Summary
Astrocytes are active partners in neural information processing [1, 2]. However, the roles of astrocytes in regulating behavior remain unclear [3, 4]. Because astrocytes have persistent circadian clock gene expression and ATP release in vitro [5–8], we hypothesized that they regulate daily rhythms in neurons and behavior. Here we demonstrated that daily rhythms in astrocytes within the mammalian master circadian pacemaker, the suprachiasmatic nucleus (SCN), determine the period of wheel-running activity. Ablating the essential clock gene, Bmal1, specifically in SCN astrocytes lengthened the circadian period of clock gene expression in the SCN and in locomotor behavior. Similarly, excision of the short-period CK1ε tau mutation specifically from SCN astrocytes resulted in lengthened rhythms in the SCN and behavior. These results indicate that astrocytes within the SCN communicate to neurons to determine circadian rhythms in physiology and in rest-activity.
Keywords: Aldh1l1, astrocyte, SCN, glia, circadian oscillator
eTOC blurb
Tso et. al. show that SCN astrocytes are synchronized circadian oscillators just like neurons. Both loss of rhythm or lengthened period in SCN astrocytes led to an increase in period in SCN neuronal and daily activity rhythms, demonstrating that astrocytes contribute to the determination of SCN output rhythm.
Results
Aldh1L1-Cre reliably labels astrocytes within the SCN
We targeted astrocytes using Aldh1L1-Cre BAC transgenic mice [9, 10] because Aldh1L1 expression in the brain is high, broad and specific to astrocytes.[9] To test the specificity and expression pattern of Aldh1L1 in the SCN, we crossed Aldh1L1-Cre/+ mice to mice carrying Cre-activated nuclear GFP transgene (LSL-GFPNLS; Supp. Table 2 details the genotypes and treatments for all experiments). Immunofluorescence staining showed that Aldh1L1-GFPNLS labels 10.9 ± 1.1 % of SCN cells (n = 6 mice, one brain section each, mean ± S.E.M.). The astrocyte marker, glial fibrillary acidic protein (GFAP), labeled 96.4 ± 1.7 % of the Aldh1L1-GFPNLS- positive cells (Figure 1a; n = 1 section each from 4 mice). None of the Aldh1L1-GFPNLS cells expressed the neuronal markers, FOX2 (a homolog of NeuN[11, 12]; Figure 1b; n = 3 brains, 762 FOX2+ cells counted) or arginine vasopressin, AVP (Supp. Fig. 1; n = 6 brains, 220 AVP+ cells counted). In summary, the Aldh1L1-Cre mouse line provides one way to specifically label astrocytes in the SCN.
Figure 1. Astrocytes targeted in the SCN with the Aldh1L1-Cre/+ mouse line.
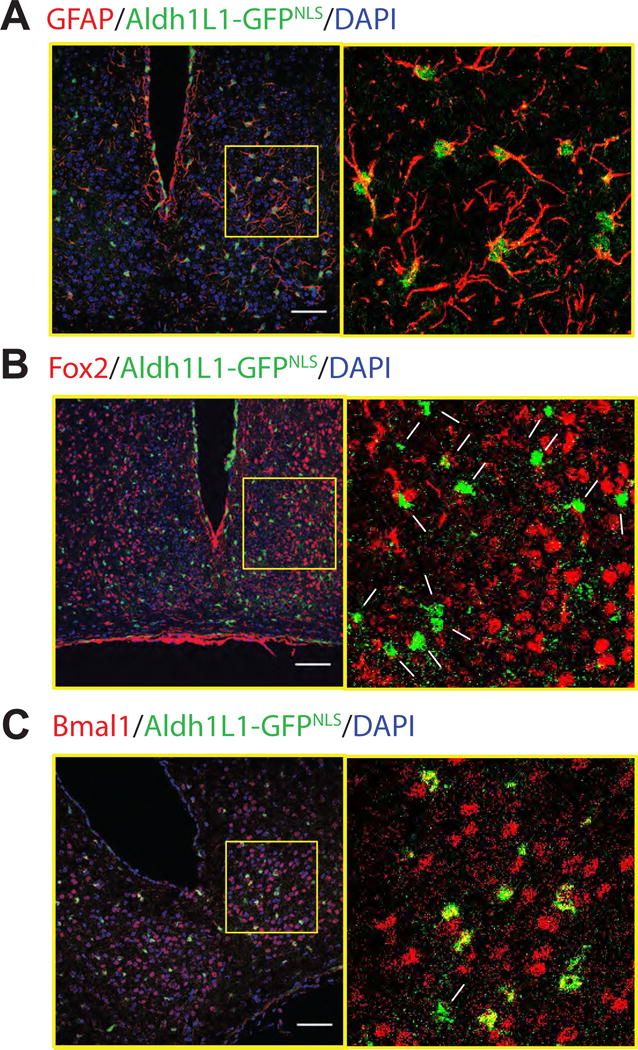
Astrocyte nuclei in the SCN were labeled in green by Cre-mediated recombination of Aldh1L1-Cre/+;LSL-GFPNLS/+ mice. Coronal brain sections were immunostained for an astroglial marker, GFAP (A), a neuronal marker, FOX2 (B) or a circadian clock protein, BMAL1(C) in red and all nuclei were counter stained with DAPI. Cells within the yellow boxes (left) were magnified (right). Filled arrowheads indicate double-labeled cells and arrows point to cells that showed no red and green colocalization. Note that Aldh1L1-positive cells reliably express the astrocyte marker, GFAP, and the circadian clock protein, BMAL1, but not the neuronal marker, FOX2. Scale bar = 75 μm. See also Figure S1.
