Abstract
Mitochondria perform diverse yet interconnected functions, producing ATP and many biosynthetic intermediates while also contributing to cellular stress responses such as autophagy and apoptosis. Mitochondria form a dynamic, interconnected network that is intimately integrated with other cellular compartments. In addition, mitochondrial functions extend beyond the boundaries of the cell and influence an organism’s physiology by regulating communication between cells and tissues. It is therefore not surprising that mitochondrial dysfunction has emerged as a key factor in a myriad of diseases, including neurodegenerative and metabolic disorders. We provide a current view of how mitochondrial functions impinge on health and disease.
Introduction
Mitochondria arose from an alpha-proteobacterium engulfed by a eukaryotic progenitor (Lane and Martin, 2010). Like their bacterial ancestor, mitochondria are comprised of two separate and functionally distinct outer (OMs) and inner membranes (IMs) that encapsulate the intermembrane space (IMS) and matrix compartments. They also contain a circular genome, mitochondrial DNA (mtDNA), that has been reduced during evolution through gene transfer to the nucleus. mtDNA is organized into discrete nucleoids in the matrix. Interestingly, the closest relatives of many mtDNA-modifying enzymes, such as mtDNA polymerase, are bacteriophage proteins (Lecrenier et al., 1997; Tiranti et al., 1997), suggesting that an infection of the mitochondrial ancestor contributed to the development of mtDNA maintenance machinery. In animals, mtDNA inheritance is almost exclusively maternal, and paternal mtDNA is actively destroyed in many species immediately after fertilization (Al Rawi et al., 2011; Sato and Sato, 2011).
Advances in proteomic, genomic, and bioinformatic approaches have provided a comprehensive inventory of mitochondrial proteins in various eukaryotes (Gaston et al., 2009; Mootha et al., 2003; Pagliarini et al., 2008; Sickmann et al., 2003). This inventory indicates that mammalian mitochondria contain over 1,500 proteins, which vary in a tissue-dependent manner. Because mtDNA encodes only 13 of these proteins, mitochondria depend on the nucleus and other cellular compartments for most of their proteins and lipids. Nuclear-encoded mitochondrial proteins are actively imported and sorted into each mitochondrial compartment (Neupert and Herrmann, 2007; Schmidt et al., 2010), followed by coordinated assembly into macromolecular complexes, comprised of subunits encoded by nuclear and mitochondrial DNA.
Although mammalian mitochondria have retained some bacterial features, it is estimated that only a small percentage of human mitochondria are derived from the original endosymbiont (Gabaldón and Huynen, 2004). However, the bacterial ancestry of mitochondria and bacteriophage-related mtDNA maintenance systems make the organelle susceptible to antimicrobial drugs: for example, mitochondrial translation is targeted by common antibiotics that block microbial ribosomes (amino-glycosides, tetracyclines) (Hutchin et al., 1993; van den Bogert and Kroon, 1981), and mtDNA maintenance is affected by antiviral nucleoside analogs (Arnaudo et al., 1991). The genetic risk factors underlying drug sensitivity of mitochondrial function are expected to be numerous, but challenging to identify.
The considerable resources a cell must provide to maintain the mitochondrial compartment underscores the varied essential roles it plays. This is further demonstrated by the fact that mitochondrial dysfunction is associated with an increasingly large proportion of human inherited disorders and is implicated in common diseases, such as neurodegenerative disorders, cardiomyopathies, metabolic syndrome, cancer, and obesity. Below we review new developments in mitochondrial biology and discuss their relevance for human disease.
Mitochondrial Defects Cause Diverse and Complex Human Diseases
Human mitochondrial disorders are a genetically heterogeneous group of different diseases, caused by mutations in mitochondrial and/or nuclear DNA, which encompass almost all fields of medicine (Ylikallio and Suomalainen, 2012). Mitochondrial diseases can affect any organ system, manifest at any age, and, depending on where the gene defect lies, be inherited from an autosome, the X chromosome, or maternally. Currently, mitochondrial disorders cannot be cured, and available treatments are directed at relieving symptoms (Suomalainen, 2011).
Mitochondrial diseases display both clinical heterogeneity and have tissue-specific manifestations, as indicated by the fact that mutations in the same mitochondrial protein complex lead to disparate disease phenotypes. For example, defects in respiratory complex I can lead to atrophy of the optic nerve in adults (Wallace et al., 1988) or subacute necrotizing encephalopathy in infants (Morris et al., 1996). The most common nuclear mutations associated with mitochondrial diseases are found in the gene encoding mitochondrial DNA polymerase γ and can manifest as early-onset hepatocerebral disorder, juvenile catastrophic epilepsy, or adult-onset ataxia-neuropathy syndrome (Euro et al., 2011; Hakonen et al., 2005; Naviaux et al., 1999; Van Goethem et al., 2001). Another example of clinical variability is exhibited by the recently characterized disease group linked to defects in mitochondrial aminoacyl-transfer RNA (tRNA) synthetases (ARS2s), whose known essential function is to join a mitochondrial tRNA with its cognate amino acid to be transfered to the ribosome for protein synthesis. ARS2 defects promote a variety of phenotypes, including cardiomyopathies, cerebral white matter disease, ovarial dysfunction, and hearing loss. (Scheper et al., 2007; Götz et al., 2011; Pierce et al., 2011) The nature of the molecular defect can often explain variations in the severity and age-of-onset of these diseases, but not the variability in tissue-specific manifestations, which may instead be defined by a patient’s complement of protective and risk alleles.
Phenotypic variability associated with mtDNA-linked diseases is also due, in part, to the high copy number of mtDNA in mammalian cells, which can consequently contain both mutant and wild-type mtDNAs populations—a situation called heteroplasmy (Holt et al., 1988). While mtDNA mutations in tRNA genes possess high clinical variability not explained by heteroplasmy, in the case of protein-coding gene mutations, the degree of heteroplasmy correlates with the severity of phenotypes. For example, for the T8993C/G mutation of mtDNA, affecting ATPase6, a low mutant load causes pigment retinopathy, ataxia, and neuropathy in adults, whereas a high mutant load causes maternally inherited Leigh syndrome in infants (Holt et al., 1990; Tatuch et al., 1992).
Heteroplasmy can be affected by segregation of mtDNA and by selective mitochondrial degradative pathways. Examples of nonrandom segregation include the nonrandom segregation of neutral mtDNA variants in mice (Battersby et al., 2005; Jokinen et al., 2010), and, in humans, the A3243G tRNALeu(UUR) mutation, whose load decreases in blood over years (Rahman et al., 2001). In mice, cells with allogenic mtDNA are recognized and targeted by the innate immune system, indicating that mitochondrial DNA-dependent antigen presentation may play a role in mtDNA selection (Ishikawa et al., 2010). Selection of mtDNA may also occur during oogenesis: in mice, mtDNA mutations in protein-coding genes are underrepresented in offspring, suggesting a mechanism that selectively eliminates cells or organelles with the most severe mutations (Fan et al., 2008; Stewart et al., 2008). Surprisingly, the fundamental molecular mechanisms underlying the process of mtDNA distribution in cells and its tissue specificity are poorly understood, given that an understanding of how the nucleoid is regulated is crucial to understanding mitochondrial diseases.
Mitochondria Are Metabolic Signaling Centers
The diverse nature of mitochondrial diseases highlights the many roles mitochondria play in cells and tissues. Mitochondria are best known for producing ATP via oxidative phosphorylation (OXPHOS). In the matrix, tricarboxylic acid cycle (TCA) enzymes generate electron carriers (NADH and FADH2), which donate electrons to the IM-localized electron transport chain (ETC). The ETC consists of four protein machines (I–IV), which through sequential redox reactions undergo conformational changes to pump protons from the matrix into the IMS. The first and largest of the respiratory complexes, complex I, is a sophisticated microscale pump consisting of 45 core subunits, whose biogenesis requires an army of assembly factors (Diaz et al., 2011; Efremov and Sazanov, 2011). The proton gradient generated by complexes I, III, and IV is released through the rotary turbine-like ATP synthase machine or complex V, which drives phosphorylation of ADP to ATP (Okuno et al., 2011; Stock et al., 1999). Beyond ATP production, the inner-membrane electrochemical potential generated by OXPHOS is a vital feature of the organelle (Mitchell, 1961). Membrane potential is harnessed for other essential mitochondrial functions, such as mitochondrial protein import (Neupert and Herrmann, 2007), and is used to trigger changes on the molecular level that alter mitochondrial behaviors in response to mitochondrial dysfunction.
Complexes I and III also generate reactive oxygen species (ROS), including oxygen radicals and hydrogen peroxide, which can damage key components of cells, including lipids, nucleic acids, and proteins (Muller et al., 2004; Murphy, 2009). ROS has been suggested to contribute to diseases associated with mitochondrial dysfunction, including neurodegeneration. For example, Leber’s hereditary optic neuropathy is associated with mutations that alter the ubiquinone binding pocket of mtDNA-encoded complex I subunits (Pätsi et al., 2008) that likely affect electron delivery from the FeS centers of complex I to ubiquinone, leading to an overreduction of FeS clusters, electron leak, and oxygen radical production.
Multiple lines of evidence indicate that mitochondrial ROS also influence homeostatic signaling pathways to control cell proliferation and differentiation and to contribute to adaptive stress signaling pathways, such as hypoxia (Hamanaka and Chandel, 2010). Observations from premature aging mouse models suggest that hematopoietic progenitors are especially sensitive to ROS and/or redox state changes that promote proliferation and prevent quiescence (Ito et al., 2004; Narasimhaiah et al., 2005). Interestingly, progeroid mtDNA Mutator mice, which accumulate mtDNA mutations, are severely anemic (Chen et al., 2009; Kujoth et al., 2005; Norddahl et al., 2011; Trifunovic et al., 2004) and have an early somatic stem cell dysfunction suppressed by n-acetyl-l-cysteine treatment. These observations imply that ROS/redox signaling affects somatic stem cell function and causes progeroid symptoms (Ahlqvist et al., 2012) and that mitochondrial dysfunction in somatic stem cells may contribute to aging-related degeneration.
In all cell types, mitochondria are the major cellular source of NADH and house parts of the pyrimidine and lipid biosynthetic pathways, including the fatty acid β-oxidation pathway, which converts long chain fatty acids to Acyl-CoA. Mitochondria also regulate cellular levels of metabolites, amino acids, and cofactors for various regulatory enzymes, including chromatin-modifying histone deacetylases. Moreover, mitochondria play a central role in metal metabolism, synthesizing heme and Fe-S clusters (Lill and Mühlenhoff, 2008), which are essential components of the major oxygen carrier, hemoglobin, as well as OXPHOS and DNA repair machinery. Mitochondria also participate in Ca2+ homeostasis, shaping the spatiotemporal distribution of this second messenger by buffering Ca2+ flux from the plasma membrane and endoplasmic reticulum (ER) (Baughman et al., 2011; De Stefani et al., 2011).
In neurons, the ability of mitochondria to modulate Ca2+ flux is essential for controlling neurotransmitter release, neurogenesis, and neuronal plasticity. In addition, mitochondria supply copious amounts of ATP as well as the TCA intermediates that serve as the building blocks for synthesis of GABA and glutamate neurotransmitters (Sibson et al., 1998; Waagepetersen et al., 2001). Compromised oxidative metabolism may therefore alter neurotransmitter levels and render the brain uniquely sensitive to oxidative energetic deficits, as has been shown for pyruvate carboxylase deficiency (Perry et al., 1985). Mitochondria-mediated lipid synthesis is also critical for neuronal function, as defects in lipoic acid synthase cause severe neonatal-onset epilepsy (Mayr et al., 2011). These additional metabolic functions of mitochondria depend, either directly or indirectly, on OXPHOS, and thus can be secondarily affected by changes in respiration and respiratory complex deficiency.
