Abstract
Background
Pathway analyses can be used to determine how host and environmental factors contribute to asthma severity.
Objective
Investigate pathways explaining asthma severity in inner-city children.
Methods
Based on medical evidence in the published literature, we developed a conceptual model to describe how eight risk-factor domains (allergen sensitization, allergic inflammation, pulmonary physiology, stress, obesity, vitamin D, environmental tobacco smoke (ETS) exposure and rhinitis severity) are linked to asthma severity. To estimate the relative magnitude and significance of hypothesized relationships among these domains and asthma severity, we applied a causal network analysis to test our model in an Inner-City Asthma Consortium study. Participants comprised 6–17 year old children (n=561) with asthma and rhinitis from 9 U.S. inner-cities who were evaluated every two months for one year. Asthma severity was measured by a longitudinal composite assessment of day and night symptoms, exacerbations, and controller usage.
Results
Our conceptual model explained 53.4% of the variance in asthma severity. An allergy pathway (linking allergen sensitization, allergic inflammation, pulmonary physiology, and rhinitis severity domains to asthma severity) and ETS exposure pathway (linking ETS exposure and pulmonary physiology domains to asthma severity) exerted significant effects on asthma severity. Among the domains, pulmonary physiology and rhinitis severity had the largest significant standardized total effects on asthma severity (−0.51 and 0.48 respectively), followed by ETS exposure (0.30) and allergic inflammation (0.22). While vitamin D had modest but significant indirect effects on asthma severity, its total effect was insignificant (0.01).
Conclusions
The standardized effect sizes generated by a causal network analysis quantify the relative contributions of different domains and can be used to prioritize interventions to address asthma severity.
Keywords: asthma, children, inner-city, allergy, sensitization, inflammation, lung function, pulmonary physiology, rhinitis, environmental tobacco smoke exposure
Graphical abstract
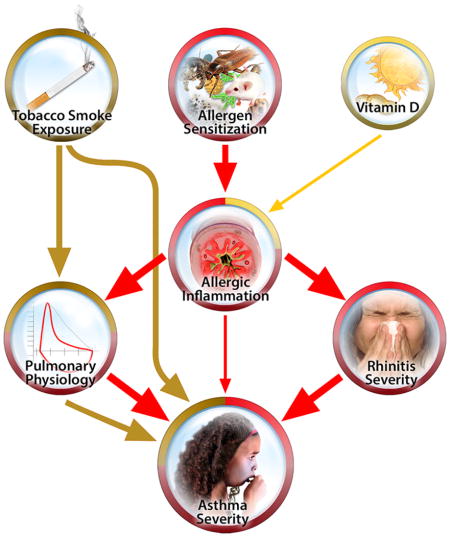
INTRODUCTION
Inner-city children experience a high burden of asthma symptoms and morbidity despite guidelines-directed care.1,2 NIH/NIAID sponsored the Inner-City Asthma Consortium (ICAC) Asthma Phenotypes in the Inner City (APIC) study to investigate how host and environmental factors contribute to asthma severity among children carefully monitored while prospectively receiving optimal, guidelines-based care. Results of the primary objectives of the APIC study are presented in the publications entitled “Asthma Phenotypes in the Inner City: Distinguishing Characteristics of Difficult-to-Control Asthma in Children”3 and “Asthma Phenotypes in Inner City Children” that appear in this issue of the Journal.4
Based on clinical and mechanistic evidence in the published literature that we have detailed in the Online Repository, we developed a conceptual model to describe how eight risk factor domains (allergen sensitization, allergic inflammation, pulmonary physiology, stress, obesity, Vitamin D, environmental tobacco smoke (ETS) exposure and rhinitis severity) are linked to asthma severity (Figure 1). This model allows us to conceptualize which domains have a direct influence on asthma severity and/or an indirect influence on asthma severity by acting through another domain(s). We then tested this conceptual model in the APIC cohort and dataset using a causal network analysis, a quantitative approach that is commonly used to describe the effects of genomics, metabolomics or biochemical pathway influences on disease phenotypes but, to our knowledge, has never been applied in the context of explaining asthma severity. Understanding the relative contributions of these risk factors to asthma severity in inner-city children, via direct and/or indirect pathways, can provide a basis for further investigations into linking mechanisms while also guiding personalized asthma management and population health policy.
FIGURE 1.
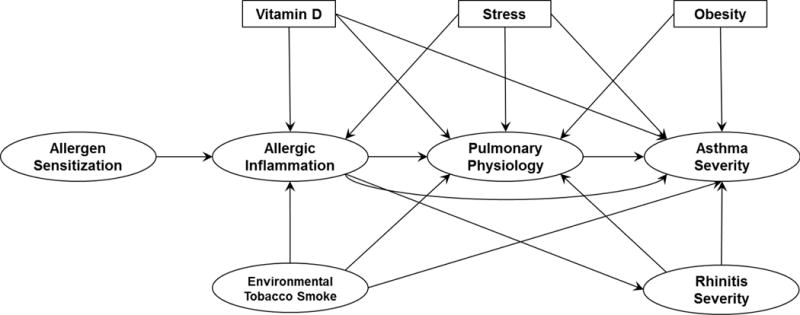
Conceptual Model of Asthma Severity. This model was generated in advance of any analyses based on observations derived from the medical literature and became the test object of this study. Oval boxes indicate domains defined by multiple observed variables, while rectangles indicate domains defined by one observed variable only. An arrow connecting two domains represents the direction of the hypothesized relationship between the two domains.
