Abstract
Traditional response criteria in MDS and AML are based on bone marrow morphology and may not accurately reflect clonal tumor burden in patients treated with non-cytotoxic chemotherapy. We used next-generation sequencing of serial bone marrow samples to monitor MDS and AML tumor burden during treatment with epigenetic therapy (decitabine and panobinostat). Serial bone marrow samples (and skin as a source of normal DNA) from 25 MDS and AML patients were sequenced (exome or 285 gene panel). We observed that responders, including those in complete remission (CR), can have persistent measurable tumor burden (i.e., mutations) for at least one year without disease progression. Using an ultra-sensitive sequencing approach, we detected extremely rare mutations (equivalent to 1 heterozygous mutant cell in 2000 non-mutant cells) months to years prior to their expansion at disease relapse. While patients can live with persistent clonal hematopoiesis in a CR or stable disease, ultimately we find evidence that expansion of a rare subclone occurs at relapse or progression. Here we demonstrate that sequencing of serial samples provides an alternative measure of tumor burden in MDS or AML patients and augments traditional response criteria that rely on bone marrow blast percentage.
Introduction
DNA hypomethylating agents such as decitabine and azacitidine are commonly used to treat myelodysplastic syndromes (MDS) and older adults with acute myeloid leukemia (AML).1–3 Although rates of complete remission (CR) are relatively low, hypomethylating agents are associated with an improved overall survival when compared to conventional care regimens.2–6 In contrast to traditional cytotoxic agents such as anthracyclines and cytarabine, maximum responses can be delayed 4–6 cycles from initiation of therapy.7 In addition, patients will invariably relapse, which underscores the need for novel therapies.
Despite being non-curative, patients treated with hypomethylating agents who achieve hematologic improvement or stable disease may derive clinical benefit from these agents, suggesting that traditional response criteria may not accurately reflect antitumor activity.8 Monitoring tumor burden in MDS can be particularly challenging where the blast percentage underestimates tumor burden and is often less than 5% of bone marrow cells.9 In patients who otherwise meet criteria for CR, the presence of persistent dysplasia is highly subjective and may also not accurately reflect tumor burden. Tracking cytogenetic abnormalities can serve as a useful adjunct in monitoring treatment responses but up to 50% of MDS and AML patients have normal karyotypes. Therefore, traditional response criteria in MDS and AML that primarily rely on bone marrow morphology (e.g., myeloblast percentage) or karyotype may not accurately reflect antitumor responses or clinical benefit.10, 11
A major advance in our understanding of MDS and AML biology has come from next-generation sequencing studies that have identified recurring somatic mutations. In MDS and AML, hematopoietic cells contain gene mutations that are variably distributed between the founding clone and daughter subclone(s).9, 12–14 A minor subclone present at diagnosis can escape eradication during initial therapy, acquire additional mutations, and ultimately contribute to relapse. We hypothesized that serially monitoring tumor burden using next-generation sequencing would provide additional information that can augment traditional measures of response. To test this hypothesis, we used next-generation sequencing of serial bone marrow samples to monitor tumor burden and to characterize the changes in the clonal structure of MDS and AML that occur during treatment with epigenetic therapy. In this study, we combined the histone deacetylase inhibitor panobinostat with decitabine in older adults with high-risk MDS or AML.15 The addition of panobinostat to a previously established decitabine regimen allowed us to evaluate the concept that two epigenetic modifiers with distinct mechanisms of action may improve the responses of patients compared to historical treatment with decitabine alone.
Materials and Methods
Patients
This trial was a phase I/II open label study of panobinostat plus decitabine in older adults with high-risk MDS or AML. The primary clinical endpoints were to determine the maximum tolerated dose and dose-limiting toxicity of panobinostat when given in combination with decitabine, and to determine the complete remission rate for the regimen. Eligible patients were age 60 years and older with high-risk MDS (IPSS Int-2 or High) or AML excluding AML with PML-RARA and core binding factor leukemias. Patients with prior treatment with either a hypomethylating agent or histone deacetylase (HDAC) inhibitor were ineligible. This study is registered at ClinicalTrials.gov #NCT00691938 and approved by the Institutional Review Board of Washington University. The study was carried out in accordance with the Declaration of Helsinki and amendments and written informed consent was obtained from all subjects prior to enrollment. Three patients enrolled in this study (PPI019, 023, 025) were reported in another publication (Welch, et. al., N Engl J Med, in press).
Treatment plan
Decitabine 20 mg/m2 was administered by intravenous infusion on days 1–5 of a 28 day cycle. Oral panobinostat was initially administered three times a week on nonconsecutive days in a 28 day cycle. Following a protocol amendment, subsequent dose levels of panobinostat were administered three times a week for the first 2 weeks only. A phase I dose escalation of panobinostat was performed using a standard 3 + 3 design ranging from 10 mg to 50 mg/daily. In the phase II, the cohort was expanded to include total of 20 patients at the selected phase I dose. Serial bone marrow samples were collected pre-study, cycle 1 day 15, after every 2 cycles of treatment, and at the time of relapse for both clinical assessment and next-generation sequencing. A subset of patients had sequencing performed on bone marrow samples obtained after study completion. Responses were assessed according to International Working Group (IWG) criteria for AML and proposed modifications to the IWG criteria for MDS.10, 11
Sequencing and SNP arrays
DNA from paired normal skin and bone marrow was enriched for a custom set of 285 recurrently mutated MDS and AML genes (RMG panel including all coding exons; Supplementary Table 1) either alone (n=24 patients) or “spiked in” to an exome sequencing reagent (n=7 patients; Enhanced Exome sequencing [ESS]). Exome sequencing data was deposited in the dbGAP database (phs000159, study version 9). Enriched libraries were sequenced on a HiSeq2000 instrument with 2×101 bp reads, as previously described.16 Paired tumor/normal variant calling for single nucleotide variants (SNVs) and indels was performed, as previously described.16, 17 Copy number alteration analysis using sequencing and SNP arrays is described in the Supplementary Methods. SNP array data was deposited in the GEO database (GSE81738). Ultra-deep error-corrected sequencing (E.D. and M.W. unpublished data) was performed on 45 serial treatment bone marrows. Briefly, ligation-based amplification probes were designed to target both DNA strands of all mutations identified by ESS or RMG sequencing interrogating a total of 384 positions covered by 2,054 amplicons. DNA (500 ng) was digested, and hybridized to probes plus 10bp degenerate oligonucleotides (molecular barcodes), and sample-specific indexes. Cases were sequenced to target 20,000× to 100,000× coverage. Data were analyzed using the Barcrawler sequencing pipeline (E.D. unpublished data), with a maximum sensitivity of 0.1% (additional details are provided in the Supplementary Methods). A complete list of the various genomic assays performed on patient samples and time-points is provided in Supplementary Table 2.