Mitochondria as Energy Sensors and Beacons
The central roles of mitochondria in metabolism position them as key actors in global energy modulation. An increased need for ATP is met by increasing mitochondrial mass and inducing OXPHOS. For example, an increase of mitochondrial mass and activity is observed after endurance exercise (Hoppeler and Fluck, 2003). The regulation of mitochondrial biogenesis is tightly coordinated with pathways that induce vascularization, enhance oxygen delivery to tissues, and enable oxygen supply to facilitate efficient mitochondrial oxidization of glucose and fat (Arany et al., 2008).
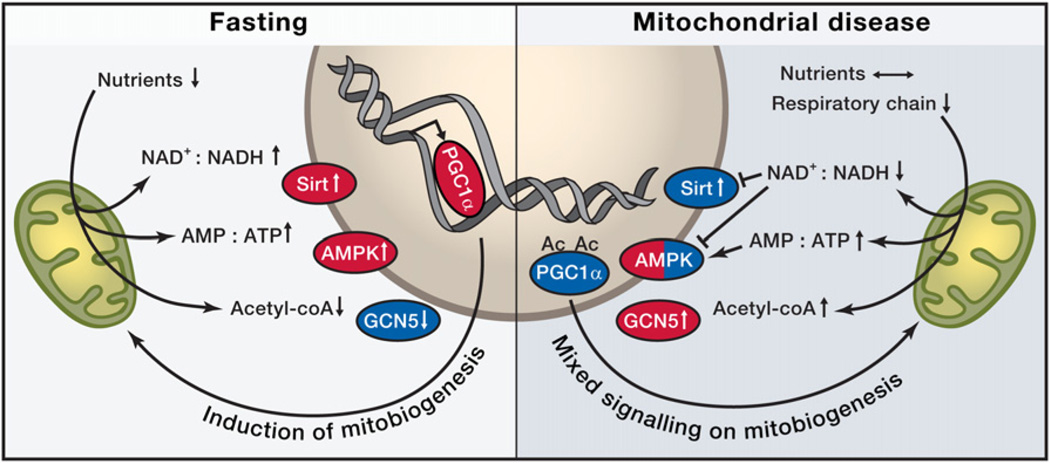
Mitochondrial metabolism is both the basis for and target of nutrient signals that ultimately orchestrate an integrated physiological response. The molecular components that sense energy status include transcription factors, hormones, cofactors, nuclear receptors, and kinases, which detect specific signals of mitochondrial activity, such as the NAD+:NADH ratio, the AMP:ATP ratio, or acetyl-CoA levels (Figure 1).
Figure 1. Nutrient Sensors in Fasting and Their Roles in Mitochondrial Disease.
Both fasting and mitochondrial disease can modify NAD+:NADH and AMP:ATP ratios through decreased nutrient availability or through reduced respiratory chain activity and have the potential to activate (red) nutrient sensors Sirtuin 1 (Sirt, an NAD+-dependent histone deacetylase) or AMP-activated kinase (AMPK) and increase mitochondrial biogenesis by activating peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1alpha). Upon decreased utilization of acetyl-coenzyme A (acetyl-coA), GCN5 (lysine acetyltransferase 2A) is activated and acetylates PGC1alpha, to inactivate it (blue). NAD+, nicotinamide adenine dinucleotide, oxidized form; NADH, nicotinamide adenine dinucleotide, reduced form; AMP, adenosine monophosphate; ATP, adenosine triphosphate; Ac, acetyl group.
Two key cellular sensors of metabolic status are the AMP-activated protein kinase (AMPK) and Sirt1, an NAD+-dependent deacetylase. AMPK is activated by an increase in AMP:ATP ratio and increased ADP concentrations, both of which accompany a decrease in caloric intake or an increase in energy expenditure (Hardie et al., 2011; Mihaylova and Shaw, 2011). Through the phosphorylation of a variety of targets, it upregulates catabolic pathways including gluconeogenesis, OXPHOS, and autophagy, while inhibiting anabolic pathways including cell growth and proliferation (Cantó et al., 2010; Carling et al., 2011). Sirt1 responds to elevated levels of NAD+ that occur upon starvation and, together with AMPK, coordinately regulates mitochondrial mass, nutrient oxidation, and ATP production to fit a cell’s particular needs via the transcription cofactor, peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α) (Cantó et al., 2009, 2010; Jäger et al., 2007; Jeninga et al., 2010; Puigserver et al., 1998; Wu et al., 1999).
Nutrient responses are likely to be highly tissue specific. In the liver, low blood lipid levels induce the nuclear PPAR-alpha receptor, which ultimately induces ketogenesis. In adipose tissue, mitochondria-derived starvation responses trigger lipolysis to provide peripheral tissues with fuels (Kharitonenkov et al., 2005; Nishimura et al., 2000). In the hypothalamus, AMPK affects neuronal plasticity and transmitter receptor activity to promote food intake and provide neuronal protection in response to hunger (Kuramoto et al., 2007; Yang et al., 2011). During a high nutritional load, multiple cell types exhibit high levels of ATP and NADH levels and the metabolic balance tips toward lipid and glycogen storage, and mitochondrial biogenesis is downregulated, increasing glycolytic ATP synthesis.
How does the interrelationship between nutrient sensing and mitochondrial function contribute to disease? Not surprisingly, alterations in mitochondrial mass and activity are contributory factors in obesity and metabolic syndrome. Comparisons between identical twin pairs discordant for obesity revealed significantly reduced mtDNA levels and decreased mitochondrial mass in the obese twin’s adipose tissue, despite identical mtDNA sequences (Pietiläinen et al., 2008). This observation indicates the importance of environmental effects in regulating mitochondrial mass and biogenesis. The discovery of active brown adipose tissue in adult humans has opened up an intriguing avenue in obesity research by clarifying the role of adaptive thermogenesis in counteracting fat storage through UCP1-mediated mitochondrial uncoupling (van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009).
Studies of the Deletor mouse provide a model to interrogate the physiological changes associated with late-onset mitochondrial disease (Tyynismaa et al., 2005). Even when these animals receive normal nutrition, their muscle cells misinterpret an OXPHOS defect and decreased ATP synthesis as starvation. Interestingly, a key regulator of anabolic processes, Akt kinase, is also activated under these conditions (Tyynismaa et al., 2010). In these mice, induction of mitochondrial biogenesis by high-fat feeding appears to be beneficial by inducing mitochondrial mass and OXPHOS activity (Ahola-Erkkilä et al., 2010). The progressive disease course of mice with cytochrome c oxidase deficiencies can similarly be delayed with treatments that increase mitochondrial biogenesis (Viscomi et al., 2011; Wenz et al., 2008; Yatsuga and Suomalainen, 2012) or that activate AMPK (Viscomi et al., 2011). In these instances, it is likely that AMPK activation leads to an increase in NAD+, triggering Sirt1 activation and subsequent PGC-1α induction of mitochondrial biogenesis (Corton et al., 1995; Golubitzky et al., 2011; Viscomi et al., 2011). Together, these studies suggest that mitochondrial biogenesis is blocked by chronic OXPHOS dysfunction and that increased mitochondrial biogenesis can be beneficial for mitochondrial disease.
Recent work has linked tumor suppressors and oncogenes directly to metabolic sensing and regulation, and has consequently indicated that altered cellular metabolism is a contributory and causative factor in cancer. Cancer cells reprogram the use of two key catabolic molecules, glucose and glutamine via signaling pathways containing known oncogenes, including myc and tumor suppressors, such as the LKB1/AMPK (Vander Heiden et al., 2009). These signaling pathways shunt glucose toward aerobic glycolysis—the so-called Warburg effect (Warburg, 1923)—and glutamine toward glutaminolysis for the purpose of producing amino acids, nucleotides and lipids that are essential for rapid proliferation. In cancer cells, mitochondria play a central role via the TCA cycle in the catabolism of glutamine. The altered metabolism of cancer cells raises the possibility that treatments that shift metabolism toward OXPHOS could be therapeutically effective against cancer. Importantly, mitochondrial metabolic enzymes have been identified as tumor suppressors. Defects in succinate dehydrogenase, fumarate hydratase, and isocitrate dehydrogenase (IDH1) cause inherited paragangliomas, pheochromocytomas, myomas, and gliomas, respectively (Baysal et al., 2000; Tomlinson et al., 2002; Yan et al., 2009). Recent intriguing findings in gliomas indicated that IDH1 mutations contribute to gliomas via multiple mechanisms, including stabilizing hypoxia-inducible factor 1, as was previously found in other tumorigenic TCA defects, and by altering the methylation of CpG islands and histones, which causes wide-ranging transcriptional consequences that contribute to oncogenesis (Turcan et al., 2012; Lu et al., 2012; Koivunen et al., 2012). The multifaceted roles of IDH1 mutations in cancer introduce an intriguing role for mitochondrial function in affecting nuclear genomic expression.
Connecting Mitochondrial Form and Function in Homeostasis and Disease
Mitochondrial form and function are intimately linked. The inner membrane is highly structured and differentiated into compositionally and functionally distinct regions (Reichert and Neupert, 2002): the inner boundary region is in close apposition to the OM and facilitates lipid trafficking, mitochondrial protein import, and respiratory complex assembly, the cristae are invaginations that penetrate into the matrix and house assembled respiratory complexes and are thought to increase the local charge density/pH to enhance ATP synthesis via OXPHOS (Strauss et al., 2008; Perkins and Frey, 2000); and crista junctions are tubules that connect the cristae to the boundary and segregate soluble intermembrane space components from the boundary regions. These junctions are restructured during apoptosis to facilitate release of proapoptotic intermembrane space proteins (Frezza et al., 2006). The biogenesis of IM domains is an active process highly dependent on the mitochondrial-specific anionic lipids, phosphotidylethanolamine and cardiolipin, whose transport and levels within mitochondria are tightly controlled by a surprisingly complex set of factors (Osman et al., 2011). Through interactions with lipids and through the formation of inner-membrane supercomplexes, abundant inner-membrane proteins, such as adenine nucleotide translocator, are also important for the structural organization of this membrane (Claypool et al., 2008). In addition, the regulated dimerization/oligomerization of ATP synthase is a major driving force for inner-membrane structure, possibly inducing and/or stabilizing the curvature of crista membranes (Paumard et al., 2002; Strauss et al., 2008). Dedicated structural assemblies have also been implicated in the organization of mitochondrial membranes (Polianskyte et al., 2009), including recent work pointing to a large conserved multiprotein Mitofilin complex (Harner et al., 2011; Hoppins et al., 2011a; von der Malsburg et al., 2011). The importance of OM/IM interactions is underscored by the observation that the Mitofilin complex, termed MitOS, also plays a role in the efficiency of mitochondrial protein import (von der Malsburg et al., 2011), components of which have been implicated in human inherited disorders, including neurological (Jin et al., 1996) and cardiac (Davey et al., 2006) syndromes. Understanding the mechanisms that contribute to the structural organization of the inner membrane will decipher its functions beyond OXPHOS, such as in mtDNA segregation, protein import, and mitochondrial dynamics (Brown et al., 2011).