METHODS
Conceptual Model of Risk Factors Contributing to Asthma Severity
A conceptual model was developed to hypothetically describe how 8 risk factor domains of allergen sensitization, allergic inflammation, pulmonary physiology, stress, obesity, Vitamin D, environmental tobacco smoke (ETS) exposure and rhinitis severity are linked to asthma severity in children (Figure 1). These domains were based on variables ascertained on at least the large majority of APIC study participants, and justified by clinical and mechanistic evidence in the published literature. The literature review substantiating the links of this model is described in the Online Repository.
Study Participants
All study participants included in this investigation were in the NIAID-sponsored ICAC APIC study. Details of the APIC cohort are reported in this issue of the Journal in Pongracic et al.3 Briefly, APIC included 6 to 17 year old children with a broad spectrum of asthma residing in low income census tracts in 9 U.S. cities (Baltimore MD, Boston MA, Chicago IL, Cincinnati OH, Dallas TX, Denver CO, Detroit MI, New York NY, Washington DC). For inclusion, study participants had to have a diagnosis of asthma by a physician and demonstrate adequate adherence to study-prescribed daily asthma controller therapies. The protocol was approved by institutional review boards from each participating center and written informed consent was obtained by the legal guardians of participating children.
Study and Assessments
APIC participants received a comprehensive assessment at Screening and had their asthma and rhinitis managed based on the National Asthma Education and Prevention Program Expert Panel Report 35 and the Allergic Rhinitis and its Impact on Asthma6,7 guidelines-derived treatment algorithms. Clinical assessment and medication adjustments occurred every two months for one year, denoted by V0, V1, V2, V3, V4, V5, and V6, as described in Pongracic et al.3 All medications (except montelukast) were provided free of charge to the study participants.
The variables used to define the domains of asthma severity and its risk factors are shown in Table I. The Asthma Severity, Rhinitis Severity, Allergen Sensitization, Allergic Inflammation, Pulmonary Physiology and ETS Exposure domains were comprised of multiple variables, while Vitamin D, Stress and Obesity domains were comprised of single variables (Table I and Table E1 in the Online Repository). The Asthma Severity domain was comprised of 4 Composite Asthma Severity Index (CASI) components (day symptoms and albuterol use, night symptoms and albuterol use, daily controller treatment step level, and exacerbations) measured at V0 – V6 and summarized using the mean and variance across all visits, 8 as well as by the Asthma Control Test / Childhood Asthma Control Test (ACT/cACT) categories (well controlled, not well controlled, very poorly controlled) at V6.9–12 The Rhinitis Severity domain included the mean and variance of both a symptom score and a medication use score across the visits. The Allergen Sensitization domain was comprised of total serum IgE, the number of allergen sensitizations, and determination of allergen sensitization to molds, dust mites, cockroaches, rodents, pets, pollen/peanut and foods as described.3 The Allergic Inflammation domain included fractional exhaled nitric oxide (FeNO) and blood eosinophil counts. The Pulmonary Physiology domain was comprised of spirometry measures (FEV1 (% predicted) and FEV1/FVC) at V0 – V6 that were summarized using the mean and variance across the visits, as well as the FEV1 bronchodilator response at V6. The ETS Exposure domain was comprised of the number of smokers in the home and urine cotinine, as measured by NicAlert™ test strips (Nymox Pharmaceutical Corp).
TABLE I.
Observed variables used to measure each domain
| Domain | Observed Variables |
|---|---|
|
| |
| Asthma Severity | Mean and variance of CASI component - Day
symptoms & albuterol use1 between V0 and V6 Mean and variance of CASI component - Night symptoms & albuterol score2 between V0 and V6 Mean and variance of CASI component -Exacerbations3 between V0 and V6 Mean and variance of controller treatment step between V0 and V6 ACT/cACT at V6 (categorized4) |
|
| |
| Rhinitis Severity | Mean and variance of rhinitis symptom
score5 between V0 and
V6 Mean and variance of rhinitis medication score6 between V0 and V6 |
|
| |
| Allergen Sensitization8 | Total serum IgE7 at V0 Number of allergic sensitizations (panel of 22)9 at V0 Sensitized to molds10 at V0 Sensitized to dust mites11 at V0 Sensitized to roaches12 at V0 Sensitized to rodents13 at V0 Sensitized to pets14 at V0 Sensitized to pollen/peanut15 at V0 |
|
| |
| Allergic Inflammation | Blood eosinophil count7 at V0 FeNO7 at V0 and V6 |
|
| |
| Pulmonary Physiology | Bronchodilator response at V6 Mean and variance of FEV1 (% predicted) between V0 and V6 Mean and variance of FEV1/FVC between V0 and V6 |
|
| |
| Environmental Tobacco Smoke Exposure | Number of smokers in the home at Screening
(categorized17) NicAlert result18 at Screening |
|
| |
| Vitamin D | Total 25-hydroxyvitamin D at V0 |
|
| |
| Stress | Caretaker Perceived Stress Scale at V0 |
|
| |
| Obesity | BMI z-score at Screening |
Composite Asthma Severity Index (CASI) component – Day symptoms includes measures of day asthma symptoms and albuterol use in the last 2 weeks (scoring range between 0 and 3).