Results
Patient characteristics and clinical response rates
A total of 52 patients with a median age of 70 years were enrolled and treated on the study protocol. (Table 1). The study population consisted of 38 patients with AML and 14 with MDS. Fifteen patients, including 14 with AML had prior therapy for their hematologic disorder (Table 1).
Table 1.
Patient Baseline Characteristics
| Patient characteristic, (n=52) | ||
|---|---|---|
| Age, years | Median (range) | 70 (61–87) |
| >70 (%) | 26 (50) | |
| >80 (%) | 6 (12) | |
| Gender | Male (%) | 25 (48) |
| Female | 27 (52) | |
| ECOG PS | 0 (%) | 25 (49) |
| 1 (%) | 21 (40) | |
| 2 (%) | 5 (12) | |
| Missing (%) | 1 (2) | |
| Diagnosis | AML (%) | 38 (74) |
| MDS (%) | 14 (27) | |
| Onset | De novo (%) | 35 (67) |
| Secondary (%) | 17 (33) | |
| WBC (/nl), median (range) | 2.7 (0.6–53.6) | |
| %Bone marrow blasts, median (range) | 19.5 (0–91) | |
| Prior therapy | Yes (%) | 15 (29) |
| No (%) | 37 (71) | |
ECOG PS: Eastern Cooperative Oncology Group performance status
In the phase I, panobinostat was escalated from 10 mg to 40 mg three times a week without reaching the maximum tolerated dose. Although no dose limiting toxicities were identified, a decision was made to reduce the dose to 40 mg for the first 2 out of 4 weeks in the dose expansion phase based on data suggesting improved tolerability.18 The most common adverse events reported during treatment include fatigue (88%) febrile neutropenia (76%), diarrhea (75%), and nausea (69%) (Supplementary Table 3).
Fifty-one patients received at least 1 dose of panobinostat and were included in the efficacy analysis (37 patients with AML and 14 with MDS). Subjects received a median of 2 cycles of treatment (range <1–12). The phase I and II efficacy results were pooled. Using IWG working group criteria, the overall rate of complete remission [CR, cytogenetic CR (CRc), morphologic CR with incomplete blood count recovery (CRi)] for AML patients (n=37) was 10% (1 CR, 1 CRc, and 2 CRi) with an additional 19% of patients achieving a morphologic leukemia-free state (mLFS, n=7). For patients with MDS, 2 of 14 achieved a CR, including one CRc. An additional 4 MDS patients achieved a marrow CR (mCR) defined as <5% blasts in the bone marrow but not meeting criteria for CR due to continued cytopenias or lack of RBC transfusion independence (Table 2). One MDS patient met criteria for hematologic improvement in both platelets and neutrophils without achieving a CR. With a median follow-up of 58 months, the median overall survival for the 51 patients who received treatment was 6.44 months. For the Phase II cohort, Dose Level 5B, the median overall survival was 3.8 months. (Supplementary Figure 1)
Table 2.
Response Evaluation
| Response | n | (%) | |
|---|---|---|---|
| AML (n=37) | CR/CRc | 2 | 5% |
| CRi | 2 | 5% | |
| mLFS | 7 | 19% | |
| TF | 26 | 70% | |
| MDS (n=14) | CR/CRc | 2 | 5% |
| CRi | 0 | 0% | |
| mCR | 4 | 11% | |
| SD | 2 | 5% | |
| Failure | 6 | 16% | |
| HI-P, HI-N | 1 | 3% |
CR: Complete Remission; CRc: Cytogenetic CR; CRi: CR with incomplete blood count recovery; TF: Treatment failure; mLFS: Morphologic leukemia-free state; mCR: marrow CR; SD: stable disease; HI-P: Hematologic improvement-platelets; HI-N: Hematologic improvement-neutrophils
Sequencing metrics
We identified 10 MDS and 15 AML patients with adequate banked skin (as a source of normal DNA) and paired bone marrow that also had at least 1 somatic mutation previously identified (data not shown). We sequenced skin and serial bone marrow samples from these 25 patients using a panel of 285 genes (including all coding exons) that are known to be recurrently mutated in AML and MDS (i.e., recurrently mutated genes; RMGs). In addition, 7 of these 25 patients also had enhanced exome sequencing (EES; exome plus RMG) of their skin and bone marrow samples from pre-study and one later time-point. Averaged coverage depth for the 285 RMG panel was 104× for skin and 160× for tumor. Averaged coverage depth for the EES was 65× for skin and 276× for tumor. For samples evaluated by ultra-deep error-corrected sequencing, we obtained 31,849× average total coverage depth (mean range 11,711–207,272×) corresponding to 4,936× average unique read family coverage depth (mean range 596–5,842×) (Supplementary Figure 2 and Supplementary Methods).