Astrocytes function as circadian oscillators in the SCN
Previous work has shown that cortical astrocyte cultures and an astrocyte-like cell line (SCN2.2) have circadian rhythms in clock gene expression and release of ATP, a potent gliotransmitter. [5–8] To determine whether SCN astrocytes possess daily rhythms in clock genes, we stained Aldh1L1-GFPNLS brain sections for BMAL1 (also called ARNTL or MOP3). We found that 85.9 ± 5.6 % (n = 6 mice perfused at ZT 2) of Aldh1L1 cells in the SCN expressed BMAL1 (Figure 1c). We next infected Aldh1L1-Cre/+ organotypic SCN slices with a novel adeno-associated virus carrying a Cre-activated bioluminescent reporter of Bmal1 transcription (AAV2/10-Bmal1ext-DIO-luc; Figure 2a). These Aldh1L1-Bmal1luc SCN slices were rhythmic with a circadian period (23.6 ± 0.2 h, n = 6; Figure 2c) and ~10-fold higher light emission than a Cre(−) littermate SCN infected with the same virus (Suppl. Table. 1). For comparison, we found similar amplitude Bmal1-luc rhythms in SCN vasoactive intestinal polypeptide (VIP) neurons (Suppl. Table 1). To measure coordination among SCN astrocytes, we imaged Aldh1L1-Bmal1luc with an ultracooled CCD camera (n = 3 SCN). We reliably detected astrocyte-shaped cells expressing the reporter of Bmal1 activity throughout the SCN (Figure 2b and data not shown). Bioluminescence in 72.8± 4.0% of cell-sized regions of interest (ROIs) was circadian and peaked at a similar time each day (Figure 2d; p < 0.0001, Rayleigh’s Test, mean vector length = 0.85±0.02). The remaining ROIs were considered arrhythmic. We conclude that astrocytes function as synchronous circadian oscillators within the SCN.
Figure 2. SCN astrocytes are functional circadian oscillators.
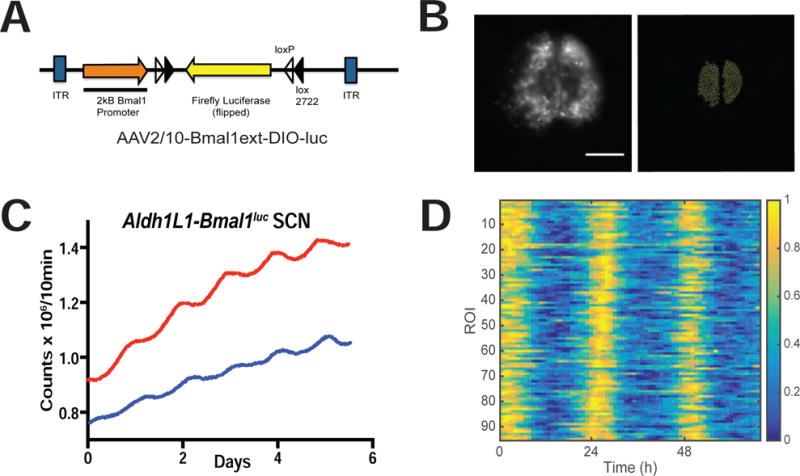
(A) Schematic of AAV-Bmal1ext-DIO-luc vector that provides a real-time, cell-type specific report of Bmal1 transcription. (B) (Left) Representative frame from a movie of bioluminescence recorded from an Aldh1L1-Bmal1luc SCN slice. Note the glowing astroglial cells throughout the bilateral SCN. Scale bar = 400μm (Right) Cell-sized regions of interest (ROIs) were used to track Bmal1 expression from astrocytes within the SCN. (C) Representative bioluminescence traces from PMT recording of two Aldh1L1-Bmal1luc SCN slices. (D) Raster plot of Bmal1 reporter expression across a representative Aldh1L1-Bmal1luc SCN slice from (B). The bioluminescence in each ROI peaked at approximately the same time daily over the three days of recording with an ultracooled CCD camera. Bioluminescence for each ROI was normalized to its maximum and pseudocolored (color bar at right). See also Table S1.
Loss of daily rhythms in SCN astrocytes lengthens circadian period in the SCN and in behavior
Using a recently developed gene editing technique [13, 14], we ablated the Bmal1 gene from targeted cells. In this strategy, short guide RNAs (sgRNAs) designed to direct the disruption of the Bmal1 gene were delivered to cells expressing the CRISPR machinery under the control of cell-type specific promoters. As a proof-of-concept, we injected a mixture of AAV carrying ubiquitously expressed pCBh-Cre and sgRNA against either LacZ (control, sgLacZ) or Bmal1 (sgBmal1; E1 and E3 denote independent sequences targeting exons 1 or 3 of the Bmal1 gene) into the SCN of LSL-Cas9/+;PER2::luc/+ mice. We found that Cas9 was subsequently expressed throughout the SCN from a GFP fusion protein co-expressed with Cas9 (data not shown). Animals that received sgBmal1 lost daily rhythms in both behavior (n= 2 of 2 mice, one for each Bmal1 sgRNA vector) and whole SCN PER2::luc (n= 12 of 12 SCN, n = 6 for each Bmal1 sgRNA vector) as predicted for global Bmal1 knockouts [15] while those that received sgLacZ (n=2 of 2 mice and 5 of 5 SCN) remained circadian (Suppl. Fig. 2).
