analysis; two SNPs failed. The average genotyping success rate across 61 SNPs among 22 study subjects was 95.0%.
Genetic QT score
A genotype score was calculated as previously described.6 Briefly, the effects of 61 common variants on QT interval in individuals of European and of African descent were previously estimated in the Arking et al. GWAS.4 We oriented the coded allele (the allele coded 0, 1 or 2) to be the QT-raising allele for each SNP, regardless of allele frequency. A “simple” score just adding up the QT-increasing alleles across the 61 variants would have a theoretical minimum of 0 QT-prolonging alleles to a maximum of122 QT-prolonging alleles, since everyone has two alleles. This approach ignores the fact that not all genetic variants have equal effects on QT interval. Our approach (taken by most others in the genetics community) is to weight each allele by the observed effect on QT from the original 2014 GWAS. This changes the scale of the score from the number of QT-prolonging alleles to the predicted QT increase on the ms scale, “predicted” not “observed” because the weights are taken from the original GWAS not the current study. A given SNP’s contribution to the QT score was weighted according to the effect estimate per coded allele. For example, rs12143842 is a C/T SNP of which the T allele has a frequency of 0.24 in individuals of European ancestry and is associated with 3.5 ms longer QT interval per allele copy. An individual homozygous for the major allele (CC) would have 0 copies of the QT-raising allele and the contribution in that individual for that SNP to the QT score would be 0 (= 3.5 * 0) ms. An individual homozygous for the minor allele (TT) would have 2 copies of the QT-raising allele and the contribution for that SNP to the QT score would be +7.0 (= 3.5 * 2) ms. This process is then repeated for all 61 SNPs and the individual SNP contributions summed. For SNPs with missing genotypes in a given individual, the contribution to the score was imputed based on the allele frequency in the general population (twice the allele frequency because every individual has two copies of each gene). For example, for rs12143842, the coded allele frequency is 0.24 and the average number of coded alleles in individuals in the general population would be 0.24 * 2 = 0.48 and thus the contribution of a missing genotype for this SNP would be 1.68 (= 3.5 * 0.48) ms. The effect of such imputation biases the genotype score toward the null.
In self-described white individuals in the current study, we used the allelic effects estimated from the prior GWAS in individuals of European ancestry (Supplementary Table 1). As reported in the Arking et al. study,4 an independent African American GWAS had a smaller sample size and therefore fewer SNPs reached stringent statistical significance (P < 5×10−8), accounting for the genome-wide multiple testing burden. However, we observed high correlation among the effects of SNPs identified in European-derived individuals with effects for the same SNPs estimated in a GWAS in 13,105 African American individuals (r = 0.60).14 We cannot tell which SNPs among these are truly associated and which are not, due to limitations of power; however, the estimates in African-American individuals for null SNPs (not truly associated) will tend to cancel each other out. Therefore, in self-described black individuals in the current study, we used the allelic effects estimated for 60 of the 61 SNPs (one SNP was unavailable) in the prior African-American GWAS.14 The European-derived and African-derived genetic QT scores were calculated in all individuals, regardless of self-described ancestry for comparison purposes, but ancestry-specific scores were tested as the primary analysis. The PLINK v1.07 statistical package was used in all QT score calculations. Genotyping, quality control and genetic QT score calculation was performed by co-investigators blinded to all clinical data including race, sex, as well as QTc or QTc response to drug.
Case-control analysis of torsade de pointes
A GWAS was previously performed on 216 individuals of European descent with drug-induced torsade de pointes collected as part of the Trans-Atlantic Alliance Against Sudden Death supported by the Fondation Leducq and the Drug-induced Arrhythmia Risk Evaluation (DARE) study, compared with 771 ancestry-matched controls.15 The control group included a sample of drug-exposed, ancestry-matched controls free of excessive QT prolongation as well as population-based controls. In the study of rare diseases, such as rare adverse drug events, with incidence well below 1%, the frequencies of common variants among population-based controls and among drug-exposed QT non-prolongers are expected to be broadly similar. In the current study of torsade cases, a diversity of potential offending agents was observed, albeit enriched for users of quinidine, sotalol and amiodarone. but considering the small number of cases, we used combined sets of drug-exposed and population-based controls to maximize the control size. Using the methods developed by Johnson and reported in Ehret et al,16 we applied an instrumental variable approach based on the weighted effects from the QT-IGC GWAS,4 on the risk of drug-induced torsade de pointes for 60 of the 68 total SNPs that were directly genotyped or imputed with imputation quality > 0.90 in the torsade study. In a sensitivity analysis, we repeated the risk score analysis using only one SNP per locus (31 index SNPs from 35 possible loci). These analyses were performed in R (R Foundation for Statistical Computing, Vienna, Austria) using the ‘gtx’ package (version 0.0.8) available at https://cran.r-project.org/web/packages/gtx/index.html.
Statistical Analysis
Personalized ECG response to drug was defined as the slope of an individual subject’s drug-induced change in ECG biomarker (Figure 2A–2C). This was calculated by inputting individual-subject baseline (triplicate ECG measurements obtained immediately prior to dosing a specific drug) and placebo corrected (time-of-day matched ECG measurement from the placebo day) change (delta-delta QTc) for each of the ECG biomarkers and plasma drug concentrations into PROC MIXED in SAS 9.3 (SAS institute, Cary, NC) with concentration as a fixed effect and subject as a random effect on concentration (i.e. with each subject having his or her own slope with an intercept set to 0). The association between biomarkers (e.g. delta-delta QTc/drug concentration slope vs. genetic QT score) was tested using the Pearson product-moment correlation coefficient in R 3.1.2. The cross-over design was not formally accounted for in the statistical analysis. P values < 0.05 were considered statistically significant.