Spectrum of gene mutations at diagnosis
The landscape of genomic alterations was highly concordant with previous studies of patients with MDS and AML (Figure 1A and Supplementary Table 4). Thirteen genes were mutated in at least 3 pre-study samples (Figure 1B) and we detected an average of 4.9 tier 1 (coding and splice sites) SNVs and indels per patient (range 1–15) when only the RMG panel was used, compared to 27.4 mutations per patient (range 9–43) using EES (Figure 1C). A total of 26 genes had tier 1 mutations in 2 or more patients (Supplementary Table 4) at pre-study banking. The vast majority of these recurrently mutated genes include those with well-established association in MDS or AML. There was no significant association between the mutational status of a gene at pre-study and achieving a complete remission using this treatment regimen.
Figure 1. Heatmap of molecular and clinical findings.
(a) Distribution of mutations in 25 patients (10 MDS, 15 AML) with at least one mutation in 16 genes or pathways in samples from any time-point. Each column represents an individual patient sample and each row represents a gene with a mutation. Mutations are indicated by colored cells and gene groups/families are indicated at the left. (b) The number of mutations in each gene present in pre-study samples is listed. Splice mutations include the splice acceptor and donor dinucleotides. (c) Number of coding mutations detected in the pre-study samples (excluding silent mutations). RMG, recurrently mutated genes in AML and MDS; Complex cytogenetics, ≥ 3 clonal abnormalities; Complete Remission, CR/CRc/CRi; Non-evaluable, received < 2 cycle of treatment; TF, transcription factors; PPI, positive patient identifier
Monitoring of tumor burden during treatment
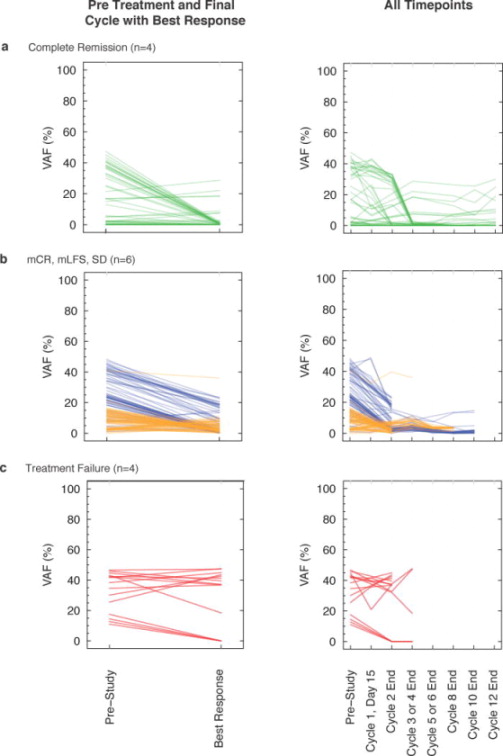
We next analyzed mutation VAFs in serial samples to monitor changes in tumor burden during treatment. We segregated patients that had a pre-study sample sequenced and received 2 or more cycles of treatment into those that achieved a complete remission (CR, CRi, or CRc; n=4) (Figure 2A), those with stable disease (SD, n=2), marrow leukemia free state (mLFS, n=2), or marrow complete remission (mCR, n=2) (Figure 2B), and those with treatment failure (n=4) (Figure 2C). As expected, the majority of mutation VAFs from patients with treatment failure did not change during treatment. While some patients with a CR had complete disappearance of VAFs at serial time-points, others had persistence of mutation VAFs. Persistence of mutations was also observed for mCR, mLFS, and SD categories. The average mutation VAF at the end of treatment was lower in patients achieving a complete remission versus those with mCR, mLFS, and SD (4.3% vs. 8.8%, respectively, p=0.03). Similarly, the average decrease in mutation VAFs from pre-study to end of treatment was greater in patients achieving a complete remission versus those that did not (21.4%% vs. 13.4%, respectively, p=0.0007). Further analysis of individual patients revealed five distinct mutation VAF patterns that were associated with different clinical responses, including i) AML patients achieving a CR or CRc (PPI013, PPI019, respectively): mutation VAFs were undetectable by cycle 2 using next-generation sequencing of the RMG panel, ii) AML with mLFS (PPI023, PPI031): mutation VAFs remained detectable but decreased to <10%, iii) MDS with CRc and mCR (PPI005, PPI035, respectively): mutation VAFs decreased to <10% and were intermittently below the level of detection, iv) MDS with mCR and CR (PPI008, PPI010, respectively) or stable disease (PPI049, PPI050): mutation VAFs were unchanged or decreased but some remained >10%, and v) AML with treatment failure (PPI006, PPI011, PPI022, PPI025): mutation VAFs were essentially unchanged and remained >30% (Supplementary Figure 3).
Figure 2. Dynamic changes in mutation VAFs during treatment.
Mutation variant allele fraction (VAF) in paired pre-study and time of best response samples from patients that achieved a complete remission (a) versus those that did not (b and c) (left panels). Each line represents a mutation that occurred in a diploid part of the genome. Serial mutation VAFs are displayed in the right panel. Stable disease (SD, n=2, blue), marrow leukemia free state (mLFS, n=2, orange), or marrow complete remission (mCR, n=2, orange). VAF, variant allele fraction.
