Abstract
Obstructive sleep apnea (OSA) is highly prevalent but very frequently undiagnosed. OSA is an independent risk factor for depression and cognitive impairment/dementia. Herein the authors review studies in the literature pertinent to the effects of OSA on the cerebral microvascular and neurovascular systems and present a model to describe the key pathophysiologic mechanisms that may underlie the associations, including hypoperfusion, endothelial dysfunction, and neuroinflammation. Intermittent hypoxia plays a critical role in initiating and amplifying these pathologic processes. Hypoperfusion and impaired cerebral vasomotor reactivity lead to the development or progression of cerebral small vessel disease (C-SVD). Hypoxemia exacerbates these processes, resulting in white matter lesions, white matter integrity abnormalities, and gray matter loss. Blood–brain barrier (BBB) hyperpermeability and neuroinflammation lead to altered synaptic plasticity, neuronal damage, and worsening C-SVD. Thus, OSA may initiate or amplify the pathologic processes of C-SVD and BBB dysfunction, resulting in the development or exacerbation of depressive symptoms and cognitive deficits. Given the evidence that adequate treatment of OSA with continuous positive airway pressure improves depression and neurocognitive functions, it is important to identify OSA when assessing patients with depression or cognitive impairment. Whether treatment of OSA changes the deteriorating trajectory of elderly patients with already-diagnosed vascular depression and cognitive impairment/dementia remains to be determined in randomized controlled trials.
Keywords: Obstructive sleep apnea, depression, cognitive impairment, intermittent hypoxemia, cerebral small vessel disease
INTRODUCTION
By the year 2040, 82.3 million Americans, or 21.7% of the U.S. population, will be over 65 years of age, and those aged 85 and older will triple from 6 million in 2013 to 14.6 million in 2040.1 Consequently, late-life illnesses that cause significant morbidity and mortality will become an increasing public health problem. Such illnesses are frequently comorbid and may have a complex effect on disease progression, prognosis, and response to treatment. For example, epidemiologic studies and clinical trials have frequently illuminated the complex relationship between vascular disease, depression, and cognitive impairment. However, certain comorbidities are just now being recognized as clinically significant, such as the relationship between obstructive sleep apnea (OSA), depression, and dementia.
Currently, 22 million Americans suffer from sleep apnea, but estimates suggest that 80% of men and 93% of women with moderate to severe OSA have not been diagnosed.2 Although the relationships between OSA and depression or cognitive impairment/dementia are increasingly recognized as a serious public health concern, our understanding of the mechanisms that underlie these associations remains incomplete. It remains unclear how OSA is linked to depression and whether common pathways link OSA to depression and cognitive impairment/dementia.
Herein, we reviewed the literature pertinent to the effects of OSA on the cerebral microvascular and neurovascular systems and present a model describing the key pathophysiologic mechanisms that may underlie these associations, including hypoperfusion, endothelial dysfunction, and neuroinflammation. Further, we also discuss recommendations on how to identify OSA in patients with neuropsychiatric comorbidities and considerations for future research on OSA.
OBSTRUCTIVE SLEEP APNEA
Diagnosis
OSA is defined as repeated complete or partial collapse of the upper airway despite an effort to breathe during sleep. Polysomnography is the gold standard diagnostic test for OSA. Home testing with a portable monitor may be used to diagnose OSA in patients at high risk for moderate to severe OSA as part of a comprehensive sleep evaluation. According to the American Academy of Sleep Medicine criteria3 for scoring respiratory events, an apneic episode is defined as complete cessation of oronasal airflow for at least 10 seconds with a 90% drop from baseline in oronasal airflow. A hypopneic episode is defined as incomplete airway obstruction for 10 seconds with a 30% drop from baseline in oronasal airflow and a 4% decrease in oxyhemoglobin saturation, or a 50% drop of oronasal airflow and 3% decrease in oxyhemoglobin saturation. The frequency of apneas and hypopneas per hour of sleep defines the apnea-hypopnea index (AHI) and determines the severity of OSA: mild (AHI of 5–15), moderate (AHI of 15–30), or severe (AHI ≥ 30).
Epidemiology
OSA affects 24% of men and 9% of women of ages 30–60 years4 and 40%–60% of older adults (65+ years).5 The prevalence of moderate to severe OSA (AHI ≥ 15) is highest6 and the sex difference lowest in older adults. The prevalence of OSA in postmenopausal women not on hormonal replacement therapy is markedly increased, resulting in the reduced sex difference.6,7
Risk Factors
Risk factors for OSA include older age, male sex, a family history of OSA, and upper airway structural abnormalities (e.g., large neck girth and craniofacial abnormalities). OSA is also associated with vascular risk factors including obesity, hyperlipidemia, glucose intolerance, alcohol, and smoking. The impact of body mass index (BMI) on OSA severity decreases with age and is negligible by age 60.8 Hormone (especially progesterone) decreases in postmenopausal women lead to reduced ventilatory drive and a greater risk of developing OSA.9
Clinical Presentations
OSA presents with snoring, frequent awakening or gasping/choking during sleep, waking in the morning with headaches, and dry mouth. Some individuals experience excessive daytime sleepiness and fatigue, which impair cognitive functioning. Individuals with untreated OSA have triple the risk of a motor vehicle accident.10 In older people (aged 60+) OSA is not associated with sleepiness and loud snoring6 but may present a more complex clinical picture, with symptoms including frequent nocturnal urination, gait disturbance,11,12 agitation,13 and postoperative delirium.14
OSA AND NEUROPSYCHIATRIC COMORBIDITIES
Depression
Prevalence
Epidemiologic studies have consistently demonstrated that OSA is associated with depression.15–18 In a cross-sectional study16 of 18,980 participants aged 15–100 years, those with major depressive disorder were five times more likely to have OSA than the general population. Data from the Veterans Health Administration Beneficiary (from 1998 to 2001) indicated that the prevalence of mood disorder was significantly higher in individuals with OSA than those without OSA (22% versus 9%, p < 0.0001).19 Furthermore, two longitudinal studies identified OSA as an independent risk factor for depression,17,18 in which the odds for developing depression were increased 2.0-fold (95% confidence interval [CI]: 1.4–2.9) in participants with mild OSA and 2.6-fold (95% CI: 1.7–3.9) in those with moderate to severe OSA.