CASI component – Night symptoms includes measures of night asthma symptoms and albuterol use in the last 2 weeks (scoring range between 0 and 3).
CASI component – Exacerbations includes hospitalizations and/or oral corticosteroid bursts in the last 2 months.
ACT is categorized as very poorly controlled (≤15), not well controlled (≥16 & ≤19), and well controlled (≥20)5,11,12,35. cACT is categorized as very poorly controlled (≤12), not well controlled (≥13 & ≤19), and well controlled (≥20) 9,10.
Rhinitis medication score is set to 0 for no medications, 5 for antihistamines only, 10 for nasal steroids only, and 15 for antihistamines and nasal steroids.
Rhinitis symptom score is based on the Modified Rhinitis Symptom Utility Index.3
Variable is log10 transformed in the structural equation model.
Positive skin prick test and/or a positive specific IgE.
Alternaria tenuis (skin prick test) or Alternaria alternata (specific IgE), Aspergillus fumigatus (both skin prick test and specific IgE), Cladosporium herbarum (specific IgE only), Dermatophagoides farinae, Dermatophagoides pteronyssinus, German cockroach, American cockroach, mouse, rat, cat, dog, oak, pecan, birch, maple, Eastern 8 tree mix, ragweed mix (giant/short; skin prick test) or short ragweed (specific Ige), Timothy grass, Kentucky Blue/June, Orchard and Timothy (K-O-T) grass mix, peanut, egg and milk.
Alternaria tenuis (skin prick test) or Alternaria alternata (specific IgE), Aspergillus fumigatus (both skin prick test and specific IgE), Cladosporium herbarum (specific IgE only).
Dermatophagoides farinae and Dermatophagoides pteronyssinus.
German cockroach and American cockroach.
Mouse and rat.
Cat and dog.
Oak, pecan, birch, maple, Eastern 8 tree mix, ragweed, Timothy grass, K-O-T grass mix and peanut.
Egg and milk.
Number of smokers in the home is categorized as 0, 1 and >1.
Analyzed as a continuous variable in the structural equation model.
Total serum IgE and allergen-specific IgE (ImmunoCAP, Phadia, Uppsala Sweden) to a panel of 20 allergens (17 common inhalant allergens and egg, milk, peanut) were measured from blood drawn at V0. Prick skin testing was performed at Screening for 12 common indoor and outdoor allergens. Allergen sensitization was defined by a wheal ≥ 3mm larger than the saline control on prick skin testing or specific IgE ≥ 0.35 kUA/L. A total of 22 allergen sensitizations were assessed and grouped in clusters (described in Pongracic et al).3 The blood sample at V0 was also used to measure blood eosinophil count (cells/mm3) and serum vitamin D concentration, determined by serum total 25-hydroxyvitamin D using high performance liquid chromatography.
Spirometry was performed at every asthma/rhinitis management visit, and bronchodilator reversibility was performed at V6. Acceptability was determined by American Thoracic Society/European Respiratory Society guidelines.13 For children under 8 years of age, American Thoracic Society Preschool Guidelines were used.14 FeNO was measured at V0 and V6 using the NIOX MINO device (Aerocrine, Stockholm Sweden).
Obesity was determined at Screening by Body Mass Index z-score based on CDC guidelines.15 Stress was measured in the caretaker at V0 using the 10-item Perceived Stress Scale.16 ETS exposure was assessed at Screening by the number of smokers in the home, and urine cotinine was measured using NicAlert™test strips (Nymox Pharmaceutical Corp). The NicAlert™test semi-quantitatively tests for cotinine using levels, which correspond to concentration ranges. Levels 0–2 (<100 ng/ml) indicate a non-user of tobacco products and levels 3–6 (>100 ng/ml) indicate a user of tobacco products. The lower limit of detection is 10 ng/ml or level 1.17 Compared to liquid chromatography-tandem mass spectrometry (LC-MS/MS), NicAlert® test strips have 91% specificity, 92% sensitivity, 94% positive predictive value, and 88% negative predictive value.18
Statistical methods
To test our conceptual model of asthma severity in Figure 1, a causal network analysis was implemented using structural equation models19 to simultaneously perform: 1) a confirmatory factor analysis of each domain in Table I to estimate the relationship between the domain of interest and each observed variable used to measure the domain; and 2) a series of regression models to estimate the standardized direct effect of each independent domain on a dependent domain (represented by arrows in Figure 1). To interpret the estimated effects from this model, note that: 1) increases in the domains of Asthma Severity, Allergen Sensitization, Allergic Inflammation, Rhinitis Severity, ETS Exposure and Obesity are interpreted as poorer outcomes while increases in the domains of Pulmonary Physiology and Vitamin D are interpreted as better outcomes; and 2) a positive direct effect is interpreted as the standard deviation increase in the dependent domain for every one standard deviation increase in the independent domain while a negative (or inverse) direct effect is interpreted as the standard deviation decrease in the dependent domain for every one standard deviation increase in the independent domain.. To account for potential confounding from demographic variables, all estimates were adjusted for age at Screening, sex and race. All models were fit using Mplus Version 7.320.
