Introduction
Transcranial direct current stimulation (tDCS), a form of non-invasive brain stimulation originally studied for its effect on motor limb physiology1, has been investigated for its use in the treatment of aphasia since 20082–3. The experimental use of tDCS for aphasia, however, began differently from those paradigms established for post stroke motor recovery, both conceptually and in method. Not only is aphasia research a relative newcomer to the field of tDCS experimentation, it has thus far been somewhat of an outlier in its limited use of tDCS autonomously.
Theoretically understood to be vastly more complex than our intricate motor systems, cortical language representation has most recently been conceptualized as a dual stream, diffuse network4–6, with language processing subcomponents evolved from non-linguistic primates7–8. In the dual stream model, human language functions are lateralized primarily in the left hemisphere, with Broca’s area comprising the left complement of a bilateral dorsal stream network devoted to naming and articulation. Conversely, Wernicke’s area constitutes the origin of a bilateral ventral stream in which semantic meaning is attached to components of speech sounds6,9–21. Additional activation in homologous right hemisphere language areas appears to be determined by lexical necessity, with increased articulatory demands activated within the bilateral dorsal stream and the decoding of unfamiliar words activated in the bilateral ventral stream network9. Complex as it may be to optimally prime the motor cortex for post-stroke limb rehabilitation using tDCS, it may be considered even more challenging to modulate the cortical plexus which encodes and produces language in all of its richness. The theoretical mechanisms of brain activation during tDCS protocols suggest that tDCS primes the brain for enhanced outcomes in behavioral therapies22, which may have led to the appeal of combining methods concurrently. The specific mechanisms by which tDCS modulates language networks however, remain equivocal. Recent literature indicates that an aggregate therapeutic impact may be generated when combining motor and cognitive resources concurrently23–24.
Herein, we will provide a broad overview of tDCS/aphasia research and suggest filling gaps in our understanding of the physiological changes induced by tDCS on language networks.
Aphasia
Aphasia is a language disorder which occurs in up to 38% of stroke survivors, often leaving them with lifelong residual deficits25–29. As such, aphasia negatively impacts stroke survivors’ safety and quality of life. People with aphasia often experience social isolation30–31, unemployment31–32, marital difficulties33, mental health issues34, and financial burdens26,35. The presence of aphasia is associated with a longer duration of hospital stay and higher risk of death35. Stroke survivors with aphasia are often concomitantly burdened with dysarthria or apraxia of speech, adding yet another level of difficulty to the already effortful task of communicating.
It has been stated that, “…one never recovers from aphasia; one recovers with aphasia36.” Similarly, recovery with aphasia is more of a fluid process than originally understood. It is now acknowledged, for example, that patients having one type of aphasia in the acute phase may present with a different form of aphasia weeks or months hence37. Many patients with aphasia have symptoms which, in fact, defy textbook categorization38–39.
Recovering with Aphasia
Prior to the last decade, aphasia literature generally conformed to the belief that recovery was limited to a 3–6-month window40–41. More recent studies, however, provide evidence to rethink this assumption41–43. In one recent example, Fiori et al. (2013) studied 7 subjects with chronic aphasia who nonetheless demonstrated multiple language improvements, with temporal stimulation improving naming of nouns, and frontal stimulation enhancing verb production44.
Patients with aphasia, furthermore, are not always ready to participate in rehabilitation within the first 3–6 months due to sensory deficits, agitation, fatigue, side effects of medications45 and disordered sleep patterns46. The reorganization of dendrites following ischemic lesions can be highly variable4. Additionally, patients may experience psychosocial issues such as depression and anxiety which make it difficult to participate optimally in speech therapy during the acute phase38.
Various forms of behavioral aphasia therapy span decades of research and include: Melodic Intonation Therapy (“MIT”)47, Constraint Induced Language Treatment (“CLT”)48, computer avatar programs such as “Aphasia Scripts”49 and “Speech Entrainment”50. Preliminary evidence suggests that increasing the intensity of speech therapy is beneficial to aphasia recovery51. This has led to the development of Intensive Comprehensive Aphasia Programs or “ICAPs”52. Advances in technology have generated a surge in computerized aphasia “apps” for home practice53 and have prompted the rise of telerehabilitation54; however, in spite of the many therapies available, no gold-standard aphasia treatment exists to-date55. What has been established, is that speech therapy to treat aphasia in any format is superior to no treatment at all43,56 and that the intensity of treatment appears to be an important factor in the extent of recovery51–52.
Medications
Medications for auxiliary use in the treatment of aphasia have had mixed success57, with most notable language improvement found with memantine, vasopressin and piracetam, as well as medications that enhance production of acetylcholine45. In their review of pharmacological treatment for aphasia, Small & Llano (2009) caution, however, that these medications are known to be helpful only with the addition of behavioral speech treatment and are not intended to replicate the benefits of speech therapy. It is likewise important to discern medications which have adverse effects on aphasia recovery, particularly since those drugs are often prescribed for other stroke related issues such as hypertension, seizures and heart disease45.