Treatment for OSA Ameliorates Depressive Symptoms
Changes in depressive symptoms with continuous positive airway pressure (CPAP) treatment suggest an association between OSA and depression. Although short-term (2–3 weeks), placebo-controlled CPAP studies did not find significant improvement in depressive symptoms,20,21 most evidence to date strongly suggests that CPAP benefits patients with mood disturbances.22–27 Most studies have focused on a middle-aged sample; less is known about depression-related benefits in elderly patients. A recently published study that included 224 elderly patients aged 70 or over with severe OSA (AHI ≥ 30) determined that at baseline, 52 patients (23.2%) had depression (Hospital Anxiety and Depression Scale on Depression score ≥ 11), 38 patients (17%) had anxiety (Hospital Anxiety and Depression Scale on Anxiety score ≥ 11), and 66 patients took antidepressants.28 After 3 months depressive (p < 0.001) and anxiety (p = 0.016) symptoms were significantly improved in the CPAP group (N = 115) versus the no CPAP group (N = 109). Thus, CPAP was found to reduce depression and anxiety in elderly patients with severe OSA.
Another study examining CPAP and depression26 reported that in 228 patients who were CPAP treatment-compliant (>5 hours per night), including 71 elderly patients (aged 65+ years, 33.1%), 118 patients (51.8%) were taking antidepressants at baseline and continued their use throughout CPAP treatment. After 3 months of CPAP treatment, AHI reduced from 46.7 (standard deviation [SD]: 27.4) to 6.5 (SD: 1.6), and the mean Patient Health Questionnaire-9 score decreased from 11.3 (SD: 6.1) to 3.7 (SD: 2.9) in the entire sample. The percentage of patients with depression (Patient Health Questionnaire-9 ≥ 10) at baseline dropped from 74.6% to 3.9% after 3 months of CPAP treatment.
One study included 20 patients with depression unresponsive to medication for over 6 months who were also symptomatic for OSA.23 Seventeen (85%) were diagnosed with OSA (AHI ≥ 10) and received CPAP treatment. After 2 months of CPAP treatment the Hamilton Rating Scale for Depression and Beck Depression Inventory scores decreased from 16.7 to 8.0 and 19.7 to 10.8, respectively.
The above findings suggest that antidepressant use did not influence the effectiveness of CPAP on depression or anxiety. Because longer treatment duration (e.g., several months minimum) is needed to show significant improvement in depressive symptoms, it is likely that the benefits of CPAP on depressive symptoms are not the direct result of eliminating apneic episodes and improving sleep fragmentation from short-term CPAP or oxygen treatment (e.g., 2–3 weeks) but may be due to its modification of the pathways linking OSA to depression.
Cognitive Impairment and Dementia
OSA-Related Cognitive Deficits
The prevalence of neurocognitive impairment in patients with OSA is not known. Deficits in attention, executive function, episodic memory, visuospatial and constructional abilities, and psychomotor speed have been identified, whereas language faculties are relatively spared.29–31 Deficits in visuospatial memory, information processing speed, and psychomotor function have been reported in some but not all studies. For example, one study of middle-aged and older adults with OSA (N = 8,059; ages 45–74 years; 55% women; 41% with less than high school education) reported that a higher AHI was associated with poorer cognitive performance on the Verbal Learning Test (verbal immediate and delayed recalls), the Verbal Fluency Test, and the Digit Symbol Test.32
OSA and the Risk of Mild Cognitive Impairment and Dementia
As part of the multicenter cohort study, Study of Osteoporotic Fractures, 298 elderly women (65+ years; mean age 82 years) enrolled in the Sleep and Cognition Study underwent overnight polysomnography.33 OSA (AHI ≥ 15) was diagnosed in 105 of the 298 women. Compared with those without OSA, patients with OSA had a higher rate of mild cognitive impairment or dementia (44.8% versus 31.1%; adjusted odds ratio: 1.85; 95% CI: 1.11–3.08). A cohort study using data from the Longitudinal Health Insurance Database in Taiwan investigated the relationship between OSA and dementia based on International Classification of Diseases, Ninth Revision codes.34 At the 5-year follow-up, the OSA group had a 1.7-fold greater risk of developing dementia than the age- and sex-matched non-OSA control group (95% CI: 1.26–2.31; p < 0.01). Specifically, the risk for dementia in men with OSA aged 50–59 years was 6-fold greater (95% CI: 1.96–18.90), and the risk in women with OSA aged 70 years or over was 3.2-fold (95% CI: 1.71–6.00) greater. In the Alzheimer’s Disease Neuroimaging Initiative,35 onset of cognitive impairment or dementia in individuals with OSA was earlier than those without OSA (age at onset: 75 versus 83; χ2 = 35.03, p < 0.01). These data suggest that OSA may advance cognitive decline in the elderly and/or increase the risk of cognitive impairment or dementia.
Effects of CPAP on Cognitive Function
Studies show that adequate CPAP therapy may improve some cognitive function in patients with OSA. A large-scale, multicenter, randomized, double-blind cohort study, the Apnea Positive Pressure Long-term Efficacy Study (APPLES), investigated the effects of CPAP on cognitive function in patients with OSA.36 Participants were randomized to active CPAP (N = 556) or sham CPAP (N = 542) groups. After 2 months of treatment, performance on working memory significantly improved in the active CPAP group compared with the sham CPAP group (p < 0.01). Those with severe OSA improved more than those with mild OSA. However, attention/psychomotor and learning/memory functions did not improve at either the 2-month or 6-month follow-up.
In examining CPAP in patients with OSA and memory impairment with T-scores (T-score of 50 represents the population mean), 58 patients with baseline mean verbal memory T-scores 2 SDs below the mean (T-score = 30.1 ± 7.3) were enrolled.37 After 3 months of CPAP treatment, the average verbal memory T-score improved to approximately 1 SD below the mean (T-score = 38.9 ± 10.1). Those patients on CPAP for 6 hours a day (68%) showed significant improvement in the verbal delayed recall test (p = 0.03). The authors suggested that long-term memory deficits might be reversible with optimized CPAP treatment. A study from the Alzheimer’s Disease Neuroimaging Initiative cohort35 found that patients on CPAP had a later onset of mild cognitive impairment or dementia than those not on CPAP (75 versus 83 years, p < 0.01), which was comparable with those without OSA. These data suggest that CPAP can protect or improve cognitive function,30,38 thus preventing or delaying the development of mild cognitive impairment and dementia.
BRAIN MORPHOLOGY IS CHANGED IN OSA
OSA and Cerebral Small Vessel Disease Risk
Cerebral small vessel disease (C-SVD) is a group of pathologic processes with various etiologies that affect small arteries and veins, arterioles, and capillaries and for which OSA may be an independent risk factor. C-SVD causes restricted blood flow in diseased small vessels, causing low perfusion pressure and hypoperfusion of the affected brain areas. In fact, chronic hypoperfusion appears to be responsible for development of ischemic C-SVD.39–44 C-SVD can be identified in structural neuroimaging (T2-weighted or fluid-attenuated inversion recovery magnetic resonance imaging [MRI]) as signal hyperintensities, whereas integrity of the white matter tracts can be detected by diffusion tensor MRI (DTI).
