ABSTRACT
Loss of cardiac macroautophagy/autophagy impairs heart function, and evidence accumulates that an increased autophagic flux may protect against cardiovascular disease. We therefore tested the protective capacity of the natural autophagy inducer spermidine in animal models of aging and hypertension, which both represent major risk factors for the development of cardiovascular disease. Dietary spermidine elicits cardioprotective effects in aged mice through enhancing cardiac autophagy and mitophagy. In salt-sensitive rats, spermidine supplementation also delays the development of hypertensive heart disease, coinciding with reduced arterial blood pressure. The high blood pressure-lowering effect likely results from improved global arginine bioavailability and protection from hypertension-associated renal damage. The polyamine spermidine is naturally present in human diets, though to a varying amount depending on food type and preparation. In humans, high dietary spermidine intake correlates with reduced blood pressure and decreased risk of cardiovascular disease and related death. Altogether, spermidine represents a cardio- and vascular-protective autophagy inducer that can be readily integrated in common diets.
Keywords: aging, autophagy, cardiovascular disease, hypertension, polyamine, spermidine
As the aged population is increasing, cardiovascular disease has reached an epidemic status and is currently the leading cause of death worldwide. Aging as well as the associated cardiovascular disease are driven by systemic metabolic deteriorations, pointing to multiorgan syndromes rather than simple diseases limited to the heart. However, current standard pharmacological therapies typically have single molecular targets with limited effects often restricted to the heart and/or the vessels, thereby underestimating the complexity of the disease. Eventually, the only remedy currently available is to combine many drugs, causing a wide array of side effects. Therefore, a potential successful preventive or therapeutic approach may require interventions with intrinsic multi-target effects. In this regard, caloric or dietary restriction has proven to trigger cardiovascular-, health-, and life span-promoting effects that are likely mediated by several, probably intersecting, mechanisms. These include, but are not limited to, the induction of protective autophagy, reduction of oxidative stress, anti-inflammatory effects, enhanced mitochondrial function as well as other systemic metabolic adaptations. However, rigorous food regimens are difficult to enforce at the population level, urging the need for so-called caloric restriction mimetics (CRM). CRM are defined as autophagy-inducing agents that mimic the beneficial metabolic effects of caloric restriction. One such CRM is the natural polyamine spermidine that we have described previously as a potent autophagy-inducing and life span-prolonging agent in simple model organisms.
In our recent study, we addressed the question whether dietary supplementation of spermidine may lead to cardioprotection and life span extension in rodent models and whether this could be translated to humans. We examined the effects of spermidine in 2 different experimental settings, namely aging and hypertension, using physiologically aged C57BL/6 wild-type mice and hypertensive Dahl salt-sensitive rats, respectively. In old mice, dietary spermidine prolongs life span and prevents age-induced hypertrophy and stiffness of the heart by improving the mechano-elastical and metabolic properties of cardiomyocytes. These effects translate into improved cardiac diastolic function (i.e., relaxation and filling properties of the heart). Although such diastolic dysfunction may not always be associated with manifest heart failure, it is one of the most important risk factors for this disease in both aged mice and humans. We therefore consider aged C57BL/6 wild-type mice as a suitable and highly relevant model of age-associated diastolic dysfunction and impending heart failure. Dietary spermidine elicits its beneficial effects on a multi-systemic level (Fig. 1), as it (i) induces cardiac autophagy and mitophagy, (ii) lowers the subclinical plasma levels of the pro-inflammatory cytokine TNF/TNF-α, (iii) increases TTN (titin) phosphorylation, a known molecular event that promotes the intrinsic elasticity of cardiomyocytes, and (iv) enhances cardiac mitochondrial volume and respiration together with an improved mitochondria-relevant metabolite composition.
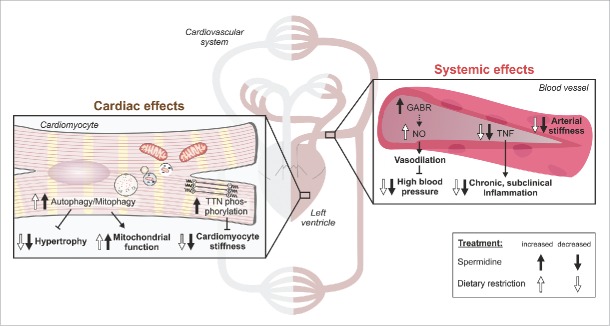
Figure 1.