The lateral organization of the OM is not as well understood, but it serves as a unique signaling platform for pathways such as BCL-2 protein-dependent apoptosis (Chipuk et al., 2010; Bogner et al., 2010) and innate antiviral immunity, which requires the regulated self-assembly of the mitochondrial localized membrane protein, MAVS, into a signaling complex essential for anti-inflammatory interferon response (Wang et al., 2011a). Recent superresolution light microscopy techniques have revealed that the OM import TOM complex is localized in clusters, whose density and distribution are regulated by growth conditions that alter mitochondrial membrane potential (Wurm et al., 2011). This observation highlights that events inside mitochondria regulate the organization and activity of complexes at the mitochondrial surface, which can influence the external structure and behavior of the organelle.
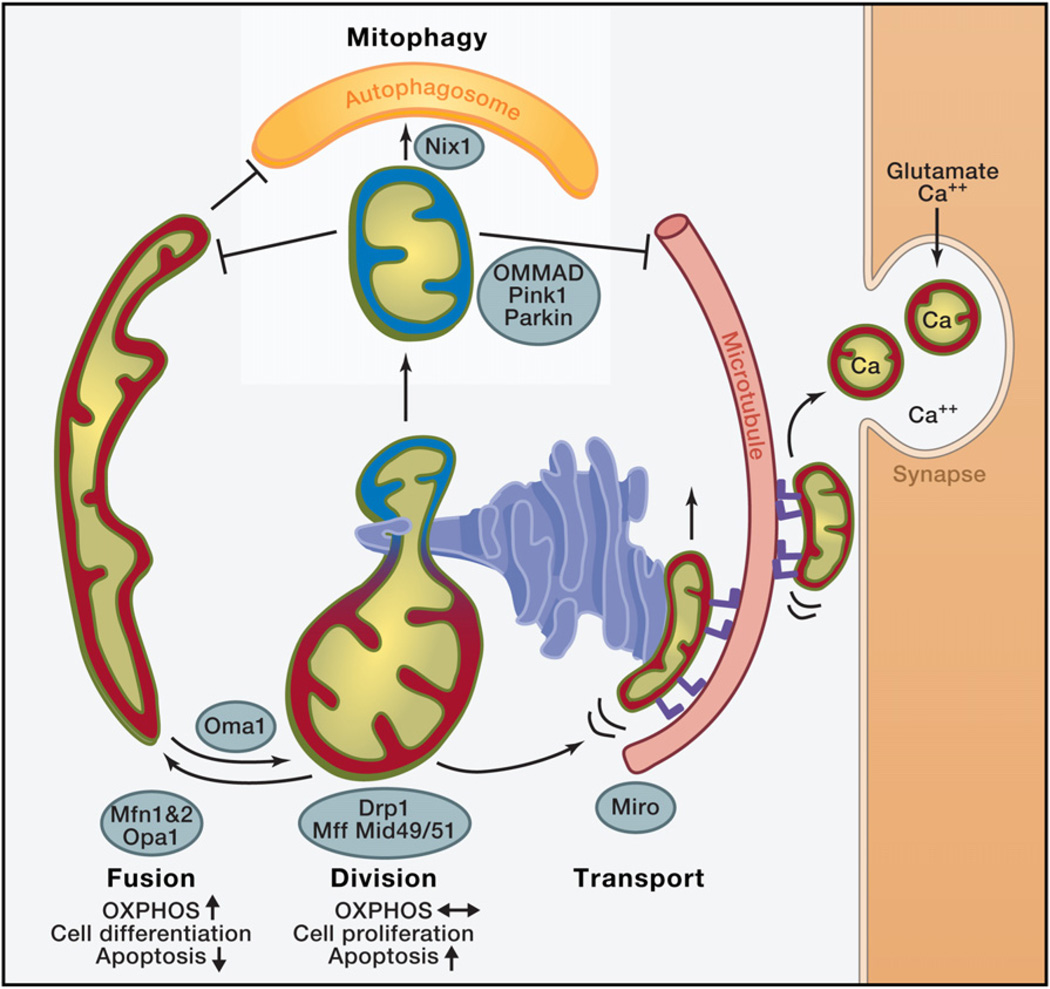
The external structure and the cellular location of mitochondria are critical for their function and depend on highly regulated activities such as mitochondrial division and fusion, motility, and tethering. These activities govern the overall shape, connectedness, and location of mitochondria within cells (Figure 2). Although little data are currently available, it is clear that the relative contributions of these activities and the molecular components that mediate them are highly tissue specific—a phenomenon that contributes to the variable manifestations of human mitochondrial diseases.
Figure 2. Roles of Mitochondrial Dynamics.
Red: mitochondria with high membrane potential, with high oxidative phosphorylation (OXPHOS) activity. Blue: Mitochondria with low membrane potential. Mitofusin 1 or 2 (MFN1, MFN2) mediate mitochondrial outer-membrane fusion in a tissue-specific manner, and OPA1 (optic atrophy gene 1) mediates inner-membrane fusion. The zinc metalloprotease OMA1 proteolytically cleaves OPA1 under low membrane potential conditions, promoting fission. Mitochondrial dynamics factors 49 and 51 or mitochondrial fission factor (Mff) recruit DRP1 onto mitochondria at sites marked by endoplasmic reticulum tubules (ER), and DRP1 mediates mitochondrial division. In cultured cells, upon a decrease in mitochondrial membrane potential, PINK1 kinase recruits Parkin, a ubiquitin E3 ligase, which ubiquitinates several mitochondrial targets, including MFN1 and Miro, to facilitate the degradation of mitochondria via mitophagy. Parkin-mediated ubiquitination triggers OMMAD, outer-mitochondrial membrane-associated degradation—a proteosomal pathway that degrades ubiquinated OM proteins in a CDC48-dependent manner. OMMAD is probably cell type-dependent and may also function in quality control. In erythrocytes, mitophagy receptor Nix1 is involved in autophagosome recruitment. ER forms close contacts with mitochondria, essential for calcium regulation in cellular microcompartments. Miro (blue feet) is a mitochondrial receptor for kinesin via Milton that facilitates the transport of mitochondria on microtubules in a Ca2+-regulated manner. Upon synaptic activity in neurons, influx of glutamate and Ca2+ halts mitochondrial transport via Miro to position them at sites of synaptic activity that require Ca2+ uptake and ATP.
In metazoans, mitochondrial motility is mediated by Miro, a conserved Ras-like GTPase that links the mitochondrial surface with the microtubule motor protein kinesin Milton (Glater et al., 2006; Hollenbeck and Saxton, 2005; Liu and Hajnóczky, 2009; Wang and Schwarz, 2009). Although the exact mechanism is not understood, Miro serves as a Ca2+ sensor that controls mitochondrial motility by virtue of its GTPase domains and its calcium binding EF hand motifs to couple an increase in cytosolic calcium to an inhibition of mitochondrial motility (Macaskill et al., 2009; Saotome et al., 2008; Wang and Schwarz, 2009). This mechanism is particularly important in neurons, where Ca2+ influx occurs at presynaptic terminals and postsynaptic dendritic spines due to glutamatergic stimulation. These local increases provide a mechanism to halt mitochondria at the site of neuronal activity, and maintain Ca2+ and energetic homeostasis. In this context, Miro may enable neurons to efficiently retain mitochondria at the sites with high Ca2+, providing a neuronal protection mechanism. Consistent with this model, the EF hands of Miro mediate glutamatergic regulation of mitochondrial motility and provide a protective mechanism against excitotoxicity (Wang and Schwarz, 2009).
Mitochondrial division and fusion are mediated by the action of large multidomain dynamin-related GTPases that function via self-assembly to remodel diverse membranes in cells (Hoppins et al., 2007). In mammals, mitochondrial division is mediated by a single dynamin-related protein, DRP1, whereas fusion requires two families of dynamin-like proteins, MFN1/MFN2 and OPA1. Evidence suggests that DRP1 divides mitochondria by forming helical structures that wrap around mitochondria (Ingerman et al., 2005; Labrousse et al., 1999; Yoon et al., 2001). Less is known about the mechanism mediating mitochondrial fusion, although it is likely that the self-assembly of the fusion dynamins contributes to membrane tethering and fusion events (DeVay et al., 2009; Griffin and Chan, 2006). The proteins that mediate mitochondrial dynamics are highly regulated and consequently integrated into cellular signaling pathways. For example, DRP1 exists as several splice variants and is modified by a plethora of posttranslational modifications, which integrate its activity with cellular events, such as apoptosis, Ca2+ signaling, hypoxic response, and the cell cycle (Strack and Cribbs, 2012).
Loss of either fusion or division activity results in dysfunctional mitochondria. One common explanation for the importance of mitochondrial fusion is the need for exchange of IMS and matrix contents, including mtDNA between mitochondria. In this manner, mitochondrial fusion may buffer partially defects and transient stresses (Chen et al., 2007, 2010; Nunnari et al., 1997). In cultured cells, stressors including UV exposure, cycloheximide treatment, and nutrient deprivation, stimulate mitochondrial fusion to generate branched and interconnected organelles and improve cell survival (Gomes et al., 2011a, 2011b; Rambold et al., 2011; Tondera et al., 2009). Mitochondrial fusion is balanced by mitochondrial division, which creates organelles of the appropriate size for transport along actin or microtubule networks. Cells that are highly polarized and dependent on mitochondrial function, such as neurons, are especially sensitive to defects in mitochondrial division (Verstreken et al., 2005). A neuronal cell-specific knockout of DRP1 in the mouse results in a decrease in neurites and defective synapse formation, while an increase in mitochondrial division in cultured neurons enhances mitochondrial mass and distribution and stimulates synapse formation (Dickey and Strack, 2011; Ishihara et al., 2009; Li et al., 2004; Wakabayashi et al., 2009).
In cells, inhibition of fusion results in OXPHOS deficiencies, mtDNA loss, and mitochondrial motility defects, and division defects also cause OXPHOS deficiencies and significant increases in ROS production (Chen et al., 2003, 2007; Hermann et al., 1998; Ishihara et al., 2009; Parone et al., 2008; Wakabayashi et al., 2009). In animals, deletions and mutations of the division and fusion machinery cause embryonic lethality, and in humans, recessive defects of DRP1 are associated with early infant mortality and cardiomyopathy (Waterham et al., 2007; Ashrafian et al., 2010) Mutations in MFN2 and OPA1 cause tissue-specific neurodegenerative diseases, Charcot Marie Tooth 2A (CMT2A) and dominant optic atrophy (DOA), respectively (Alexander et al., 2000; Delettre et al., 2000; Züchner et al., 2004). These pathogenic conditions emphasize the important physiological roles and differential requirement of mitochondrial dynamics in different cell types.