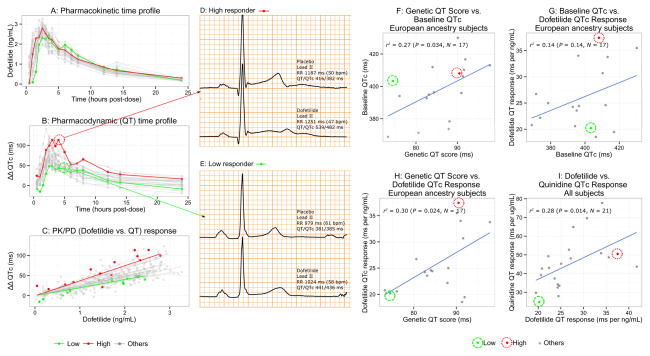
Figure 2. Pharmacokinetic-Pharmacodyamic Response and Genetic QT Score.
(A) Pharmacokinetic (PK) time profile shows plasma dofetilide concentration at each of the 15 time-points post-dose (dots) for each subject (lines). Example subjects are shown in red (dofetilide high responder) and green (low responder) throughout. (B) Pharmacodynamic (PD) time profile shows baseline- and placebo-corrected changes from baseline in heart rate corrected QT (ΔΔQTc) at 15 time-points (dots) after a single oral dose of dofetilide for each subject (lines). (C) PK/PD response plot showing the measures of ΔΔQTc from the ECGs and the corresponding time-matched dofetilide plasma concentration. Solid lines show each subject’s QTc concentration-dependent response, the slope of which was tested in genetic QT score analyses. ECG examples show lead II and QT/QTc measures of (D) a high responder subject (red line and dots in A, B and C panels) during placebo (top ECG) and dofetilide (bottom ECG) and (E) a low responder subject (green line and dots in A, B and C panels) during placebo (top ECG) and dofetilide (bottom ECG). Note that while lead II is shown, QT measurements are from the global vector magnitude lead as described in the Methods. Correlations between (F) genetic QT score vs. baseline QTc in white subjects, (G) baseline QTc vs. dofetilide QTc response in white subjects, (H) genetic QT score vs. dofetilide QTc response in white subjects and (I) dofetilide QTc response vs. quinidine QTc response in all subjects are shown. Each dot represents a subject’s value. The scale of the QT genetic score is in ms of predicted QT effect for the variants in aggregate, as described in the Methods.
Results
The drug study included 17 self-described white, 4 black and 1 Asian subject free of electrolyte abnormality, concomitant medication use, or clinically apparent cardiovascular disease (Table 1). The white group included 8 men and 9 women of mean age 26 years. The European genetic score explained 27% of the variability in baseline QTc in white subjects (P = 0.03, Figure 2F). The African American genetic score was also correlated with baseline QTc in black subjects (P = 0.03), although the small sample size limits precise estimation of the effect (Supplementary Table 2).
Table 1.
Baseline characteristics
| Group | All | White | Black | Asian |
|---|---|---|---|---|
| Age (years) | 26.9 ± 5.5 | 25.7 ± 5.3 | 30.3 ± 3.8 | 35.0 |
| BMI (kg/m2) | 23.1 ± 2.7 | 22.5 ± 2.7 | 25.3 ± 1.0 | 23.1 |
| QTc (ms) | 395.9 ± 17.1 | 398.0 ± 17.2 | 389.5 ± 19.0 | 385.5 |
| European genetic QT score (ms) | 86.3 ± 6.4 | 85.8 ± 6.9 | 88.8 ± 5.2 | 84.2 |
| African genetic QT score (ms) | 53.1 ± 4.8 | 53.4 ± 5.2 | 51.9 ± 3.4 | 51.3 |
| Total subjects (n) | 22 | 17 | 4 | 1 |
| Female (n) | 11 | 9 | 2 | 0 |
Age, body mass index (BMI), QTc and genetic QT score values reported as mean ± standard deviation.
Baseline QTc was not a significant predictor of drug-induced QTc prolongation for any of the drugs in 17 white subjects, potentially due to limited power (Figure 2G, Supplementary Figure 1). However, there was a significant correlation between the genetic QT score and drug-induced QTc prolongation (Table 2, Supplementary Figure 1). Among white subjects, European genetic score explained 30% of the variability (P = 0.02) in response to dofetilide (Figure 2h), 23% in response to quinidine (P = 0.06) and 27% in response to ranolazine (P = 0.03). Among 4 black subjects, a significant correlation existed between baseline QTc and response to dofetilide (P = 0.04, Supplementary Table 2), and between the African American genetic score and response to dofetilide (P = 0.03, Table 2), but not for quinidine or ranolazine.
Table 2.
Correlations between common genetic variant QT score and drug-induced QTc slope response
| Genetic QT score vs. treatment (white subjects) | r [95% CI] | P | N | r2 |
|---|---|---|---|---|
| Genetic score vs. Baseline QTc | 0.52 [0.05 to 0.80] | 0.03 | 17 | 0.27 |
| Genetic score vs. Dofetilide QTc slope | 0.55 [0.09 to 0.81] | 0.02 | 17 | 0.30 |
| Genetic score vs. Quinidine QTc slope | 0.48 [−0.03 to 0.79] | 0.06 | 16 | 0.23 |
| Genetic score vs. Ranolazine QTc slope | 0.52 [0.05 to 0.80] | 0.03 | 17 | 0.27 |
|
|
||||
| Genetic QT score vs. treatment (black or African American subjects) | r [95% CI] | P | N | r2 |
|
| ||||
| Genetic score vs. Baseline QTc | 0.97 [0.11 to 1.00] | 0.03 | 4 | 0.94 |
| Genetic score vs. Dofetilide QTc slope | 0.97 [0.12 to 1.00] | 0.03 | 4 | 0.94 |
| Genetic score vs. Quinidine QTc slope | 0.18 [−0.94 to 0.97] | 0.82 | 4 | 0.03 |
| Genetic score vs. Ranolazine QTc slope | 0.55 [−0.87 to 0.99] | 0.45 | 4 | 0.30 |
Fig I in the Data Supplement shows the corresponding correlation plots.