In cases where our conceptual model indicated that the association between the independent and dependent domains could be mediated by another domain, we tested for mediated effects using the approach of Baron and Kenny,21 which splits the estimated total effect of the independent domain on the dependent domain into a sum of two parts: 1) The indirect effect of the independent domain on the dependent domain acting through a mediating domain or pathway; and 2) The direct effect of the independent domain on the dependent domain, adjusting for the effect of any other domains that are hypothesized to be associated with the dependent domain. Evidence of mediation was found if the following three effects were statistically significant at the 0.05 significance level: 1) The total effect of the independent domain on the dependent domain; 2) The direct effect of the independent domain on the mediating domain or pathway; and 3) The direct effect of the mediating domain or pathway on the dependent domain. In cases where our conceptual model only indicated an indirect effect between distal domains (and so no test of mediation could be conducted), only the indirect effect between the domains was estimated.
Because controller treatment step level is partially determined by measures of Pulmonary Physiology, a sensitivity analysis was performed in which Asthma Severity was re-defined by removing the mean and variance of controller treatment step level between V0 and V6.
RESULTS
Participant Characteristics
To ensure adequate longitudinal data on clinical asthma severity and rhinitis severity, of 717 APIC participants enrolled, 98 participants (13.7%) were excluded because they did not complete at least 4 of the 6 Evaluation & Management visits, and 40 additional participants (5.6%) were excluded because they did not have rhinitis. Participants without rhinitis were excluded because rhinitis severity, a domain in our hypothetical model of asthma severity, was not measured. An additional 18 study participants (2.5%) were excluded due to missing race classification, Caretaker Perceived Stress Scale at V0, BMI z-score at Screening or total 25-hydroxyvitamin D at V0. This resulted in an analytic sample size of 561 participants (78.2% of the APIC cohort). Characteristics of the 561 participants are reported in Table E1 in the Online Repository. Overall, the median age at Screening was 10.8 years, 57.8% were male and 64.4% self-identified as non-Hispanic Black while 28.5% self-identified as Hispanic. Additionally, 55.1% were from households with annual income less than $15,000.
Domains
Standardized estimates of the effect of each domain on each of the observed variables comprising each domain are shown in Figures E1A – E1F in the Online Repository. All variables were significantly associated with their respective domains except for one variable in Asthma Severity (variability in controller treatment step across V0 – V6).
Pathways
Figure 2 provides standardized estimates of the direct effects of each independent domain on each dependent domain for all pathways in our conceptual model using the APIC data; significant indirect effects are also indicated by the thick arrows. To address potential confounding arising from demographic covariates, all estimates were adjusted for age, sex and race. Table E2 in the Online Repository provides standardized estimates of the effects of age, sex and race on each domain. This model accounted for 53.4% of the variation in Asthma Severity. Root Mean Square Error of Approximation, a commonly used measure of how well a structural equation model fits the observed data, was 0.063 (90% CI: (0.060, 0.067)), indicating an adequate fit.22 Interpretation of these results by pathways is discussed in the following sections.
FIGURE 2.
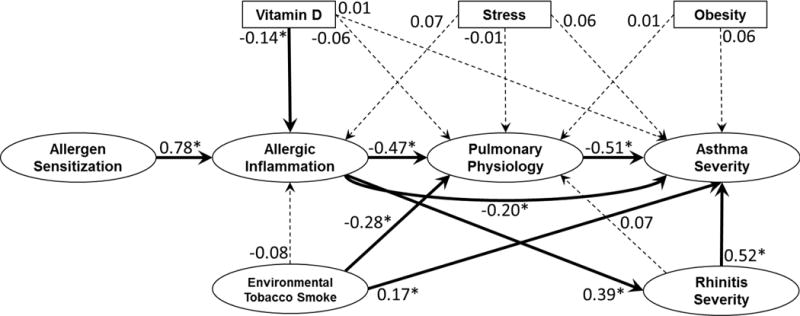
Direct and Indirect Effects of All Pathways. Estimates are standardized direct effects that are interpreted as the standard deviation increase in the dependent domain for every one standard deviation increase in the independent domain. Estimates with associated p-values that are less than 0.05 are denoted by * and solid lines. Thick lines further denote statistically significant pathways by tests of indirect effects and mediation. Statistically insignificant estimates are denoted by dashed lines. All estimates are adjusted for age, sex and race.
Allergy Pathway
Figure 3A highlights the pathways to Asthma Severity through Allergen Sensitization, Allergic Inflammation, Pulmonary Physiology and Rhinitis Severity. Our results showed that:
Allergen Sensitization was positively associated with Allergic Inflammation. Specifically, for every 1 standard deviation increase in Allergen Sensitization, Allergic Inflammation increased by 0.78 standard deviations (direct effect: 0.78±0.04; p<0.001). Comparing this standardized estimate to all others, this association is the strongest one detected.
Allergic Inflammation was inversely associated with Pulmonary Physiology (direct effect: −0.47±0.05; p<0.001) and positively associated with Rhinitis Severity (direct effect: 0.39±0.06; p<0.001).
Pulmonary Physiology was inversely associated with Asthma Severity (direct effect: −0.51±0.06; p<0.001).
Rhinitis Severity was positively associated with Asthma Severity (direct effect: 0.52±0.06; p<0.001).
FIGURE 3.