Neuroimaging
Neuroimaging studies are essential to understanding the substructures of language and the physiological impact of tDCS. As noted by Saur et al. (2006)58 and Geranmayeh et al., (2014)59, before the advent of fMRIs, language was considered domain specific. The two hypotheses which predominated the literature at that time were the “perilesional hypothesis” and “laterality shift hypothesis.” The suggestion that language laterality to the right hemisphere is maladaptive led to the “disinhibition hypothesis,” which stated that transcallosal inhibition is responsible for poor recovery59. These divergent views of language recovery could be used to justify a particular treatment; or, in the case of tDCS, each view might accompany differing recommendations for montage and polarity. Laska et al., (2011)60 and Meinzer et al. (2013)61 recommend caution however, in the interpretation of fMRI language activation measurements. They note that positive changes in functional language ability may not always correlate with neuroimaging data61.
tDCS for Aphasia Rehabilitation
Experimentation with alternate forms of physiological intervention for aphasia, such as non-invasive brain stimulation, began in the 1980s with transcranial magnetic stimulation (TMS). TMS targets cortical areas via electromagnetic current and has the ability to transiently induce speech arrest, providing opportunities to explore the neural connectivity of language in the brain62. Additionally, TMS supplies a method for mapping the brain, which can be used in conjunction with other brain imaging technologies (e.g., EEG, fMRI, etc.) At the turn of this century, a new form of non-invasive brain stimulation emerged in the field of stroke recovery, transcranial direct current stimulation (tDCS).
Unlike TMS, tDCS uses a low-intensity current of 1–2 mA to modulate (excite or inhibit) neuronal activity63. It has been explored in stroke rehabilitation as a method for encouraging brain plasticity, with results often lasting beyond the initial period of stimulation62. tDCS also has the advantage of being portable, with built-in sham control, making it suitable for clinical experimentation during behavioral therapies.
The first experiments examining the effects of tDCS on the human motor cortex appeared promising64–65. Nitche and Paulus extended their exploration of the effects of tDCS on human motor recovery to include adjunctive fine-motor training65; however, it was not until 2007 that tDCS was combined outright with physical therapy for stroke66. Results suggested that tDCS might prime the brain as an adjuvant to behavioral motor-limb therapies, optimizing recovery. Subsequent neuronavigation using TMS allowed researchers the opportunity to more precisely map specific cortical areas, providing the chance to explore the effects of various tDCS stimulation intensities and polarities (e.g., excitatory or inhibitory stimulation).
In 2008, 2 studies emerged which looked at the effects of tDCS on language abilities, with 1 study experimenting on healthy subjects2 and 1 on patients with aphasia3. In the majority of subsequent tDCS/aphasia studies, tDCS was paired with language training (Figure 1), possibly because stand-alone tDCS treatment was not viewed to provide the same level of consistent language improvement9. Aphasia studies regarding other forms of non-invasive brain stimulation such as TMS may have provided further justification for combining tDCS with language training67. Of note, while most tDCS/aphasia studies included sham stimulation along with behavioral intervention, sham stimulation, when used in combination with behavioral therapy, cannot tell us what tDCS does autonomously. As a result, we know something about the effects of tDCS on language behavior, but an understanding of the physiological underpinnings of tDCS on language networks remains elusive.
Figure 1. 2014–2015 publications.
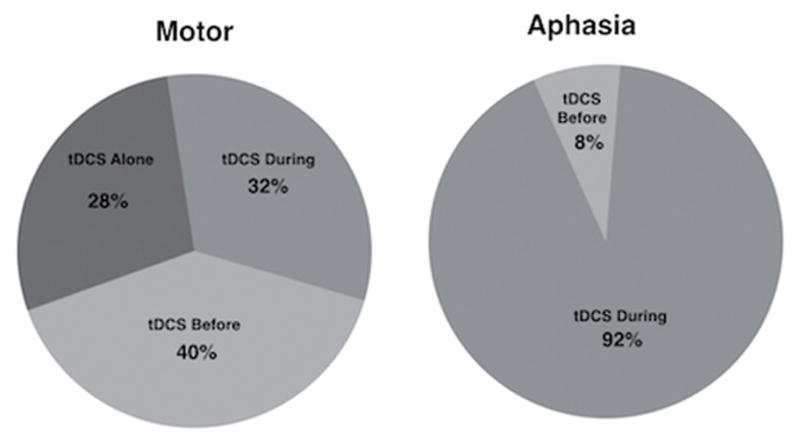
Differences in use of stand-alone tDCS, as well as timing (before or during therapy), can be seen during post-stroke motor-limb vs. aphasia studies in a recent 2-year period.
Source: PubMed. (Source criteria: tDCS/stroke, tDCS/motor, tDCS/motor/stroke, tDCS/aphasia, tDCS/language)
Cipollari and colleagues (2015) in their recent study combining TMS and EEG to measure the physiological effects of tDCS on aphasia treatment, sought to address the limited amount of literature on the neurophysiology of tDCS on language areas68. There are several unique elements in this study, including the use of right homologous language areas, the type of therapeutic intervention (MIT) and the severity level of subjects. tDCS was used to increase activity in the right inferior frontal gyrus (IFG) as it is implicated in prosodic aspects of language function. They discovered via TMS-EEG that right-hemisphere anodal stimulation likely enhanced the effects of MIT. Previously, Wirth et al. (2011) had used EEG to measure the effect of anodal tDCS over the left prefrontal cortex of healthy subjects and similarly noted improved naming compared with sham stimulation69. In spite of some limitations, these studies have taken a positive step in the direction of exploring the physiological effects of tDCS on language substrates.
tDCS/Aphasia Literature Reviews
Reviews of tDCS/aphasia literature are numerous35,43,63,70 (Figure 2), yet recent meta analyses provide conflicting evidence of the effectiveness of tDCS for aphasia. One recent meta-analysis found statistically significant improvements in people with aphasia using tDCS71, while another meta-analysis reported some promise using cathodal stimulation over the contralateral hemisphere, but found no statistical significance regarding the effects of tDCS for aphasia overall56.