We next investigated how response to one QT-prolonging drug predicted the response to other QT-prolonging drugs, combining subjects of all races together. There were significant correlations between all drug-drug relationships, with response to each drug explaining 24–29% of the variability in response to each of the other drugs (Figure 2I, Table 3).
Table 3.
Correlations between responses to different drugs
| Drug A vs. Drug B by ECG measure (all subjects) | r [95% CI] | P | N | r2 |
|---|---|---|---|---|
| QTc | ||||
| Dofetilide vs. Quinidine | 0.53 [0.13 to 0.78] | 0.01 | 21 | 0.28 |
| Dofetilide vs. Ranolazine | 0.49 [0.09 to 0.76] | 0.02 | 22 | 0.24 |
| Quinidine vs. Ranolazine | 0.53 [0.13 to 0.78] | 0.01 | 21 | 0.29 |
| J-Tpeakc | ||||
| Dofetilide vs. Quinidine | 0.46 [0.03 to 0.74] | 0.04 | 21 | 0.21 |
| Dofetilide vs. Ranolazine | 0.54 [0.15 to 0.78] | 0.009 | 22 | 0.29 |
| Quinidine vs. Ranolazine | 0.51 [0.11 to 0.77] | 0.02 | 21 | 0.26 |
| Tpeak-Tend | ||||
| Dofetilide vs. Quinidine | 0.72 [0.41 to 0.88] | <0.001 | 21 | 0.52 |
| Dofetilide vs. Ranolazine | 0.44 [0.03 to 0.73] | 0.04 | 22 | 0.20 |
| Quinidine vs. Ranolazine | 0.57 [0.19 to 0.80] | 0.007 | 21 | 0.33 |
Drug A vs. Drug B correlations computed comparing the slopes of each measure.
While hERG potassium channel block prolongs both J-Tpeakc and Tpeak-Tend intervals, additional inward current block from L-type calcium or late sodium current block can shorten the J-Tpeakc interval.8,17 Thus, Tpeak-Tend may be a more specific marker for hERG potassium channel block than the entire QT interval.7,11 Genetic QT score was not associated with baseline Tpeak-Tend or drug-induced change in Tpeak-Tend (Supplementary Table 3). Response to each of the two strongest hERG potassium channel blocking drugs (dofetilide and quinidine) explained 52% of the variability in the response to the other (P < 0.001, Table 3). Baseline Tpeak-Tend was also correlated with drug-induced QTc prolongation for dofetilide and quinidine, but not ranolazine (Supplementary Table 4) and baseline Tpeak-Tend was correlated with drug-induced Tpeak-Tend prolongation for all three drugs (Supplementary Table 5).
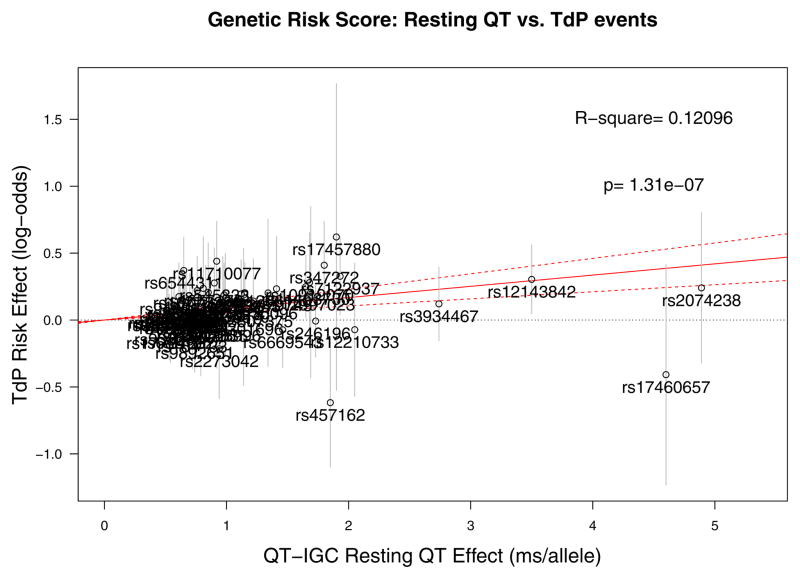
To test the relevance of the genetic risk score’s impact on quantitative QT response to drug exposure to the outcome for which QT response is a surrogate, we examined a previously published GWAS of drug-induced torsade de pointes.15 From a GWAS in 216 individuals with drug-induced torsade de pointes of European descent compared to 771 ancestry-matched controls, 60 of 68 possible QT SNPs had adequate imputation quality or were directly genotyped and available for analysis (Supplementary Table 6). Increasing genetic QT risk score was associated with significantly increased risk of drug-induced torsade de pointes (P = 1.3 × 10−7), explaining 12.1% of variation in risk (Figure 3).
Figure 3. Validation of genotype score in cases of drug-induced torsade de pointes.
Instrumental variable analysis of effect of 60 SNPs associated with resting QTc, using effect estimates from the QT-IGC GWAS (x axis) in milliseconds of predicted QT interval per allele as a predictor of log odds ratio of drug-induced torsade de pointes (y axis). Individual labels represent SNPs used in the analysis, and error bars correspond to the standard error of the log odds ratio of drug-induced torsade de pointes. For example, the QT-raising allele of SNP rs12143842 is associated with a 3.5 ms longer QT interval (Supplementary Table 1) and a ln(OR) of 0.30, corresponding to an OR of 1.35 for torsade de pointes risk (Supplementary Table 6). The overall R-square and p value reflect the effect on torsade de pointes risk of all variants combined in the score.
In a sensitivity analysis restricted to one SNP per locus, for which 31 SNPs at 35 loci were available, the genetic risk score explained a smaller proportion of variance in drug-induced QT prolongation and significance was attenuated (Supplementary Table 7), but it remained a significant predictor of torsade risk (P = 3 × 10−6, r2 = 9.6%, Supplementary Figure 2).