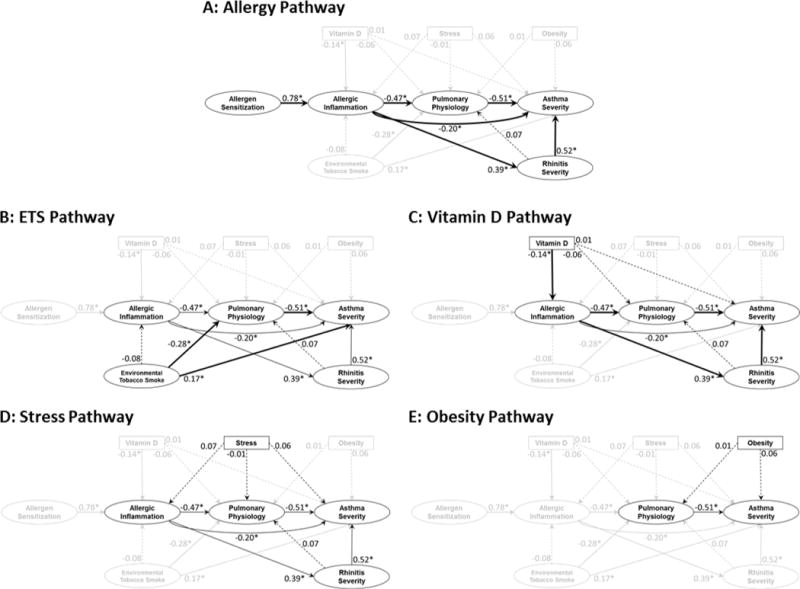
Pathways to Asthma Severity. Estimates are standardized direct effects that are interpreted as the standard deviation increase in the dependent domain for every one standard deviation increase in the independent domain. Estimates with associated p-values that are less than 0.05 are denoted by * and solid lines. Thick lines further denote statistically significant pathways by tests of indirect effects and mediation. Statistically insignificant estimates are denoted by dashed lines. All estimates are adjusted for age, sex and race.
Analysis of indirect effects within the Allergy Pathway domains on Asthma Severity showed that Allergen Sensitization was positively associated with Asthma Severity via Allergic Inflammation, Pulmonary Physiology and Rhinitis Severity such that the standardized total effect was 0.18±0.05; (p<0.001; Table E3 in the Online Repository). While Allergic Inflammation was inversely associated with Asthma Severity (direct effect: −0.20±0.08; p=0.01), the total effect of Allergic Inflammation on Asthma Severity was positive (0.22±0.06; p<0.001; Table E4 in the Online Repository) because the effect of Allergic Inflammation on Asthma Severity was mediated by Pulmonary Physiology and Rhinitis Severity (Table E4 in the Online Repository).
ETS Pathway
Figure 3B highlights the pathways from ETS exposure to Asthma Severity. Our results showed that ETS exposure was inversely associated with Pulmonary Physiology (direct effect: −0.28±0.07; p<0.001) and positively associated with Asthma Severity (direct effect: 0.17±0.08; p=0.03). Additionally, the total effect of ETS exposure on Asthma Severity was mediated by Pulmonary Physiology (Table E4 in the Online Repository). There was no evidence of an indirect effect of ETS exposure on Asthma Severity via Allergic Inflammation.
Vitamin D Pathway
Figure 3C highlights the pathways from Vitamin D to Asthma Severity. Our results showed that Vitamin D was inversely associated with Allergic Inflammation (direct effect: −0.14±0.06; p=0.02), but not directly associated with Pulmonary Physiology (−0.06±0.05; p=0.23) or Asthma Severity (0.01±0.05; p=0.82). Mediation analyses (Table E4 in the Online Repository) revealed modest indirect associations between Vitamin D and Asthma Severity via Allergic Inflammation and Pulmonary Physiology (−0.04±0.02; p=0.03), and through Allergic Inflammation and Rhinitis Severity (−0.03±0.01; p=0.04). However, the total effect of Vitamin D on Asthma Severity was insignificant (0.01±0.05; p=0.89).
Stress Pathway
Figure 3D highlights the pathways from Stress to Asthma Severity. Our results showed that Stress was not directly associated with Allergic Inflammation (0.07±0.05; p=0.19), Pulmonary Physiology (−0.01±0.05; p=0.90), or Asthma Severity (0.06±0.05; p=0.22), nor were there significant indirect or total effects on Asthma Severity (Table E4 in the Online Repository).
Obesity Pathway
Figure 3E highlights the pathways from Obesity to Asthma Severity. Our results showed that Obesity was not directly associated with Pulmonary Physiology (0.01±0.05; p=0.77) or Asthma Severity (0.06±0.04; p=0.11), nor were there significant indirect or total effects on Asthma Severity (Table E4 in the Online Repository). While previous literature in the inner city has suggested that the effect of obesity on asthma control was significant in females but not in males,23 a similar effect was not seen in national data.24 Because this effect was not consistent within the literature, we tested if the direct effect of Obesity on Asthma Severity differed by sex by including an interaction between sex and Obesity. However, our results showed no evidence that the effect of Obesity on Asthma Severity differed by sex (p=0.42) and so we did not account for this effect in our final model.
Sensitivity Analysis
Because controller treatment step at each visit was partially determined by measures included in the Pulmonary Physiology domain, a sensitivity analysis that re-defined Asthma Severity without the mean and variance of controller treatment step across V0 and V6 was performed and showed similar results (results not shown).