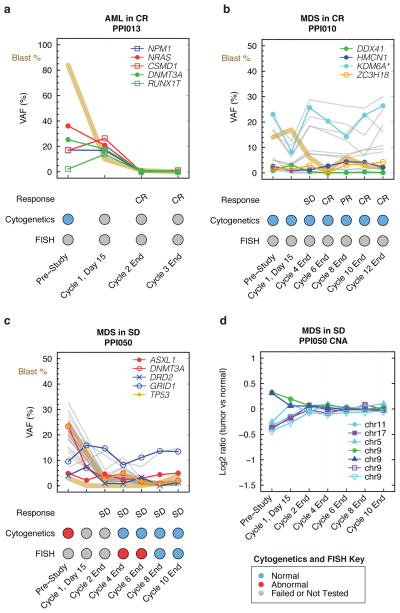
Sequencing, cytogenetic, FISH, and copy number alteration data (Supplementary Tables 5–7) for 3 representative cases with different mutation VAF patterns are shown in Figure 3. A cytogenetically normal AML patient (PPI013; AML with CR) started treatment with a blast count >80% (Figure 3A). Sequencing of the initial bone marrow sample demonstrated 5 mutations in recurrently mutated genes with a mean VAF of 24%. The patient achieved a CR with a normal blast count by the end of cycle 2. VAFs for these mutations became undetectable at cycle 2 by sequencing of the RMG panel, consistent with the clinical response. We also observed that the blast percentage decreases prior to dramatic changes in mutation VAFs at cycle 1 day 15, suggesting that the differentiation of blasts could falsely underestimate tumor burden (Figure 3A; see also PPI005 in Supplementary Figure 3).
Figure 3. Persistence of mutation VAFs in complete remission and stable disease.
(a) An AML patient with normal cytogenetics (PPI013) achieved a CR at the end of cycle 2. The blast % decreased at cycle 1 day 15 prior to a decrease in 5 mutation VAFs, and normalized by the end of cycle 2. The molecular and morphologic response was similar in this patient. (b) An MDS patient with normal cytogenetics (PPI010) achieved a CR at the end of cycle 6 and 12. Mutations VAFs remained detectable throughout treatment, including a mutation in KDM6A that was present with a copy number adjusted VAF ~20–30% (i.e., ~40–60% of cells harboring a mutation). The molecular and morphologic response was discordant in this patient with a normal karyotype. (c) An MDS patient with abnormal cytogenetics (PPI050) had persistent stable disease throughout treatment. Cytogenetics and FISH normalized by cycle 8. While there was an initial decrease in mutations VAFs, they remained detectable throughout treatment. A mutation in ASXL1 was unchanged during treatment and may represent a clone that is independent of the malignant clone (i.e., clonal hematopoiesis of indeterminate significance). The molecular and morphologic response was similar and the patient remained with cytopenias despite have a low level of detectable mutations. (d) Copy number alterations detected by RMG sequencing for PPI050 are shown. Copy number gains have a log2 ratio of tumor versus normal >0 and losses <0. The altered regions are not detected by the end of cycle 2, including del(5). CR, complete remission; SD, stable disease; PR, partial remission; CNA, copy number alterations; VAF, variant allele fraction; FISH, fluorescent in situ hybridization; *copy number adjusted VAF.
A cytogenetically normal MDS patient with RAEB-2 (PPI010; MDS with CR) presented with a blast count of 14% and a mean mutation VAF of 6.5% across multiple clones (Figure 3B). The patient had stable disease at the end of cycle 4 with a blast count of 6% and a mean VAF of 7.3%. The patient completed the study in CR with a blast count of 2%, however, the mean mutation VAF was 8.7% across multiple clones, indicating that ~17% of tumor cells were present in the bone marrow despite having achieved a morphologic and clinical CR.
The third patient (PPI050; MDS with SD), an MDS patient with RCMD and complex cytogenetics [including del(5q)], began the study with a blast count of 3% and a mean VAF of 20% (Figure 3C). The patient achieved stable disease (SD) by the end of cycle 2 with a blast count of <5% and mean VAF of 5.2% and completed the study in SD with a blast count of <5% and a mean VAF of 4.2%. This patient also had clonal cytogenetic findings that were variably detected during treatment. While conventional cytogenetics (20 metaphases) did not detect clonal findings beyond the pre-study time-point, fluorescence in situ hybridization studies (FISH) performed after cycle 4 and 6 showed low-level persistence of del(5) (9% and 1.33% of cells, respectively) consistent with sequencing results. However, cytogenetics, FISH and copy number alterations based on RMG gene-panel sequencing were negative at cycles 8 and 10 while sequencing remaining positive (Figure 3C-D). This patient had incomplete recovery of blood counts, despite having very low tumor burden based on FISH and sequencing. Collectively, the data indicate that there can be discrepancies between clinical and molecular response in patients treated with this regimen.
Complex patterns of clonal evolution
We have previously shown that MDS and AML samples contain both founding clone mutations (present in every cell of the tumor), as well as subclonal mutations, which occur in only a fraction of the tumor cells (and have lower VAFs). We identified dynamic changes in the size of distinct subclones by tracking mutation VAFs in serial samples. “Falling” subclones with diminishing VAFs indicate specific susceptibility to therapy, while “rising” subclones indicate a relative growth advantage and suggest treatment resistance.
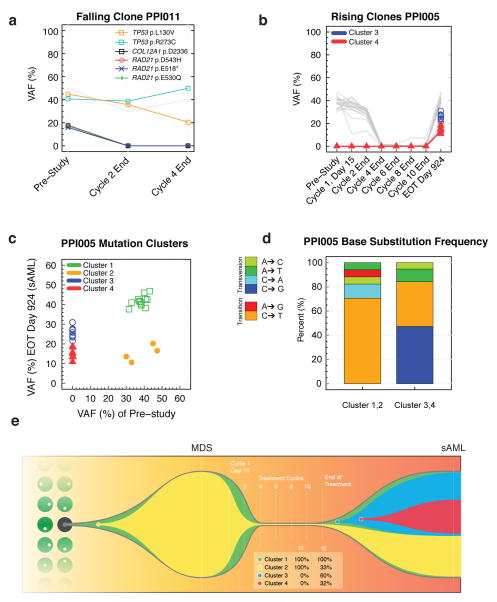
AML patient PPI011 did not achieve a complete remission, but sequencing revealed selective clearance of a subclone harboring TP53 and RAD21 mutations (Figure 4A). The founding clone persisted and contained biallelic TP53 mutations. These cells subsequently acquired a deletion of the TP53 L130V allele at the end of cycle 4. While not curative in this patient, the falling subclone containing TP53 and RAD21 mutations identifies a combination of mutations that may be sensitive to panobinostat and decitabine treatment.