To test the necessity of astrocytes’ molecular clocks in behavioral rhythms, we injected AAV carrying sgBmal1 into the SCN of Aldh1L1-Cre/+; LSL-Cas9/+ mice (Astro-Bmal1−/−) and Cre(−) littermate controls (Figure 3a). Before AAV injection, LSL-Cas9/+ and Aldh1L1-Cre/+; LSL-Cas9/+ mice showed similar wheel-running periods in constant darkness (23.79 ± 0.05h, n = 9 and 23.75 ± 0.08 h, n = 12, Student’s t-test, n.s.). Strikingly, CRISPR-mediated loss of Bmal1 in SCN astrocytes significantly lengthened their locomotor periodicity (Figure 3b,e; Controls vs. Aldh1L1-Bmal1−/−: sgBmal1_E1: 23.76±0.12h, n = 5 vs. 24.22±0.10h, n = 6; sgBmal1_E3: 23.75±0.08 h, n = 4 vs. 24.38±0.13 h, n = 3; p < 0.01; 1-way ANOVA with Sidak multiple comparison test). Loss of Bmal1 in SCN astrocytes was confirmed by immunohistochemistry (Figures 3c and 3d; 2.5±1.2% of cells were BMAL1- and Aldh1L1-positive cells and nearly 97.7±0.1% of Aldh1L1-Cas9 cells were GFAP positive; Bmal1: n = 9; GFAP: n = 6). In Aldh1L1-Bmal1−/− SCN, the period of PER2::luc rhythms was also significantly lengthened compared to Cre(−) littermate controls (Figures 3f and g; 24.63±0.14h, n = 6 vs. 25.82±0.41h, n = 5, p< 0.05, Student’s t-test). Using a CRISPR-independent approach, we again found period lengthening of circadian rhythms in flox Bmal1 mice injected with AAV8-GFAP-Cre-GFP compared to flox Bmal1 mice injected with a control AAV8-GFAP-GFP (23.7±0.09h, n = 5 vs 23.07±0.07h, n=7; p<0.01, t-test),We conclude that loss of Bmal1 in SCN astrocytes lengthens circadian rhythms in SCN neurons and behavior.
Figure 3. Loss of Bmal1 in SCN astrocytes lengthens circadian period in vitro and in vivo.
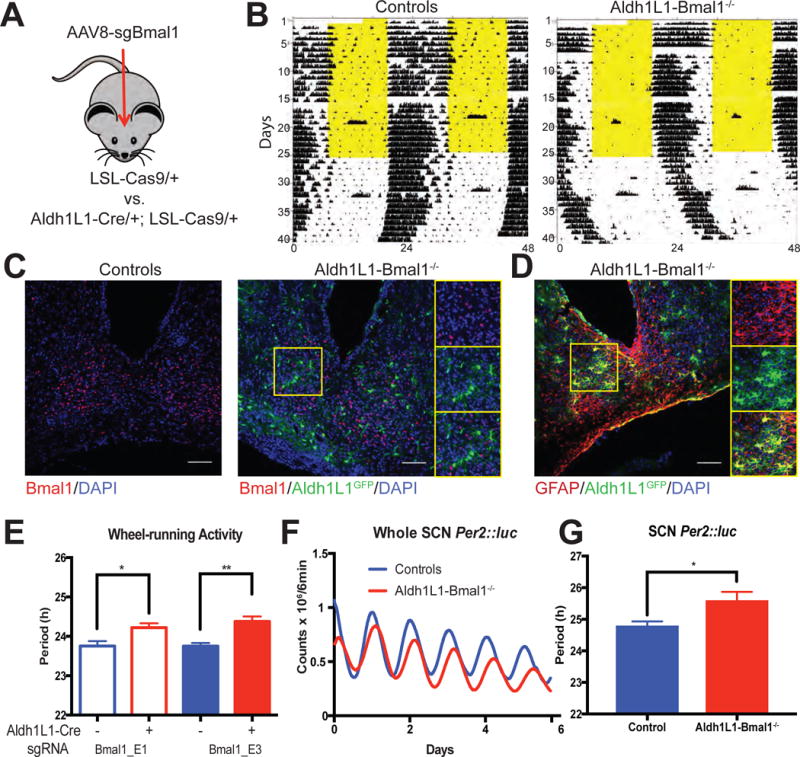
(A) Schematic showing how CRISPR was used to delete the Bmal1 gene in SCN astroglia. (B) Representative locomotor activity of a LSL-Cas9/+ littermate control and an Aldh1L1-Cre/+; LSL-Cas9/+ mouse both injected with AAV-sgBmal1_E3 into bilateral SCN. Each line shows wheel running (black ticks) over two days with the second day’s data replotted on the line below. The mice were less active in the light (yellow) in the 12h: 12h light:dark cycle of the first 25 days of recording and showed free-running rhythms in constant darkness. (C) Coronal brain sections immunostained for Bmal1 (red) and GFP (green) with insets showing the abundant BMAL1 staining in the control and lack of BMAL1 in Aldh1L1 cells in mouse with targeted deletion. (D) GFAP staining (red) reliably colocalized with Cre-activated GFP (green) in this representative SCN of an Aldh1L1-Cas9 mouse. (E) Bmal1 ablation in Aldh1L1 cells by either of two independent guide RNAs (E1 or E3) in the SCN increased the circadian period of locomotor activity compared to Cre(−) controls. * : p< 0.05; ** : p < 0.01; 1-way ANOVA with Sidak multiple comparison test. (F) Representative PER2::luc traces from the cultured SCN of a Aldh1L1-Cre/+; LSL-Cas9/+; PER2::luc/+ mouse (red) and a LSL-Cas9/+; PER2::luc/+ littermate control (blue) both injected with sgBmal1_E3 into bilateral SCN in vivo. (G) Loss of Bmal1 lengthened circadian period in the isolated SCN. * : p < 0.05; t-test. Scale bars = 75 μm. Error bars: mean ± sem. See also figure S2.