DISCUSSION
We sought to integrate host and environmental factors known to influence asthma severity into pathways so that we can focus on important pathophysiologic aspects of the disease and target interventions for improvement of asthma management. To our knowledge, this is the first study to develop and test a pathways construct of asthma severity and determine the relative contributions of these factors and their pathways using a structural equation modeling approach. We had the benefit of applying this analytical approach to a large cohort of children with asthma living in 9 geographically distributed U.S. inner cities, which was designed to define phenotypic characteristics of asthma. The APIC study differed from most asthma phenotypes studies in that it included prospective, standardized guidelines-based asthma and rhinitis management aimed at achieving optimal control of both lower and nasal airways disease activity. The APIC study also included participants with a wide range of asthma severity (i.e., not limited to children with severe asthma), allowing for wide ranges of all outcomes of interest in our model.3,4 Characterizations of asthma and rhinitis severity were based on conventional composite assessments of day and night symptoms, exacerbations, and controller usage.5–7,25 Altogether, this pathways construct accounted for 53.4% of the observed variance in asthma severity in this study, a remarkable effect considering the complexity of this condition.
Our model supported the concept that multiple pathways contribute significantly to asthma severity and, importantly, that allergen sensitization is perhaps the originating domain in this pathophysiologic chain. Based on the model:
Allergy acts through more than one pathway to exert its effect on asthma severity
Our analysis verified sequential links to asthma severity, from allergen sensitization to allergic inflammation, and subsequently through pulmonary physiology or through rhinitis severity to asthma severity. This implies that conventional management does not adequately mitigate the effects of allergen sensitization on downstream airways inflammation, obstruction and dysfunction. We can infer that more focus should be directed to interventions to suppress the allergy pathway to ameliorate asthma above and beyond the improvement achieved by current guidelines-based care.
ETS exposure exerts significant direct and indirect (via pulmonary physiology) effects on asthma severity
There are significant direct and indirect effects of ETS exposure on asthma severity. ETS exposure was based on two semi-quantitative measures: number of smokers in the home by self-report and urine cotinine (a nicotine metabolite) by NicAlert test strips as an indicator of any ETS exposure. Studies using both measures have ranged from finding a high correlation between them, while other studies have found substantial discordance, with health effects being associated with one measure or the other, leading some investigators to conclude that both measures are useful and relevant when investigating the impact of ETS exposure on respiratory disease in children.26 Accordingly, ETS exposure assessments for this study included both and have been compared in Table E5 in the Online Repository. We found a significant association between self-reported smokers in the home and urine cotinine levels (p<0.001); however, urine cotinine was detectable in 80% of study participants reporting no tobacco smokers in the home. This reiterates the importance of both assessments of as well as measures to reduce ETS exposure to improve asthma in the context of guidelines-based care.
Rhinitis severity had a remarkably strong direct effect on asthma severity
This supports the relevance of the “one airway” concept of disease (“from nostrils to alveoli”) for rhinitis in asthma6,27 and represents an important observation for investigators and clinicians that are studying and aiming to optimize asthma control. It is interesting that rhinitis severity not directly impact pulmonary physiology, as some of the proposed mechanisms of the nose-lung interaction would predict.22 The effect may be driven by the physiologic consequences of oral breathing that is quite common in children in the presence of rhinitis. By losing the filtering and other air-conditioning capacity of the nasal passages, the lower airways may be rendered more susceptible to environmental stimuli, including allergens, irritants and infectious agents.
Other pathways contributed less to asthma severity
While Vitamin D had modest but significant indirect effects on asthma severity mediated by allergic inflammation, pulmonary physiology and rhinitis severity, the direct and total effect of Vitamin D on asthma severity were both insignificant. We also tested multiple pathways from stress to asthma severity and found them to be insignificant. Similarly, obesity was not significantly associated with asthma severity, nor was the effect of obesity on asthma severity modified by gender (data not shown). The lack of evidence regarding the effect of these domains on asthma severity does not mean that these are insignificant factors and might have been limited by our choice of variable(s) used to represent these domains or the conceptual model itself. For example, our choice of caretaker Perceived Stress Scale is a standard measure often used and recommended to assess the effect of stress on asthma.16,28 While other aspects of psychosocial stress not measured by this scale might have greater effects on asthma severity, they were not obtained in APIC. As well, our study sample consisted primarily of Black or Hispanic children with higher BMIs. Consequently, our inability to detect significant effects of BMI on asthma severity may be due to a sample that is not representative of other study populations where this type of effect has been detected.
An important element of this analysis is that we can report standardized total, as well as direct and indirect effects of each domain on asthma severity. Comparing the standardized total effect sizes on asthma severity (Table E4 in the Online Repository), we found that pulmonary physiology and rhinitis severity had the largest standardized total effect sizes (−0.51 and 0.48, respectively, p<0.001), followed by ETS exposure (0.30, p<0.001) and allergic inflammation (0.22, p<0.001). We were also able to determine whether some domains were significant mediators of the effects of others. Indeed, as we had hypothesized in constructing the model, the APIC dataset confirmed an indirect effect of allergen sensitization on asthma severity via allergic inflammation, which then branched out into effects on pulmonary physiology and rhinitis severity; and the ETS effect on asthma severity is partially mediated through pulmonary physiology.