In the first community-based study of the relationship between OSA and cerebral white matter morphology,45 533 older adults (age 60 ± 7.5 years) with neither cardiovascular nor neurologic disease underwent overnight polysomnography. Most participants (57.5%) did not have OSA, whereas 32% had mild and 10.5% moderate to severe OSA. The study found that (1) AHI was positively correlated with the severity of white matter hyperintensities (WMHs) on MRI (r = 0.17, p = 0.0001); (2) moderate to severe OSA was associated with twice the risk of having WMHs versus those without OSA (odds ratio: 2.03; 95% CI: 1.02–4.05) after adjusting for age, sex, BMI, drinking, smoking, hyperlipidemia, and history of diabetes mellitus; and (3) 89% of WMHs in patients with OSA were in the frontal lobes. Among patients aged 65 or over (N = 120, 24% of total participants), 69% of them had more severe white matter changes measured by a four-point age-related white matter change scale46 compared with 8% of all participants. The authors suggested that moderate to severe OSA was an independent risk factor for the development of white matter changes in the middle-aged or older general population.
DTI studies also demonstrate that OSA is linked to white matter integrity abnormalities,47–50 whereas a further study identified functional white matter impairment in OSA.51 A study investigating white matter integrity changes before and after CPAP treatment in patients with severe OSA included 17 male patients with untreated OSA (AHI ≥ 30) and 15 matched healthy control subjects (AHI < 5).50 Notably, these patients were relatively young (mean age of 43) and had a normal BMI and no medical comorbidity. Initial DTI demonstrated lower fractional anisotropy (a measure positively correlated with white matter integrity) in patients with OSA versus control subjects. Specifically, multiple areas of subcortical tracts of the superior and inferior parietal lobe, including the superior longitudinal fascicle, and of deep frontal white matter, involving the arcuate fascicle, were degraded. After 12 months of CPAP treatment, DTI demonstrated significant improvements in measures of white matter fiber integrity as well as clinical improvement in memory, attention, and executive functions. The authors suggested that white matter integrity abnormalities associated with OSA might be reversible with adequate CPAP therapy.
White Matter Changes and Depression
Substantial evidence supports an association between depression and C-SVD. Initial reports relating depression and subcortical WMHs seen on MRI were in elderly patients with depression.52,53 Compared with age- and sex-matched healthy control subjects, patients with depression but no history of neurologic illness had higher rates of moderate to severe deep and WMHs, and PVHs, and subcortical gray matter hyperintensities. The “vascular depression” hypothesis54 proposed that cerebrovascular disease such as C-SVD may predispose, precipitate, or perpetuate some geriatric depressive syndromes. Postmortem DTI studies supported this hypothesis, demonstrating that disruption of neural connectivity between subcortical structures and their frontal projections contributed to the risk of depression, especially when dorsolateral prefrontal cortical white matter was involved.55–57 Vascular depression is now considered a validated subtype of depression.58,59
In a longitudinal study, the AGES-Reykjavik Study,60 1,949 participants (mean age 75 ± 5 years; 57% women) free of dementia and depression were followed for 5 years. Baseline and follow-up MRI and depressive symptoms measured with the 15-item Geriatric Depression Scale61 were compared. The study reported the new-onset depression rate was 10% based on Geriatric Depression Scale score ≥ 6 at follow-up or new use of antidepressant medication. The new onset of depression was found to be associated with the progression of C-SVD, such as an increase of volume of WMHs (p < 0.007), new Virchow-Robin spaces (p < 0.001), new subcortical infarcts (p < 0.02), or any new infarcts (p < 0.01) after adjusting for cofounders, including cognitive function, demographic, and cardiovascular factors. Another controlled study investigated whether C-SVD–associated depression was mediated by white matter damage.62 Patients with evidence of C-SVD on MRI underwent additional DTI. The results showed that white matter damage was significantly associated with depressive symptoms in patients with C-SVD (β = 0.25, p = 0.03; 95% CI: 0.03–0.47). These data support vascular depression hypothesis.
Cerebral Microvascular Regulatory Impairment and Depression
In a prospective epidemiologic study of older adults (aged 65+ years), the Rotterdam Study,63 cerebral vasomotor reactivity (CVR) was measured with CO2-enhanced transcranial Doppler ultrasonography and depression was measured with both Center for Epidemiologic Studies Depression Scale (score ≥ 16) and Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria for depressive disorders. At baseline, participants were free of a history of depression, stroke, and dementia, and the mean duration of follow-up was 4 years. All analyses were adjusted for sociodemographic data, cardiovascular risk factors, and new onset of stroke. The study found that new-onset of depression met both Center for Epidemiologic Studies Depression Scale score ≥ 16 and Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria, including major depression, dysthymic disorder, and minor depression, and was associated with a reduction in mean blood flow velocity (odds ratio: 0.83; 95% CI: 0.69–0.98; p = 0.032) and CRV (odds ratio: 0.66; 95% CI: 0.53–0.83; p < 0.001).
