ABSTRACT
Zika virus (ZIKV) infections occur in areas where dengue virus (DENV), West Nile virus (WNV), yellow fever virus (YFV), and other viruses of the genus Flavivirus cocirculate. The envelope (E) proteins of these closely related flaviviruses induce specific long-term immunity, yet subsequent infections are associated with cross-reactive antibody responses that may enhance disease susceptibility and severity. To gain a better understanding of ZIKV infections against a background of similar viral diseases, we examined serological immune responses to ZIKV, WNV, DENV, and YFV infections of humans and nonhuman primates (NHPs). Using printed microarrays, we detected very specific antibody responses to primary infections with probes of recombinant E proteins from 15 species and lineages of flaviviruses pathogenic to humans, while high cross-reactivity between ZIKV and DENV was observed with 11 printed native viruses. Notably, antibodies from human primary ZIKV or secondary DENV infections that occurred in areas where flavivirus is endemic broadly recognized E proteins from many flaviviruses, especially DENV, indicating a strong influence of infection history on immune responses. A predictive algorithm was used to tentatively identify previous encounters with specific flaviviruses based on serum antibody interactions with the multispecies panel of E proteins. These results illustrate the potential impact of exposure to related viruses on the outcome of ZIKV infection and offer considerations for development of vaccines and diagnostics.
KEYWORDS: Zika, cross-reactivity, flavivirus, humoral immunity, protein microarray
INTRODUCTION
Zika disease is spread to humans by transfer of Zika virus (ZIKV) primarily through the bites of infected Aedes aegypti or Aedes albopictus mosquitoes (1, 2) and secondarily by sexual (3) or vertical (4–6) transmission. The majority of ZIKV infections are asymptomatic or mild with low-grade fever, arthralgia, conjunctivitis, and rash (7), while a lower frequency of cases may result in congenital microcephaly via in utero infections in infants and Guillain-Barré syndrome in adults (4–6, 8). Prior to the first reported outbreaks in the Pacific Islands in 2007 (9) and 2013 to 2014 (10, 11), only sporadic human cases of ZIKV were documented in Africa and Southeast Asia (10, 11). However, the number of confirmed human cases has increased dramatically over the past 9 years as ZIKV has spread to regions with naive populations, leading to the current epidemic in Brazil and another 58 countries with ongoing ZIKV transmission (12).
The single-stranded (plus-strand) genomic RNA of ZIKV and other Flavivirus species (flaviviruses) encodes a nonsegmented open reading frame that is cleaved during and after translation into three structural proteins (capsid [C], envelope [E], and membrane [M] proteins) that are incorporated into the virus, and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) that are necessary for replication. The overall molecular organization of the mature ZIKV from cryo-electron microscopy (cryo-EM) structures (13, 14) is very similar to the closely related dengue virus (DENV) and West Nile virus (WNV) (15, 16), as well as more distantly related tick-borne encephalitis virus (TBEV) and Japanese encephalitis virus (JEV) (17, 18). However, infectious particles also exhibit structural heterogeneity from immature to mature forms (19) within species, which may affect the protective potency of antibodies. Heterodimers of E and M proteins displayed on the outer surfaces of the virus (13, 14) undergo extensive conformational changes that facilitate infection of cells, and these proteins are primary targets for circulating antibodies (20). Further, the E proteins of many flavivirus strains harbor a potentially glycosylated, four-residue loop, and deletions of this feature in ZIKV are selected against in vivo (21). The NS1 protein, which is secreted by infected cells (22), is another important antigen that may be involved in immune evasion and pathogenesis. The specificities of antibodies interacting with NS1 are likely to be affected by a balance of surface features that are conserved among flaviviruses as well as the diverse electrostatic characteristics (23).
The four DENV serotypes (DENV serotype 1 [DENV1] to DENV4) are loosely categorized by cross-neutralization with polyclonal antibodies (24). Serological immune responses protect from reinfection with the same (homotypic) virus, while cross-reactive antibodies generated from previous infections with another (heterotypic) DENV serotype may enhance disease outcomes (25–28). Although most infections are mild and self-limiting, dengue disease can progress to hemorrhagic fever, capillary leakage, and dengue shock syndrome (27, 28). Secondary heterotypic infection can in some cases lead to increased risk of severe dengue disease, possibly because antibodies to one serotype may enhance infections with heterologous serotypes (antibody-dependent enhancement [ADE]) by promoting viral entry and infection through Fc receptor-expressing cells (27–30). The extent of disease enhancement in human ZIKV infections due to preexisting flavivirus antibodies is not well documented. While there is a higher risk of more-severe disease from secondary DENV infections, among flaviviruses, severe neurological pathologies may be uniquely associated with ZIKV infections during fetal development, with considerable uncertainty remaining regarding potential long-term health effects.