Figure 4. Differential sensitivity of tumor clones during treatment.
(a) An AML patient (PPI011) with treatment failure harbors a subclone defined by three somatic RAD21 mutations (colored lines) that become undetectable by the end of treatment (i.e., falling clone). Additional mutations (grey) define the founding clone that is treatment-resistant and contains two TP53 mutations. (b) An MDS patient who achieved CR at cycle 4 progressed to secondary AML (sAML) 924 days after the end of treatment on this study. Two subclones (red and blue) emerge and are detectable in the sAML sample. These subclones were not detected at pre-study or during the treatment cycles (i.e., rising clones). (c) The mutation VAFs from MDS patient PPI005 are shown at the pre-study MDS stage and after progression to sAML at 924 days after the end of treatment. Mutation VAFs were adjusted for chromosomal copy number. Unsupervised clustering of individual mutations identified four distinct mutation clusters representing clones, two of which (red and blue) are specific to the sAML sample. (d) Spectrum of single base substitutions in cluster 1 and 2 (present in initial pre-study MDS) versus clusters 3 and 4 (detectable in sAML). The sAML-specific clusters show a greater proportion of C-G transversions that are associated with decitabine treatment, suggesting that some mutations are caused by the treatment. (e) For patient PPI005, the tumor phylogeny was inferred using the clonevol package (https://github.com/hdng/clonevol, manuscript in preparation). Two models are possible, differing only in whether subclone 4 is derived from subclone 3 or 4 (Supplementary Figure 6). The model assigned higher likelihood was used to produce the above plot summarizing the clonal evolution from the MDS stage to the sAML. Residual non-mutant normal cells are not depicted at MDS or sAML time-points (i.e., percentage of cells with cluster mutations represents tumor cells only). Cells in clone 1 contain cluster 1 mutations. Clone 1 (green) is the founding clone and is present in nearly all bone marrow cells at MDS and sAML time points; clone 2 (yellow) is similarly present in almost all cells at the MDS pre-study time-point, but is present in only 33% of cells in the secondary AML time-point. Clones 3 and 4 (blue and red) are not detected in the pre-study MDS sample but emerge at sAML and are present in 60% and 32% of bone marrow cells, respectively. EOT, end of treatment. VAF, variant allele fraction.
We also observed that progression from MDS to secondary AML (sAML) is sometimes characterized by the expansion of a rare subclone (i.e., rising subclone) (Figure 4B). We performed whole exome sequencing on a bone marrow sample obtained 924 days after the end of treatment when this patient presented with sAML. Clustering of mutations present in the pre-study and sAML samples reveal that while the sAML is derived from the same founding clone, it also contains new subclonal mutations (Figure 4C). While C to T transitions are most common in the founding clone, the new subclones are dominated by C to G transversions (p=0.03) (Figure 4D). Coupled with the knowledge that decitabine can induce C to G transversions,19 this altered mutation spectrum suggests that many of these rising subclone mutations may have been caused by decitabine treatment. The overall pattern of clonal evolution of PPI005 is complex and characterized by the fall of the MDS founding clone (green) and a subclone (yellow) during treatment, followed by the return of the founding clone (green) and emergence of 3 subclones at sAML progression (yellow, blue and red) (Figure 4E).
Transient response of TP53 mutant clones is common
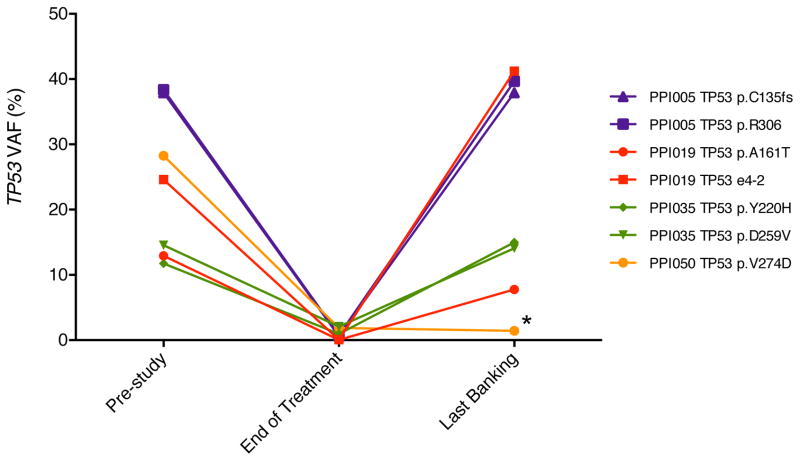
We next attempted to identify mutations that are predictive of a response to treatment by searching for genes that consistently showed a decrease in their mutation VAFs during treatment, regardless of whether present in a founding clone or subclone. TP53 and SRSF2 were the only genes mutated in at least 3 patients that had follow-up sequencing at cycle 2 or later, allowing us to correlate mutation status with clinical response. While there was a consistent decrease in TP53 VAFs during treatment (Figure 5), this was not associated with obtaining a complete remission at the treatment dose and schedule of drugs used in this study. TP53 mutation VAFs decreased by an average of 26.5-fold in 4 patients; however, the VAF subsequently increased during relapse in 3 of 4 patients with follow-up samples. Only PPI050 has not relapsed and has since been maintained on single-agent decitabine (Figure 5). Two of the 3 patients with SRSF2 mutations had a decrease in their VAFs, but neither VAF dropped below 10% (Supplementary Figure 4)
Figure 5. TP53 mutation VAFs decrease during treatment.