Figure 2. tDCS-Stroke Publications.
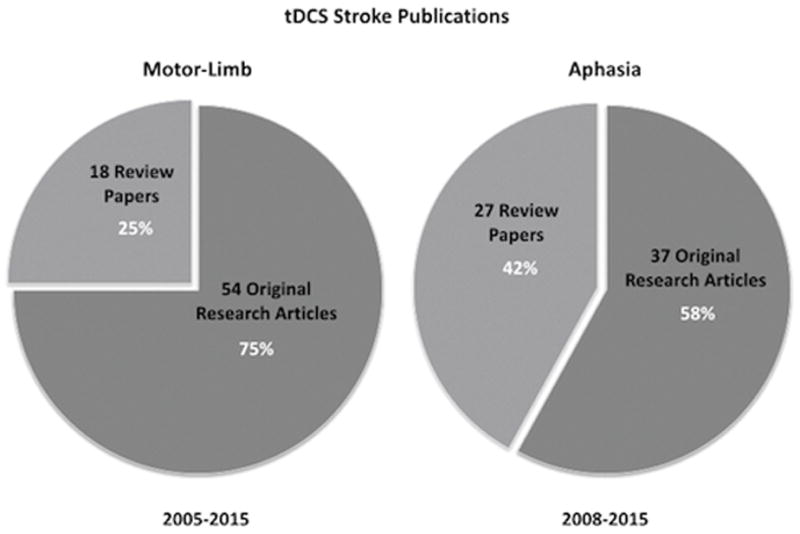
tDCS Stroke publications by type, for motor-limb and aphasia. Source: PubMed. (Search criteria: tDCS/motor/stroke, tDCS/motor/stroke/review, tDCS/aphasia, tDCS/aphasia/review)
One common critique across tDCS/aphasia literature reviews, is a paucity of functional communication measures. In a Cochrane systematic review, Elsner and colleagues (2013) found that primary functional measures did not provide adequate information about whether tDCS promotes greater functional recovery than speech therapy alone56. Measures in recent aphasia/tDCS studies focus on naming as the central measure of language improvement3,9,37. Clinicians have experienced first-hand, however, the patient with aphasia who scores poorly on naming tasks, yet passes important functional communication milestones such as ordering a meal in a restaurant, which are difficult to quantify. Future studies may wish to address whether tDCS promotes gains in functional daily communication, as well as naming tasks.
A Motor-Language Connection and tDCS
Prior views of language representation in the brain held to the notion that each subset of language function operates in discrete modules4,72. It is now understood that language operations shift fluidly throughout the brain and are tied to many other brain functions57,72. Pullvermüller & Berthier, (2008) report that belief in a modular language system encouraged separation of linguistic tasks in speech treatment, so that naming and syntax, for example, would not be addressed together. They note that fMRI studies have changed our view of the modular concept. The authors recommend combining language and action tasks simultaneously, to strengthen language recovery43. One example of the additive effects of combined motor-language training were noted in a set of 2 combined studies, using 23 and 40 healthy adults respectively, in which simultaneous training on language-motor tasks had a beneficial effect on both semantic and motor performance25. These findings correspond with a 2009 report by Harnish et al. of combined motor and language improvement after arm training exercises in subjects with chronic aphasia73 as well as the informal observation of Glover et al. (2002) during a study in young children with hemiplegia74.
Primaßhin et al. (2016) published a recent collection of 4 aphasia case studies which further demonstrated parallel motor-language recovery systems at work. The authors reported that motor and language improvements are additive in stroke recovery, rather than serving to compete for neural resources24. In another study which looked at “pantomime” skills in people with aphasia, van Nispen and colleagues (2016) found that semantic deficits associated with aphasia also appear to have a negative impact on the kinesthetic representation of the distinctive features of objects75. In their 2012 review, Roby-Brami et al. reported that brain areas which underlie the motion of reaching and grasping are connected with visual pathways as part of a “dynamic system” of networks which communicate via mirror neurons with Broca’s area76.
Cumulatively, these recent papers present the possibility that motor and language rehabilitation work well when combined. This presents an intriguing possibility for the direction of future tDCS/aphasia research. Both language and motor functions may be modulated via tDCS, for example, by targeting the supplementary motor area77 and cerebellum78. Supporting the rationale for this view, Hertrich and colleagues (2016), looked at the role of the supplementary motor area (SMA) in language function and noted that the anterior portion of the SMA (or pre-SMA) was important for “context integration” and language processing77. Similarly, in a recent proof-of-concept study, Turkeletaub et al. (2016) reported that tDCS modulation of the cerebellum may enhance verbal fluency78.
Discussion
Speech-language pathologists strive to use evidence-based practices in the treatment of aphasia and rely on experts’ findings to justify the integration of new treatment strategies. We know that the study of tDCS for aphasia rehabilitation is safe79; and, that when combined with speech-language therapy, it can sometimes be beneficial80. We know that we are not stimulating modular language substrates with tDCS, but rather, an interconnected web of language activity4,43,57. Additionally, we know that we are far from understanding the mechanisms of what tDCS does physiologically in the brain to promote language recovery4. We believe it is therefore crucial to investigate the biological mechanisms of tDCS upon language networks. Like attempted pharmacological treatments for aphasia, tDCS has been reported to produce neurochemical changes, such as changes in N-methyl-D-aspartate (NMDA) receptor activity. Unlike pharmacological treatments however, tDCS has no known serious side effects79. tDCS could therefore be beneficial to aphasic patients when concerns arise regarding multiple drug interactions23.