Beyond the potential role of antibodies in exacerbating disease, there are great challenges for developing accurate serological tests for ZIKV infections. Assays were recently developed and approved by the FDA under emergency use authorization for the detection of viral RNA or specific IgM and neutralizing antibodies in the biological fluids from patients with suspected Zika disease (31, 32). However, negative results for viremia do not exclude ZIKV infection, as circulating virus levels are highest several days before the onset of symptoms and begin to decline early in the acute phase of infection (33). In addition, interpretation of ZIKV antibody test results is complicated due to suspected cross-reactivity with other flaviviruses, especially for individuals who have had previous flavivirus exposures. Infections caused by DENV, WNV, and yellow fever virus (YFV) are spread by common mosquito vectors, circulate in similar areas, and present early disease symptoms that are identical to those of ZIKV infections (25–28). Infected individuals may have high levels of antibodies to multiple flaviviruses that hinder conclusive determination of the virus responsible for the most recent infection (29). Thus, laboratory methods that can better differentiate clinical infections and facilitate accurate disease surveillance are integral to an effective public health response to the current Zika disease epidemic and to future outbreaks. Toward this goal, we examined serological immune responses to commonly encountered infections by using a microarray of viruses and isolated protein antigens representing major phylogenetic lineages of flaviviruses. Our results demonstrate that primary human and rhesus macaque antibody responses to infection are highly specific for envelope proteins from the etiological agent, while responses to whole viruses are most cross-reactive. We further show that antibody recognition of isolated viral antigens can be used to resolve complex infection histories.
RESULTS
Antibody responses to ZIKV.
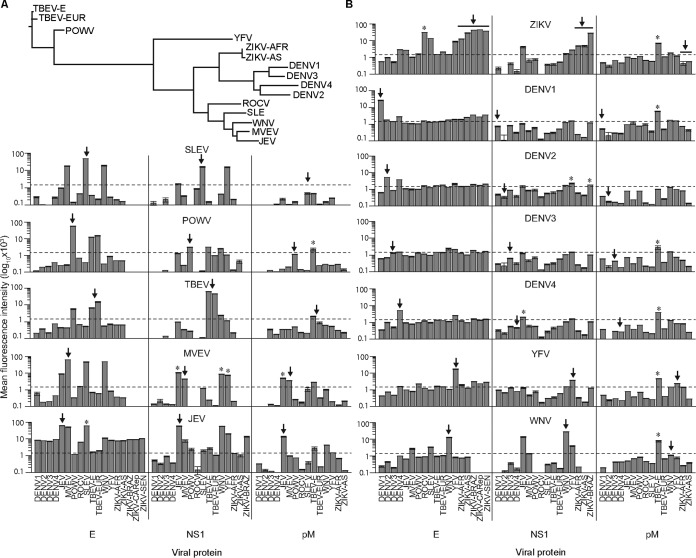
To examine serological immune responses, we developed a flavivirus-focused microarray comprising mature and immature forms (34) of DENV1 to DENV4 and ZIKV (five Asian isolates and six African lineage isolates), along with recombinant protein antigens from 15 isolates (Table 1 and Fig. 1A). Equivalent densities of recombinant proteins or viruses were deposited by inkjet printing on microarray surfaces coated with a thin layer of nitrocellulose. The printed antigens were evaluated with mouse antisera against 12 flaviviruses, representing antibodies produced by a noninfectious route. The E antigens from all flaviviruses except DENV3 were detected by mouse antibodies (IgG) that were produced in response to the corresponding virus, as shown in Fig. 1B, with evidence of significant E cross-reactivity for JEV antisera. The NS1 proteins from all except DENV1 to DENV4 were detected by the mouse antivirus sera, whereas the M antigen was only weakly recognized at best. We utilized the microarray to examine sera from nonhuman primates (NHPs) (Macaca mulatta) that were challenged subcutaneously with either African or Asian isolates of ZIKV (Table 1; see Fig. S1A in the supplemental material) (33) (Zika Open-Research Portal [https://zika.labkey.com]). The African and Asian lineages of ZIKV share ∼95% of E-protein amino acid sequences (35) or about the same level of similarity found among E proteins of individual DENV serotypes. Serum antibody binding to virus particles and E antigens of ZIKV, as measured by the microarray, was substantially elevated (Fig. 2A and B) 21 to 28 days postinfection (dpi), while no antibody recognition was observed for NS1 or M proteins (Fig. 2B), suggesting that anti-NS1 and anti-M antibodies represent a small proportion of the humoral immune response to infection during the time points examined. Antibody recognition was more robust for mature virus particles (∼2-fold higher) than for immature virus particles, which may display different conformations of E and M proteins (13). Within the assay, the highest antibody interactions were detected by ZIKV E proteins in comparison to mature ZIKV (Fig. 2A and B). However, a direct quantitative relationship between virus and recombinant protein cannot be determined by these results, because the complex nature of the native virus precludes printing equal molar amounts of available antigen. Only minor differences were observed in antibody responses to individual African and Asian lineage ZIKV antigens for both virus and E protein (Fig. 2), consistent with the conserved amino acid sequences and a single ZIKV serotype, as recently reported by others (36). The E-protein-specific IgM responses were detected by 3 dpi, coinciding with the rise of virus, peaked by 11 dpi, and subsided thereafter (Fig. 2C). The corresponding IgG responses were delayed compared to IgM, consistent with a naive immune response, and displayed increasing levels through 28 dpi (Fig. 2C). Further, there were no apparent differences in the magnitude or kinetics of humoral immune responses to the different amounts of virus used for challenges (Fig. S2), suggesting that levels of IgG and IgM were increasing in tandem with virus replication.
TABLE 1.
Flavivirus strains used for production of whole viruses and recombinant proteins
| Virusa | IDb | Isolate | Country | Yr | Host | GenBank accession no. |
|---|---|---|---|---|---|---|
| ZIKV | ||||||
| Asian lineage | H/PF/2013c | French Polynesia | 2013 | Human | KJ776791 | |
| 1 | SV0127/14 | Thailand | 2014 | Human | KU681081 | |
| 2 | CPC-0740d | Philippines | 2012 | Human | KU681082 | |
| 3 | VABC59 | USA (Puerto Rico) | 2015 | Human | KU501215 | |
| 4 | SPH2015 | Brazil | 2015 | Human | KU321639 | |
| 5 | YAPd | Micronesia | 2007 | Human | EU545988 | |
| African lineage | 1 | MR-766c,d | Uganda | 1947 | Macaca mulatta | KU955594 |
| 2 | IBH30656 | Nigeria | 1968 | Human | HQ234500 | |
| 3 | DAKAR41525 | Senegal | 1984 | Aedes africanus | KU955591 | |
| 4 | DAKAR 41662 | Senegal | 1984 | A. africanus | KU955592 | |
| 5 | ARB7701 | Central Africa | 1976 | A. africanus | KF268950 | |
| 6 | ArD_41519 | Senegal | 1984 | A. africanus | HQ234501 | |
| DENV1 | HAWAII | USA | 1944 | Human | KM204119 | |
| DENV2 | NGC | New Guinea | 1944 | Human | KM204118 | |
| DENV3 | H87 | Philippines | 1956 | Human | M93130 | |
| DENV4 | H241 | Philippines | 1956 | Human | AY947539 | |
| WNV | NY99 | USA | 1999 | Owl | NC_009942 | |
| YFV | 17-D-204 | USA | 1985 | Vaccine | JX503529 | |
| JEV | SA14-14-2 | South Korea | 2006 | Vaccine | JN604986 | |
| SLEV | PARTON | USA | 1933 | Human | EF158070 | |
| MVEV | 1-51 | Australia | 1952 | Human | NC_000943 | |
| ROCV | SPH34675 | Brazil | 1975 | Human | AY632542 | |
| POWV | LB | Canada | 1958 | Human | NC_003687 | |
| TBEV-E | SOFJIN-HO | Russia | 1937 | Human | AB062064 | |
| TBEV-EUR | NEUDOERFL | Austria | 1971 | Ixodes ricinus | NC_001672 |
Virus abbreviations: ZIKV, Zika virus; DENV, dengue virus; WNV, West Nile virus; YFV, yellow fever virus; JEV, Japanese encephalitis virus; SLEV, St. Louis encephalitis virus; MVEV, Murray Valley encephalitis virus; ROCV, Rocio virus; POWV, Powassan virus; TBEV-E, tick-borne encephalitis virus, Eastern strain; TBEV-EUR, tick-borne encephalitis virus, European strain.