Discussion
Drug-induced QT prolongation and torsade de pointes have resulted in the withdrawal of several drugs from the market and over 150 are listed on CredibleMeds.org as being associated with QT prolongation and/or torsade de pointes.18 However, the incidence of torsade de pointes is low and only a small number of patients develop drug-induced long QT syndrome. The present pilot study provides a link between common genetic variants and drug-induced QT prolongation and demonstrates how GWAS results can be leveraged to define personalized pharmacodynamic response to drugs. Moreover, our finding that these same common genetic variants influence risk of drug-induced torsade de pointes confirms the potential clinical relevance of the genetic QT score.
A genetic component of long QT syndrome has been recognized since the 1950s,19 with the molecular basis of rare genetic variants causing congenital long QT syndrome being identified in the 1990s. However, not all individuals with congenital long QT syndrome variants have prolonged QT intervals at baseline, a hallmark of incomplete penetrance of the genetic abnormality. Recent GWASs have identified more than 60 common genetic variants that individually have small effects on QT at baseline (e.g. 1–3 ms), but in aggregate may have a larger effect. Individual SNPs at the NOS1AP locus and at KCNE1 have been associated with increased risk of acquired long QT syndrome.20,21 Indeed, in the present study we demonstrated that a weighted combination of 61 common genetic variants explained 27% of the variability in baseline QTc. This common genetic variability may help explain the incomplete penetrance of congenital long QT syndrome,22–24 but also why only certain individuals without recognized congenital long QT syndrome develop drug-induced long QT syndrome and torsade de pointes.
Previous reports have suggested that patients developing drug-induced long QT syndrome with one drug are more likely to develop drug-induced long QT syndrome with exposure to other drugs.25 In addition, Kannankeril et al. studied the effects of quinidine on drug-induced QTc and Tpeak-Tend prolongation in first-degree relatives of patients who developed drug-induced long QT syndrome, including torsade de pointes, compared to relatives of patients who tolerated QT-prolonging therapy.26 Having a relative with drug-induced long QT syndrome was associated with exaggerated Tpeak-Tend prolongation, but not QTc prolongation compared to having a drug-tolerant relative, although the sample size was limited.26 This is consistent with our recent findings that global Tpeak-Tend measured in the vector magnitude lead may be a more specific biomarker than QT prolongation for hERG potassium channel block,7,8,11 and in the present study response to the two strongest hERG blockers (dofetilide and quinidine) explained 52% of the variability in response to the other. However, the genetic QT score was not associated with baseline or drug-induced Tpeak-Tend prolongation. This is not surprising as the common genetic variants were selected for association with the whole QT interval not just the Tpeak-Tend component. Nonetheless, the relationship between Tpeak-Tend measurements at baseline and individual-subject drug response suggests that further study should investigate the relationship between Tpeak-Tend, common genetic variants and risk.
Repolarization reserve, as originally proposed,27,28 suggests that there are multiple redundant mechanisms that contribute to repolarization, such that minor alterations (e.g. from genetic variants) may not be detectable at baseline. However, in the presence of additional insults such as hypokalemia or exposure to a drug, reduced repolarization reserve can be unmasked, resulting in an extreme drug response that can lead to ventricular arrhythmias.29 This model has been considered largely in the context of Mendelian Long QT Syndromes (LQTS), in which some ion channel mutation carriers only manifest life-threatening arrhythmia following drug exposure. While cases of subclinical Mendelian long QT syndrome exposed by the development of torsade de pointes on drug challenge are well recognized, these appear to represent a minority of cases of drug-induced long QT syndrome.30–32 Our genetic and drug A vs. drug B response findings strongly support that a significant proportion of repolarization reserve27,28 in apparently healthy subjects has a genetic basis and that a relatively modest number of common variants—many in genes without an established role in Mendelian long QT syndromes—in aggregate plays a substantial role. That the genetic QT score is associated with increased risk of drug-induced torsade de pointes supports the clinical relevance of these variants and confirms the established relationship between QT prolongation after drug exposure and torsade de pointes risk. However, precise quantification of risk of torsade de pointes will be challenging due to the rarity of the outcome and the modest sample size of existing case-control collections.
The present study is limited by the small sample size, especially in African Americans, and attempted replication is needed to confirm the findings in individuals of European descent, to provide more precise estimates of effects and to perform adequately powered tests in individuals of African and other non-European ancestries. The study was conducted in healthy volunteers as opposed to patients, in whom sources of variation in QT response may be greater. However, the study represents a proof of principle that common genetic variants in aggregate influence QT response by administering multiple QT-prolonging drugs to the same subjects in a phase 1 clinical trial unit with pharmacokinetic-pharmacodynamic modeling to precisely define personalized response. We have imputed results for missing genotypes, although this is expected to bias results to the null. Additionally, aggregating individual effects of variants in genes in diverse pathways does not establish which variants drive the risk of QT prolongation and torsade. We took a genetic risk score approach to maximize power under a model in which QT-prolonging alleles generally increase QT prolongation following drug exposure. Ultimately, much larger sample sizes, including, for example, individuals with the underlying cardiovascular diseases for which anti-arrhythmic medications such as those examined here are prescribed, will be required to establish which variants contribute to the predictive ability of the score and the relative explanatory power of a genetic risk score when set against other clinical predictors of QT interval response.
Individualized prediction of risk of adverse response to medication is needed. Our finding that a simple genetic risk score comprised of 61 common variants explains a substantial proportion of variation in QT response to multiple drugs highlights the opportunity to translate GWAS findings to clinical care. Genetic risk scores will be expanded as more genetic variants are identified. The current study highlights the value of genetic studies of continuous, quantitative cardiovascular traits measured in very large sample sizes to identify variants that have meaningful effects on clinical outcomes captured in much smaller samples. Studies to examine whether preemptive, pre-prescription genotyping leads to a reduction in serious adverse events are warranted.
Supplementary Material
Clinical Perspective.
What is new?