Inclusion of behavioral training in the majority of tDCS/aphasia studies may inhibit an understanding of what tDCS does autonomously to language functions. It is true that a tDCS/aphasia experiment without language training would divest aphasic subjects of concomitant therapy; but conversely, it might enable scientists to develop improved tDCS paradigms that can later be combined with behavioral treatments. Further, while there are a number of studies which examine the effects of tDCS on healthy motor physiology, its effects on healthy language networks has not been as thoroughly explored (Figure 3).
Figure 3. Timeline of tDCS Limb vs. Language Studies.
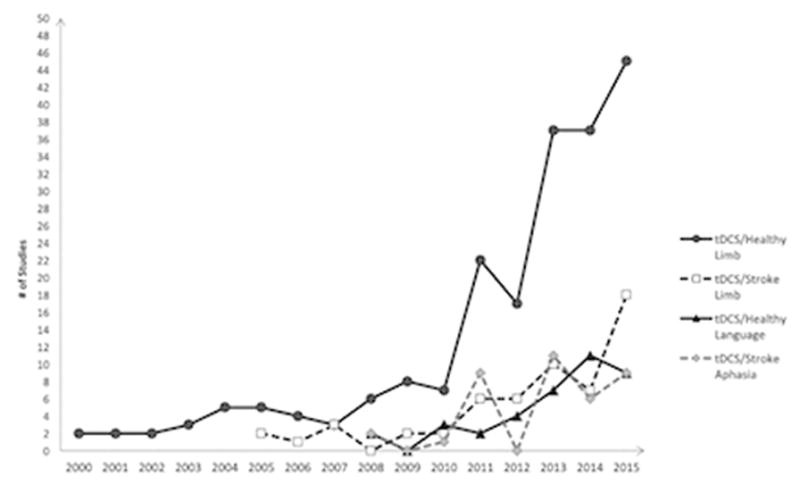
Considerable data has been collected regarding the effects of tDCS on healthy motor physiology vs. the effects of tDCS upon healthy language networks. Source: PubMed. (Search criteria: tDCS/motor, tDCS/motor/stroke, tDCS/aphasia, tDCS/language)
Scientific literature contains a wealth of substantive reviews on progress within the field of tDCS/aphasia research; however, the number of review papers has been disproportionately high when compared with the number of experimental studies conducted (Figure 2). The number of tDCS/aphasia review papers has even exceeded those in tDCS/motor-limb literature, in spite of its later origin. This suggests that in tDCS/stroke rehabilitation literature as a whole, there is a great deal of important discussion about the merits of tDCS for aphasia, with a correspondingly smaller number of original research studies supporting the debate.
Hauser, Chomsky and Fitch, described the architecture of language as having both sensory-motor functions, found even in primates, and a subsystem which generates an expanding syntax from conceptual representations81. According to Hauser and colleagues (2002), this subsystem then grafts grammatical principles onto the phonological system, resulting in meaningful speech. Inter-connectivity of language to other areas of brain function however, continues to be revealed in surprising ways. Language is a dyadic or interactive process82, which can be seen in the context of social communication, as well as in the synergy of neuronal connections in cortical language areas which extend toward many other physical and mental human functions24,57,76. The question of whether similar mechanisms are at work in post-stroke motor recovery and aphasia is not new. For example, in a retrospective analysis of 21 stroke patients with aphasia, Lazar et al. (2010) suggested the possibility that multimodal brain regions could impact recovery for post-stroke limb deficits and aphasia concurrently83. tDCS studies have found a relationship between speech and hand recovery84, as well as implicit motor learning85. As noted by Dipper et al. (2015), nonlinguistic components in the rehabilitation of aphasia are increasingly becoming affirmed84. Future studies may wish to investigate whether tDCS of shared motor/language areas could provide similar effects, by simultaneously targeting language and motor systems, toward overall improved functional outcomes.
On a final note, in light of the diversity of languages in tDCS/aphasia protocols63, it is interesting to consider the findings of recent neuro-linguistic studies which contend that language processing is activated in differing brain regions among speakers of languages that are structurally or morphologically dissimilar (e.g., Mandarin or Hebrew, compared with English, for example)86–88. Future tDCS-aphasia studies may therefore wish to compare tDCS montage, polarity and outcomes across linguistically disparate languages, as well as in bilingual versus monolingual speakers.
New Opportunities
This broad overview of tDCS-aphasia literature yields considerable promise. From this we see the following plausible opportunities for further experimentation:
-
tDCS modulation of
diffuse motor areas which are thought to interface with perisylvian language areas (e.g., the cerebellum, supplementary motor area, etc.);
cortical language or language-motor areas with adjunctive multidisciplinary paradigms of restorative therapy (physical, occupational and speech);
cortical language or language-motor areas using functional language outcome measures (e.g., taking a phone message, ordering in a restaurant, etc.);
cortical language or language-motor areas combined with more intensive, circumscribed aphasia treatment; and
bilingual subjects, especially wherein the languages spoken are linguistically divergent.