Four patients harbored somatic mutations inTP53 with VAFs >5% in the pre-study or cycle 1 time-point, completed at least 2 cycles of treatment, and had no evidence of TP53 loss of heterozygosity. These patients showed a mean VAF decrease of 26.5-fold at the end of treatment. All 4 patients had follow-up bone marrows obtained after completing the study, and 3 of 4 showed a subsequent increase in TP53 VAFs; the remaining patient (PPI050) continued on single agent decitabine after completing the study (indicated by *). Mutations are indicated in standard p-syntax.
Mutations persist at low levels during clinical complete remission
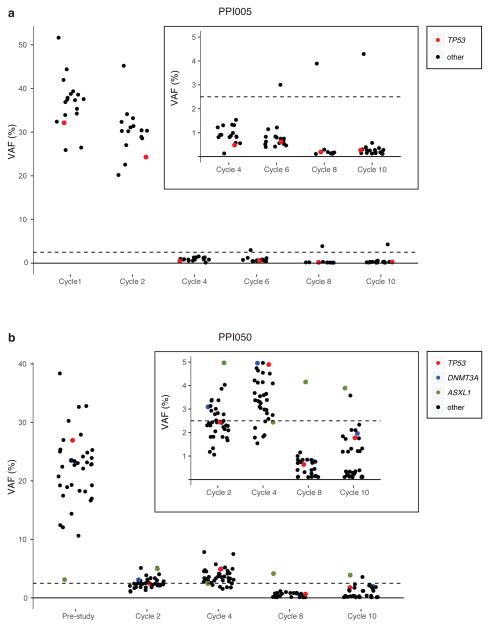
Next, we investigated the extent to which clonal mutations were cleared under this regimen using an ultra-deep error corrected sequencing approach with a sensitivity of 0.1% VAF (see Supplementary Methods). We sequenced serial samples from 8 patients using ultra-deep error corrected sequencing, including 4 patients achieving complete remissions (CR, CRc, or CRi) or marrow CR (Supplementary Table 2). In patient PPI005, nearly all mutations detected in the initial pre-study MDS sample could be detected in subsequent treatment time points using this approach, often with VAFs much lower than that detectable by standard sequencing (i.e., ~2% VAF). The TP53 p.R306* mutation, present with a VAF of 37% at the start of treatment was not detected at treatment cycle 8 using standard sequencing (0 variant reads out of 113 unique reads). Using ultra-deep error corrected sequencing, we confirmed its presence with a VAF of 0.16% (6 unique read families out of 3686 total read families passing filter) (Figure 6A, inset). We further validated these findings using droplet digital PCR (Supplementary Figure 5). Thus, even during clinical and molecular CR (based on standard sequencing, cytogenetics, and FISH) ultra-deep error corrected sequencing provided evidence of persistent disease.
Figure 6. Persistence of rare tumor cells are detected using ultra-deep error corrected sequencing.
(a) An MDS patient (PPI005) achieved CR at the end of cycle 4, consistent with absence of mutation detection by standard sequencing platforms (conservative sensitivity indicated by the dashed line at 2% VAF). Somatic mutations were detected at all treatment time-points using ultra-deep error corrected sequencing and high sequence coverage, including during CR when many mutations are present with VAFs <1% (inset panel). Canonical gene mutations are indicated by colored circles. (b) An MDS patient with stable disease (no CR) shows similar persistence of detectable somatic mutations using ultra-deep error corrected sequencing across all treatment time-points, including those with VAFs <1% (inset). Mutations were detectable at cycles 8 and 10 when cytogenetics and FISH were negative. The ASXL1 mutation (green) does not change with treatment and is likely present in a nonmalignant clone distinct from the founding clone. VAF, variant allele fraction.
We then demonstrated persistence and eventual expansion of clonal mutations, including the TP53 mutation, at progression to secondary AML, 924 days after the end of treatment. At secondary AML transformation new progression-specific (i.e., rising subclone) mutations were detected using whole exome sequencing (Figure 4B). Patient PPI050 showed a similar persistence of MDS mutations using ultra-deep error corrected sequencing despite mutation VAFs being below the threshold of detection using standard non-error corrected next-generation sequencing (Figure 6B, inset). While the majority of mutations travel together as a single clone in this patient, the ASXL1 variant, initially present with a VAF of 4.2%, appears to represent a distinct clone that is not affected by chemotherapy, and it probably represents a non-malignant clone.
Discussion
In this paper, we describe our results combining decitabine and panobinostat in older adults with high-risk MDS or AML. Although the combination was well tolerated by most patients, the addition of panobinostat did not improve rates of CR/CRi in comparison to prior studies of decitabine alone with reported CR rates of 13–18% in phase 3 studies.20, 21 Despite the low overall rates of CR/CRi, we were able to gain important insights into the clonal dynamics of MDS and AML during treatment with epigenetic therapy. We observed that blast percentage frequently underestimates the burden of disease in these patients, particularly in MDS, and that molecular responses can be discordant with clinical response criteria. Using a sensitive barcoded sequencing technique, we were able to reliably identify low-level persistence of mutations in patients with clinical complete remissions. Many of these mutations were not detected using standard next-generation sequencing. In addition, rare tumor cells were identified months to years before patients presented with a morphologic relapse. Collectively, detection and serial tracking of mutation VAFs provided a unique molecular signature for each patient, and allowed for the dynamic monitoring of tumor clones and detection of measurable residual disease.