Circadian period of SCN astrocytes regulates circadian period of the SCN and of locomotor behavior
Because BMAL1 is a transcription factor with circadian and non-circadian functions, we utilized the CK1ε tau mutation as an independent method to conditionally manipulate daily timing in astrocytes. This point mutation in exon 4 of the CK1ε gene shortens the period of wheel-running activity under constant darkness (CK1εtau/tau: ~20h, CK1εtau/+: ~22h)[16, 17]. The phenotype can be reversed when exon 4 is floxed out by Cre recombinase so that CK1ε+/− or CK1ε−/− mice have a 24h period [16]. We found that Cre (−) littermates (termed CK1εtau/+) showed a ~22h period as previously reported while Aldh1L1-Cre/+; CK1εtau/+; PER2::luc/+ (referred to as Aldh1L1-CK1εtau/+) animals showed a significantly lengthened period (Figure 4a–b; 22.21 ± 0.13 h, n = 9 vs. 23.42 ± 0.10 h, n = 11, Student’s t-test, p < 0.0001). The SCN of Aldh1L1- CK1ε tau/+ mice also showed lengthened PER2::luc rhythms (Figure 4c–d; 22.1 ± 0.18 h vs. 26.06 ± 0.68 h, n = 5 SCN per group, Student’s t-test, p < 0.01), indicating the behavioral period directly results from the lengthened rhythm within the SCN.
Figure 4. Loss of CK1ε in SCN astrocytes lengthens circadian period in vivo and in vitro.
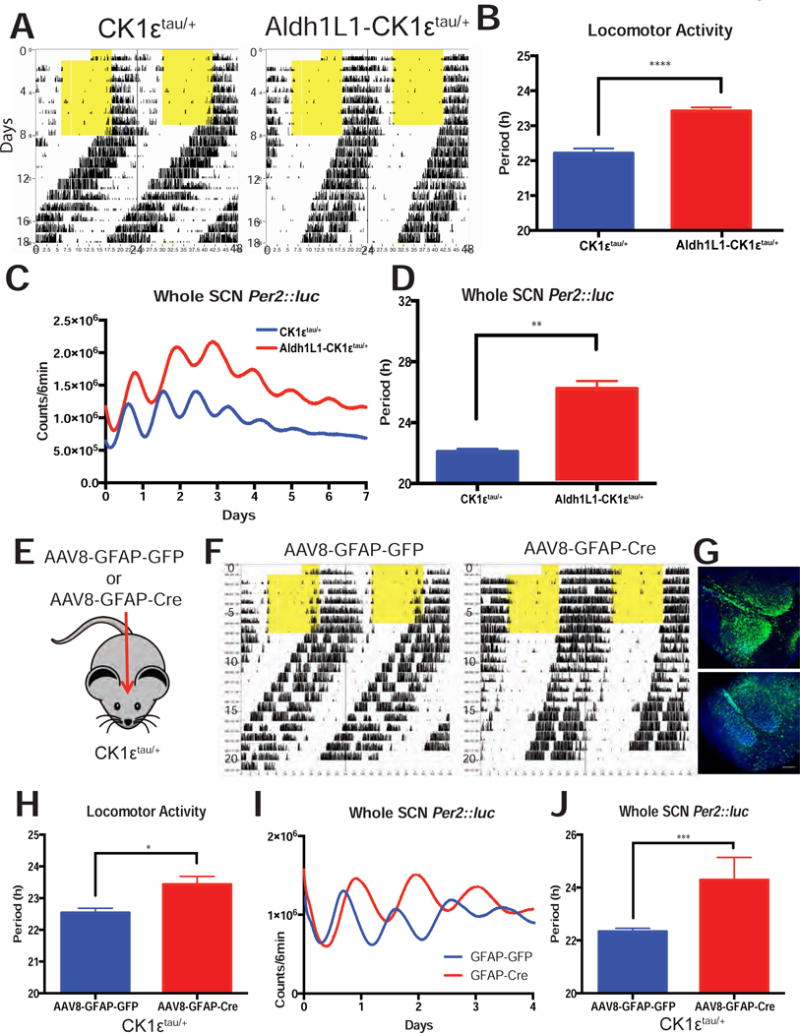
(A) Representative actograms of Aldh1L1- CK1εtau/+ and CK1εtau/+ littermates showing how locomotor activity free-ran in constant darkness with (B) a longer circadian period in mice where the CK1εtau/+ mutation was removed from astrocytes (p < 0.0001, t-test). (C) Representative PER2::luc recordings from the isolated SCN of Aldh1L1- CK1εtau/+ and CK1εtau/+ littermates with (d) a longer circadian period in SCN where the CK1εtau/+ mutation was removed from astrocytes (p < 0.01, t-test). (E) Schematic of a second method to change circadian timing in astrocytes using GFAP AAV injection to CK1εtau/+ SCN in vivo. (F) Representative actograms of two CK1εtau/+ mice injected with either AAV8-GFAP-GFP or AAV8-GFAP-Cre-GFP with (G) successful viral targeting to the SCN. (H) The circadian period of locomotor activity of AAV8-GFAP-Cre injected CK1εtau/+ mice was approximately 1 h longer than controls (p < 0.05, t-test). (I) Representative whole SCN PER2::luc recording from CK1εtau/+ animals injected with either AAV8-GFAP-GFP or AAV8-GFAP-Cre-GFP. (J) Period of PER2::luc recordings from AAV8-GFAP-Cre; CK1εtau/+ SCN was approximately 2 h longer than controls (p < 0.001, t-test). Error bars: mean ± sem.