In a clinical study CVR was assessed with acetazolamide-enhanced, transcranial Doppler ultrasound in patients with major depression showing that CVR was significantly reduced in depressed patients compared with healthy control subjects in the absence of vascular risk factors (p < 0.05).64 The same group of investigators conducted a subsequent study and found that CVR was significantly reduced in acutely depressed patients compared with nondepressed control subjects. On the 21-month follow-up, CVR in the depressed group significantly improved with antidepressants, whereas CVR in the nondepressed control group remained unchanged.65 The epidemiologic and clinical data demonstrate that cerebral microvascular regulatory impairment plays an important role in the development of depression and supports the vascular depression hypothesis.63–65
Gray Matter Loss and Cognitive Impairment
Sixteen patients with newly diagnosed moderate to severe OSA (age 56 ± 7 years, 13 men) and 14 control subjects (age 58 ± 5 years, 9 men) underwent MRI and voxel-based morphometry and neuropsychological testing. Compared with control subjects, patients with OSA performed worse on the Verbal Learning Test (immediate and delayed recalls), the Stroop Test, and the Digit Span Backward Test. The density of cortical gray matter, right hippocampus, and right and left caudate were decreased in patients with OSA compared with control subjects.66 Another study reported that regional gray matter loss, often unilateral, was found in multiple sites of the brain, including the frontal and parietal cortex, temporal lobe, anterior cingulate, hippocampus, and cerebellum. The extent of gray matter volume loss increases correlated positively with OSA severity.67 The hippocampal atrophy in older patients with OSA suggests that degenerative processes have taken place. Nevertheless, results from previous volumetric brain morphometry studies showed significant gray matter reduction in multiple cortical and subcortical regions in patients with OSA compared with healthy control subjects.68–71
MECHANISMS LINKING OSA TO VASCULAR DEPRESSION AND COGNITIVE IMPAIRMENT
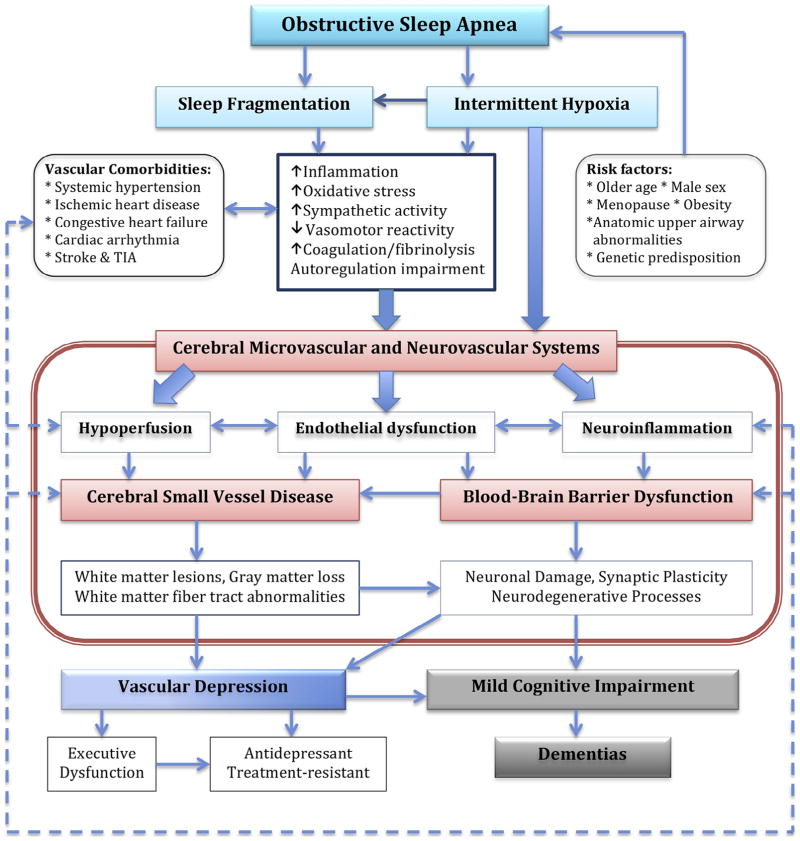
OSA causes nocturnal intermittent hypoxemia and sleep fragmentation (arousals) in response to oxygen desaturation. Emerging evidence indicates that OSA, vascular depression, and cognitive impairment/dementia are linked to several pathologic processes in the cerebral microvascular and neurovascular systems. We present a model describing the key pathophysiologic mechanisms that underlie those associations for which intermittent hypoxia plays a critical role, including hypoperfusion, endothelial dysfunction, and neuroinflammation (Fig. 1).
FIGURE 1.
A model to describe the pathophysiologic mechanisms underlying the association between obstructive sleep apnea, vascular depression, and cognitive impairment/dementia. TIA: transient ischemic attack.
Hypoperfusion
In OSA there are profound changes in nocturnal intracranial hemodynamics and oxygen saturation. During an apneic episode, cerebral blood flow velocity increases progressively and then drops sharply below baseline at a termination of an episode. There is a linear correlation between cerebral blood flow velocity and mean systemic blood pressure.72,73 In a healthy individual the cerebral autoregulation mechanism protects the brain by maintaining cerebral perfusion during blood pressure changes. However, this system is impaired in patients with OSA, resulting in cerebral hypoperfusion in the regions with poor collateral circulation, such as the terminal arterial territories.74 Chronic hypoperfusion in long penetrating small arteries and arterioles is likely responsible for the initial ischemic changes in white matter and gray matter.39
In patients with moderate to severe OSA, increased cerebral blood flow to meet the oxygen demand may not be able to compensate for oxygen desaturation.75,76 Thus, chronic hypoxemia promotes the progression of C-SVD,77 resulting in lacunar infarcts, white matter lesions and white matter fiber tract abnormalities, and gray matter loss.73,78 Anatomically, certain regions of the brain are particularly vulnerable to prolonged hypoxic-ischemic injury, such as the prefrontal and frontal lobes,79,80 basal ganglia,81 and hippocampus.82,83 Damage to these brain regions is associated with abnormal myelin and axonal integrity,84 resulting in mood disturbance and cognitive deficits in patients with OSA.48,66,70 There is evidence that prolonged hypoxic-ischemic damage to the frontal and prefrontal cortex is associated with executive dysfunction in patients with moderate to severe OSA47,85 that improved with CPAP treatment.86
The ischemic change in cerebral microvascular structures related to depression can be explained by the “hypoperfusion” mechanism of vascular depression.87 This hypothesis suggests that impairment in hemodynamics and cerebral autoregulation leads to cerebral perfusion deficits, resulting in altered regional brain function and WMHs. Also, the “disconnection” mechanism of vascular depression suggests that damage to specific fiber tracts and neural circuits, especially the frontostriatal and limbic systems, results in disrupted neural connections that regulate mood and cognition.87 Specifically, greater damage to the uncinate and superior longitudinal fasciculi was associated with more severe depression and executive dysfunction. In addition, ischemic damage to the medial cortex and lateral prefrontal cortex and subcortical and temporal structures is associated with antidepressant treatment-resistance. Thus, OSA alone or comorbid with C-SVD may contribute to the development and progression of vascular depression.
Endothelial Dysfunction and CVR
In OSA repetitive intracranial blood flow surges during apneic episodes cause damage to the endothelial cells of small arteries and arterioles, which results in decreased endothelial vasodilator production (e.g., nitric oxide [NO]).88,89 NO plays a critical role in the regulation of cerebral blood flow in response to hypercapnia (CO2-dependent) through chemoregulatory mechanisms to maintain a hemostatic microenvironment.74,90,91 Because the availability of NO is decreased in OSA,89,92,93 the vasodilatory capacity of CVR in response to hypercapnia due to hypoxemia is compromised.94–96 CPAP therapy significantly increases circulating NO metabolites (serum nitrite/nitrate) in patients, demonstrating the association between OSA and CVR.89 Impaired CVR leads to poor microvascular blood flow during apneas and hypopneas, resulting in the development or progression of deep and periventricular WMHs and high grades of deep and periventricular white matter lesions (e.g., grades 2 and 3) according to the Fazekas scale.97,98 These evidence suggests that NO plays a crucial role in acute hemodynamic regulation and long-term vascular remodeling in OSA.89
Epidemiologic and clinical studies have shown that reduced cerebral blood flow because of impaired CVR is a risk factor for depression in the absence of cardiovascular risk factors, cardiac disease, and stroke.63–65,99 Because OSA causes impaired CVR and subsequent ischemic damage of white matter and white matter fiber tracts, OSA is a risk factor for vascular depression in some patients. Treatment of OSA may prevent further damage to cerebral small arteries and arterioles, which may reduce the risk of developing or exacerbating vascular depression.