We demonstrated that a genetic risk score comprised of multiple independent genetic variants that have previously been found to be associated with QT interval duration are collectively associated with the degree of drug-induced QT prolongation.
In addition, the genetic risk score was associated with drug-induced torsade de pointes in a case-control cohort.
What are the clinical implications?
If our results are confirmed in real-world collections of drug-exposed patients with larger sample sizes, the genetic risk score (updated as new variants are discovered) could potentially be used to individualize assessment of risks and benefits of drugs with high risk for drug-induced arrhythmias.
Acknowledgments
The authors thank the staff of Spaulding Clinical Research and Frontage Laboratories for data collection. The opinions presented here are those of the authors. No official support or endorsement by the FDA is intended nor should be inferred. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the U.S. Department of Health and Human Services.
Funding/Support: The drug intervention project was supported in part by Food and Drug Administration’s Critical Path Initiative and FDA’s Chief Scientist Challenge Grant and by a research fellowship from the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the Food and Drug Administration, as well as institutional support from Massachusetts General Hospital and funding from the NIH (HL124262). Dr. Rosenberg was supported by a Postdoctoral Fellowship Award of the Heart Rhythm Society. Dr. Kosova was supported by the Cell and Molecular Training for Cardiovascular Biology training grant of the MGH CVRC (T32HL7208). Support for the previously published drug-induced torsade de pointes GWAS was provided by the Leducq Foundation-supported “Alliance Against Sudden Death”, U19HL65962, P50GM115305, and a collaboration with the International Serious Adverse Events Consortium, whose membership included Abbott, Amgen, Astra-Zeneca, Cerner, Daiichi-Sankyo, GlaxoSmithKline, Merck, Novartis, Pfizer, Takeda, and the Welcome Trust. The DARE study was funded by the British Heart Foundation under special project grant SP/02/001.
Footnotes
Subject codes: [5] Arrhythmias, clinical electrophysiology, drugs, [109] Clinical genetics, [146] Genomics
Disclosures: None of the authors has any conflicts of interest to report.
References
- 1.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 3.Newton-Cheh C, Eijgelsheim M, Rice KM, de Bakker PI, Yin X, Estrada K, Bis JC, Marciante K, Rivadeneira F, Noseworthy PA, Sotoodehnia N, Smith NL, Rotter JI, Kors JA, Witteman JC, Hofman A, Heckbert SR, O’Donnell CJ, Uitterlinden AG, Psaty BM, Lumley T, Larson MG, Stricker BH. Common variants at ten loci influence QT interval duration in the QTGEN Study. Nat Genet. 2009;41:399–406. doi: 10.1038/ng.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arking DE, Pulit SL, Crotti L, van der Harst P, Munroe PB, Koopmann TT, Sotoodehnia N, Rossin EJ, Morley M, Wang X, Johnson AD, Lundby A, Gudbjartsson DF, Noseworthy PA, Eijgelsheim M, Bradford Y, Tarasov KV, Dorr M, Muller-Nurasyid M, Lahtinen AM, Nolte IM, Smith AV, Bis JC, Isaacs A, Newhouse SJ, Evans DS, Post WS, Waggott D, Lyytikainen LP, Hicks AA, Eisele L, Ellinghaus D, Hayward C, Navarro P, Ulivi S, Tanaka T, Tester DJ, Chatel S, Gustafsson S, Kumari M, Morris RW, Naluai AT, Padmanabhan S, Kluttig A, Strohmer B, Panayiotou AG, Torres M, Knoflach M, Hubacek JA, Slowikowski K, Raychaudhuri S, Kumar RD, Harris TB, Launer LJ, Shuldiner AR, Alonso A, Bader JS, Ehret G, Huang H, Kao WH, Strait JB, Macfarlane PW, Brown M, Caulfield MJ, Samani NJ, Kronenberg F, Willeit J, Smith JG, Greiser KH, Meyer Zu Schwabedissen H, Werdan K, Carella M, Zelante L, Heckbert SR, Psaty BM, Rotter JI, Kolcic I, Polasek O, Wright AF, Griffin M, Daly MJ, Arnar DO, Holm H, Thorsteinsdottir U, Denny JC, Roden DM, Zuvich RL, Emilsson V, Plump AS, Larson MG, O’Donnell CJ, Yin X, Bobbo M, D’Adamo AP, Iorio A, Sinagra G, Carracedo A, Cummings SR, Nalls MA, Jula A, Kontula KK, Marjamaa A, Oikarinen L, Perola M, Porthan K, Erbel R, Hoffmann P, Jockel KH, Kalsch H, Nothen MM, den Hoed M, Loos RJ, Thelle DS, Gieger C, Meitinger T, Perz S, Peters A, Prucha H, Sinner MF, Waldenberger M, de Boer RA, Franke L, van der Vleuten PA, Beckmann BM, Martens E, Bardai A, Hofman N, Wilde AA, Behr ER, Dalageorgou C, Giudicessi JR, Medeiros-Domingo A, Barc J, Kyndt F, Probst V, Ghidoni A, Insolia R, Hamilton RM, Scherer SW, Brandimarto J, Margulies K, Moravec CE, del Greco MF, Fuchsberger C, O’Connell JR, Lee WK, Watt GC, Campbell H, Wild SH, El Mokhtari NE, Frey N, Asselbergs FW, Mateo Leach I, Navis G, van den Berg MP, van Veldhuisen DJ, Kellis M, Krijthe