We next infected CK1ε tau/+ mice with a virus (AAV8-GFAP-Cre-GFP) to remove the tau mutation specifically in GFAP(+) astrocytes of the SCN (Figure 4e). Animals infected with the GFAP-Cre virus, like Aldh1L1- CK1εtau/+ mice, had a lengthened period in locomotor activity (Figures 4f,h; 22.54 ± 0.14 h vs. 23.43 ± 0.25 h, n = 4 per group, Student’s t-test, p < 0.05) and in isolated SCN PER2::luc rhythms (Figures 4I–J; 22.34 ± 0.12 h vs. 25.08 ± 0.41 h, n=4 per group, Student’s t-test, p < 0.001). AAV infection was confirmed by GFP fluorescence in the SCN (Figure 4g). Taken together, these results show that two independent methods that increased the period of SCN astrocytes relative to the rest of the body lengthened the periods of SCN and behavioral rhythms.
Discussion
Here, three independent manipulations of clock genes in astrocytes similarly changed daily rhythms in the SCN and in behavior. Although previous publications implicated astrocytes in SCN function (reviewed in [18, 19]), we lacked the tools to target these glial cells directly. Using promoters for GFAP and Aldh1L1 to drive reporters and manipulate clock genes, we were able to control astrocytes specifically within the SCN in vivo and in vitro. A conditional, real-time reporter of Bmal1 transcription revealed synchronous circadian rhythms in astrocytes within SCN slice cultures. This is consistent with the reported rhythms in extracellular ATP attributed to astroglia in the isolated SCN [6, 7], but differs from a study that did not find daily cycles in Per1-luc expression in SCN astrocytes [20]. That report was based on an absence of immunohistochemical evidence for colocalization of GFAP and luciferase and may have been confounded by low or anti-phase levels of the two proteins. Furthermore, circadian rhythms have been reported in Per1-luc, PER2::luc, Bmal1-luc and ATP release in cultured cortical astrocytes [5, 8] and in PER levels in Drosophila glial cells. [21, 22] We conclude that astrocytes are functional circadian oscillators within the SCN. It will be important to investigate phase differences between daily rhythms in SCN neurons and astrocytes and whether oscillations of SCN neuronal firing[23], neuropeptide release [24] or metabolic demand [25] are coupled to astrocyte rhythms.
In this study, we found evidence that circadian clocks in SCN astrocytes, like in SCN neurons, are modulators of daily rhythms in the SCN and behavior. Loss of rhythm in SCN astrocytes through Bmal1 deletion resulted in lengthened circadian period of rest-activity rhythms. A similar 1-hour increase in period was recently reported for mice in which AVP neurons had Bmal1 knocked out[26]. Thus, in contrast to the arrhythmicity produced by ablation of the clock in many SCN neurons[27, 28], Bmal1-deletion in a small proportion of SCN cells appears to change the period of the SCN and behavior. Specifically, loss of rhythmicity in the 20% of SCN cells that express AVP or the 10% cells that express Aldh1L1 or GFAP suffices to lengthen SCN periodicity. This is consistent with the results of genetic chimera mice where the circadian phenotype scaled with the fraction of SCN cells homozygous for the dominant negative form of CLOCK[29]. We conclude that astrocytes likely play as important a role as any neuronal cell class in circadian timekeeping in the SCN and behavior.