In OSA the disruption of NO pathways causes a cascade of neuronal metabolic deficiencies, resulting in destabilizing neurons, synapses, and neurotransmission, which in turn leads to synaptic loss and neuronal damage.100,101 In some patients depleted NO leads to the neurodegenerative state characterized by the formation of amyloid angiopathy, senile plaques, and neurofibrillary tangles.102,103 Thus, OSA may contribute to the development of cognitive impairment as well as depression.
Blood–Brain Barrier and Neuroinflammation
In OSA repetitive hypoxia and reoxygenation stimulates endothelial cells to produce excessive reactive oxygen species, especially during reoxygenation, which promotes oxidative stress.104,105 Oxidative stress causes BBB hyperpermeability106 and neuroinflammation,107 resulting in plasma proteins leaking into the arteriolar walls and perivascular spaces (Virchow-Robin spaces).108 A consequence of this is the accumulation of macrophages and fibrosis in the arteriolar walls, leading to the development or progression of C-SVD, which can cause white matter damage and lacunar infarction.109,110 Also, accumulation of plasma proteins (e.g., Aβ amyloid) in the small arterial walls and perivascular space results in neurotoxicity, which initiates the onset or contributes to the progression of neurodegeneration.111–113 Finally, inflammation at the blood–brain barrier (BBB) leads to altered transport of molecules across the barrier, resulting in progressive synaptic plasticity and neuronal dysfunction and loss.114,115 Together, OSA may play an important role in the pathogenesis of depression and cognitive impairment through neuroinflammation and BBB hyperpermeability.
CONCLUSIONS
OSA may initiate or amplify the pathologic processes of C-SVD and BBB dysfunction, resulting in the development or exacerbation of depressive symptoms and cognitive deficits. In elderly patients with vascular risk factors, OSA may put further stress on the already compromised cerebral microvascular and neurovascular systems. Given the evidence that adequate treatment of OSA with CPAP improves depression and neurocognitive functions, it is important to identify OSA when assessing patients with depression or cognitive impairment.
RECOMMENDATIONS FOR CLINICIANS
Patients may have OSA symptoms for years and yet remain undiagnosed. In many cases because symptoms of depression overlap with symptoms of OSA, treating the depression may cause OSA to be overlooked.116 Patients with untreated OSA may present to psychiatric clinics with complaints of sleep disturbances, daytime sleepiness, fatigue, increased irritability or agitation, and chronically depressed mood. In middle-aged and older adults with a subjective cognitive decline and clinical evidence of cognitive impairment and dementia, clinicians should routinely inquire about OSA symptoms.
Several validated questionnaires are available for screening for OSA in clinical settings, such as the Berlin and Stop-Bang questionnaires.117 The Berlin screening questionnaire118 is most commonly used. It assess the severity of snoring, fatigue, and hypertension. The Stop-Bang questionnaire has the highest methodologic quality and sensitivity in predicting moderate (AHI ≥ 15) and severe (AHI ≥ 30) OSA.117 The Stop-Bang has eight yes/no questions: STOP (loud Snore, Tired, Observed apnea, and high blood Pressure) and BANG (BMI, Age, Neck size, and Gender).119,120 Patients with a high probability of having OSA on screening should receive a comprehensive sleep evaluation by a sleep specialist.
FUTURE RESEARCH DIRECTIONS
Among primary sleep disorders, OSA causes the most serious morbidity and mortality. There is strong evidence that patients with untreated moderate to severe OSA have increased rates of depression, cognitive impairment, and dementia. Clinical studies have demonstrated that treatment for OSA (e.g., CPAP and oxygen therapy) improves cerebral microvascular perfusion and oxygenation, reduces vascular inflammatory and immune responses, and repairs BBB functionality. Therefore, successful treatment of OSA may prevent further cerebral microvascular and neurovascular damage. Large-scale studies are needed to examine whether treatment of OSA changes the deteriorating trajectory of elderly patients with depression, cognitive impairment, and dementia.
Studies are needed to elucidate the impact of OSA on the development of, treatment response to, and prognosis of vascular depression and cognitive impairment. Treatment of OSA improves cerebral microvascular perfusion, oxygenation, and hypertension and reduces vascular inflammatory responses and thus may reduce further cerebral microstructure damage. It is compelling to study whether the effective treatment of OSA prevents the development of depression and cognitive impairment and/or changes the deteriorating trajectory of patients with vascular depression, cognitive impairment, and dementia.
Acknowledgments
Supported by grant T32 MH020004 from the National Institute of Mental Health.