Linking Mitochondrial Dynamics with Apoptosis and Autophagy
Mitochondrial division and fusion also impinge on apoptosis by mechanisms that are not yet fully understood (Martinou and Youle, 2011). During apoptosis, mitochondria dramatically fragment as a consequence of an increased recruitment of DRP1 to mitochondria, which is key to the positive regulatory role DRP1 plays in Bax/Bak-mediated mitochondrial outer-membrane permeabilization (MOMP) (Frank et al., 2001; Jagasia et al., 2005; Wasiak et al., 2007). Although DRP1’s positive role in apoptosis is independent of its role in the regulation of mitochondrial structure per se, mitochondrial shape is likely to be an important factor in MOMP (Cassidy-Stone et al., 2008; Montessuit et al., 2010). In contrast, mitochondrial fusion protects cells from apoptotic cell death, and activation of apoptosis coordinately inhibits fusion activity (Lee et al., 2004; Olichon et al., 2003; Sugioka et al., 2004). This protection is in part due to the role of OPA1 in the integrity of crista junctions and its ability to limit the release of proapoptotic IMS components (Cipolat et al., 2006; Frezza et al., 2006). Conversely, BCL-2 proteins play regulatory roles in mitochondrial dynamics in healthy cells, where they stimulate fusion (Cleland et al., 2011; Hoppins et al., 2011b; Karbowski et al., 2006; Rolland et al., 2009). The regulatory network formed by BCL-2 proteins and mitochondrial dynamics proteins may be a contributory factor in the human neurodegenerative diseases associated with mutations in MFN2 and OPA1 (Olichon et al., 2007). Furthermore, the roles of BCL-2 in regulating mitochondrial dynamics and in tumors as an antiapoptotic factor link mitochondrial fission and fusion to cancer.
Mitochondrial dynamics are also closely integrated with the mitophagy quality control pathway (Twig et al., 2008; Youle and Narendra, 2011). PINK1, an IMS-localized Ser/Thr kinase, and Parkin, a cytoplasmic E3 ubiquitin ligase, regulate mitophagy in cultured cell models and in fruit-fly muscle. Together these proteins collaborate to sense and trigger the removal of “damaged” mitochondria (Clark et al., 2006; Greene et al., 2003; Narendra et al., 2008; Park et al., 2006). Loss of membrane potential inhibits the degradation of PINK1 and reroutes it to the surface of mitochondria, where it accumulates and recruits Parkin (Kim et al., 2008; Lin and Kang, 2008; Matsuda et al., 2010; Narendra et al., 2010; Vives-Bauza et al., 2010; Ziviani et al., 2010). On the mitochondrial surface Parkin ubiquitinates a specific subset ofOMproteins, resulting in their proteasomal degradation by a mechanism that resembles ER-associated degradation pathway (Neutzner et al., 2007; Yoshii et al., 2011; Ziviani et al., 2010; Chan et al., 2011; Heo et al., 2010; Tanaka et al., 2010; Xu et al., 2011). Another mitophagy pathway functions in erythrocyte development, where upon reticulocyte maturation mitochondria are actively eliminated. This mitophagy pathway is dependent on Nix, an OM-associated BH3-only member of BCL-2 family proteins, suggesting that Nix functions as a mitophagy receptor (Sandoval et al., 2008; Schweers et al., 2007). This raises the possibility that other tissue- or condition-specific mitophagy receptors exist. The presence of such receptors and their functions remain to be elucidated. Given the nature of mitophagy, such receptors could, in addition to contributing to quality control, also dramatically impact mtDNA segregation.
Recent studies specifically connect PINK1/Parkin-mediated autophagy with mitochondrial dynamics and motility, providing evidence that Parkin ubiquitinates MFN1, MFN2, and Miro in cultured cells, leading to their degradation and consequently altering mitochondrial behavior (Chan et al., 2011; Gegg et al., 2010; Poole et al., 2010; Tanaka et al., 2010; Wang et al., 2011b; Ziviani et al., 2010). In this context, multiple OM-associated ubiquitin ligases have been identified whose substrates and roles are largely unknown (Anton et al., 2011; Braschi et al., 2009; Durr et al., 2006; Nakamura et al., 2006; Neutzner et al., 2008; Tang et al., 2011). Loss of membrane potential also attenuates mitochondrial fusion via OMA1-mediated cleavage of integral membrane isoforms of OPA1 (Ehses et al., 2009; Head et al., 2009). Consistently, mitophagy is attenuated in cells with decreased mitochondrial division and/or increased fusion activities likely because larger organelles are occluded from autophagosomes. Indeed, nutrient starvation in cultured cells induces the formation of a hyperfused mitochondrial network, which protects mitochondria from elimination via mitophagy (Gomes et al., 2011a; Rambold et al., 2011). In flies, attenuation of mitochondrial fusion or stimulation of mitochondrial division can rescue phenotypes associated with PINK1 or Parkin mutants, and loss of division exacerbates these phenotypes and causes lethality (Deng et al., 2008; Poole et al., 2008). Thus, evidence is consistent with the idea that mitophagy is a pathway that coordinately regulates mitochondrial structure and motility to effectively segregate damaged mitochondria from a healthy network in cells, which facilitates their degradation.
The relevance of the PINK1/Parkin mitochondrial turnover pathway in animal models has not yet been established; however, defects in this pathway have been suggested to play a role in the development of Parkinson’s disease (PD), where a role of PINK1 and Parkin were originally characterized as their mutant forms cause familial early-onset PD (Kitada et al., 1998; Valente et al., 2004). The association of PINK1/Parkin to PD points to their essential role in maintenance of dopaminergic neurons, the cell type in substantia nigra of mesencephalon that most severely degenerates in PD. Data from cell culture models suggest the intriguing possibility that defective mitochondrial quality control contributes more generally to Parkinson-like phenotypes, potentially explaining why mtDNA mutation accumulate in substantia nigra neurons (Bender et al., 2006; Kraytsberg et al., 2006). A recent mouse study, however, questions this simple model. In the PD Mitopark mouse model, progressive depletion of mtDNA in dopaminergic neurons does not lead to the accumulation of mitochondrial Parkin, and loss of Parkin does not affect neurological disease progression (Sterky et al., 2011). This raises the possibility that PINK1/Parkin contribute to PD by mechanisms other than mitophagy. Parkin has been implicated in nonneuronal mediated lipid uptake regulation, raising the possibility that altered lipid metabolism contributes to Parkin-linked PD (Kim et al., 2011). Additionally, mitochondrial dysfunction is linked to PD by early toxicological studies on MPTP, a compound that is metabolized into a complex I inhibitor, MPP+. MPP+ selectively accumulates in dopaminergic cells and causes symptoms of PD in humans (Davis et al., 1979; Langston et al., 1983; Vyas et al., 1986). Gene defects that lead to mtDNA mutations, such as dominant mutations of mitochondrial DNA polymerase gamma, also cause PD (Luoma et al., 2004). These observations highlight the complex multifactorial nature of neurodegeneration and point to the need for additional animal studies to elucidate physiological roles of mitophagy and its contribution to PD.
Altered mitochondrial dynamics have also been implicated in neurodegeneration. In cell culture models for neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis, mitochondria typically fragment in response to the expression of misfolded proteins (Cho et al., 2010; Costa et al., 2010; Lutz et al., 2009; Shirendeb et al., 2012; Song et al., 2011; Wang et al., 2009). Although it is not clear whether mitochondrial fragmentation is a cause or a consequence of the pathogenic process, inhibition of mitochondrial division attenuates disease-associated phenotypes in multiple models of neurodegenerative disease (Cassidy-Stone et al., 2008; Cui et al., 2010; Lackner and Nunnari, 2010). Inhibiting division may also attenuate the ubiquitin dependent turnover of outer-membrane proteins and mitophagy, which would allow essential behaviors like mitochondrial motility to be retained. In this context, it is possible that the mitochondrial motility defects associated with absent or altered MFN1 and MFN2 proteins result from the targeted degradation of Miro (Baloh et al., 2007; Chen et al., 2005).
The role of mitochondrial division and its potential as a therapeutic target for neurodegeneration needs to be further explored in relevant animal models. In addition, as mitochondrial division is essential in mammals, this pathway may have limited therapeutic potential for neurodegeneration. However, more acute ischemic reperfusion injuries and drug toxicities are also associated with increased mitochondrial fragmentation in cultured cells models and in animal models of myocardial infarction and drug induced renal toxicity, inhibition of mitochondrial division has shown therapeutic promise (Brooks et al., 2009; Ong et al., 2010).
The Roles of Interorganellar Contacts in Mitochondrial Biology
Mitochondrial distribution and dynamics are influenced by intimate physical connections between the mitochondrial outer membrane and diverse intracellular membranes, such as the plasma membrane, peroxisomes, ER, autophagosome, s and lysosomes, termed mitochondria-associated membranes (MAMs) (Figure 3). MAMs create unique environments or platforms for the localization and activity of components that function in shared interorganellar functions, such as Ca2+ homeostasis and lipid biosynthesis (Hayashi et al., 2009; Rizzuto and Pozzan, 2006; Voelker, 2009). Physical tethers are also thought to be important to stably position mitochondria at specific locations within cells, for example, in the axons and dendrites of neurons or in muscle fibers for efficient energy utilization (García-Pérez et al., 2011; Kang et al., 2008).
Figure 3. Interorganellar Communication.
Ub, ubiquitin; red and blue, proteins in mitochondrial outer membrane are PINK1, a mitochondrial kinase, and the E3 ubiquitin ligase Parkin, recruited onto mitochondria by PINK1; MDV, mitochondria-derived vesicle; NAD, nicotinamide adenine dinucleotide, oxidized form; NADH, nicotinamide adenine dinucleotide, reduced form; AMP, adenosine monophosphate; ATP, adenosine triphosphate; ER, endoplasmic reticulum, tubules of which are marking sites of mitochondrial division; MAVS, mitochondrial antiviral signaling, which is activated by viral RNA; MAM, mitochondrial-associated endoplasmic reticulum membrane.
Communication of mitochondria with intracellular structures also occurs via small vesicles that bud off of mitochondria in a DRP1-independent manner (Neuspiel et al., 2008). Interestingly, treatment of cells with antimycin A, an inhibitor of complex III, stimulates the biogenesis of vesicles that carry mitochondrial cargo that fuse with lysosomes, suggesting that this pathway functions in quality control (Soubannier et al., 2012).
A role for ER mitochondrial contacts has been shown in both mitochondrial division and in apoptosis, which has broader implications for understanding how mitochondrial dysfunction contributes to disease. Ca2+ release at ER-mitochondrial contacts may sensitize mitochondria to apoptotic effectors (Breckenridge et al., 2003; Iwasawa et al., 2011; Tabas and Ron, 2011). During mitochondrial division, ER tubules wrap around and likely constrict mitochondria and mark sites of mitochondrial division—a process conserved from yeast to mammals (Friedman et al., 2011). In this context, the observation that Bax colocalizes with DRP1 at sites of mitochondrial constriction during apoptosis (Karbowski et al., 2002; Nechushtan et al., 2001) raises the possibility that Bax-dependent MOMP occurs at and depends on regions of ER-mitochondria contact. ER stress and mitochondrial dysfunction have been implicated in a shared set of diseases associated with altered mitochondrial dynamics. Thus, these observations raise the possibility that alterations in ER-mitochondrial contacts are a contributory factor in human disease (Schon and Przedborski, 2011).