BP, Franco OH, Hofman A, Kors JA, Uitterlinden AG, Witteman JC, Kedenko L, Lamina C, Oostra BA, Abecasis GR, Lakatta EG, Mulas A, Orru M, Schlessinger D, Uda M, Markus MR, Volker U, Snieder H, Spector TD, Arnlov J, Lind L, Sundstrom J, Syvanen AC, Kivimaki M, Kahonen M, Mononen N, Raitakari OT, Viikari JS, Adamkova V, Kiechl S, Brion M, Nicolaides AN, Paulweber B, Haerting J, Dominiczak AF, Nyberg F, Whincup PH, Hingorani AD, Schott JJ, Bezzina CR, Ingelsson E, Ferrucci L, Gasparini P, Wilson JF, Rudan I, Franke A, Muhleisen TW, Pramstaller PP, Lehtimaki TJ, Paterson AD, Parsa A, Liu Y, van Duijn CM, Siscovick DS, Gudnason V, Jamshidi Y, Salomaa V, Felix SB, Sanna S, Ritchie MD, Stricker BH, Stefansson K, Boyer LA, Cappola TP, Olsen JV, Lage K, Schwartz PJ, Kaab S, Chakravarti A, Ackerman MJ, Pfeufer A, de Bakker PI, Newton-Cheh C Consortium H, Consortium CA, Consortium C, eMC, Dcct/Edic. Genetic association study of QT interval highlights role for calcium signaling pathways in myocardial repolarization. Nat Genet. 2014;46:826–836. doi: 10.1038/ng.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeufer A, Sanna S, Arking DE, Muller M, Gateva V, Fuchsberger C, Ehret GB, Orru M, Pattaro C, Kottgen A, Perz S, Usala G, Barbalic M, Li M, Putz B, Scuteri A, Prineas RJ, Sinner MF, Gieger C, Najjar SS, Kao WH, Muhleisen TW, Dei M, Happle C, Mohlenkamp S, Crisponi L, Erbel R, Jockel KH, Naitza S, Steinbeck G, Marroni F, Hicks AA, Lakatta E, Muller-Myhsok B, Pramstaller PP, Wichmann HE, Schlessinger D, Boerwinkle E, Meitinger T, Uda M, Coresh J, Kaab S, Abecasis GR, Chakravarti A. Common variants at ten loci modulate the QT interval duration in the QTSCD Study. Nat Genet. 2009;41:407–414. doi: 10.1038/ng.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noseworthy PA, Havulinna AS, Porthan K, Lahtinen AM, Jula A, Karhunen PJ, Perola M, Oikarinen L, Kontula KK, Salomaa V, Newton-Cheh C. Common genetic variants, QT interval, and sudden cardiac death in a Finnish population-based study. Circ Cardiovas Genet. 2011;4:305–311. doi: 10.1161/CIRCGENETICS.110.959049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johannesen L, Vicente J, Mason J, Sanabria C, Waite-Labott K, Hong M, Guo P, Lin J, JSS, Galeotti L, Florian J, Ugander M, Stockbridge N, Strauss D. Differentiating Drug-Induced Multichannel Block on the Electrocardiogram: Randomized Study of Dofetilide, Quinidine, Ranolazine, and Verapamil. Clin Pharmacol Ther. 2014;96:549–558. doi: 10.1038/clpt.2014.155. [DOI] [PubMed] [Google Scholar]
- 8.Vicente J, Johannesen L, Mason JW, Crumb WJ, Pueyo E, Stockbridge N, Strauss DG. Comprehensive T wave morphology assessment in a randomized clinical study of dofetilide, quinidine, ranolazine, and verapamil. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001615. pii: e001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badilini F, Vaglio M, Sarapa N. Automatic extraction of ECG strips from continuous 12-lead holter recordings for QT analysis at prescheduled versus optimized time points. Ann Noninvasive Electrocardiol. 2009;14:S22–S29. doi: 10.1111/j.1542-474X.2008.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guldenring D, Finlay DD, Bond RR, Kennedy A, McLaughlin J, Galeotti L, Strauss DG. The derivation of the spatial QRS-T angle and the spatial ventricular gradient using the Mason-Likar 12-lead electrocardiogram. J Electrocardiol. 2015;48:1045–1052. doi: 10.1016/j.jelectrocard.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Johannesen L, Vicente J, Gray R, Galeotti L, Loring Z, Garnett C, Florian J, Ugander M, Stockbridge N, Strauss D. Improving the Assessment of Heart Toxicity for All New Drugs through Translational Regulatory Science. Clin Pharmacol Ther. 2014;95:501–508. doi: 10.1038/clpt.2013.238. [DOI] [PubMed] [Google Scholar]
- 12.Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, Mietus JE, Moody GB, Peng CK, Stanley HE. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101:E215–220. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 13.Johannesen L, Vicente J, Hosseini M, Strauss DG. Automated Algorithm for J-Tpeak and Tpeak-Tend Assessment of Drug-Induced Proarrhythmia Risk. PLoS One. 2016;11:e0166925. doi: 10.1371/journal.pone.0166925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JG, Avery CL, Evans DS, Nalls MA, Meng YA, Smith EN, Palmer C, Tanaka T, Mehra R, Butler AM. Impact of ancestry and common genetic variants on QT interval in African Americans. Circ Cardiovasc Genet. 