References
- 1.Profile of Older Americans—2014 Administration on Aging. U.S. Department of Health and Human Services; Dec 10, 2014. aoa.acl.gov/aging_statistics. [Google Scholar]
- 2.Young T, Evans L, Finn L, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 3.Iber CA-IS, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. Westchester, Illinois: American Academy of Sleep Medicine; 2007. p. 1. [Google Scholar]
- 4.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 5.Ancoli-Israel S, Kripke DF, Klauber MR, et al. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 7.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 8.Tishler PV, Larkin EK, Schluchter MD, et al. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289:2230–2237. doi: 10.1001/jama.289.17.2230. [DOI] [PubMed] [Google Scholar]
- 9.Keefe DL, Watson R, Naftolin F. Hormone replacement therapy may alleviate sleep apnea in menopausal women: a pilot study. Menopause. 1999;6:196–200. doi: 10.1097/00042192-199906030-00004. [DOI] [PubMed] [Google Scholar]
- 10.George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax. 2001;56:508–512. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celle S, Annweiler C, Camicioli R, et al. Sleep-related breathing disorders and gait variability: a cross-sectional preliminary study. BMC Pulm Med. 2014;14:140. doi: 10.1186/1471-2466-14-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allali G, Perrig S, Cleusix M, et al. Gait abnormalities in obstructive sleep apnea and impact of continuous positive airway pressure. Respir Physiol Neurobiol. 2014;201:31–33. doi: 10.1016/j.resp.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Gehrman PR, Martin JL, Shochat T, et al. Sleep-disordered breathing and agitation in institutionalized adults with Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:426–433. [PubMed] [Google Scholar]
- 14.Flink BJ, Rivelli SK, Cox EA, et al. Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology. 2012;116:788–796. doi: 10.1097/ALN.0b013e31824b94fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheaton AG, Perry GS, Chapman DP, et al. Sleep disordered breathing and depression among U.S. adults: National Health and Nutrition Examination Survey, 2005–2008. Sleep. 2012;35:461–467. doi: 10.5665/sleep.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohayon MM. The effects of breathing-related sleep disorders on mood disturbances in the general population. J Clin Psychiatry. 2003;64:1195–1200. doi: 10.4088/jcp.v64n1009. quiz, 1274-1196. [DOI] [PubMed] [Google Scholar]
- 17.Chen YH, Keller JK, Kang JH, et al. Obstructive sleep apnea and the subsequent risk of depressive disorder: a population-based follow-up study. J Clin Sleep Med. 2013;9:417–423. doi: 10.5664/jcsm.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peppard PE, Szklo-Coxe M, Hla KM, et al. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166:1709–1715. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 19.Sharafkhaneh A, Giray N, Richardson P, et al. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28:1405–1411. doi: 10.1093/sleep/28.11.1405. [DOI] [PubMed] [Google Scholar]
- 20.Lee IS, Bardwell W, Ancoli-Israel S, et al. Effect of three weeks of continuous positive airway pressure treatment on mood in patients with obstructive sleep apnoea: a randomized placebo-controlled study. Sleep Med. 2012;13:161–166. doi: 10.1016/j.sleep.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bardwell WA, Norman D, Ancoli-Israel S, et al. Effects of 2-week nocturnal oxygen supplementation and continuous positive airway pressure treatment on psychological symptoms in patients with obstructive sleep apnea: a randomized placebo-controlled study. Behav Sleep Med. 2007;5:21–38. doi: 10.1207/s15402010bsm0501_2. [DOI] [PubMed] [Google Scholar]
- 22.El-Sherbini AM, Bediwy AS, El-Mitwalli A. Association between obstructive sleep apnea (OSA) and depression and the effect of continuous positive airway pressure (CPAP) treatment. Neuropsychiatr Dis Treat. 2011;7:715–721. doi: 10.2147/NDT.S26341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11:552–557. doi: 10.1016/j.sleep.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz DJ, Kohler WC, Karatinos G. Symptoms of depression in individuals with obstructive sleep apnea may be amenable to treatment with continuous positive airway pressure. Chest. 2005;128:1304–1309. doi: 10.1378/chest.128.3.1304. [DOI] [PubMed] [Google Scholar]
- 25.Gagnadoux F, Le Vaillant M, Goupil F, et al. Depressive symptoms before and after long-term CPAP therapy in patients with sleep apnea. Chest. 2014;145:1025–1031. doi: 10.1378/chest.13-2373. [DOI] [PubMed] [Google Scholar]
- 26.Edwards C, Mukherjee S, Simpson L, et al. Depressive symptoms before and after treatment of obstructive sleep apnea in men and women. J Clin Sleep Med. 2015;11:1029–1038. doi: 10.5664/jcsm.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Means MK, Lichstein KL, Edinger JD, et al. Changes in depressive symptoms after continuous positive airway pressure treatment for obstructive sleep apnea. Sleep Breath. 2003;7:31–42. doi: 10.1007/s11325-003-0031-x. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Garcia MA, Chiner E, Hernandez L, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J. 2015;46:142–151. doi: 10.1183/09031936.00064214. [DOI] [PubMed] [Google Scholar]
- 29.Aloia MS, Arnedt JT, Davis JD, et al. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772–785. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 30.Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology. 2013;18:61–70. doi: 10.1111/j.1440-1843.2012.02255.x. [DOI] [PubMed] [Google Scholar]
- 31.Wallace A, Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013;36:203–220. doi: 10.5665/sleep.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramos AR, Tarraf W, Rundek T, et al. Obstructive sleep apnea and neurocognitive function in a Hispanic/Latino population. Neurology. 2015;84:391–398. doi: 10.1212/WNL.0000000000001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang WP, Liu ME, Chang WC, et al. Sleep apnea and the risk of dementia: a population-based 5-year follow-up study in Taiwan. PLoS ONE. 2013;8:e78655. doi: 10.1371/journal.pone.0078655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osorio RS, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84:1964–1971. doi: 10.1212/WNL.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2012;35:1593–1602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmerman ME, Arnedt JT, Stanchina M, et al. Normalization of memory performance and positive airway pressure adherence in memory-impaired patients with obstructive sleep apnea. Chest. 2006;130:1772–1778. doi: 10.1378/chest.130.6.1772. [DOI] [PubMed] [Google Scholar]
- 38.Crawford-Achour E, Dauphinot V, Martin MS, et al. Protective effect of long-term CPAP therapy on cognitive performance in elderly patients with severe OSA: the PROOF Study. J Clin Sleep Med. 2015;11:519–524. doi: 10.5664/jcsm.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 40.Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986;19:253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- 41.Fazekas F, Niederkorn K, Schmidt R, et al. White matter signal abnormalities in normal individuals: correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke. 1988;19:1285–1288. doi: 10.1161/01.str.19.10.1285. [DOI] [PubMed] [Google Scholar]