Used for challenge of M. mulatta.
FIG 1.
Phylogenetic relationships and recognition of microarrayed antigens by virus-specific antibody standards. (A) The phylogenies of flaviviruses examined in this study were inferred from an alignment of amino acid sequences from envelope (E) proteins. (B) Microarrays of E, nonstructural protein 1 (NS1), and premembrane (pM) proteins probed with mouse polyclonal antibodies generated against each virus shown (centered labels above each row of bar graphs). Antibody binding data are shown as log10-transformed mean fluorescence intensities (±standard errors of the means [SEM] [error bars]), and the arrows indicate the virus-specific antigens. Heterologous antigens that exhibit increased recognition compared to the virus-specific antigen are labeled with an asterisk (P < 0.05, one-way ANOVA with Tukey's range test). Virus abbreviations: YFV, yellow fever virus; SLEV, St. Louis encephalitis virus; DENV, dengue virus; DENV1, dengue virus serotype 1; POWV, Powassan virus; TBEV-E, tick-borne encephalitis virus, Eastern strain; TBEV-EUR, tick-borne encephalitis virus, European strain; MVEV, Murray Valley encephalitis virus; WNV, West Nile virus; ZIKV, Zika virus; ZIKV-AFR, ZIKV from Africa; ZIKV-AS, ZIKV from Asia; JEV, Japanese encephalitis virus; ROCV, Rocio virus.
FIG 2.
Specificity and kinetics of the humoral immune response to ZIKV. (A and B) ZIKV-challenged nonhuman primate (NHP) IgG recognition of ZIKV particles harvested early (48 h) or late (144 h) postinfection of HEK293T cells (A) and ZIKV proteins (envelope [E], nonstructural protein 1 [NS1], and premembrane protein [pM]) (B) from five Asian (AS) and six African (AFR) lineages (Table 1). ZIKV-specific antibody responses are denoted by scatter plots with center horizontal lines representing the mean binding of serum antibodies from NHPs challenged with either an AFR (n = 3) (circles) or AS (n = 3) (squares) lineage ZIKV at 0 to 2 days postinfection (dpi) (open symbols) and 21 to 28 dpi (filled symbols). Error bars indicate SEM. Statistically significant differences between mean antibody binding of all ZIKV-challenged NHPs to ZIKV antigens at 0 to 2 dpi and 21 to 28 dpi were calculated using a one-tailed Student's t test (*, P < 7.5e−5; ns, not significant), while no significant differences were observed between mean antibody binding of ZIKV-AS- and ZIKV-AFR-challenged groups to AS and AFR ZIKV antigens at 21 to 28 dpi (two-tailed Student's t test). (C) IgM and IgG binding profiles to ZIKV particles (harvest at 144 h) and ZIKV E protein are compared to viral load (Zika Open-Research Portal [https://zika.labkey.com]) from preinfection (day 0) to 28 dpi for ZIKV-challenged NHPs (n = 9). Second-order (IgM), third-order (IgG), and fourth-order (viral load) polynomial curves were fitted to the data, with fitted lines and shading under the curve consistent with data point colors.
Cross-reactivity of antibodies from primary flavivirus infections.