Are circadian rhythms in astrocytes sufficient or necessary for daily rhythms in the SCN or behavior? Several recent studies have found that Bmal1-deletion or PER2 overexpression in SCN neurons abolished circadian rhythms in locomotion[28, 30, 31]. We therefore posit that having a molecular clock in astrocytes is not sufficient to sustain behavioral rhythms. Our finding that mice remain circadian after loss of Bmal1 in astrocytes also argues against the necessity of an astrocyte circadian clock. However, increasing the intrinsic period of SCN astrocytes by deletion of CK1ε reliably lengthens behavioral period. This is a striking result because increasing period in a subpopulation of SCN neurons does not necessarily drive behavioral rhythms to longer periods. For example, using drivers that include SCN AVP neurons (AVP, NMS or Scg2) to alter clock gene expression can increase locomotor period [27, 31, 32], but overexpression of ClockΔ19 in SCN VIP neurons (~10% of SCN neurons) does not lengthen behavioral period[31]. Furthermore, manipulations that would be predicted to increase cell-intrinsic period by at least 4h, even when targeted to 40% of SCN cells (e.g. with Drd1a) also tend to have smaller and less reliable effects on behavioral period [33]. Remarkably, we found that deletion of CK1ε in astrocytes which has the cell-autonomous effect of increasing period by about 2 h, sufficed to increase the behavioral period by about 1.5 h. Thus, our data argues that clocks in SCN astrocytes likely play a more important role than some SCN neurons in determining behavioral periodicity.
Our findings also highlighted that glia in mammals and Drosophila may play fundamentally different roles in their circadian circuits. In contrast to our findings in SCN astrocytes, glial-specific knockdown of the essential clock gene, Per, did not change PER expression in neurons or locomotor activity rhythms in flies [21, 34]. Since PER expression was absent from glia cells in those flies, it is possible that clock-less astrocytes in the fly brain can be driven to oscillate by circadian neurons so that behavior remains intact. Alternatively, it may be that loss of PER in flies is not equivalent to loss of Bmal1 in mice. The consequence of the loss of the Bmal1 ortholog, Cyc, is yet to be tested in glial cells in flies. Notably, in flies, loss-of-function mutation in the glial-specific, clock-controlled gene, ebony [35] or glial-specific perturbations of membrane potential, vesicular release or intracellular Ca2+ storage can lead to behavioral arrhythmicity. This suggests that dysregulation of glial physiology can interfere with daily rhythms in physiology and behavior in flies. Future studies should test whether and which cellular functions of SCN astrocytes are necessary for rhythmicity in the SCN and behavior.
Since our experiments ablating BMAL1 or rescuing the tau mutation in SCN astrocytes all resulted in a remarkably similar period lengthening in vitro and in vivo, it is likely that any genetic or environmental disruptions of daily rhythms in astroglia will alter daily rhythms in behavior. That is, it is unlikely that the period lengthening phenotype reflects functions of BMAL1 and CK1ε outside of circadian rhythms. This is supported by our prediction and finding that removal of the CK1ε tau mutation from SCN astrocytes would lengthen the period close to 24h. Thus our data strongly argue that the astrocyte circadian clock regulates daily behaviors.
Why do genetic manipulations predicted to abolish or increase period in SCN astrocytes both result in a similar long period in the SCN and behavior? It could be that loss of BMAL1 or CK1ε similarly impacts a signal (or signals) from astrocytes (e.g. a diffusible factor, structural change or a metabolic precursor) that influences daily rhythms in SCN neurons. Based our data, this signal is not normally necessary for rhythmicity in neurons, but its levels are likely clock-controlled and accelerate the period of the neuronal clock either directly or by modulating neuron-neuron interactions. Several testable predictions can be made with this proposed model: (1) arrhythmicity in astrocytes caused by disruption of either the positive (e.g. BMAL1 or CLOCK) or negative limb (e.g. PERs or CRYs) of the circadian transcription-translation feedback loop will always lengthen period; (2) combining SCN astrocytes with intrinsically different circadian periods with WT neurons will draw behavioral period towards the astrocyte period; (3) blocking signaling from SCN neurons to astrocytes will alter daily behavior and the phase relationship between rhythms in SCN neurons and astrocytes and/or between SCN neurons.
In summary, we found that SCN astrocytes have persistent daily rhythms in gene expression that modulate the period of SCN and rest-activity rhythms. These daily rhythms intrinsic to astrocytes may also contribute to other behaviors including sleep. [4, 36]
Experimental Procedures
Animals
Mice were housed in 12h:12h light:dark cycle with ad libitum food and water unless otherwise stated. Transgenic mice used here were Aldh1L1-Cre/+ (Jax: 023748, founders were a gift from D. Rowitch, HHMI/UCSF), LSL-Cas9-eGFP (Jax: 024858), LSL-GFPNLS (Jax: 008516), CK1εtau/tau (gift from M. Butler, OSHU, [16]), VIP-IRES-Cre (Jax: 010908), Bmal1f/f (Jax: 007668) and PER2::luc (gift from J. Takahashi, HHMI/UT Southwestern). All procedures were approved by the Animal Care and Use Committee of Washington University and conformed to US National Institutes of Health guidelines.