Spermidine improves cardiovascular health similar to caloric restriction. Comparison of the molecular and cellular changes induced by dietary spermidine relevant to cardiovascular health is depicted. Spermidine-induced effects are compared with similar effects known for caloric restriction. White arrows illustrate that a process is known to be up- or downregulated in response to caloric restriction, while black arrows show the changes elicited by dietary spermidine. GABR, global arginine bioavailability ratio; NO, nitric oxide; TTN, titin. Note: parts of this figure are modified versions of Wikimedia commons “A kreislauf01.svg” (Jörg Rittmeister, commons.wikimedia.org/wiki/File:Akreislauf01.svg) and “Blood vessels-en.svg” (Kelvinsong, commons.wikimedia.org/wiki/File:Blood_vessels-en.svg) both licensed under the Creative Commons Attribution-Share Alike 3.0 unported license (creativecommons.org/licenses/by-sa/3.0/).
Importantly, dietary spermidine fails to exert its heart-protective effects in mice with autophagy-deficient cardiomyocytes, arguing for autophagy as a pivotal mechanism underlying spermidine-induced cardioprotection. Autophagy may act through the detoxification of long-lived damaged proteins or protein aggregates that otherwise compromise the functionality of cardiomyocytes, and/or by degrading defective mitochondria (mitophagy), thus ensuring the ‘metabolic fitness’ of cardiac cells. Autophagy may also be involved in yet undefined signaling processes that affect the molecular events essential for cardiovascular health. Future research will elucidate, which of the described molecular events induced by spermidine (including phosphorylation of TTN and the reduction of circulating levels of TNF) are autophagy-dependent. Alternatively, primarily autophagy-independent mechanisms may also act in an additive or synergistic manner to autophagy.
In hypertensive rats, dietary spermidine delays the onset of salt-induced hypertension, again preserving diastolic function via refinement of myocardial compliance (i.e., elasticity). This effect protects animals from the progression to heart failure, which is observed in control rats that are not fed spermidine. The high blood pressure-lowering (anti-hypertensive) effect of spermidine can be, at least partly, explained by an increase of global arginine bioavailability. Arginine is known as the sole source for biosynthesis of the vasodilator nitric oxide. Our results suggest that dietary spermidine administration manipulates the intersecting cellular routes of arginine- and polyamine-metabolism. As a result of this manipulation, arginine may be favored for nitric oxide production instead of being used for polyamine biosynthesis. However, several questions regarding the anti-hypertensive effect of spermidine remain to be answered in future studies. For instance, will the anti-hypertensive effect of spermidine be reproducible in other modalities (i.e., etiologies) of hypertension? Can spermidine reverse already developed hypertension? Finally, it should also be addressed whether spermidine and other CRM share a common mechanism that underlies the blood pressure-lowering effects, specifically addressing the role of autophagy in blood pressure regulation.
In humans, spermidine-rich diet, as assessed by food questionnaires, is associated with reduced blood pressure and decreased risk of heart failure and cardiovascular disease. Hence, it will be crucial to test—in interventional prospective clinical studies—for additive or synergistic effects of dietary spermidine as an adjuvant in the current standard management strategies against cardiovascular disease. For example, supplementation of spermidine or spermidine-rich natural food extracts along with anti-hypertensive or heart-supporting drugs in the setting of hypertension or heart failure may help to reduce the number and/or the dose of combined medications and, thus, alleviate their potential adverse effects.
In summary, the multi-faceted beneficial effects elicited by dietary spermidine, phenocopy many of the known molecular, metabolic, and physiological consequences of caloric restriction (Fig. 1). Hence, the CRM spermidine can be a novel and feasible strategy in the combat against the dominant cause of death worldwide: heart disease.
Disclosure of potential conflicts of interest
F.M., T.E., DC-G., S.J.S. and S. Stekovic have equity interests in TLL, a company founded in 2016 that will develop natural food extracts.
Funding
FM is grateful to the Austrian Science Fund FWF (Austria) for grants P23490-B20, P 29262, P24381, P 29203 P 27893, I1000 and ‘SFB Lipotox’ (F3012), as well as to BMWFW and the Karl-Franzens University for grant ‘Unkonventionelle Forschung’ and grant DKplus Metabolic and Cardiovascular Diseases (W1226). We acknowledge support from NAWI Graz. S. Sedej is supported by the Austrian Science Fund FWF through grant P27637-B28 and by a grant from the Austrian Heart Foundation (Österreichischer Herzfonds). M.A. was trained within the frame of the Ph.D Program Molecular Medicine of the Medical University of Graz. The project is supported by the Leducq Foundation (G.K.). W.A.L. is supported by SFB1002, TPA08, from the German Research Foundation. S.K. is supported by an excellence initiative (Competence Centers for Excellent Technologies - COMET) of the Austrian Research Promotion Agency FFG: “Research Center of Excellence in Vascular Aging – Tyrol, VASCage” (K-Project No. 843536) funded by the BMVIT, BMWFW, Wirtschaftsagentur Wien and Standortagentur Tirol.