Conclusion
In eight short years, aphasia literature has developed information, both theoretical and practical, on methods for combining tDCS with behavioral therapy for post-stroke aphasia. New data suggests a direct connection between neural motor-limb networks and speech-language systems, opening the door to methods for combining physical and cognitive resources in stroke recovery, through both tDCS and behavioral therapies. While the neurophysiological underpinnings of tDCS on language substrates require further exploration, available data support that continued tDCS/aphasia research may assist in the creation of stronger therapies, providing brain recovery from this common debilitating disorder.
Acknowledgments
Funding Sources
This work was supported by NICHD of the NIH, under award number R01HD069776.
Footnotes
Disclosures
The authors have no conflicts of interest or disclosures to report.
References
- 1.Nitsche M, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flöel A, Rösser N, Michka O, Knecht S, Breitenstein C. Noninvasive Brain Stimulation Improves Language Learning. Journal of Cognitive Neuroscience. 2008;20:1415–1422. doi: 10.1162/jocn.2008.20098. [DOI] [PubMed] [Google Scholar]
- 3.Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic-Sposta S, et al. Improved naming after transcranial direct current stimulation in aphasia. Journal of Neurology, Neurosurgery & Psychiatry. 2008;79:451–453. doi: 10.1136/jnnp.2007.135277. [DOI] [PubMed] [Google Scholar]
- 4.Thiel A, Zumbansen A. The pathophysiology of post-stroke aphasia: A network approach. RNN. 2016;34:507–518. doi: 10.3233/rnn-150632. [DOI] [PubMed] [Google Scholar]
- 5.Cogan G, Thesen T, Carlson C, Doyle W, Devinsky O, Pesaran B. Sensory–motor transformations for speech occur bilaterally. Nature. 2014;507:94–98. doi: 10.1038/nature12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 7.Rauschecker J. Ventral and dorsal streams in the evolution of speech and language. Frontiers in Evolutionary Neuroscience. 2012:4. doi: 10.3389/fnevo.2012.00007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rauschecker J, Scott S. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nature Neuroscience. 2009;12:718–724. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandars M, Cloutman L, Woollams A. Taking Sides: An Integrative Review of the Impact of Laterality and Polarity on Efficacy of Therapeutic Transcranial Direct Current Stimulation for Anomia in Chronic Poststroke Aphasia. Neural Plasticity. 2016:1–21. doi: 10.1155/2016/8428256.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrod S, Pickering M. Dual-stream accounts bridge the gap between monkey audition and human language processing. Physics of Life Reviews. 2016;16:69–70. doi: 10.1016/j.plrev.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Sammler D, Grosbras M, Anwander A, Bestelmeyer P, Belin P. Dorsal and Ventral Pathways for Prosody. Current Biology. 2015;25:3079–3085. doi: 10.1016/j.cub.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Musso M, Weiller C, Horn A, Glauche V, Umarova R, Hennig J, et al. A single dual-stream framework for syntactic computations in music and language. NeuroImage. 2015;117:267–283. doi: 10.1016/j.neuroimage.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Corballis M. What’s left in language? Beyond the classical model. Annals of the New York Academy of Sciences. 2015;1359:14–29. doi: 10.1111/nyas.12761. [DOI] [PubMed] [Google Scholar]
- 14.Chang E, Raygor K, Berger M. Contemporary model of language organization: an overview for neurosurgeons. Journal of Neurosurgery. 2015;122:250–261. doi: 10.3171/2014.10.jns132647. [DOI] [PubMed] [Google Scholar]
- 15.Tippett D, Niparko J, Hillis A. Aphasia: Current Concepts in Theory and Practice. J Neurol Transl Neurosci. 2014;2:1042. [PMC free article] [PubMed] [Google Scholar]
- 16.Bezgin G, Rybacki K, van Opstal AJ, Bakker R, Shen K, Vakorin VA, et al. Auditory–prefrontal axonal connectivity in the macaque cortex: Quantitative assessment of processing streams. Brain and Language. 2014;135:73–84. doi: 10.1016/j.bandl.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Berthier ML, Froudist Walsh S, Dávila G, Nabrozidis A, Juárez Y, Ruiz de Mier R, Gutiérrez A, et al. Dissociated repetition deficits in aphasia can reflect flexible interactions between left dorsal and ventral streams and gender-dimorphic architecture of the right dorsal stream. Frontiers in Human Neuroscience. 2013:7. doi: 10.3389/fnhum.2013.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cloutman L, Binney R, Morris D, Parker G, Lambon Ralph M. Using in vivo probabilistic tractography to reveal two segregated dorsal ‘language-cognitive’ pathways in the human brain. Brain and Language. 2013;127:230–240. doi: 10.1016/j.bandl.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nozari N, Dell G. How damaged brains repeat words: A computational approach. Brain and Language. 2013;126:327–337. doi: 10.1016/j.bandl.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dell G, Schwartz M, Nozari N, Faseyitan O, Branch Coslett H. Voxel-based lesion-parameter mapping: Identifying the neural correlates of a computational model of word production. Cognition. 2013;128:380–396. doi: 10.1016/j.cognition.