Molecular profiling of tumors has contributed greatly to our understanding of the pathogenesis of hematologic malignancies.12, 17, 22, 23 Most tumors have several recognized driver mutations that are variably distributed between the founding clone and subclones, yielding an enormous number of combinations that might be important to tumorigenesis and outcomes. Prior studies have also suggested that mutations in genes encoding epigenetic modifiers, including DNMT3A or TET2, are predictive of response to hypomethylating agents.24–27 The small sample size of this study limited our ability to comprehensively study the impact of many genotypes on response. However, we did observe that subclones with TP53 mutations show marked, but not durable, responses to treatment with panobinostat and decitabine. Collectively, there remains a large degree of variability in clinical outcomes based on single gene mutation status (categorized simply as absent or present). This lack of predictive power may reflect the combinatorial complexity of mutations in AML and MDS. While baseline mutations have some predictive ability in MDS and AML, our goal was to assess the feasibility and utility of monitoring tumor burden by serially monitoring mutations and clones during treatment. The serial sequencing of samples from the same patient allowed us to make several clinically relevant observations.
First, mutations frequently persist using this treatment regimen, even during a complete remission. The low-level mutation VAFs we detect in remission may represent persistence of mutant hematopoietic stem and progenitor cells (HSPCs) following epigenetic therapy, as previously shown using FISH and flow sorted HSPCs.28, 29 Ultra-deep error corrected sequencing that we used here may allow for the detection and tracking of these rare cells without isolating HSPCs. Second, tracking individual mutation VAFs may not accurately reflect tumor burden. The persistence of the KDM6A mutation in this subject achieving a CR suggests that the abnormal blast count and cytopenias were not driven by the clone containing the KDM6A mutation. Whether this mutation resides in the hematopoietic stem cell or a later progenitor is not clear, but it is a long lived cell as the clone persists for at least 12 months. The persistence of an ASXL1 mutation in PPI050 likely represents an unrelated clonal hematopoiesis of indeterminate potential (CHIP) mutation rather than a tumor clone.30–33 Sequencing serial samples was necessary to decipher this possibility. Similarly, the persistence of clonal mutations does not necessarily indicate a clinical benefit is not obtainable. For example, PPI010 achieved a durable CR and PPI050 had a lasting SD with persistent mutation VAFs. Finally, we observed that tracking mutation VAFs only present in a subclone can underestimate the tumor burden in a patient (e.g., RAD21 mutations in PPI011). Ultimately, tracking every clone with serial sampling will overcome these limitations.
Sequencing results provide an objective measure of tumor burden that may complement traditional response criteria and help guide treatment decisions. For PPI023 and PPI031 with mLFS, sequencing results indicate that the lack of hematopoietic recovery may be related to drug toxicity rather than tumor burden, suggesting a delay in treatment may be justified. Recently, Merlevede et. al. reported that mutation VAFs were largely unchanged in monocytes from CMML patients that responded to hypomethylating agents and achieved a marrow CR (i.e., decrease in bone marrow blasts to <5% without hematologic improvement).34 Our data for patients with a marrow CR are similar, reinforcing the discordance between marrow blast percentage and mutation clearance. Sequencing results also indicate that decitabine can induce mutations during the course of treatment. PPI005 progressed to secondary AML, and two subclones emerged. These subclones contained new C to G transversions, a signature of decitabine exposure.19 A larger set of secondary AML samples arising after decitabine treatment will need to be sequenced to address whether decitabine-induced mutations may influence disease progression. This observation may have implications, since hypomethylating agents have been proposed and tested as maintenance therapy for AML.35 The contribution of panobinostat to the mutational and clonal changes is not known.
The development of effective therapies for patients with MDS or AML has been hampered by the lack of surrogate trial endpoints that can predict clinical benefit. Historically, phase 2 studies in high-risk MDS and AML have relied on CR as encouraging evidence of antitumor activity but almost uniformly have failed in phase 3 studies. By performing serial sequencing of samples obtained during the course of therapy, we gained important clinical insights into the tumor response to epigenetic therapy. As sequencing technologies continue to mature, we believe that serial analyses may provide important information that is complementary to traditional measures of outcome in clinical trials. Indeed, clearance of mutation VAFs after induction therapy is predictive of outcomes in AML.16 Given the clonal complexity of hematologic cancers, platforms that can identify and track all clones may be necessary to inform clinical practice. Ultimately, incorporating serial sequencing studies into clinical trial design may allow us monitor tumor burden and better evaluate agents at earlier stage and guide strategies for subsequent drug development.
Supplementary Material
Acknowledgments
Support was provided by Novartis, NIH/NCI K23CA140707 (GLU) and a SPORE in Leukemia (P50CA171963 to EJD, DCL, TAG, and MJW), ICTS-CTSA (EJD), Gabrielle’s Angel Foundation (MJW), Leukemia and Lymphoma Society Scholar Award (MJW), and the Lottie Caroline Hardy Trust (TAG, MJW). Support for procurement of human samples was provided by an NIH/NCI grant (P01 CA101937). Technical assistance was provided by the Alvin J. Siteman Cancer Center Tissue Procurement Core supported by an NCI Cancer Center Support Grant (P30CA91842).
Footnotes
Conflict of interest statement: GLU has received compensation as a consultant for Novartis. The remaining authors report no relevant conflicts of interest.
Supplementary information is available at Leukemia’s website.