Organismal Roles of Mitochondria
Recent studies demonstrate that a defect in mitochondrial function in one tissue has consequences for the whole organism and have expanded our view of mitochondria beyond their cell autonomous roles. In mouse models of mitochondrial disease and in human patients, OXPHOS-deficient skeletal muscle secretes FGF21, a cytokine that enters the blood and circulates (Suomalainen et al., 2011; Tyynismaa et al., 2010) (Figure 4). FGF21 is a fasting-related hormone, which induces ketogenesis in the liver and mobilizes lipids from adipose tissue for oxidation (Badman et al., 2009; Hotta et al., 2009; Kharitonenkov et al., 2005). In mitochondrial disease, FGF21 is constitutively secreted from pseudostarving OXPHOS-deficient muscle fibers, resulting in chronic lipid recruitment from adipose tissue and metabolic derangement (Figure 4). Another muscle-secreted cytokine, irisin, was recently shown to mediate the differentiation of white adipose cells to brown fat in response to exercise and PGC-1alpha-induced mitochondrial biogenesis in skeletal muscle (Boström et al., 2012). A non-cell-autonomous mitochondrial regulatory pathway was also reported in C. elegans: a tissue-specific RNA interference-mediated knockdown of cytochrome c oxidase subunit in neurons causes a local cellular stress response in neurons that is also communicated to the gut (Durieux et al., 2011). The cellular response is an unfolded protein stress response pathway specific to mitochondria (UPRmt) that also exists in mammals (Haynes and Ron, 2010). UPRmt originates in mitochondria from the accumulation of unassembled respiratory complex subunits and is communicated to the nucleus via an unknown mechanism where it culminates in the regulated expression of mitochondrial protein chaperones, such as HSP-60 (Benedetti et al., 2006; Haynes et al., 2007; Haynes and Ron, 2010; Yoneda et al., 2004; Zhao et al., 2002). The mechanism by which activation of the UPRmt is propagated in a non-cell-autonomous manner is also not known, but has been speculated to occur via a “mitokine” that signals OXPHOS deficiency to the whole organism. In addition to peptides, candidates for long range signaling molecules include metabolites and amino acids, whose levels can be easily sensed by over considerable distances by cells and tissues. The finding that a single dysfunctional tissue or cell can tune or reprogram the whole organism via secreted signaling molecules is a new concept in mitochondrial disease. These relatively unexplored pathways are likely an essential part of pathogenesis and by their secretory nature are attractive targets for therapy.
Figure 4. Organismal Effects of Mitochondrial Respiratory Chain Deficiency.
Skeletal muscle interprets mitochondrial OXPHOS defect as a starvation response in the presence of normal nutrition. The defective muscle fibers secrete FGF21, a hormone-like cytokine, to blood circulation, mobilizing lipids from storage fat, affecting whole-organismal lipid metabolism as a chronic response.
Several outstanding questions are raised by these observations. Are only some tissues capable of initiating whole-organism energy metabolic reprogramming? In humans, brain-specific mitochondrial disorders show low FGF21 levels (Suomalainen et al., 2011), suggesting that brain tissue is not the source for FGF21 secretion. Does chronic starvation produce harmful signals that influence disease progression? Is signaling limited to energy deficiency, or can other organelles induce cytokine reprogramming as well? Answers to these questions will provide crucial insight into the tissue specific manifestations of mitochondrial disorders.
Perspective
Mitochondrial function and behavior are central to the physiology of humans and, consequently, “mitochondrial dysfunction” has been implicated in a wide range of diseases that encompass all aspects of medicine. The complexity of mitochondrial functions and thus “mitochondrial dysfunction,” however, are challenges to unravel, but these challenges must be met to determine whether mitochondrial manipulation can be harnessed therapeutically. Continued basic biological approaches are critical so that we can understand on a molecular level known pathways and characterize new pathways that impact mitochondrial behavior and functions. The development of animal models that faithfully mimic human mitochondrial disease mutations is also essential to understand the physiological significance of these pathways, to unravel the highly tissue specific functions and regulation of mitochondria, and to develop therapeutics (Johnson et al., 2007a, 2007b; Tyynismaa and Suomalainen, 2009). These systems provide the opportunity to determine how “mitochondrial dysfunction” regulates or alters key pathways, which is another critical piece of the puzzle of mitochondrial-linked diseases. Systems-based approaches, such as mapping the genetic interactions between genes encoding mitochondrial proteins, will be required to elucidate the interactions between mitochondrial functions. The first mitochondrial focused map has now been constructed in yeast and reveals the dense and significant connections between mitochondrial localized pathways distributed in different mitochondrial compartments (Hoppins et al., 2011a). Recent technological developments will allow for systems based biochemical, metabolic and genomic approaches, which will provide invaluable insight into mitochondrial biology. These approaches will enable the construction of a complete mitochondrial network map that will be invaluable for understanding the role of “mitochondrial dysfunction” in human disease. The utilization of next-generation sequencing technology advances that exploit the mitochondrial proteome has and will continue to greatly accelerate these advances (Calvo et al., 2012; Tyynismaa et al., 2012). Sequencing advances will continue to lead to the identification of novel mitochondrial proteins and pathways and have already enabled more streamlined diagnosis and the opportunity for genetic counseling for patients with mitochondrial diseases. In combination with intelligent strategies to screen the rich repertoire of existing small molecule libraries, these approaches hold the promise of future cures.
ACKNOWLEDGMENTS
The authors would like to express thanks to members of the Nunnari lab for discussions and manuscript editing. J.N. is supported by National Institutes of Health grants R01GM062942 and R01GM097432. A.S. is supported by the Sigrid Jusélius Foundation, Jane and Aatos Erkko Foundation, Academy of Finland, European Research Council, University of Helsinki, and Helsinki University Central Hospital. The authors wish to thank Helena Schmidt for figure art.
REFERENCES
- Ahlqvist KJ, Hämäläinen RH, Yatsuga S, Uutela M, Terzioglu M, Götz A, Forsström S, Salven P, Angers-Loustau A, Kopra OH, et al. Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. Cell Metab. 2012;15:100–109. doi: 10.1016/j.cmet.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Ahola-Erkkilä S, Carroll CJ, Peltola-Mjösund K, Tulkki V, Mattila I, Seppänen-Laakso T, Oresic M, Tyynismaa H, Suomalainen A. Ketogenic diet slows down mitochondrial myopathy progression in mice. Hum. Mol. Genet. 2010;19:1974–1984. doi: 10.1093/hmg/ddq076. [DOI] [PubMed] [Google Scholar]
- Al Rawi S, Louvet-Vallée S, Djeddi A, Sachse M, Culetto E, Hajjar C, Boyd L, Legouis R, Galy V. Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science. 2011;334:1144–1147. doi: 10.1126/science.1211878. [DOI] [PubMed] [Google Scholar]
- Alexander C, Votruba M, Pesch UAE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat. Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- Anton F, Fres JM, Schauss A, Pinson B, Praefcke GJ, Langer T, Escobar-Henriques M. Ugo1 and Mdm30 act sequentially during Fzo1-mediated mitochondrial outer membrane fusion. J. Cell Sci. 2011;124:1126–1135. doi: 10.1242/jcs.073080. [DOI] [PubMed] [Google Scholar]
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1 alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- Arnaudo E, Dalakas M, Shanske S, Moraes CT, DiMauro S, Schon EA. Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet. 1991;337:508–510. doi: 10.1016/0140-6736(91)91294-5. [DOI] [PubMed] [Google Scholar]
- Ashrafian H, Docherty L, Leo V, Towlson C, Neilan M, Steeples V, Lygate CA, Hough T, Townsend S, Williams D, et al. A mutation in the mitochondrial fission gene Dnm1l leads to cardiomyopathy. PLoS Genet. 2010;6:e1001000. doi: 10.1371/journal.pgen.1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150:4931–4940. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloh RH, Schmidt RE, Pestronk A, Milbrandt J. Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J. Neurosci. 2007;27:422–430. doi: 10.1523/JNEUROSCI.4798-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battersby BJ, Redpath ME, Shoubridge EA. Mitochondrial DNA segregation in hematopoietic lineages does not depend on MHC presentation of mitochondrially encoded peptides. Hum. Mol. Genet. 2005;14:2587–2594. doi: 10.1093/hmg/ddi293. [DOI] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- Benedetti C, Haynes CM, Yang Y, Harding HP, Ron D. Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics. 2006;174:229–239. doi: 10.1534/genetics.106.061580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogner C, Leber B, Andrews DW. Apoptosis: embedded in membranes. Curr. Opin. Cell Biol. 2010;22:845–851. doi: 10.1016/j.ceb.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al. A PGC1-a-dependent myokine that drives brown-fat-like development of white fat and thermo-genesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braschi E, Zunino R, McBride HM. MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep. 2009;10:748–754. doi: 10.1038/embor.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608–8618. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Tkachuk AN, Shtengel G, Kopek BG, Bogenhagen DF, Hess HF, Clayton DA. Superresolution fluorescence imaging of mitochondrial nucleoids reveals their spatial range, limits, and membrane interaction. Mol. Cell. Biol. 2011;31:4994–5010. doi: 10.1128/MCB.05694-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo SE, Compton AG, Hershman SG, Lim SC, Lieber DS, Tucker EJ, Laskowski A, Garone C, Liu S, Jaffe DB, et al. Molecular diagnosis of infantile mitochondrial disease with targeted next-generation sequencing. Sci. Transl. Med. 2012;4:ra10. doi: 10.1126/scitranslmed.3003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling D, Mayer FV, Sanders MJ, Gamblin SJ. AMP-activated protein kinase: nature’s energy sensor. Nat. Chem. Biol. 2011;7:512–518. doi: 10.1038/nchembio.610. [DOI] [PubMed] [Google Scholar]
- Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev. Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RL, Hess S, Chan DC. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum. Mol. Genet. 2011;20:1726–1737. doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Chen ML, Logan TD, Hochberg ML, Shelat SG, Yu X, Wilding GE, Tan W, Kujoth GC, Prolla TA, Selak MA, et al. Erythroid dysplasia, megaloblastic anemia, and impaired lymphopoiesis arising from mitochondrial dysfunction. Blood. 2009;114:4045–4053. doi: 10.1182/blood-2008-08-169474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol. Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Lipton SA. Mitochondrial dynamics in cell death and neurodegeneration. Cell. Mol. Life Sci. 2010;67:3435–3447. doi: 10.1007/s00018-010-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D’Adamio L, et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- Claypool SM, Oktay Y, Boontheung P, Loo JA, Koehler CM. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J. Cell Biol. 2008;182:937–950. doi: 10.1083/jcb.200801152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland MM, Norris KL, Karbowski M, Wang C, Suen DF, Jiao S, George NM, Luo X, Li Z, Youle RJ. Bcl-2 family interaction with the mitochondrial morphogenesis machinery. Cell Death Differ. 2011;18:235–247. doi: 10.1038/cdd.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- Costa V, Giacomello M, Hudec R, Lopreiato R, Ermak G, Lim D, Malorni W, Davies KJ, Carafoli E, Scorrano L. Mitochondrial fission and cristae disruption increase the response of cell models of Huntington’s disease to apoptotic stimuli. EMBO Mol Med. 2010;2:490–503. doi: 10.1002/emmm.201000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Tang X, Christian WV, Yoon Y, Tieu K. Perturbations in mitochondrial dynamics induced by human mutant PINK1 can be rescued by the mitochondrial division inhibitor mdivi-1. J. Biol. Chem. 2010;285:11740–11752. doi: 10.1074/jbc.M109.066662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey KM, Parboosingh JS, McLeod DR, Chan A, Casey R, Ferreira P, Snyder FF, Bridge PJ, Bernier FP. Mutation of DNAJC19, a human homologue of yeast inner mitochondrial membrane co-chaperones, causes DCMA syndrome, a novel autosomal recessive Barth syndrome-like condition. J. Med. Genet. 2006;43:385–393. doi: 10.1136/jmg.2005.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GC, Williams AC, Markey SP, Ebert MH, Caine ED, Reichert CM, Kopin IJ. Chronic Parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res. 1979;1:249–254. doi: 10.1016/0165-1781(79)90006-4. [DOI] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delettre C, Lenaers G, Griffoin J-M, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat. Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- Deng H, Dodson MW, Huang H, Guo M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc. Natl. Acad. Sci. USA. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVay RM, Dominguez-Ramirez L, Lackner LL, Hoppins S, Stahlberg H, Nunnari J. Coassembly of Mgm1 isoforms requires cardiolipin and mediates mitochondrial inner membrane fusion. J. Cell Biol. 2009;186:793–803. doi: 10.1083/jcb.200906098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz F, Kotarsky H, Fellman V, Moraes CT. Mitochondrial disorders caused by mutations in respiratory chain assembly factors. Semin. Fetal Neonatal Med. 2011;16:197–204. doi: 10.1016/j.siny.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey AS, Strack S. PKA/AKAP1 and PP2A/Bb2 regulate neuronal morphogenesis via Drp1 phosphorylation and mitochondrial bioenergetics. J. Neurosci. 2011;31:15716–15726. doi: 10.1523/JNEUROSCI.3159-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durr M, Escobar-Henriques M, Merz S, Geimer S, Langer T, Westermann B. Nonredundant roles of mitochondria-associated F-box proteins Mfb1 and Mdm30 in maintenance of mitochondrial morphology in yeast. Mol. Biol. Cell. 2006;17:3745–3755. doi: 10.1091/mbc.E06-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efremov RG, Sazanov LA. Respiratory complex I: ‘steam engine’ of the cell? Curr. Opin. Struct. Biol. 2011;21:532–540. doi: 10.1016/j.sbi.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, Martinou JC, Westermann B, Rugarli EI, Langer T. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J. Cell Biol. 2009;187:1023–1036. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euro L, Farnum GA, Palin E, Suomalainen A, Kaguni LS. Clustering of Alpers disease mutations and catalytic defects in biochemical variants reveal new features of molecular mechanism of the human mitochondrial replicase, Pol γ. Nucleic Acids Res. 2011;39:9072–9084. doi: 10.1093/nar/gkr618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Waymire KG, Narula N, Li P, Rocher C, Coskun PE, Vannan MA, Narula J, Macgregor GR, Wallace DC. A mouse model of mitochondrial disease reveals germline selection against severe mtDNA mutations. Science. 2008;319:958–962. doi: 10.1126/science.1147786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaldón T, Huynen MA. Prediction of protein function and pathways in the genome era. Cell. Mol. Life Sci. 2004;61:930–944. doi: 10.1007/s00018-003-3387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pérez C, Schneider TG, Hajnóczky G, Csordás G. Alignment of sarcoplasmic reticulum-mitochondrial junctions with mitochondrial contact points. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H1907–H1915. doi: 10.1152/ajpheart.00397.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston D, Tsaousis AD, Roger AJ. Predicting proteomes of mitochondria and related organelles from genomic and expressed sequence tag data. Methods Enzymol. 2009;457:21–47. doi: 10.1016/S0076-6879(09)05002-2. [DOI] [PubMed] [Google Scholar]
- Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum. Mol. Genet. 2010;19:4861–4870. doi: 10.1093/hmg/ddq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubitzky A, Dan P, Weissman S, Link G, Wikstrom JD, Saada A. Screening for active small molecules in mitochondrial complex I deficient patient’s fibroblasts, reveals AICAR as the most beneficial compound. PLoS ONE. 2011;6:e26883. doi: 10.1371/journal.pone.0026883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 2011a;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes LC, Di Benedetto G, Scorrano L. Essential amino acids and glutamine regulate induction of mitochondrial elongation during autophagy. Cell Cycle. 2011b;10:2635–2639. doi: 10.4161/cc.10.16.17002. [DOI] [PubMed] [Google Scholar]
- Götz A, Tyynismaa H, Euro L, Ellonen P, Hyötyläinen T, Ojala T, Hämä-läinen RH, Tommiska J, Raivio T, Oresic M, et al. Exome sequencing identifies mitochondrial alanyl-tRNA synthetase mutations in infantile mitochondrial cardiomyopathy. Am. J. Hum. Genet. 2011;88:635–642. doi: 10.1016/j.ajhg.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl. Acad. Sci. USA. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EE, Chan DC. Domain interactions within Fzo1 oligomers are essential for mitochondrial fusion. J. Biol. Chem. 2006;281:16599–16606. doi: 10.1074/jbc.M601847200. [DOI] [PubMed] [Google Scholar]
- Hakonen AH, Heiskanen S, Juvonen V, Lappalainen I, Luoma PT, Rantamaki M, Goethem GV, Lofgren A, Hackman P, Paetau A, et al. Mitochondrial DNA polymerase W748S mutation: a common cause of autosomal recessive ataxia with ancient European origin. Am. J. Hum. Genet. 2005;77:430–441. doi: 10.1086/444548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Carling D, Gamblin SJ. AMP-activated protein kinase: also regulated by ADP? Trends Biochem. Sci. 2011;36:470–477. doi: 10.1016/j.tibs.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Harner M, Körner C, Walther D, Mokranjac D, Kaesmacher J, Welsch U, Griffith J, Mann M, Reggiori F, Neupert W. The mitochondrial contact site complex, a determinant of mitochondrial architecture. EMBO J. 2011;30:4356–4370. doi: 10.1038/emboj.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes CM, Ron D. The mitochondrial UPR - protecting organelle protein homeostasis. J. Cell Sci. 2010;123:3849–3855. doi: 10.1242/jcs.075119. [DOI] [PubMed] [Google Scholar]
- Haynes CM, Petrova K, Benedetti C, Yang Y, Ron D. ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev. Cell. 2007;13:467–480. doi: 10.1016/j.devcel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J. Cell Biol. 2009;187:959–966. doi: 10.1083/jcb.200906083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo JM, Livnat-Levanon N, Taylor EB, Jones KT, Dephoure N, Ring J, Xie J, Brodsky JL, Madeo F, Gygi SP, et al. A stress-responsive system for mitochondrial protein degradation. Mol. Cell. 2010;40:465–480. doi: 10.1016/j.molcel.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann GJ, Thatcher JW, Mills JP, Hales KG, Fuller MT, Nunnari J, Shaw JM. Mitochondrial fusion in yeast requires the transmembrane GTPase Fzo1p. J. Cell Biol. 1998;143:359–373. doi: 10.1083/jcb.143.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J. Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988;331:717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Holt IJ, Harding AE, Petty RK, Morgan-Hughes JA. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am. J. Hum. Genet. 1990;46:428–433. [PMC free article] [PubMed] [Google Scholar]
- Hoppeler H, Fluck M. Plasticity of skeletal muscle mitochondria: structure and function. Med. Sci. Sports Exerc. 2003;35:95–104. doi: 10.1249/01.MSS.0000043292.99104.12. [DOI] [PubMed] [Google Scholar]
- Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu. Rev. Biochem. 2007;76:751–780. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- Hoppins S, Collins SR, Cassidy-Stone A, Hummel E, Devay RM, Lackner LL, Westermann B, Schuldiner M, Weissman JS, Nunnari J. A mitochondrial-focused genetic interaction map reveals a scaffold-like complex required for inner membrane organization in mitochondria. J. Cell Biol. 2011a;195:323–340. doi: 10.1083/jcb.201107053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppins S, Edlich F, Cleland MM, Banerjee S, McCaffery JM, Youle RJ, Nunnari J. The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Mol. Cell. 2011b;41:150–160. doi: 10.1016/j.molcel.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y, Nakamura H, Konishi M, Murata Y, Takagi H, Matsumura S, Inoue K, Fushiki T, Itoh N. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150:4625–4633. doi: 10.1210/en.2009-0119. [DOI] [PubMed] [Google Scholar]
- Hutchin T, Haworth I, Higashi K, Fischel-Ghodsian N, Stoneking M, Saha N, Arnos C, Cortopassi G. A molecular basis for human hypersensitivity to aminoglycoside antibiotics. Nucleic Acids Res. 1993;21:4174–4179. doi: 10.1093/nar/21.18.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J. Cell Biol. 2005;170:1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat. Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Toyama-Sorimachi N, Nakada K, Morimoto M, Imanishi H, Yoshizaki M, Sasawatari S, Niikura M, Takenaga K, Yonekawa H, Hayashi J. The innate immune system in host mice targets cells with allogenic mitochondrial DNA. J. Exp. Med. 2010;207:2297–2305. doi: 10.1084/jem.20092296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- Iwasawa R, Mahul-Mellier A-L, Datler C, Pazarentzos E, Grimm S. Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. EMBO J. 2011;30:556–568. doi: 10.1038/emboj.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagasia R, Grote P, Westermann B, Conradt B. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature. 2005;433:754–760. doi: 10.1038/nature03316. [DOI] [PubMed] [Google Scholar]
- Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1 alpha. Proc. Natl. Acad. Sci. USA. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeninga EH, Schoonjans K, Auwerx J. Reversible acetylation of PGC-1: connecting energy sensors and effectors to guarantee metabolic flexibility. Oncogene. 2010;29:4617–4624. doi: 10.1038/onc.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, May M, Tranebjaerg L, Kendall E, Fontán G, Jackson J, Subramony SH, Arena F, Lubs H, Smith S, et al. A novel X-linked gene DDP shows mutations in families with deafness (DFN-1), dystonia, mental deficiency and blindness. Nat. Genet. 1996;14:177–180. doi: 10.1038/ng1096-177. [DOI] [PubMed] [Google Scholar]
- Johnson DT, Harris RA, Blair PV, Balaban RS. Functional consequences of mitochondrial proteome heterogeneity. Am. J. Physiol. Cell Physiol. 2007a;292:C698–C707. doi: 10.1152/ajpcell.00109.2006. [DOI] [PubMed] [Google Scholar]
- Johnson DT, Harris RA, French S, Blair PV, You J, Bemis KG, Wang M, Balaban RS. Tissue heterogeneity of the mammalian mitochondrial proteome. Am. J. Physiol. Cell Physiol. 2007b;292:C689–C697. doi: 10.1152/ajpcell.00108.2006. [DOI] [PubMed] [Google Scholar]
- Jokinen R, Marttinen P, Sandell HK, Manninen T, Teerenhovi H, Wai T, Teoli D, Loredo-Osti JC, Shoubridge EA, Battersby BJ. Gimap3 regulates tissue-specific mitochondrial DNA segregation. PLoS Genet. 2010;6:e1001161. doi: 10.1371/journal.pgen.1001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Tian JH, Pan PY, Zald P, Li C, Deng C, Sheng ZH. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, et al. FGF-21 as a novel metabolic regulator. J. Clin. Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Park J, Kim S, Song S, Kwon SK, Lee SH, Kitada T, Kim JM, Chung J. PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem. Biophys. Res. Commun. 2008;377:975–980. doi: 10.1016/j.bbrc.2008.10.104. [DOI] [PubMed] [Google Scholar]