2012;5:647–655. doi: 10.1161/CIRCGENETICS.112.962787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behr ER, Ritchie MD, Tanaka T, Kaab S, Crawford DC, Nicoletti P, Floratos A, Sinner MF, Kannankeril PJ, Wilde AA, Bezzina CR, Schulze-Bahr E, Zumhagen S, Guicheney P, Bishopric NH, Marshall V, Shakir S, Dalageorgou C, Bevan S, Jamshidi Y, Bastiaenen R, Myerburg RJ, Schott JJ, Camm AJ, Steinbeck G, Norris K, Altman RB, Tatonetti NP, Jeffery S, Kubo M, Nakamura Y, Shen Y, George AL, Jr, Roden DM. Genome wide analysis of drug-induced torsades de pointes: lack of common variants with large effect sizes. PLoS One. 2013;8:e78511. doi: 10.1371/journal.pone.0078511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International Consortium for Blood Pressure Genome-Wide Association S; Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O’Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sober S, Parsa A, Luan J, Arora P, Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden JF, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, van der Harst P, Kao WH, Sjogren M, Vinay DG, Alexander M, Tabara Y, Shaw-Hawkins S, Whincup PH, Liu Y, Shi G, Kuusisto J, Tayo B, Seielstad M, Sim X, Nguyen KD, Lehtimaki T, Matullo G, Wu Y, Gaunt TR, Onland-Moret NC, Cooper MN, Platou CG, Org E, Hardy R, Dahgam S, Palmen J, Vitart V, Braund PS, Kuznetsova T, Uiterwaal CS, Adeyemo A, Palmas W, Campbell H, Ludwig B, Tomaszewski M, Tzoulaki I, Palmer ND, Aspelund T, Garcia M, Chang YP, O’Connell JR, Steinle NI, Grobbee DE, Arking DE, Kardia SL, Morrison AC, Hernandez D, Najjar S, McArdle WL, Hadley D, Brown MJ, Connell JM, Hingorani AD, Day IN, Lawlor DA, Beilby JP, Lawrence RW, Clarke R, Hopewell JC, Ongen H, Dreisbach AW, Li Y, Young JH, Bis JC, Kahonen M, Viikari J, Adair LS, Lee NR, Chen MH, Olden M, Pattaro C, Bolton JA, Kottgen A, Bergmann S, Mooser V, Chaturvedi N, Frayling TM, Islam M, Jafar TH, Erdmann J, Kulkarni SR, Bornstein SR, Grassler J, Groop L, Voight BF, Kettunen J, Howard P, Taylor A, Guarrera S, Ricceri F, Emilsson V, Plump A, Barroso I, Khaw KT, Weder AB, Hunt SC, Sun YV, Bergman RN, Collins FS, Bonnycastle LL, Scott LJ, Stringham HM, Peltonen L, Perola M, Vartiainen E, Brand SM, Staessen JA, Wang TJ, Burton PR, Soler Artigas M, Dong Y, Snieder H, Wang X, Zhu H, Lohman KK, Rudock ME, Heckbert SR, Smith NL, Wiggins KL, Doumatey A, Shriner D, Veldre G, Viigimaa M, Kinra S, Prabhakaran D, Tripathy V, Langefeld CD, Rosengren A, Thelle DS, Corsi AM, Singleton A, Forrester T, Hilton G, McKenzie CA, Salako T, Iwai N, Kita Y, Ogihara T, Ohkubo T, Okamura T, Ueshima H, Umemura S, Eyheramendy S, Meitinger T, Wichmann HE, Cho YS, Kim HL, Lee JY, Scott J, Sehmi JS, Zhang W, Hedblad B, Nilsson P, Smith GD, Wong A, Narisu N, Stancakova A, Raffel LJ, Yao J, Kathiresan S, O’Donnell CJ, Schwartz SM, Ikram MA, Longstreth WT, Jr, Mosley TH, Seshadri S, Shrine NR, Wain LV, Morken MA, Swift AJ, Laitinen J, Prokopenko I, Zitting P, Cooper JA, Humphries SE, Danesh J, Rasheed A, Goel A, Hamsten A, Watkins H, Bakker SJ, van Gilst WH, Janipalli CS, Mani KR, Yajnik CS, Hofman A, Mattace-Raso FU, Oostra BA, Demirkan A, Isaacs A, Rivadeneira F, Lakatta EG, Orru M, Scuteri A, Ala-Korpela M, Kangas AJ, Lyytikainen LP, Soininen P, Tukiainen T, Wurtz P, Ong RT, Dorr M, Kroemer HK, Volker U, Volzke H, Galan P, Hercberg S, Lathrop M, Zelenika D, Deloukas P, Mangino M, Spector TD, Zhai G, Meschia JF, Nalls MA, Sharma P, Terzic J, Kumar MV, Denniff M, Zukowska-Szczechowska E, Wagenknecht LE, Fowkes FG, Charchar FJ, Schwarz PE, Hayward C, Guo X, Rotimi C, Bots ML, Brand E, Samani NJ, Polasek O, Talmud PJ, Nyberg F, Kuh D, Laan M, Hveem K, Palmer LJ, van der Schouw YT, Casas JP, Mohlke KL, Vineis P, Raitakari O, Ganesh SK, Wong TY, Tai ES, Cooper RS, Laakso M, Rao DC, Harris TB, Morris RW, Dominiczak AF, Kivimaki M, Marmot MG, Miki T, Saleheen D, Chandak GR, Coresh J, Navis G, Salomaa V, Han BG, Zhu X, Kooner JS, Melander O, Ridker PM, Bandinelli S, Gyllensten UB, Wright AF, Wilson JF, Ferrucci L, Farrall M, Tuomilehto J, Pramstaller PP, Elosua R, Soranzo N, Sijbrands EJ, Altshuler D, Loos RJ, Shuldiner AR, Gieger C, Meneton P, Uitterlinden AG, Wareham NJ, Gudnason V, Rotter JI, Rettig R, Uda M, Strachan DP, Witteman JC, Hartikainen AL, Beckmann JS, Boerwinkle E, Vasan RS, Boehnke M, Larson MG, Jarvelin MR, Psaty BM, Abecasis GR, Chakravarti A, Elliott P, van Duijn CM, Newton-Cheh C, Levy D, Caulfield MJ, Johnson T consortium CA, Consortium CK, KidneyGen C, EchoGen c, consortium C-H. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vicente J, Johannesen L, Hosseini M, Mason JW, Sager PT, Pueyo E, Strauss DG. Electrocardiographic Biomarkers for Detection of Drug-Induced Late Sodium Current Block. PLoS One. 2016;11:e0163619. doi: 10.1371/journal.pone.0163619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woosley RL, Heise CW, Romero KA. QTdrugs List. AZCERT, Inc; 1822 Innovation Park Dr., Oro Valley, AZ 85755: www.CredibleMeds.org. [Accession Date November 1, 2016] [Google Scholar]