References
- 1.Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2009 Feb 1;28(4):556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 2.Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002 May 15;20(10):2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006 Apr 15;106(8):1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 4.Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015 Jul 16;126(3):291–299. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010 Feb 1;28(4):562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 6.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009 Mar;10(3):223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverman LR, Fenaux P, Mufti GJ, Santini V, Hellstrom-Lindberg E, Gattermann N, et al. Continued azacitidine therapy beyond time of first response improves quality of response in patients with higher-risk myelodysplastic syndromes. Cancer. 2011 Jun 15;117(12):2697–2702. doi: 10.1002/cncr.25774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gore SD, Fenaux P, Santini V, Bennett JM, Silverman LR, Seymour JF, et al. A multivariate analysis of the relationship between response and survival among patients with higher-risk myelodysplastic syndromes treated within azacitidine or conventional care regimens in the randomized AZA-001 trial. Haematologica. 2013 Jul;98(7):1067–1072. doi: 10.3324/haematol.2012.074831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walter MJ, Shen D, Ding L, Shao J, Koboldt DC, Chen K, et al. Clonal architecture of secondary acute myeloid leukemia. N Engl J Med. 2012 Mar 22;366(12):1090–1098. doi: 10.1056/NEJMoa1106968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003 Dec 15;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006 Jul 15;108(2):419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 12.Walter MJ, Shen D, Shao J, Ding L, White BS, Kandoth C, et al. Clonal diversity of recurrently mutated genes in myelodysplastic syndromes. Leukemia. 2013 Jun;27(6):1275–1282. doi: 10.1038/leu.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012 Jul 20;150(2):264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012 Jan 26;481(7382):506–510. doi: 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atadja P. Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett. 2009 Aug 8;280(2):233–241. doi: 10.1016/j.canlet.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Klco JM, Miller CA, Griffith M, Petti A, Spencer DH, Ketkar-Kulkarni S, et al. Association Between Mutation Clearance After Induction Therapy and Outcomes in Acute Myeloid Leukemia. JAMA. 2015 Aug 25;314(8):811–822. doi: 10.1001/jama.2015.9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Research TCGA. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013 May 30;368(22):2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.San-Miguel JF, Richardson PG, Gunther A, Sezer O, Siegel D, Blade J, et al. Phase Ib study of panobinostat and bortezomib in relapsed or relapsed and refractory multiple myeloma. J Clin Oncol. 2013 Oct 10;31(29):3696–3703. doi: 10.1200/JCO.2012.46.7068. [DOI] [PubMed] [Google Scholar]
- 19.Jackson-Grusby L, Laird PW, Magge SN, Moeller BJ, Jaenisch R. Mutagenicity of 5-aza-2′-deoxycytidine is mediated by the mammalian DNA methyltransferase. Proc Natl Acad Sci U S A. 1997 Apr 29;94(9):4681–4685. doi: 10.1073/pnas.94.9.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012 Jul 20;30(21):2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lubbert M, Suciu S, Baila L, Ruter BH, Platzbecker U, Giagounidis A, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011 May 20;29(15):1987–1996. doi: 10.1200/JCO.2010.30.9245. [DOI] [PubMed] [Google Scholar]
- 22.Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014 Feb;28(2):241–247. doi: 10.1038/leu.2013.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013 Nov 21;122(22):3616–3627. doi: 10.1182/blood-2013-08-518886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metzeler KH, Walker A, Geyer S, Garzon R, Klisovic RB, Bloomfield CD, et al. DNMT3A mutations and response to the hypomethylating agent decitabine in acute myeloid leukemia. Leukemia. 2012 May;26(5):1106–1107. doi: 10.1038/leu.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itzykson R, Kosmider O, Cluzeau T, Mansat-De Mas V, Dreyfus F, Beyne-Rauzy O, et al. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia. 2011 Jul;25(7):1147–1152. doi: 10.1038/leu.2011.71. [DOI] [PubMed] [Google Scholar]
- 26.Bejar R, Lord A, Stevenson K, Bar-Natan M, Perez-Ladaga A, Zaneveld J, et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood. 2014 Oct 23;124(17):2705–2712. doi: 10.1182/blood-2014-06-582809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traina F, Visconte V, Elson P, Tabarroki A, Jankowska AM, Hasrouni E, et al. Impact of molecular mutations on treatment response to DNMT inhibitors in myelodysplasia and related neoplasms. Leukemia. 2014 Jan;28(1):78–87. doi: 10.1038/leu.2013.269. [DOI] [PubMed] [Google Scholar]
- 28.Craddock C, Quek L, Goardon N, Freeman S, Siddique S, Raghavan M, et al. Azacitidine fails to eradicate leukemic stem/progenitor cell populations in patients with acute myeloid leukemia and myelodysplasia. Leukemia. 2013 Apr;27(5):1028–1036. doi: 10.1038/leu.2012.312. [DOI] [PubMed] [Google Scholar]
- 29.Will B, Zhou L, Vogler TO, Ben-Neriah S, Schinke C, Tamari R, et al. Stem and progenitor cells in myelodysplastic syndromes show aberrant stage-specific expansion and harbor genetic and epigenetic alterations. Blood. 2012 Sep 6;120(10):2076–2086. doi: 10.1182/blood-2011-12-399683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014 Dec;20(12):1472–1478. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014 Dec 25;371(26):2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. The N Engl J Med. 2014 Dec 25;371(26):2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKerrell T, Park N, Moreno T, Grove CS, Ponstingl H, Stephens J, et al. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell reports. 2015 Mar 3;10(8):1239–1245. doi: 10.1016/j.celrep.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merlevede J, Droin N, Qin T, Meldi K, Yoshida K, Morabito M, et al. Mutation allele burden remains unchanged in chronic myelomonocytic leukaemia responding to hypomethylating agents. Nat Commun. 2016;7:10767. doi: 10.1038/ncomms10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boumber Y, Kantarjian H, Jorgensen J, Wen S, Faderl S, Castoro R, et al. A randomized study of decitabine versus conventional care for maintenance therapy in patients with acute myeloid leukemia in complete remission. Leukemia. 2012 Nov;26(11):2428–2431. doi: 10.1038/leu.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.