The E proteins of ZIKV and DENV have a high degree of structural similarity that may contribute to shared antibody epitopes. We examined NHPs (M. mulatta) challenged independently with DENV1 to DENV4 (Fig. S1A) (37). For virus, antibodies (30 dpi) from NHPs infected with any DENV serotype were highly cross-reactive to heterologous DENV serotypes and ZIKV (Fig. 3A and Table 1). Further, DENV2 and DENV3 antibodies displayed substantially higher reactivity with the heterologous ZIKV, while IgG from ZIKV-challenged NHPs was more specific for ZIKV at the virus level, with a lower overall level of cross-reactivity toward DENV1 to -4 (Fig. 3A). In contrast with viruses, the E proteins presented antibody recognition profiles that were very specific for the challenge virus (Fig. 3A) and minimal antibody recognition of E proteins from 10 more distantly related flaviviruses (Fig. 4A). DENV-challenged NHPs exhibited the highest antibody binding to the E protein from the DENV challenge serotype, and antibodies from ZIKV-challenged NHPs essentially bound only to ZIKV E antigens. A principal-component analysis (PCA) of antibody bound by DENV and ZIKV E antigens differentiated serotype-specific DENV and ZIKV infection sera due to the higher degree of homotypic E recognition (Fig. 3B). In contrast to the E-antigen results, PCA based on IgG recognition of virus only enabled distinction of ZIKV- from DENV-challenged sera, whereas DENV serotype-specific clusters were not evident (Fig. 3B). Furthermore, antibodies from NHPs challenged with African or Asian lineage ZIKV were not differentiated by E protein or virus (Fig. 2 and 3B). We also considered YFV, both as a nearest neighbor of ZIKV and DENV (Fig. 1A) and because vaccination against yellow fever is common in many countries with a high prevalence of dengue. While serum antibodies from NHPs vaccinated with the 17D YFV strain (Fig. S1A) (38) predominantly recognized the E antigen of YFV (Fig. 4A), a modest level of cross-reactivity was evident with several other E proteins, including those of ZIKV and tick-borne encephalitis virus (TBEV).
FIG 3.
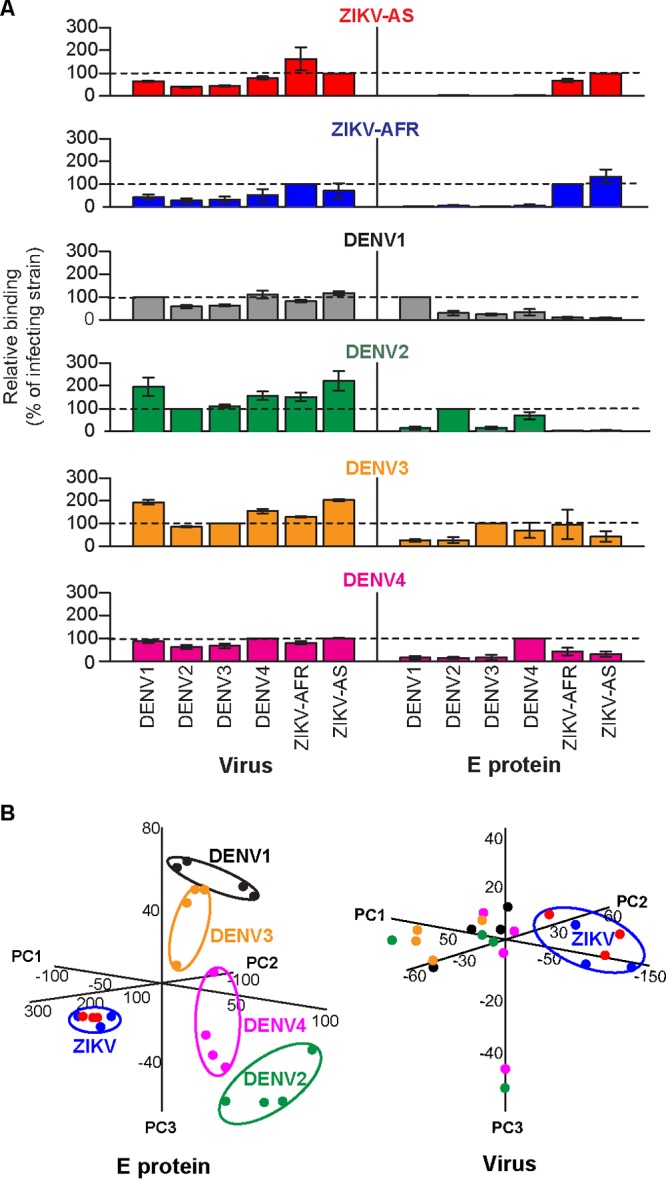
Differentiation of nonhuman primates challenged with ZIKV or DENV by specific IgG binding to E antigens. (A) Binding of convalescent-phase serum antibodies from nonhuman primates (NHPs) challenged with either an Asian (H/PF) (n = 3) (red) or African (MR-766) (n = 3) (royal blue) lineage ZIKV, or DENV (n = 4 each for the DENV1 [black], DENV2 [green], DENV3 [orange] groups; n = 3 for the DENV4 group [magenta]) to whole viruses (144 h) and E proteins. Values shown are antibody binding signals relative to the virus used for challenge (±SEM). (B) Principal-component analyses of relative IgG binding to E proteins and viruses (144 h) by NHP antibodies. Individual data points and virus-specific clusters are colored according to the challenge virus as in panel A. PC1, principal component 1.