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kümmerer D, Hartwigsen G, Kellmeyer P, Glauche V, Mader I, Klöppel S, et al. Damage to ventral and dorsal language pathways in acute aphasia. Brain. 2013;136:619–629. doi: 10.1093/brain/aws354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hickok G. The functional neuroanatomy of language. Physics of Life Reviews. 2009;6:121–143. doi: 10.1016/j.plrev.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimulation. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Primaßin A, Scholtes N, Heim S, Huber W, Neuschäfer M, Binkofski F, et al. Determinants of Concurrent Motor and Language Recovery during Intensive Therapy in Chronic Stroke Patients: Four Single-Case Studies. Frontiers in Neurology. 2015:6. doi: 10.3389/fneur.2015.00215.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez A, McCabe M, Nocera J, Reilly J. Concurrent Word Generation and Motor Performance: Further Evidence for Language-Motor Interaction. PLoS ONE. 2012;7:e37094. doi: 10.1371/journal.pone.0037094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellis C, Urban S. Age and aphasia: a review of presence, type, recovery and clinical outcomes. Topics in Stroke Rehabilitation. 2016:1–10. doi: 10.1080/10749357.2016.1150412.. [DOI] [PubMed] [Google Scholar]
- 27.Demeyere N, Riddoch MJ, Slavkova ED, Jones K, Reckless I, Mathieson P, et al. Domain-specific versus generalized cognitive screening in acute stroke. Journal of Neurology. 2015;263:306–315. doi: 10.1007/s00415-015-7964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jani M, Gore G. Occurrence of communication and swallowing problems in neurological disorders: analysis of forty patients. NeuroRehabilitation. 2014;35:719–27. doi: 10.3233/NRE-141165. [DOI] [PubMed] [Google Scholar]
- 29.Berthier M. Poststroke Aphasia. Drugs & Aging. 2005;22:163–182. doi: 10.2165/00002512-200522020-00006. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, Fowler R, Rodney D, Cherney L, Small S. IMITATE: An intensive computer-based treatment for aphasia based on action observation and imitation. Aphasiology. 2010;24:449–465. doi: 10.1080/02687030802714157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacquet-Andrieu A. The Aphasic Patient: Vulnerability and/or Exclusion. Cult Med Psychiatry. 2014;38:60–76. doi: 10.1007/s11013-014-9363-1. [DOI] [PubMed] [Google Scholar]
- 32.Niemi J, Koivuselkà-Sallinen P, Sarajärvi L. Grammatical Morphology in Aphasia: A Case of Errata or Reader Misinterpretations? Cortex. 1988;24:579–582. doi: 10.1016/s0010-9452(88)80052-2. [DOI] [PubMed] [Google Scholar]
- 33.Visser-Meily A, Post M, van de Port I, Maas C, Forstberg-Warleby G, Lindeman E. Psychosocial Functioning of Spouses of Patients With Stroke From Initial Inpatient Rehabilitation to 3 Years Poststroke: Course and Relations With Coping Strategies. Stroke. 2008;40:1399–1404. doi: 10.1161/strokeaha.108.516682. [DOI] [PubMed] [Google Scholar]
- 34.Quinn TJ, Paolucci S, Sunnerhagen KS, Sivenius J, Walker MF, Toni D, et al. European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Evidence-based stroke rehabilitation: an expanded guidance document from the European Stroke Organisation (ESO) guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Journal of Rehabilitation Medicine. 2009;41:99–111. doi: 10.2340/16501977-0301. [DOI] [PubMed] [Google Scholar]
- 35.Flowers HL, Skoretz SA, Silver FL, Rochon E, Fang J, Flamand-Roze C, et al. Poststroke Aphasia Frequency, Recovery, and Outcomes: A Systematic Review and Meta-Analysis. Archives of Physical Medicine and Rehabilitation. 2016 doi: 10.1016/j.apmr.2016.03.006.. [DOI] [PubMed] [Google Scholar]
- 36.Sarno M, Levita E. Recovery in treated aphasia in the first year post-stroke. Stroke. 1979;10:663–670. doi: 10.1161/01.str.10.6.663. [DOI] [PubMed] [Google Scholar]
- 37.Fridriksson J, Baker J, Moser D. Cortical mapping of naming errors in aphasia. Human Brain Mapping. 2009;30:2487–2498. doi: 10.1002/hbm.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charidimou A, Kasselimis D, Varkanitsa M, Selai C, Potagas C, Evdokimidis I. Why Is It Difficult to Predict Language Impairment and Outcome in Patients with Aphasia after Stroke? J Clin Neurol. 2014;10:75. doi: 10.3988/jcn.2014.10.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demonet J. Renewal of the Neurophysiology of Language: Functional Neuroimaging. Physiological Reviews. 2005;85:49–95. doi: 10.1152/physrev.00049.2003. [DOI] [PubMed] [Google Scholar]
- 40.Nicholas M, Helm-Estabrooks N, Ward-Lonergan J, Morgan A. Evolution of severe aphasia in the first two years post onset. Archives of Physical Medicine and Rehabilitation. 1993;74:830–836. doi: 10.1016/0003-9993(93)90009-y. [DOI] [PubMed] [Google Scholar]
- 41.Demeurisse G, Demol O, Derouck M, de Beuckelaer R, Coekaerts M, Capon A. Quantitative study of the rate of recovery from aphasia due to ischemic stroke. Stroke. 1980;11:455–458. doi: 10.1161/01.str.11.5.455. [DOI] [PubMed] [Google Scholar]
- 42.Nouwens F, Visch-Brink E, Van de Sandt-Koenderman M, Dippel D, Koudstaal P, de Lau L. Optimal timing of speech and language therapy for aphasia after stroke: more evidence needed. Expert Review of Neurotherapeutics. 2015;15:885–893. doi: 10.1586/14737175.2015.1058161. [DOI] [PubMed] [Google Scholar]