- 42.van Swieten JC, van den Hout JH, van Ketel BA, et al. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain. 1991;114(Pt 2):761–774. doi: 10.1093/brain/114.2.761. [DOI] [PubMed] [Google Scholar]
- 43.Markus HS, Lythgoe DJ, Ostegaard L, et al. Reduced cerebral blood flow in white matter in ischaemic leukoaraiosis demonstrated using quantitative exogenous contrast based perfusion MRI. J Neurol Neurosurg Psychiatry. 2000;69:48–53. doi: 10.1136/jnnp.69.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 45.Kim H, Yun CH, Thomas RJ, et al. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep. 2013;36:709–715. doi: 10.5665/sleep.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 47.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 48.Macey PM, Kumar R, Woo MA, et al. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–977. [PMC free article] [PubMed] [Google Scholar]
- 49.Chen HL, Lu CH, Lin HC, et al. White matter damage and systemic inflammation in obstructive sleep apnea. Sleep. 2015;38:361–370. doi: 10.5665/sleep.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castronovo V, Scifo P, Castellano A, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep. 2014;37:1465–1475. doi: 10.5665/sleep.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zimmerman ME, Aloia MS. A review of neuroimaging in obstructive sleep apnea. J Clin Sleep Med. 2006;2:461–471. [PubMed] [Google Scholar]
- 52.Coffey CE, Figiel GS, Djang WT, et al. Leukoencephalopathy in elderly depressed patients referred for ECT. Biol Psychiatry. 1988;24:143–161. doi: 10.1016/0006-3223(88)90270-3. [DOI] [PubMed] [Google Scholar]
- 53.Coffey CE, Figiel GS, Djang WT, et al. Subcortical hyperintensity on magnetic resonance imaging: a comparison of normal and depressed elderly subjects. Am J Psychiatry. 1990;147:187–189. doi: 10.1176/ajp.147.2.187. [DOI] [PubMed] [Google Scholar]
- 54.Alexopoulos GS, Meyers BS, Young RC, et al. “Vascular depression” hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 55.Bae JN, MacFall JR, Krishnan KR, et al. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry. 2006;60:1356–1363. doi: 10.1016/j.biopsych.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 56.Taylor WD, MacFall JR, Payne ME, et al. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry. 2004;161:1293–1296. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- 57.Sheline YI, Price JL, Vaishnavi SN, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165:524–532. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sneed JR, Rindskopf D, Steffens DC, et al. The vascular depression subtype: evidence of internal validity. Biol Psychiatry. 2008;64:491–497. doi: 10.1016/j.biopsych.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 60.van Sloten TT, Sigurdsson S, van Buchem MA, et al. Cerebral small vessel disease and association with higher incidence of depressive symptoms in a general elderly population: the AGES-Reykjavik Study. Am J Psychiatry. 2015;172:570–578. doi: 10.1176/appi.ajp.2014.14050578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 62.Brookes RL, Herbert V, Lawrence AJ, et al. Depression in small-vessel disease relates to white matter ultrastructural damage, not disability. Neurology. 2014;83:1417–1423. doi: 10.1212/WNL.0000000000000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Direk N, Koudstaal PJ, Hofman A, et al. Cerebral hemodynamics and incident depression: the Rotterdam Study. Biol Psychiatry. 2012;72:318–323. doi: 10.1016/j.biopsych.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 64.de Castro AG, Bajbouj M, Schlattmann P, et al. Cerebrovascular reactivity in depressed patients without vascular risk factors. J Psychiatr Res. 2008;42:78–82. doi: 10.1016/j.jpsychires.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 65.Lemke H, de Castro AG, Schlattmann P, et al. Cerebrovascular reactivity over time-course from major depressive episode to remission. J Psychiatr Res. 2010;44:132–136. doi: 10.1016/j.jpsychires.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 66.Yaouhi K, Bertran F, Clochon P, et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. J Sleep Res. 2009;18:36–48. doi: 10.1111/j.1365-2869.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 67.Macey PM, Henderson LA, Macey KE, et al. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:1382–1387. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- 68.Huynh NT, Prilipko O, Kushida CA, et al. Volumetric brain morphometry changes in patients with obstructive sleep apnea syndrome: effects of CPAP treatment and literature review. Front Neurol. 2014;5:58. doi: 10.3389/fneur.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joo EY, Tae WS, Lee MJ, et al. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep. 2010;33:235–241. doi: 10.1093/sleep/33.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weng HH, Tsai YH, Chen CF, et al. Mapping gray matter reductions in obstructive sleep apnea: an activation likelihood estimation meta-analysis. Sleep. 2014;37:167–175. doi: 10.5665/sleep.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morrell MJ, McRobbie DW, Quest RA, et al. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med. 2003;4:451–454. doi: 10.1016/s1389-9457(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 72.Klingelhofer J, Hajak G, Sander D, et al. Assessment of intracranial hemodynamics in sleep apnea syndrome. Stroke. 1992;23:1427–1433. doi: 10.1161/01.str.23.10.1427. [DOI] [PubMed] [Google Scholar]
- 73.Balfors EM, Franklin KA. Impairment of cerebral perfusion during obstructive sleep apneas. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1587–1591. doi: 10.1164/ajrccm.150.6.7952619. [DOI] [PubMed] [Google Scholar]
- 74.Urbano F, Roux F, Schindler J, et al. Impaired cerebral autoregulation in obstructive sleep apnea. J Appl Physiol. 2008;105:1852–1857. doi: 10.1152/japplphysiol.90900.2008. [DOI] [PubMed] [Google Scholar]
- 75.Muraki M, Kitaguchi S, Ichihashi H, et al. Apnoea-hypopnoea index during rapid eye movement and non-rapid eye movement sleep in obstructive sleep apnoea. J Int Med Res. 2008;36:906–913. doi: 10.1177/147323000803600506. [DOI] [PubMed] [Google Scholar]
- 76.Hayakawa T, Terashima M, Kayukawa Y, et al. Changes in cerebral oxygenation and hemodynamics during obstructive sleep apneas. Chest. 1996;109:916–921. doi: 10.1378/chest.109.4.916. [DOI] [PubMed] [Google Scholar]
- 77.Lloyd EE, Durgan DJ, Martini SR, et al. Pathological effects of obstructive apneas during the sleep cycle in an animal model of cerebral small vessel disease. Hypertension. 2015;66:913–917. doi: 10.1161/HYPERTENSIONAHA.115.05764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miyamoto O, Auer RN. Hypoxia, hyperoxia, ischemia, and brain necrosis. Neurology. 2000;54:362–371. doi: 10.1212/wnl.54.2.362. [DOI] [PubMed] [Google Scholar]
- 79.Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63:246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peng W, Chen R, Jiang Z, et al. Correlation between cognitive function and hippocampal atrophy and cerebral white matter lesions in patients with obstructive sleep apnea hypopnea syndrome. Zhonghua Yi Xue Za Zhi. 2014;94:724–728. [PubMed] [Google Scholar]
- 81.Hawker K, Lang AE. Hypoxicischemic damage of the basal ganglia. Case reports and a review of the literature. Mov Disord. 1990;5:219–224. doi: 10.1002/mds.870050306. [DOI] [PubMed] [Google Scholar]