FIG 4.

Antibody specificity of primary and secondary flavivirus infections. Relative binding (±SEM) of convalescent-phase serum antibodies from nonhuman primate (NHP) and human flavivirus infections to 15 flavivirus E proteins is shown. (A) Sera from primary infections are indicated by color as follows: gray, DENV-challenged NHPs (individual data for each NHP group are overlaid in a scatter plot; n = 4 each for the DENV1 [black], DENV2 [green], and DENV3 [orange] groups and n = 3 for the DENV4 group [magenta]); green, human (Hu) rDEN2Δ30 (n = 8) (primary infection); red, pooled African and Asian lineage ZIKV NHPs (n = 6); white, YFV-vaccinated NHPs (n = 3). (B) Sera from confirmed human flaviviral infections with unknown infection histories are indicated by color as follows: gray, DENV (individual data are overlaid in a scatter plot; the colors correspond to the most recent DENV infection); green, DENV2 (n = 5); orange, DENV3 (n = 2); red, ZIKV (n = 4); white, YFV vaccination (n = 13); cyan, WNV (n = 20). (C) Predicted infection histories of human secondary DENV (gray in panel B) and primary ZIKV (red in panel B) infections, based on a supervised SVM classifier. Individual human sera are shown at the bottom (Z for ZIKV, D2 for DENV2, and D3 for DENV3; virus followed by serum identification [ID] number), with probability values for each viral class (left) gradient colored from low to high (white to royal blue) (right). Predicted infection histories are designated by colored bars above serum ID (DENV1 [black], DENV4 [magenta], no prediction [no bar]).
The animals in the ZIKV, DENV, and YFV infection studies we examined were domestically bred in isolation from most infectious diseases. Therefore, it was important to compare results from the naive backgrounds of animal disease models with primary infections of humans without documented prior exposures to flaviviruses. Dengue human infection models were recently developed to assess the efficacy of live attenuated DENV vaccines (39). Human challenges with the attenuated DENV2 strain rDEN2Δ30 (40) result in a mild disease, with viremia, rash, and neutropenia. We examined sera collected from flavivirus-naive subjects 28 days after challenge (103 PFU) with rDEN2Δ30 by subcutaneous injection (Fig. S1B) (40). Among the extended panel of E proteins (Table 1), human antibody responses to rDEN2Δ30 resulted in specific recognition of the E protein from DENV2 and to a lesser degree from DENV4 (Fig. 4A and Fig. S3A), which is most similar to DENV2 among all other flaviviruses (Fig. 1A). Low levels of neutralizing antibodies against other DENV serotypes were previously reported for individuals challenged with rDEN2Δ30 (41). For viruses, extensive human antibody cross-reactivity was again noted for other DENV serotypes and ZIKV strains (Fig. S3A). These results indicated that the NHP DENV challenge model replicated the antigen specificity profile of human antibody responses to primary infection.
Antibody cross-reactivity between flaviviruses could be influenced by homology of sequences and structures, as well as the abundance and degree of cross-reactive antibodies in polyclonal sera. For example, cross-reactivity could be due to a small population of antibodies that exhibit high levels of specificity for heterologous E proteins, or it may be due to a larger population of antibodies that exhibit broad cross-reactivity. Although the highest recognition of the homologous E protein was common for antibodies from primary infections, we observed differences in the amount of total antibody across virus species (Fig. 5). Comparing results obtained with all E proteins, cross-reactive antibodies were not detected for ZIKV, while DENV1 and DENV2 antibodies recognized other DENV serotypes. Antibodies from DENV3-infected and YFV-vaccinated NHPs exhibited the lowest binding to the respective E proteins, while a high level of DENV2 E-specific antibodies interacted with the DENV2 E protein (Fig. 5). The lower levels of DENV3 and YFV antibodies that were specific for the cognate E protein, compared to DENV2 for example, contributed to the appearance of an overall higher level of background cross-reactivity (Fig. 3A and 4A). Vaccination with the live-attenuated 17D strain results in low levels of viremia that mimic a true YFV infection, and titers of specific antibodies are also lower than those in wild-type YFV infections (42). In addition, antibodies from NHPs challenged with ZIKV (33) and DENV (43) exhibited neutralizing antibody titers (33, 43) that directly correlated (R2 > 0.99) with the E-antibody recognition pattern we observed (Fig. 5).