This investigation has limitations. Because this is an observational study, the direct, indirect and total effects are estimates of statistical association rather than causation or clinical relevance. Exclusion of 18 participants with missing covariate data could also limit the generalizability of these findings. Compared to included participants, excluded participants had lower rhinitis symptom scores, lower variance of FEV1 (% predicted), higher mean FEV1/FVC, lower variance of FEV1/FVC and lower CASI night symptoms & albuterol use. Their influence on the results is limited because they represent only 18/579=3.1% of participants in our study. The domains, and the observed variables used to measure each domain, could be missing potentially important host and environmental factors such as other relevant environmental exposures and aspects of diet/nutrition. For example, cockroach allergen sensitization with high levels of exposure has been shown to have greater effects on asthma severity in inner-city children than sensitization alone,29 so including allergen exposure in this model could have strengthened the allergy pathway. Since allergen measurements in house dust samples were obtained in only a subset of APIC participants, we did not include allergen exposure in this model. Some of our variables may lack adequate precision, validity and/or domain representation to observe significant or strong pathway associations. For example, the Stress domain is represented by the 10-question caretaker Perceived Stress Score (PSS), which was recommended in the NIH/AHRQ-sponsored Asthma Outcomes Workshop Expert Report as a brief, well-validated, and simply administered measure of global stress.28 However, the PSS does not necessarily capture other psychosocial mediators of asthma outcomes that are common in inner-city living and have been linked to asthma severity, such as violence and severely negative life events.30–33 Some potential associations were not tested (e.g., stress affecting asthma severity via BMI and/or ETS exposure) and could be explored in future work. Family history of asthma was not included in our conceptual model since, to our knowledge, family history of asthma has not been associated with asthma severity as it has with asthma prevalence. We recognize that clear evidence of the genetic contribution to asthma severity exists, such as the association of the CDHR3 gene locus to severe asthma exacerbations in young children.34 Another potential limitation of this study is its conduct in children with asthma living in U.S. inner cities; extrapolation to children living in other locales and/or to adults would benefit from validation in those populations.
In conclusion, our causal network analysis is a rigorous investigative approach to develop and test a literature-based model of essential factors underlying asthma severity. This approach provides standardized effect sizes that are useful comparators of the relative contributions of different domains and can be used as a basis for prioritizing interventions to improve disease severity and management. With our complete model explaining more than half of the variability in asthma severity, the strong contributors are high priority targets for improving asthma outcomes in addition to standard care: allergen sensitization, allergic inflammation, ETS exposure, pulmonary physiology and rhinitis. These findings demonstrate the usefulness of causal network analysis in formulating a blueprint for pathway-targeted interventional studies to improve future guidelines-directed care.
Supplementary Material
Capsule Summary.
This analysis demonstrates how allergen sensitization and inflammation, pulmonary physiology, environmental tobacco smoke exposure and rhinitis severity explain asthma severity, and may serve as a roadmap to improve asthma outcomes.
Acknowledgments
Acknowledgements: We are grateful to the APIC study participants and their families who gave of themselves to be our investigational partners; our study staff personnel who are dedicated to our inner-city asthma mission and clinical research excellence; and Christine Sorkness and Patrick Heinritz in the ICAC Administrative Center (Madison, WI) and Samuel Arbes, Michelle Walter, and Herman Mitchell at Rho, Inc. for their leadership, commitment to excellence despite all of the challenges, and their legacies in inner-city asthma research.
Funding Sources: This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract numbers HHSN272200900052C and HHSN272201000052I, and 1UM1AI114271-01. Additional support was provided by the National Center for Research Resources, and National Center for Advancing Translational Sciences, National Institutes of Health, under grants NCRR/NIH UL1TR000451, UL1RR025780, UL1TR000075 and NCATS/NIH UL1TR000154, UL1TR001082, UL1TR000077-04, UL1TR000040, UL1TR000150, and UL1TR001105. Glaxo SmithKline (GSK) provided Ventolin, Flovent, Advair and Flonase under a clinical trial agreement with NIH NIAID; GSK did not have a role in the development or approval of the protocol, conduct of the trial, data analysis, manuscript preparation, or the decision to submit for publication.
Abbreviations
- NIH
National Institutes of Health
- NIAID
National Institutes of Allergy and Infectious Diseases
- ICAC
Inner City Asthma Consortium
- APIC
Asthma Phenotypes in the Inner City
- ETS
Environmental Tobacco Smoke
- CASI
Composite Asthma Severity Index
- ACT/cACT
Asthma Control Test/Childhood Asthma Control Test
- FeNO
Fractional exhaled Nitric Oxide
- FEV1
Forced Expiratory Volume in the first second
- FEV1/FVC
Ratio of Forced Expiratory Volume in the first second to forced vital capacity
- IgE
Immunoglobulin E
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Implications: In children with asthma and rhinitis, allergen sensitization, allergic inflammation, pulmonary physiology, environmental tobacco smoke exposure and rhinitis severity are related through specific pathways to asthma severity.