Viral Vectors
We produced viruses carrying cell-type specific Cre drivers or guide RNAs to delete Bmal1 in targeted cells. The pCBh-Cre construct was modified from AAV:ITR-U6-sgRNA(backbone)-pCBh-Cre-WPRE-hGHpA-ITR (Feng Zhang, Harvard, purchased from Addgene) by replacing the Cre sequence with mCherry-NLS from pcDNA3.1-Peredox plasmid (Gary Yellen, Harvard) to facilitate tracking of the virus (hereafter referred as sgBB). The following DNA sequences were inserted at the designated site of the sgRNA scaffold of sgBB:
|
| |
| Name | Sense Sequence |
|
| |
| AAV:ITR-U6-sgLacZ-pCBh-mCherry-NLS-WPRE-hGHpA-ITR | 5′-TGCGAATACGCCCACGCGAT |
|
| |
| AAV:ITR-U6-sgBmal1_E1-pCBh-mCherry-NLS-WPRE-hGHpA-ITR | 5′-GTGTGGACTGCAATCGCAAG |
|
| |
| AAV:ITR-U6-sgBmal1_E3_pCBh-mCherry-NLS-WPRE-hGHpA-ITR | 5′-GTAGATAAACTCACCGTGCTA |
|
| |
All experimental sgRNAs had a score >=89 from the CRISPR design tool (http://crispr.mit.edu/) with no apparent off-target sites and were designed to target genomic DNA sequences 5′ of bHLH and PAS domains of the Bmal1 gene. AAV2/8 sgRNA and pCBh-Cre vectors were generated by Hope Center Viral Vector Core at Washington University.
AAV2/10 Bmal1-DIO-luc was made by replacing Bmal1 cDNA in AAV2/10-Bmal1-DIO-Bmal1 [26]and generated by the Hope Center Viral Vector Core. AAV8-GFAP-GFP and AAV8-GFAP-Cre-GFP were from University of North Carolina Viral Vector Core.
AAV Infection
We injected viruses (0.4μl/side) into the bilateral SCN in vivo (Coordinates (in mm) from Bregma: A/P 0 M/L ±0.2 D/V −5.6) using a Hamilton Neuros Syringe. It should be noted that under this injection protocol, some mCherry-NLS expression from the sgBmal1 AAV (Fig. 3) was observed in neighboring hypothalamic areas along the third ventricle. Therefore we cannot rule out loss of Bmal1 in some extra-SCN astrocytes. However, in the independent experiment using GFAP-Cre-GFP or GFAP-GFP AAVs, GFP expression was largely restricted to SCN (for example Fig. 4g) indicating that changes to circadian timing in SCN astrocytes suffices to produce a circadian phenotype. We also infected P5–P6 SCN slices (250μm coronal) after 2 days in vitro with 1μl of AAV added directly on top of the SCN slice. (Fig.2) The mice and the slices were allowed at least 2 weeks for recovery and viral expression prior to data collection.
Supplementary Material
Highlights.
SCN astrocytes are functional, synchronized circadian oscillators
Loss of Bmal1 in SCN astrocytes lengthened the period of SCN neurons and behavior
Reversal of the tau mutation specifically in SCN astrocytes also lengthened period
Acknowledgments
We thank Dr. Andrew Liu for providing the Bmal1:luc NIH 3T3 cell line that was used in preliminary testing of the sgRNA constructs. We thank Drs. Erik Musiek and Hal Gainer for sharing antibodies. We thank Herzog lab members for discussions and S. Allu for assistance in data analysis. Bmal1 sgRNA AAV vectors were generated by the Hope Center Viral Vector Core with support from a Hope Center Just-in-time Grant. This work is supported by NINDS grant 095367.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
C.F.T. and E.D.H designed research. C.F.T., T.S., A.G. and T.P. performed research. C.F.T., A.G.,T.P. and E.D.H. analyzed data. C.F.T. and E.D.H. wrote the paper. AAV2/10-Bmal1-DIO-luc is an unpublished reagent generated by M. M.
References
- 1.Chung WS, Welsh CA, Barres BA, Stevens B. Do glia drive synaptic and cognitive impairment in disease? Nat Neurosci. 2015;18:1539–1545. doi: 10.1038/nn.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuchero JB, Barres BA. Glia in mammalian development and disease. Development. 2015;142:3805–3809. doi: 10.1242/dev.129304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci. 2016;19:182–189. doi: 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- 4.Haydon PG, Nedergaard M. How do astrocytes participate in neural plasticity? Cold Spring Harb Perspect Biol. 2014;7:a020438. doi: 10.1101/cshperspect.a020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marpegan L, Swanstrom AE, Chung K, Simon T, Haydon PG, Khan SK, Liu AC, Herzog ED, Beaule C. Circadian regulation of ATP release in astrocytes. J Neurosci. 2011;31:8342–8350. doi: 10.1523/JNEUROSCI.6537-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkeen JF, Womac AD, Earnest DJ, Zoran MJ. Mitochondrial calcium signaling mediates rhythmic extracellular ATP accumulation in suprachiasmatic nucleus astrocytes. J Neurosci. 2011;31:8432–8440. doi: 10.1523/JNEUROSCI.6576-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Womac AD, Burkeen JF, Neuendorff N, Earnest DJ, Zoran MJ. Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes. Eur J Neurosci. 2009;30:869–876. doi: 10.1111/j.1460-9568.2009.06874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prolo LM, Takahashi JS, Herzog ED. Circadian rhythm generation and entrainment in astrocytes. J Neurosci. 2005;25:404–408. doi: 10.1523/JNEUROSCI.4133-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molofsky AV, Kelley KW, Tsai HH, Redmond SA, Chang SM, Madireddy L, Chan JR, Baranzini SE, Ullian EM, Rowitch DH. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature. 