- Kim KY, Stevens MV, Akter MH, Rusk SE, Huang RJ, Cohen A, Noguchi A, Springer D, Bocharov AV, Eggerman TL, et al. Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. J. Clin. Invest. 2011;121:3701–3712. doi: 10.1172/JCI44736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Koivunen P, Lee S, Duncan CG, Lopez G, Lu G, Ramkissoon S, Losman JA, Joensuu P, Bergmann U, Gross S, et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012 doi: 10.1038/nature10898. Published online February 15, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat. Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Kuramoto N, Wilkins ME, Fairfax BP, Revilla-Sanchez R, Terunuma M, Tamaki K, Iemata M, Warren N, Couve A, Calver A, et al. Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007;53:233–247. doi: 10.1016/j.neuron.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol. Cell. 1999;4:815–826. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- Lackner LL, Nunnari J. Small molecule inhibitors of mitochondrial division: tools that translate basic biological research into medicine. Chem. Biol. 2010;17:578–583. doi: 10.1016/j.chembiol.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane N, Martin W. The energetics of genome complexity. Nature. 2010;467:929–934. doi: 10.1038/nature09486. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Lecrenier N, Van Der Bruggen P, Foury F. Mitochondrial DNA polymerases from yeast to man: a new family of polymerases. Gene. 1997;185:147–152. doi: 10.1016/s0378-1119(96)00663-4. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol. Biol. Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lill R, Mühlenhoff U. Maturation of iron-sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu. Rev. Biochem. 2008;77:669–700. doi: 10.1146/annurev.biochem.76.052705.162653. [DOI] [PubMed] [Google Scholar]
- Lin W, Kang UJ. Characterization of PINK1 processing, stability, and subcellular localization. J. Neurochem. 2008;106:464–474. doi: 10.1111/j.1471-4159.2008.05398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu X, Hajnóczky G. Ca2+-dependent regulation of mitochondrial dynamics by the Miro-Milton complex. Int. J. Biochem. Cell Biol. 2009;41:1972–1976. doi: 10.1016/j.biocel.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, Edwards CR, Khanin R, Figueroa ME, Melnick A, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. [February 15, 2012];Nature. 2012 doi: 10.1038/nature10860. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, Chalmers RM, Oldfors A, Rautakorpi I, Peltonen L, Majamaa K, et al. Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet. 2004;364:875–882. doi: 10.1016/S0140-6736(04)16983-3. [DOI] [PubMed] [Google Scholar]
- 124.Lutz AK, Exner N, Fett ME, Schlehe JS, Kloos K, Lämmermann K, Brunner B, Kurz-Drexler A, Vogel F, Reichert AS, et al. Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J. Biol. Chem. 2009;284:22938–22951. doi: 10.1074/jbc.M109.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61:541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mayr JA, Zimmermann FA, Fauth C, Bergheim C, Meierhofer D, Radmayr D, Zschocke J, Koch J, Sperl W. Lipoic acid synthetase deficiency causes neonatal-onset epilepsy, defective mitochondrial energy metabolism, and glycine elevation. Am. J. Hum. Genet. 2011;89:792–797. doi: 10.1016/j.ajhg.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 131.Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, Basañez G, Meda P, Martinou JC. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mootha VK, Bunkenborg J, Olsen JV, Hjerrild M, Wisniewski JR, Stahl E, Bolouri MS, Ray HN, Sihag S, Kamal M, et al. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell. 2003;115:629–640. doi: 10.1016/s0092-8674(03)00926-7. [DOI] [PubMed] [Google Scholar]
- 133.Morris AA, Leonard JV, Brown GK, Bidouki SK, Bindoff LA, Woodward CE, Harding AE, Lake BD, Harding BN, Farrell MA, et al. Deficiency of respiratory chain complex I is a common cause of Leigh disease. Ann. Neurol. 1996;40:25–30. doi: 10.1002/ana.410400107. [DOI] [PubMed] [Google Scholar]
- 134.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 135.Murphy MP. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nakamura N, Kimura Y, Tokuda M, Honda S, Hirose S. MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep. 2006;7:1019–1022. doi: 10.1038/sj.embor.7400790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Narasimhaiah R, Tuchman A, Lin SL, Naegele JR. Oxidative damage and defective DNA repair is linked to apoptosis of migrating neurons and progenitors during cerebral cortex development in Ku70-deficient mice. Cereb. Cortex. 2005;15:696–707. doi: 10.1093/cercor/bhh171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Naviaux RK, Nyhan WL, Barshop BA, Poulton J, Markusic D, Karpinski NC, Haas RH. Mitochondrial DNA polymerase gamma deficiency and mtDNA depletion in a child with Alpers’ syndrome. Ann. Neurol. 1999;45:54–58. doi: 10.1002/1531-8249(199901)45:1<54::aid-art10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 141.Nechushtan A, Smith CL, Lamensdorf I, Yoon SH, Youle RJ. Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J. Cell Biol. 2001;153:1265–1276. doi: 10.1083/jcb.153.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu. Rev. Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- 143.Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, Andrade-Navarro MA, McBride HM. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr. Biol. 2008;18:102–108. doi: 10.1016/j.cub.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 144.Neutzner A, Youle RJ, Karbowski M. Outer mitochondrial membrane protein degradation by the proteasome. Novartis Found. Symp. 2007;287:4–14. discussion 14–20. [PubMed] [Google Scholar]
- 145.Neutzner A, Benard G, Youle RJ, Karbowski M. Role of the ubiquitin conjugation system in the maintenance of mitochondrial homeostasis. Ann. N Y Acad. Sci. 2008;1147:242–253. doi: 10.1196/annals.1427.012. [DOI] [PubMed] [Google Scholar]
- 146.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta. 2000;1492:203–206. doi: 10.1016/s0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 147.Norddahl GL, Pronk CJ, Wahlestedt M, Sten G, Nygren JM, Ugale A, Sigvardsson M, Bryder D. Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell Stem Cell. 2011;8:499–510. doi: 10.1016/j.stem.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 148.Nunnari J, Marshall W, Straight A, Murray A, Sedat JW, Walter P. Mitochondrial transmission during mating in Saccharomyces cerevisiae is determined by mitochondrial fusion and fission and the intramitochondrial segregation of mitochondrial DNA. Mol. Biol. Cell. 1997;8:1233–1242. doi: 10.1091/mbc.8.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Okuno D, Iino R, Noji H. Rotation and structure of FoF1-ATP synthase. J. Biochem. 2011;149:655–664. doi: 10.1093/jb/mvr049. [DOI] [PubMed] [Google Scholar]
- 150.Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J. Biol. Chem. 2003;278:7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- 151.Olichon A, Landes T, Arnauné-Pelloquin L, Emorine LJ, Mils V, Guichet A, Delettre C, Hamel C, Amati-Bonneau P, Bonneau D, et al. Effects of OPA1 mutations on mitochondrial morphology and apoptosis: relevance to ADOA pathogenesis. J. Cell. Physiol. 2007;211:423–430. doi: 10.1002/jcp.20950. [DOI] [PubMed] [Google Scholar]
- 152.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 153.Osman C, Voelker DR, Langer T. Making heads or tails of phospholipids in mitochondria. J. Cell Biol. 2011;192:7–16. doi: 10.1083/jcb.201006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 156.Parone PA, Da Cruz S, Tondera D, Mattenberger Y, James DI, Maechler P, Barja F, Martinou JC. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE. 2008;3:257. doi: 10.1371/journal.pone.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pätsi J, Kervinen M, Finel M, Hassinen IE. Leber hereditary optic neuropathy mutations in the ND6 subunit of mitochondrial complex I affect ubiquinone reduction kinetics in a bacterial model of the enzyme. Biochem. J. 2008;409:129–137. doi: 10.1042/BJ20070866. [DOI] [PubMed] [Google Scholar]
- 158.Paumard P, Vaillier J, Coulary B, Schaeffer J, Soubannier V, Mueller DM, Brèthes D, di Rago JP, Velours J. The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 2002;21:221–230. doi: 10.1093/emboj/21.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Perkins GA, Frey TG. Recent structural insight into mitochondria gained by microscopy. Micron. 2000;31:97–111. doi: 10.1016/s0968-4328(99)00065-7. [DOI] [PubMed] [Google Scholar]
- 160.Perry TL, Haworth JC, Robinson BH. Brain amino acid abnormalities in pyruvate carboxylase deficiency. J. Inherit. Metab. Dis. 1985;8:63–66. doi: 10.1007/BF01801666. [DOI] [PubMed] [Google Scholar]
- 161.Pierce SB, Chisholm KM, Lynch ED, Lee MK, Walsh T, Opitz JM, Li W, Klevit RE, King MC. Mutations in mitochondrial histidyl tRNA synthetase HARS2 cause ovarian dysgenesis and sensorineural hearing loss of Perrault syndrome. Proc. Natl. Acad. Sci. USA. 2011;108:6543–6548. doi: 10.1073/pnas.1103471108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pietiläinen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, Keränen H, Suomalainen A, Götz A, Suortti T, Yki-Järvinen H, et al. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med. 2008;5:e51. doi: 10.1371/journal.pmed.0050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Polianskyte Z, Peitsaro N, Dapkunas A, Liobikas J, Soliymani R, Lalowski M, Speer O, Seitsonen J, Butcher S, Cereghetti GM, et al. LACTB is a filament-forming protein localized in mitochondria. Proc. Natl. Acad. Sci. USA. 2009;106:18960–18965. doi: 10.1073/pnas.0906734106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc. Natl. Acad. Sci. USA. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Poole AC, Thomas RE, Yu S, Vincow ES, Pallanck L. The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS ONE. 2010;5:e10054. doi: 10.1371/journal.pone.0010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 167.Rahman S, Poulton J, Marchington D, Suomalainen A. Decrease of 3243 A—>G mtDNA mutation from blood in MELAS syndrome: a longitudinal study. Am. J. Hum. Genet. 2001;68:238–240. doi: 10.1086/316930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc. Natl. Acad. Sci. USA. 2011;108:10190–10195. doi: 10.1073/pnas.1107402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reichert AS, Neupert W. Contact sites between the outer and inner membrane of mitochondria-role in protein transport. Biochim. Biophys. Acta. 2002;1592:41–49. doi: 10.1016/s0167-4889(02)00263-x. [DOI] [PubMed] [Google Scholar]
- 170.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 171.Rolland SG, Lu Y, David CN, Conradt B. The BCL-2-like protein CED-9 of C elegans promotes FZO-1/Mfn1,2- and EAT-3/Opa1-dependent mitochondrial fusion. J. Cell Biol. 2009;186:525–540. doi: 10.1083/jcb.200905070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, Rizzuto R, Hajnóczky G. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc. Natl. Acad. Sci. USA. 2008;105:20728–20733. doi: 10.1073/pnas.0808953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sato M, Sato K. Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science. 2011;334:1141–1144. doi: 10.1126/science.1210333. [DOI] [PubMed] [Google Scholar]
- 175.Scheper GC, van der Klok T, van Andel RJ, van Berkel CG, Sissler M, Smet J, Muravina TI, Serkov SV, Uziel G, Bugiani M, et al. Mitochondrial aspartyl-tRNA synthetase deficiency causes leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation. Nat. Genet. 2007;39:534–539. doi: 10.1038/ng2013. [DOI] [PubMed] [Google Scholar]