References
- 1.Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065–72. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teach SJ, Gergen PJ, Szefler SJ, et al. Seasonal risk factors for asthma exacerbations among inner-city children. J Allergy Clin Immunol. 2015;135:1465–73e5. doi: 10.1016/j.jaci.2014.12.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pongracic JA, Krouse RZ, Babineau DC, et al. Asthma Phenotypes in the Inner City: Distinguishing Characteristics of Difficult-to-Control Asthma in Children. J Aller Clin Immunol Submitted simultaneously. doi: 10.1016/j.jaci.2016.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoratti EM, Krouse RZ, Babineau DC, et al. Asthma phenotypes in inner-city children. J Aller Clin Immunol Submitted simultaneously. doi: 10.1016/j.jaci.2016.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. U.S. Department of Health and Human Services; 2007. http://www.nhlbi.nih.gov/guidelines/asthma/ [Google Scholar]
- 6.Bousquet J, Van Cauwenberge P, Khaltaev N, Aria Workshop G, World Health O. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108:S147–334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 7.Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–76. doi: 10.1016/j.jaci.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 8.Wildfire JJ, Gergen PJ, Sorkness CA, et al. Development and validation of the Composite Asthma Severity Index–an outcome measure for use in children and adolescents. The Journal of allergy and clinical immunology. 2012;129:694–701. doi: 10.1016/j.jaci.2011.12.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu AH, Zeiger R, Sorkness C, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007;119:817–25. doi: 10.1016/j.jaci.2006.12.662. [DOI] [PubMed] [Google Scholar]
- 10.Liu AH, Zeiger RS, Sorkness CA, et al. The Childhood Asthma Control Test: retrospective determination and clinical validation of a cut point to identify children with very poorly controlled asthma. J Allergy Clin Immunol. 2010;126:267–73. 73 e1. doi: 10.1016/j.jaci.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549–56. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Schatz M, Mosen DM, Kosinski M, et al. Validity of the Asthma Control Test completed at home. Am J Manag Care. 2007;13:661–7. [PubMed] [Google Scholar]
- 13.Standardization of Spirometry, 1994 Update. American Thoracic Society. American journal of respiratory and critical care medicine. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 14.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. The European respiratory journal. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital and health statistics Series 11, Data from the national health survey. 2002:1–190. [PubMed] [Google Scholar]
- 16.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of health and social behavior. 1983;24:385–96. [PubMed] [Google Scholar]
- 17.NicAlert®: Product Insert (Urine Samples) 2016 Accessed 2132016 at http://www.nymox.com/default.action?itemid=47.
- 18.Yeh E, Levasseur G, Kaiserman MJ. Evaluation of urinary cotinine immunoassay test strips used to assess smoking status. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2011;13:1045–51. doi: 10.1093/ntr/ntr127. [DOI] [PubMed] [Google Scholar]
- 19.MacKinnon DP. Introduction to statistical mediation analysis. New York: Lawrence Erlbaum Associates; 2008. [Google Scholar]
- 20.Muthén LKM BO. Mplus User’s Guide. 7th. Los Angeles, CA: Muthén & Muthén; pp. 1998–2015. [Google Scholar]
- 21.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 22.MacCallum RC, Brown MW, Sugawara HM. Power Analysis and Determination of Sample Size for Covariance Structure Modeling. Psychological Methods. 1996;1:130–49. [Google Scholar]
- 23.Kattan M, Kumar R, Bloomberg GR, et al. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010;125:584–92. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visness CM, London SJ, Daniels JL, et al. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999–2006. The Journal of asthma : official journal of the Association for the Care of Asthma. 2010;47:822–9. doi: 10.3109/02770903.2010.489388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA) 2015. 2015 Accessed October 10, 2015, 2015, at http://www.ginasthma.org.
- 26.Gergen PJ. Environmental tobacco smoke as a risk factor for respiratory disease in children. Respiration physiology. 2001;128:39–46. doi: 10.1016/s0034-5687(01)00263-8. [DOI] [PubMed] [Google Scholar]
- 27.Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003;111:1171–83. doi: 10.1067/mai.2003.1592. quiz 84. [DOI] [PubMed] [Google Scholar]
- 28.Rand CS, Wright RJ, Cabana MD, et al. Mediators of asthma outcomes. J Allergy Clin Immunol. 2012;129:S136–41. doi: 10.1016/j.jaci.2011.12.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. The New England journal of medicine. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 30.Sandberg S, Jarvenpaa S, Penttinen A, Paton JY, McCann DC. Asthma exacerbations in children immediately following stressful life events: a Cox’s hierarchical regression. Thorax. 2004;59:1046–51. doi: 10.1136/thx.2004.024604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandberg S, Paton JY, Ahola S, et al. The role of acute and chronic stress in asthma attacks in children. Lancet. 2000;356:982–7. doi: 10.1016/S0140-6736(00)02715-X. [DOI] [PubMed] [Google Scholar]
- 32.Wright RJ, Mitchell H, Visness CM, et al. Community violence and asthma morbidity: the Inner-City Asthma Study. Am J Public Health. 2004;94:625–32. doi: 10.2105/ajph.94.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright RJ, Steinbach SF. Violence: an unrecognized environmental exposure that may contribute to greater asthma morbidity in high risk inner-city populations. Environmental health perspectives. 2001;109:1085–9. doi: 10.1289/ehp.011091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonnelykke K, Sleiman P, Nielsen K, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nature genetics. 2014;46:51–5. doi: 10.1038/ng.2830. [DOI] [PubMed] [Google Scholar]
- 35.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. The Journal of allergy and clinical immunology. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