2014;509:189–194. doi: 10.1038/nature13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Underwood JG, Boutz PL, Dougherty JD, Stoilov P, Black DL. Homologues of the Caenorhabditis elegans Fox-1 protein are neuronal splicing regulators in mammals. Mol Cell Biol. 2005;25:10005–10016. doi: 10.1128/MCB.25.22.10005-10016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KK, Adelstein RS, Kawamoto S. Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. J Biol Chem. 2009;284:31052–31061. doi: 10.1074/jbc.M109.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, Dahlman JE, Parnas O, Eisenhaure TM, Jovanovic M, et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell. 2014;159:440–455. doi: 10.1016/j.cell.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, Sur M, Zhang F. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol. 2015;33:102–106. doi: 10.1038/nbt.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng QJ, Logunova L, Maywood ES, Gallego M, Lebiecki J, Brown TM, Sladek M, Semikhodskii AS, Glossop NR, Piggins HD, et al. Setting clock speed in mammals: the CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron. 2008;58:78–88. doi: 10.1016/j.neuron.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ralph MR, Menaker M. A mutation of the circadian system in golden hamsters. Science. 1988;241:1225–1227. doi: 10.1126/science.3413487. [DOI] [PubMed] [Google Scholar]
- 18.Evans JA. Collective timekeeping among cells of the master circadian clock. J Endocrinol. 2016;230:R27–49. doi: 10.1530/JOE-16-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson FR. Glial cell modulation of circadian rhythms. Glia. 2011;59:1341–1350. doi: 10.1002/glia.21097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- 21.Ng FS, Tangredi MM, Jackson FR. Glial cells physiologically modulate clock neurons and circadian behavior in a calcium-dependent manner. Curr Biol. 2011;21:625–634. doi: 10.1016/j.cub.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zerr DM, Hall JC, Rosbash M, Siwicki KK. Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J Neurosci. 1990;10:2749–2762. doi: 10.1523/JNEUROSCI.10-08-02749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz WJ, Gainer H. Suprachiasmatic nucleus: use of 14C-labeled deoxyglucose uptake as a functional marker. Science. 1977;197:1089–1091. doi: 10.1126/science.887940. [DOI] [PubMed] [Google Scholar]
- 26.Mieda M, Ono D, Hasegawa E, Okamoto H, Honma K, Honma S, Sakurai T. Cellular clocks in AVP neurons of the SCN are critical for interneuronal coupling regulating circadian behavior rhythm. Neuron. 2015;85:1103–1116. doi: 10.1016/j.neuron.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Hong HK, Chong JL, Song W, Song EJ, Jyawook AA, Schook AC, Ko CH, Takahashi JS. Inducible and reversible Clock gene expression in brain using the tTA system for the study of circadian behavior. PLoS Genet. 2007;3:e33. doi: 10.1371/journal.pgen.0030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izumo M, Pejchal M, Schook AC, Lange RP, Walisser JA, Sato TR, Wang X, Bradfield CA, Takahashi JS. Differential effects of light and feeding on circadian organization of peripheral clocks in a forebrain Bmal1 mutant. Elife. 2014;3 doi: 10.7554/eLife.04617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Low-Zeddies SS, Takahashi JS. Chimera analysis of the Clock mutation in mice shows that complex cellular integration determines circadian behavior. Cell. 2001;105:25–42. doi: 10.1016/s0092-8674(01)00294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen R, Schirmer A, Lee Y, Lee H, Kumar V, Yoo SH, Takahashi JS, Lee C. Rhythmic PER abundance defines a critical nodal point for negative feedback within the circadian clock mechanism. Mol Cell. 2009;36:417–430. doi: 10.1016/j.molcel.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee IT, Chang AS, Manandhar M, Shan Y, Fan J, Izumo M, Ikeda Y, Motoike T, Dixon S, Seinfeld JE, et al. Neuromedin s-producing neurons act as essential pacemakers in the suprachiasmatic nucleus to couple clock neurons and dictate circadian rhythms. Neuron. 2015;85:1086–1102. doi: 10.1016/j.neuron.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mieda M, Okamoto H, Sakurai T. Manipulating the Cellular Circadian Period of Arginine Vasopressin Neurons Alters the Behavioral Circadian Period. Curr Biol. 2016;26:2535–2542. doi: 10.1016/j.cub.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Smyllie NJ, Chesham JE, Hamnett R, Maywood ES, Hastings MH. Temporally chimeric mice reveal flexibility of circadian period-setting in the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 2016;113:3657–3662. doi: 10.1073/pnas.1511351113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson FR, Ng FS, Sengupta S, You S, Huang Y. Glial cell regulation of rhythmic behavior. Methods Enzymol. 2015;552:45–73. doi: 10.1016/bs.mie.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suh J, Jackson FR. Drosophila ebony activity is required in glia for the circadian regulation of locomotor activity. Neuron. 2007;55:435–447. doi: 10.1016/j.neuron.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira JF, Sardinha VM, Guerra-Gomes S, Araque A, Sousa N. Do stars govern our actions? Astrocyte involvement in rodent behavior. Trends Neurosci. 2015;38:535–549. doi: 10.1016/j.tins.2015.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