- 43.Pulvermüller F, Berthier M. Aphasia therapy on a neuroscience basis. Aphasiology. 2008;22:563–599. doi: 10.1080/02687030701612213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiori V, Cipollari S, Di Paola M, Razzano C, Caltagirone C, Marangolo P. tDCS stimulation segregates words in the brain: evidence from aphasia. Frontiers in Human Neuroscience. 2013:7. doi: 10.3389/fnhum.2013.00269.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Small S, Llano D. Biological approaches to aphasia treatment. Current Neurology and Neuroscience Reports. 2009;9:443–450. doi: 10.1007/s11910-009-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seitz R, Donnan G. Recovery Potential After Acute Stroke. Frontiers in Neurology. 2015:6. doi: 10.3389/fneur.2015.00238.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albert M, Sparks R, Helm N. Melodic Intonation Therapy for Aphasia. Archives of Neurology. 1973;29:130–131. doi: 10.1001/archneur.1973.00490260074018. [DOI] [PubMed] [Google Scholar]
- 48.Pulvermüller F, Neininger B, Elbert T, Mohr B, Rockstroh B, Koebbel P, et al. Constraint-Induced Therapy of Chronic Aphasia After Stroke. Stroke. 2001;32:1621–1626. doi: 10.1161/01.str.32.7.1621. [DOI] [PubMed] [Google Scholar]
- 49.Cherney L, Kaye R, van Vuuren S. Acquisition and Maintenance of Scripts in Aphasia: A Comparison of Two Cuing Conditions. American Journal of Speech-Language Pathology. 2014;23:S343. doi: 10.1044/2014_ajslp-13-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fridriksson J, Basilakos A, Hickok G, Bonilha L, Rorden C. Speech entrainment compensates for Broca’s area damage. Cortex. 2015;69:68–75. doi: 10.1016/j.cortex.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carpenter J, Cherney L. Increasing aphasia treatment intensity in an acute inpatient rehabilitation programme: a feasibility study. Aphasiology. 2015;30:542–565. doi: 10.1080/02687038.2015.1023695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Babbitt E, Worrall L, Cherney L. Structure, Processes, and Retrospective Outcomes From an Intensive Comprehensive Aphasia Program. American Journal of Speech-Language Pathology. 2015;24:S854. doi: 10.1044/2015_ajslp-14-0164. [DOI] [PubMed] [Google Scholar]
- 53.Stark B, Warburton E. Improved language in chronic aphasia after self-delivered iPad speech therapy. Neuropsychological Rehabilitation. 2016:1–14. doi: 10.1080/09602011.2016.1146150.. [DOI] [PubMed] [Google Scholar]
- 54.Cherney L, van Vuuren S. Telerehabilitation, Virtual Therapists, and Acquired Neurologic Speech and Language Disorders. Seminars in Speech and Language. 2012;33:243–258. doi: 10.1055/s-0032-1320044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlaug G, Norton A, Marchina S, Zipse L, Wan C. From singing to speaking: facilitating recovery from nonfluent aphasia. Future Neurology. 2010:657–665. doi: 10.2217/fnl.10.44.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (TDCS) for improving activities in patients after stroke. Physiotherapy. 2015;101:e359–e360. doi: 10.1016/j.physio.2015.03.573. [DOI] [Google Scholar]
- 57.Cahana-Amitay D, Albert M, Oveis A. Psycholinguistics of aphasia pharmacotherapy: Asking the right questions. Aphasiology. 2013;28:133–154. doi: 10.1080/02687038.2013.818099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saur D. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- 59.Geranmayeh F, Brownsett S, Wise R. Task-induced brain activity in aphasic stroke patients: what is driving recovery? Brain. 2014;137:2632–2648. doi: 10.1093/brain/awu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laska A, Kahan T, Hellblom A, Murray V, von Arbin M. A Randomized Controlled Trial on Very Early Speech and Language Therapy in Acute Stroke Patients with Aphasia. Cerebrovascular Diseases Extra. 2011;1:66–74. doi: 10.1159/000329835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meinzer M, Beeson PM, Cappa S, Crinion J, Kiran S, Saur D, et al. Neuroimaging in Aphasia Treatment Research Workshop. Neuroimaging in aphasia treatment research: Consensus and practical guidelines for data analysis. NeuroImage. 2013;73:215–224. doi: 10.1016/j.neuroimage.2012.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pascual-Leone A, Gates J, Dhuna A. Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology. 1991;41:697–702. doi: 10.1212/wnl.41.5.697. [DOI] [PubMed] [Google Scholar]
- 63.Otal B, Dutta A, Foerster Á, Ripolles O, Kuceyeski A, Miranda PC, et al. Opportunities for Guided Multichannel Non-invasive Transcranial Current Stimulation in Poststroke Rehabilitation. Frontiers in Neurology. 2016:7. doi: 10.3389/fneur.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nitsche M, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nitsche M, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 66.Hesse S, Werner C, Schonhardt E, Bardeleben A, Jenrich W, Kirker S. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: a pilot study. Restor Neurol Neurosci. 2007;25:9–15. [PubMed] [Google Scholar]