- 176.Schmidt O, Pfanner N, Meisinger C. Mitochondrial protein import: from proteomics to functional mechanisms. Nat. Rev. Mol. Cell Biol. 2010;11:655–667. doi: 10.1038/nrm2959. [DOI] [PubMed] [Google Scholar]
- 177.Schon EA, Przedborski S. Mitochondria: the next (neurode) generation. Neuron. 2011;70:1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, Kundu M, Opferman JT, Cleveland JL, Miller JL, Ney PA. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc. Natl. Acad. Sci. USA. 2007;104:19500–19505. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shirendeb UP, Calkins MJ, Manczak M, Anekonda V, Dufour B, McBride JL, Mao P, Reddy PH. Mutant huntingtin’s interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington’s disease. Hum. Mol. Genet. 2012;21:406–420. doi: 10.1093/hmg/ddr475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc. Natl. Acad. Sci. USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sickmann A, Reinders J, Wagner Y, Joppich C, Zahedi R, Meyer HE, Schönfisch B, Perschil I, Chacinska A, Guiard B, et al. The proteome of Saccharomyces cerevisiae mitochondria. Proc. Natl. Acad. Sci. USA. 2003;100:13207–13212. doi: 10.1073/pnas.2135385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Song W, Chen J, Petrilli A, Liot G, Klinglmayr E, Zhou Y, Poquiz P, Tjong J, Pouladi MA, Hayden MR, et al. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat. Med. 2011;17:377–382. doi: 10.1038/nm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Soubannier V, McLelland GL, Zunino R, Braschi E, Rippstein P, Fon EA, McBride HM. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr. Biol. 2012;22:135–141. doi: 10.1016/j.cub.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 184.Sterky FH, Lee S, Wibom R, Olson L, Larsson NG. Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc. Natl. Acad. Sci. USA. 2011;108:12937–12942. doi: 10.1073/pnas.1103295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stewart JB, Freyer C, Elson JL, Wredenberg A, Cansu Z, Trifunovic A, Larsson NG. Strong purifying selection in transmission of mammalian mitochondrial DNA. PLoS Biol. 2008;6:e10. doi: 10.1371/journal.pbio.0060010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Stock D, Leslie AG, Walker JE. Molecular architecture of the rotary motor in ATP synthase. Science. 1999;286:1700–1705. doi: 10.1126/science.286.5445.1700. [DOI] [PubMed] [Google Scholar]
- 187.Strack S, Cribbs JT. Allosteric modulation of Drp1 assembly and mitochondrial fission by the variable domain. J. Biol. Chem. 2012 doi: 10.1074/jbc.M112.342105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Strauss M, Hofhaus G, Schröder RR, Kühlbrandt W. Dimer ribbons of ATP synthase shape the inner mitochondrial membrane. EMBO J. 2008;27:1154–1160. doi: 10.1038/emboj.2008.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J. Biol. Chem. 2004;279:52726–52734. doi: 10.1074/jbc.M408910200. [DOI] [PubMed] [Google Scholar]
- 190.Suomalainen A. Therapy for mitochondrial disorders: little proof, high research activity, some promise. Semin. Fetal Neonatal Med. 2011;16:236–240. doi: 10.1016/j.siny.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 191.Suomalainen A, Elo JM, Pietiläinen KH, Hakonen AH, Sevastianova K, Korpela M, Isohanni P, Marjavaara SK, Tyni T, Kiuru-Enari S, et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol. 2011;10:806–818. doi: 10.1016/S1474-4422(11)70155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J. Cell Biol. 2010;191:1367–1380. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tang F, Wang B, Li N, Wu Y, Jia J, Suo T, Chen Q, Liu YJ, Tang J. RNF185, a novel mitochondrial ubiquitin E3 ligase, regulates autophagy through interaction with BNIP1. PLoS ONE. 2011;6:e24367. doi: 10.1371/journal.pone.0024367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tatuch Y, Christodoulou J, Feigenbaum A, Clarke JT, Wherret J, Smith C, Rudd N, Petrova-Benedict R, Robinson BH. Heteroplasmic mtDNA mutation (T——G) at 8993 can cause Leigh disease when the percentage of abnormal mtDNA is high. Am. J. Hum. Genet. 1992;50:852–858. [PMC free article] [PubMed] [Google Scholar]
- 196.Tiranti V, Savoia A, Forti F, D’Apolito M-F, Centra M, Rocchi M, Zeviani M. Identification of the gene encoding the human mitochondrial RNA polymerase (h-mtRPOL) by cyberscreening of the Expressed Sequence Tags database. Hum. Mol. Genet. 1997;6:615–625. doi: 10.1093/hmg/6.4.615. [DOI] [PubMed] [Google Scholar]
- 197.Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, et al. Multiple Leiomyoma Consortium. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 198.Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, et al. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 2009;28:1589–1600. doi: 10.1038/emboj.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S, Oldfors A, Wibom R, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 200.Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, Campos C, Fabius AWM, Lu C, Ward PS, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. [February 15, 2012];Nature. 2012 doi: 10.1038/nature10866. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tyynismaa H, Suomalainen A. Mouse models of mitochondrial DNA defects and their relevance for human disease. EMBO Rep. 2009;10:137–143. doi: 10.1038/embor.2008.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tyynismaa H, Mjosund KP, Wanrooij S, Lappalainen I, Ylikallio E, Jalanko A, Spelbrink JN, Paetau A, Suomalainen A. Mutant mitochondrial helicase Twinkle causes multiple mtDNA deletions and a late-onset mitochondrial disease in mice. Proc. Natl. Acad. Sci. USA. 2005;102:17687–17692. doi: 10.1073/pnas.0505551102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tyynismaa H, Carroll CJ, Raimundo N, Ahola-Erkkilä S, Wenz T, Ruhanen H, Guse K, Hemminki A, Peltola-Mjøsund KE, Tulkki V, et al. Mitochondrial myopathy induces a starvation-like response. Hum. Mol. Genet. 2010;19:3948–3958. doi: 10.1093/hmg/ddq310. [DOI] [PubMed] [Google Scholar]
- 205.Tyynismaa H, Sun R, Ahola-Erkkilä S, Almusa H, Pöyhönen R, Korpela M, Honkaniemi J, Isohanni P, Paetau A, Wang L, Suomalainen A. Thymidine kinase 2 mutations in autosomal recessive progressive external ophthalmoplegia with multiple mitochondrial DNA deletions. Hum. Mol. Genet. 2012;21:66–75. doi: 10.1093/hmg/ddr438. [DOI] [PubMed] [Google Scholar]
- 206.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 207.van den Bogert C, Kroon AM. Tissue distribution and effects on mitochondrial protein synthesis of tetracyclines after prolonged continuous intravenous administration to rats. Biochem. Pharmacol. 1981;30:1706–1709. doi: 10.1016/0006-2952(81)90403-2. [DOI] [PubMed] [Google Scholar]
- 208.Van Goethem G, Dermaut B, Löfgren A, Martin JJ, Van Broeckhoven C. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat. Genet. 2001;28:211–212. doi: 10.1038/90034. [DOI] [PubMed] [Google Scholar]
- 209.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 210.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 212.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 213.Viscomi C, Bottani E, Civiletto G, Cerutti R, Moggio M, Fagiolari G, Schon EA, Lamperti C, Zeviani M. In vivo correction of COX deficiency by activation of the AMPK/PGC-1α axis. Cell Metab. 2011;14:80–90. doi: 10.1016/j.cmet.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl. Acad. Sci. USA. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Voelker DR. Genetic and biochemical analysis of non-vesicular lipid traffic. Annu. Rev. Biochem. 2009;78:827–856. doi: 10.1146/annurev.biochem.78.081307.112144. [DOI] [PubMed] [Google Scholar]
- 216.von der Malsburg K, Müller JM, Bohnert M, Oeljeklaus S, Kwiatkowska P, Becker T, Loniewska-Lwowska A, Wiese S, Rao S, Milenkovic D, et al. Dual role of mitofilin in mitochondrial membrane organization and protein biogenesis. Dev. Cell. 2011;21:694–707. doi: 10.1016/j.devcel.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 217.Vyas I, Heikkila RE, Nicklas WJ. Studies on the neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: inhibition of NAD-linked substrate oxidation by its metabolite, 1-methyl-4-phenylpyridinium. J. Neurochem. 1986;46:1501–1507. doi: 10.1111/j.1471-4159.1986.tb01768.x. [DOI] [PubMed] [Google Scholar]
- 218.Waagepetersen HS, Sonnewald U, Gegelashvili G, Larsson OM, Schousboe A. Metabolic distinction between vesicular and cytosolic GABA in cultured GABAergic neurons using 13C magnetic resonance spectroscopy. J. Neurosci. Res. 2001;63:347–355. doi: 10.1002/1097-4547(20010215)63:4<347::AID-JNR1029>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 219.Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J. Cell Biol. 2009;186:805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ, 2nd, Nikoskelainen EK. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 221.Wang X, Schwarz TL. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J. Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang C, Liu X, Wei B. Mitochondrion: an emerging platform critical for host antiviral signaling. Expert Opin. Ther. Targets. 2011a;15:647–665. doi: 10.1517/14728222.2011.561321. [DOI] [PubMed] [Google Scholar]
- 224.Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011b;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Warburg O. The prime cause and prevention of cancer. Biochem. Z. 1923;142:317. [Google Scholar]
- 226.Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J. Cell Biol. 2007;177:439–450. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, Leonard JV. A lethal defect of mitochondrial and peroxisomal fission. N. Engl. J. Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 228.Wenz T, Diaz F, Spiegelman BM, Moraes CT. Activation of the PPAR/PGC-1alpha pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype. Cell Metab. 2008;8:249–256. doi: 10.1016/j.cmet.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 229.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 230.Wurm CA, Neumann D, Lauterbach MA, Harke B, Egner A, Hell SW, Jakobs S. Nanoscale distribution of mitochondrial import receptor Tom20 is adjusted to cellular conditions and exhibits an inner-cellular gradient. Proc. Natl. Acad. Sci. USA. 2011;108:13546–13551. doi: 10.1073/pnas.1107553108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Xu S, Peng G, Wang Y, Fang S, Karbowski M. The AAA-ATPase p97 is essential for outer mitochondrial membrane protein turnover. Mol. Biol. Cell. 2011;22:291–300. doi: 10.1091/mbc.E10-09-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yatsuga S, Suomalainen A. Effect of bezafibrate treatment on late-onset mitochondrial myopathy in mice. Hum. Mol. Genet. 2012;21:526–535. doi: 10.1093/hmg/ddr482. [DOI] [PubMed] [Google Scholar]
- 235.Ylikallio E, Suomalainen A. Mechanisms of mitochondrial diseases. Ann. Med. 2012;44:41–59. doi: 10.3109/07853890.2011.598547. [DOI] [PubMed] [Google Scholar]
- 236.Yoneda T, Benedetti C, Urano F, Clark SG, Harding HP, Ron D. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J. Cell Sci. 2004;117:4055–4066. doi: 10.1242/jcs.01275. [DOI] [PubMed] [Google Scholar]
- 237.Yoon Y, Pitts KR, McNiven MA. Mammalian dynamin-like protein DLP1 tubulates membranes. Mol. Biol. Cell. 2001;12:2894–2905. doi: 10.1091/mbc.12.9.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yoshii SR, Kishi C, Ishihara N, Mizushima N. Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane. J. Biol. Chem. 2011;286:19630–19640. doi: 10.1074/jbc.M110.209338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21:4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ziviani E, Tao RN, Whitworth AJ. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc. Natl. Acad. Sci. USA. 2010;107:5018–5023. doi: 10.1073/pnas.0913485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Züchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat. Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]