- 82.Bennett SA, Tenniswood M, Chen JH, et al. Chronic cerebral hypoperfusion elicits neuronal apoptosis and behavioral impairment. Neuroreport. 1998;9:161–166. doi: 10.1097/00001756-199801050-00033. [DOI] [PubMed] [Google Scholar]
- 83.Feng J, Wu Q, Zhang D, et al. Hippocampal impairments are associated with intermittent hypoxia of obstructive sleep apnea. Chin Med J. 2012;125:696–701. [PubMed] [Google Scholar]
- 84.Kumar R, Pham TT, Macey PM, et al. Abnormal myelin and axonal integrity in recently diagnosed patients with obstructive sleep apnea. Sleep. 2014;37:723–732. doi: 10.5665/sleep.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saunamaki T, Jehkonen M. A review of executive functions in obstructive sleep apnea syndrome. Acta Neurol Scand. 2007;115:1–11. doi: 10.1111/j.1600-0404.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 86.Olaithe M, Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36:1297–1305. doi: 10.5665/sleep.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Faraci FM, Breese KR, Heistad DD. Cerebral vasodilation during hypercapnia. Role of glibenclamide-sensitive potassium channels and nitric oxide. Stroke. 1994;25:1679–1683. doi: 10.1161/01.str.25.8.1679. [DOI] [PubMed] [Google Scholar]
- 89.Ip MS, Lam B, Chan LY, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–2171. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- 90.Lavi S, Gaitini D, Milloul V, et al. Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2006;291:H1856–H1861. doi: 10.1152/ajpheart.00014.2006. [DOI] [PubMed] [Google Scholar]
- 91.Lavi S, Egbarya R, Lavi R, et al. Role of nitric oxide in the regulation of cerebral blood flow in humans: chemoregulation versus mechanoregulation. Circulation. 2003;107:1901–1905. doi: 10.1161/01.CIR.0000057973.99140.5A. [DOI] [PubMed] [Google Scholar]
- 92.Atkeson A, Jelic S. Mechanisms of endothelial dysfunction in obstructive sleep apnea. Vasc Health Risk Manag. 2008;4:1327–1335. doi: 10.2147/vhrm.s4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lurie A. Endothelial dysfunction in adults with obstructive sleep apnea. Adv Cardiol. 2011;46:139–170. doi: 10.1159/000325108. [DOI] [PubMed] [Google Scholar]
- 94.Jimenez Caballero PE, Coloma Navarro R, Segura Martin T, et al. Cerebral hemodynamic changes at basilar artery in patients with obstructive sleep apnea syndrome. A case-control study. Acta Neurol Scand. 2014;129:80–84. doi: 10.1111/ane.12156. [DOI] [PubMed] [Google Scholar]
- 95.Suzuki Y, Fujita M, Mizutani N, et al. Role of nitric oxide in the control of cerebral microcirculation under physiological and pathological conditions. Clin Hemorheol Microcirc. 2000;23:307–312. [PubMed] [Google Scholar]
- 96.Furtner M, Staudacher M, Frauscher B, et al. Cerebral vasoreactivity decreases overnight in severe obstructive sleep apnea syndrome: a study of cerebral hemodynamics. Sleep Med. 2009;10:875–881. doi: 10.1016/j.sleep.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 97.Fu JH, Lu CZ, Hong Z, et al. Relationship between cerebral vasomotor reactivity and white matter lesions in elderly subjects without large artery occlusive disease. J Neuroimaging. 2006;16:120–125. doi: 10.1111/j.1552-6569.2006.00030.x. [DOI] [PubMed] [Google Scholar]
- 98.Bakker SL, de Leeuw FE, de Groot JC, et al. Cerebral vasomotor reactivity and cerebral white matter lesions in the elderly. Neurology. 1999;52:578–583. doi: 10.1212/wnl.52.3.578. [DOI] [PubMed] [Google Scholar]
- 99.Lavoie KL, Pelletier R, Arsenault A, et al. Association between clinical depression and endothelial function measured by forearm hyperemic reactivity. Psychosom Med. 2010;72:20–26. doi: 10.1097/PSY.0b013e3181c2d6b8. [DOI] [PubMed] [Google Scholar]
- 100.Tong XK, Hamel E. Regional cholinergic denervation of cortical microvessels and nitric oxide synthase-containing neurons in Alzheimer’s disease. Neuroscience. 1999;92:163–175. doi: 10.1016/s0306-4522(98)00750-7. [DOI] [PubMed] [Google Scholar]
- 101.Toda N, Okamura T. Cerebral blood flow regulation by nitric oxide in Alzheimer’s disease. J Alzheimers Dis. 2012;32:569–578. doi: 10.3233/JAD-2012-120670. [DOI] [PubMed] [Google Scholar]
- 102.de la Torre JC, Stefano GB. Evidence that Alzheimer’s disease is a microvascular disorder: the role of constitutive nitric oxide. Brain Res Brain Res Rev. 2000;34:119–136. doi: 10.1016/s0165-0173(00)00043-6. [DOI] [PubMed] [Google Scholar]
- 103.Togo T, Katsuse O, Iseki E. Nitric oxide pathways in Alzheimer’s disease and other neurodegenerative dementias. Neurol Res. 2004;26:563–566. doi: 10.1179/016164104225016236. [DOI] [PubMed] [Google Scholar]
- 104.Yamauchi M, Kimura H. Oxidative stress in obstructive sleep apnea: putative pathways to the cardiovascular complications. Antioxid Redox Signal. 2008;10:755–768. doi: 10.1089/ars.2007.1946. [DOI] [PubMed] [Google Scholar]
- 105.Lavie L. Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 106.Skoog I, Wallin A, Fredman P, et al. A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer’s disease and vascular dementia. Neurology. 1998;50:966–971. doi: 10.1212/wnl.50.4.966. [DOI] [PubMed] [Google Scholar]
- 107.Jelic S, Le Jemtel TH. Inflammation, oxidative stress, and the vascular endothelium in obstructive sleep apnea. Trends Cardiovasc Med. 2008;18:253–260. doi: 10.1016/j.tcm.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 108.Grinberg LT, Thal DR. Vascular pathology in the aged human brain. Acta Neuropathol. 2010;119:277–290. doi: 10.1007/s00401-010-0652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wardlaw JM. Blood-brain barrier and cerebral small vessel disease. J Neurol Sci. 2010;299:66–71. doi: 10.1016/j.jns.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 110.Topakian R, Barrick TR, Howe FA, et al. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry. 2010;81:192–197. doi: 10.1136/jnnp.2009.172072. [DOI] [PubMed] [Google Scholar]
- 111.Zlokovic BV, Deane R, Sallstrom J, et al. Neurovascular pathways and Alzheimer amyloid beta-peptide. Brain Pathol. 2005;15:78–83. doi: 10.1111/j.1750-3639.2005.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sagare AP, Bell RD, Zlokovic BV. Neurovascular defects and faulty amyloid-beta vascular clearance in Alzheimer’s disease. J Alzheimers Dis. 2013;33(suppl 1):S87–S100. doi: 10.3233/JAD-2012-129037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lim DC, Pack AI. Obstructive sleep apnea and cognitive impairment: addressing the blood-brain barrier. Sleep Med Rev. 2014;18:35–48. doi: 10.1016/j.smrv.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 116.Lamberg L. Depression treatment may overlook severe sleep disorder. Psychiatr News. 2010;45:18. [Google Scholar]
- 117.Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57:423–438. doi: 10.1007/s12630-010-9280-x. [DOI] [PubMed] [Google Scholar]
- 118.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 119.Chung F, Subramanyam R, Liao P, et al. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth. 2012;108:768–775. doi: 10.1093/bja/aes022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chung F, Yang Y, Brown R, et al. Alternative scoring models of STOP-bang questionnaire improve specificity to detect undiagnosed obstructive sleep apnea. J Clin Sleep Med. 2014;10:951–958. doi: 10.5664/jcsm.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]