- 67.Hoffman P, Crutch S. Knowing what and where: TMS evidence for the dual neural basis of geographical knowledge. Cortex. 2016;75:151–159. doi: 10.1016/j.cortex.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cipollari S, Veniero D, Razzano C, Caltagirone C, Koch G, Marangolo P. Combining TMS-EEG with transcranial direct current stimulation language treatment in aphasia. Expert Review of Neurotherapeutics. 2015;15:833–845. doi: 10.1586/14737175.2015.1049998. [DOI] [PubMed] [Google Scholar]
- 69.Wirth M, Abdel Rahman R, Kuenecke J, Koenig T, Horn H, Sommer W, et al. Effects of transcranial direct current stimulation (tDCS) on behavior and electrophysiology of language production. Neuropsychologia. 2011;49:3989–3998. doi: 10.1016/j.neuropsychologia.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 70.Shah P, Szaflarski J, Allendorfer J, Hamilton R. Induction of neuroplasticity and recovery in post-stroke aphasia by non-invasive brain stimulation. Frontiers in Human Neuroscience. 2013:7. doi: 10.3389/fnhum.2013.00888.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shah-Basak P, Wurzman R, Purcell J, Gervits F, Hamilton R. Fields or flows? A comparative metaanalysis of transcranial magnetic and direct current stimulation to treat post-stroke aphasia. RNN. 2016;34:537–558. doi: 10.3233/rnn-150616. [DOI] [PubMed] [Google Scholar]
- 72.Blumstein S, Amso D. Dynamic Functional Organization of Language: Insights from Functional Neuroimaging. Perspectives on Psychological Science. 2013;8:44–48. doi: 10.1177/1745691612469021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harnish S, Meinzer M, Trinastic J, Page S. Language improves in chronic aphasia after motor therapy for upper extremity hemiparesis: a case series [abstract] Archives of Physical Medicine and Rehabilitation. 2009;90:E16. [Google Scholar]
- 74.Glover JE, Mateer CA, Yoell C, Speed S. The effectiveness of constraint induced movement therapy in two young children with hemiplegia. Pediatric Rehabilitation. 2002;5:125–131. doi: 10.1080/1363849021000039326. [DOI] [PubMed] [Google Scholar]
- 75.van Nispen K, van de Sandt-Koenderman M, Mol L, Krahmer E. Pantomime Production by People With Aphasia: What Are Influencing Factors? Journal of Speech-Language and Hearing Research. 2016;59:745. doi: 10.1044/2015_jslhr-l-15-0166. [DOI] [PubMed] [Google Scholar]
- 76.Roby-Brami A, Hermsdorfer J, Roy A, Jacobs S. A neuropsychological perspective on the link between language and praxis in modern humans. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;367:144–160. doi: 10.1098/rstb.2011.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hertrich I, Dietrich S, Ackermann H. The role of the supplementary motor area for speech and language processing. Neuroscience & Biobehavioral Reviews. 2016;68:602–610. doi: 10.1016/j.neubiorev.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 78.Turkeltaub P, Swears M, D’Mello A, Stoodley C. Cerebellar tDCS as a novel treatment for aphasia? Evidence from behavioral and resting-state functional connectivity data in healthy adults. RNN. 2016;34:491–505. doi: 10.3233/rnn-150633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimulation. 2016;9:641–661. doi: 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holland R, Crinion J. Can tDCS enhance treatment of aphasia after stroke? Aphasiology. 2012;26:1169–1191. doi: 10.1080/02687038.2011.616925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hauser M, Chomsky N, Fitch WT. The Faculty of Language: What Is It, Who Has It, and How Did It Evolve? Science. 2002;298:1569–1579. doi: 10.1126/science.298.5598.1569. [DOI] [PubMed] [Google Scholar]
- 82.Preisig BC, Eggenberger N, Zito G, Vanbellingen T, Schumacher R, Hopfner S, et al. Perception of co-speech gestures in aphasic patients: A visual exploration study during the observation of dyadic conversations. Cortex. 2015;64:157–168. doi: 10.1016/j.cortex.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 83.Lazar R, Minzer B, Antoniello D, Festa J, Krakauer J, Marshall R. Improvement in Aphasia Scores After Stroke Is Well Predicted by Initial Severity. Stroke. 2010;41:1485–1488. doi: 10.1161/strokeaha.109.577338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dipper L, Pritchard M, Morgan G, Cocks N. The language–gesture connection: Evidence from aphasia. Clinical Linguistics & Phonetics. 2015;29:748–763. doi: 10.3109/02699206.2015.1036462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Conway C, Pisoni D. Neurocognitive Basis of Implicit Learning of Sequential Structure and Its Relation to Language Processing. Annals of the New York Academy of Sciences. 2008;1145:113–131. doi: 10.1196/annals.1416.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ge J, Peng G, Lyu B, Wang Y, Yan Z, Zhendong N, et al. Cross-language differences in the brain network subserving intelligible speech. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2972–2977. doi: 10.1073/pnas.1416000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bick AS, Goelman G, Frost R. Hebrew brain vs. English brain: Language modulates the way it is processed. Journal of Cognitive Neuroscience. 2011;23:2280–2290. doi: 10.1162/jocn.2010.21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khachatryan E, Vanhoof G, Beyens H, Goeleven A, Thijs V, Van Hulle MM. Language processing in bilingual aphasia: a new insight into the problem. Wiley Interdisciplinary Reviews: Cognitive Science. 2016:180–196. doi: 10.1002/wcs.1384.. [DOI] [PubMed] [Google Scholar]