- 19.Moss AJ. Long QT Syndrome. Jama. 2003;289:2041–2044. doi: 10.1001/jama.289.16.2041. [DOI] [PubMed] [Google Scholar]
- 20.Jamshidi Y, Nolte IM, Dalageorgou C, Zheng D, Johnson T, Bastiaenen R, Ruddy S, Talbott D, Norris KJ, Snieder H, George AL, Marshall V, Shakir S, Kannankeril PJ, Munroe PB, Camm AJ, Jeffery S, Roden DM, Behr ER. Common variation in the NOS1AP gene is associated with drug-induced QT prolongation and ventricular arrhythmia. J Am Coll Cardiol. 2012;60:841–850. doi: 10.1016/j.jacc.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaab S, Crawford DC, Sinner MF, Behr ER, Kannankeril PJ, Wilde AA, Bezzina CR, Schulze-Bahr E, Guicheney P, Bishopric NH, Myerburg RJ, Schott JJ, Pfeufer A, Beckmann BM, Martens E, Zhang T, Stallmeyer B, Zumhagen S, Denjoy I, Bardai A, Van Gelder IC, Jamshidi Y, Dalageorgou C, Marshall V, Jeffery S, Shakir S, Camm AJ, Steinbeck G, Perz S, Lichtner P, Meitinger T, Peters A, Wichmann HE, Ingram C, Bradford Y, Carter S, Norris K, Ritchie MD, George AL, Jr, Roden DM. A large candidate gene survey identifies the KCNE1 D85N polymorphism as a possible modulator of drug-induced torsades de pointes. Circ Cardiovasc Genet. 2012;5:91–99. doi: 10.1161/CIRCGENETICS.111.960930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crotti L, Lundquist AL, Insolia R, Pedrazzini M, Ferrandi C, De Ferrari GM, Vicentini A, Yang P, Roden DM, George AL, Jr, Schwartz PJ. KCNH2-K897T is a genetic modifier of latent congenital long-QT syndrome. Circulation. 2005;112:1251–1258. doi: 10.1161/CIRCULATIONAHA.105.549071. [DOI] [PubMed] [Google Scholar]
- 23.Crotti L, Monti MC, Insolia R, Peljto A, Goosen A, Brink PA, Greenberg DA, Schwartz PJ, George AL., Jr NOS1AP is a genetic modifier of the long-QT syndrome. Circulation. 2009;120:1657–1663. doi: 10.1161/CIRCULATIONAHA.109.879643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomas M, Napolitano C, De Giuli L, Bloise R, Subirana I, Malovini A, Bellazzi R, Arking DE, Marban E, Chakravarti A, Spooner PM, Priori SG. Polymorphisms in the NOS1AP gene modulate QT interval duration and risk of arrhythmias in the long QT syndrome. J Am Coll Cardiol. 2010;55:2745–2752. doi: 10.1016/j.jacc.2009.12.065. [DOI] [PubMed] [Google Scholar]
- 25.Lazzara R. Antiarrhythmic drugs and torsade de pointes. Eur Heart J. 1993;14(Suppl H):88–92. doi: 10.1093/eurheartj/14.suppl_h.88. [DOI] [PubMed] [Google Scholar]
- 26.Kannankeril PJ, Roden DM, Norris KJ, Whalen SP, George AL, Jr, Murray KT. Genetic susceptibility to acquired long QT syndrome: pharmacologic challenge in first-degree relatives. Heart Rhythm. 2005;2:134–140. doi: 10.1016/j.hrthm.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 27.Roden DM. Long QT syndrome: reduced repolarization reserve and the genetic link. J Intern Med. 2006;259:59–69. doi: 10.1111/j.1365-2796.2005.01589.x. [DOI] [PubMed] [Google Scholar]
- 28.Roden DM, Abraham RL. Refining repolarization reserve. Heart rhythm : the official journal of the Heart Rhythm Society. 2011;8:1756–1757. doi: 10.1016/j.hrthm.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaab S, Hinterseer M, Nabauer M, Steinbeck G. Sotalol testing unmasks altered repolarization in patients with suspected acquired long-QT-syndrome--a case-control pilot study using i.v. sotalol. Eur Heart Journal. 2003;24:649–657. doi: 10.1016/s0195-668x(02)00806-0. [DOI] [PubMed] [Google Scholar]
- 30.Paulussen AD, Gilissen RA, Armstrong M, Doevendans PA, Verhasselt P, Smeets HJ, Schulze-Bahr E, Haverkamp W, Breithardt G, Cohen N, Aerssens J. Genetic variations of KCNQ1, KCNH2, SCN5A, KCNE1, and KCNE2 in drug-induced long QT syndrome patients. J Mol Med (Berl) 2004;82:182–188. doi: 10.1007/s00109-003-0522-z. [DOI] [PubMed] [Google Scholar]
- 31.Itoh H, Sakaguchi T, Ding WG, Watanabe E, Watanabe I, Nishio Y, Makiyama T, Ohno S, Akao M, Higashi Y, Zenda N, Kubota T, Mori C, Okajima K, Haruna T, Miyamoto A, Kawamura M, Ishida K, Nagaoka I, Oka Y, Nakazawa Y, Yao T, Jo H, Sugimoto Y, Ashihara T, Hayashi H, Ito M, Imoto K, Matsuura H, Horie M. Latent genetic backgrounds and molecular pathogenesis in drug-induced long-QT syndrome. Circ Arrhythm Electrophysiol. 2009;2:511–523. doi: 10.1161/CIRCEP.109.862649. [DOI] [PubMed] [Google Scholar]
- 32.Weeke P, Mosley JD, Hanna D, Delaney JT, Shaffer C, Wells QS, Van Driest S, Karnes JH, Ingram C, Guo Y, Shyr Y, Norris K, Kannankeril PJ, Ramirez AH, Smith JD, Mardis ER, Nickerson D, George AL, Jr, Roden DM. Exome sequencing implicates an increased burden of rare potassium channel variants in the risk of drug-induced long QT interval syndrome. J Am Coll Cardiol. 2014;63:1430–1437. doi: 10.1016/j.jacc.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.