FIG 5.
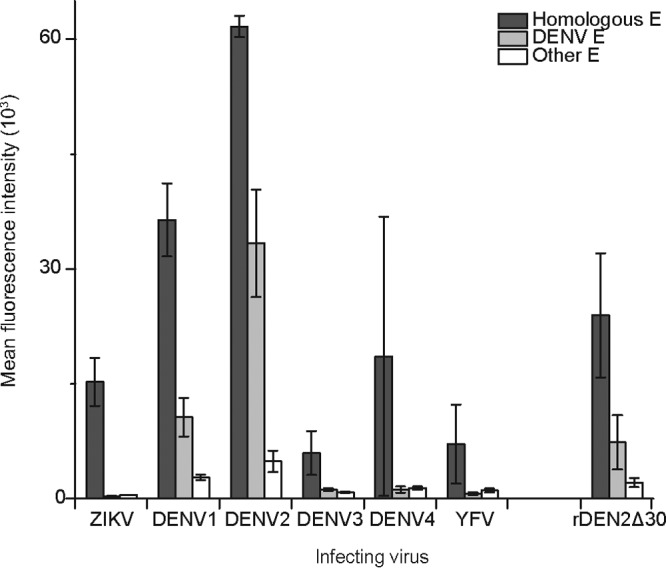
Quantitative comparisons of antibodies directed to the infecting virus versus all other flaviviruses. Antibody recognition of microarrayed E proteins displayed as mean fluorescence intensity (±SEM). Antibodies from primary flavivirus infections of NHPs (ZIKV, DENV1 to DENV4, and YFV) and humans (rDEN2Δ30) exhibited significantly decreased recognition of heterologous E antigens compared to virus-specific E (dark gray) (P < 0.05 by one-way ANOVA with Tukey's range test). DENV E proteins are separated from all other flavivirus E proteins (including YFV, SLEV, POWV, TBEV, MVEV, WNV, JEV, and ROCV) to show DENV antibody cross-reactivity between serotypes.
Secondary flavivirus infections.
The antibodies of flavivirus-naive NHPs and humans to primary flavivirus infection were highly specific to the E protein of the challenge virus. Because increased levels of antibody cross-reactivity would be expected for flavivirus-primed individuals with secondary flavivirus infection, we next examined human sera after one or more flavivirus exposures. In contrast to results obtained with primary infections, IgG from DENV2 or DENV3 infections occurring in Peru prior to the Zika epidemic (here defined as secondary DENV infections) (Fig. S1C) collectively interacted with several E proteins, including those from ZIKV (Fig. 4B), suggesting that antibodies from previous infections, possibly DENV4 (based on the amount of IgG bound), dominated immune responses to other flaviviruses. Despite expectations, sera from secondary DENV2 infections did not correlate with primary DENV2 infections (Fig. S3B), providing additional evidence of previous dengue infection in these samples. Moreover, although principally recognizing the E protein of YFV, antibodies from human 17D vaccinations (Fig. S1D) were less specific than those from primary NHP vaccinations, as E proteins from DENV4 and several other flaviviruses were also targeted (Fig. 4B). It is possible that the less-specific YFV responses were a result of declining antibody titers, as the sera were collected up to 118 days after vaccination. We further noted that serological responses from WNV infections that occurred in North America (Fig. S1D), a region with only a small incidence of dengue, exhibited elevated antibody interactions with E proteins from WNV and a few other flaviviruses, but only a low level of interactions with DENV antigens (Fig. 4B). Finally, we examined primary ZIKV infections from the Dominican Republic (Fig. S1C), a Caribbean country where dengue is endemic. Antibodies from ZIKV infections interacted to a greater extent with E proteins from DENV than from ZIKV (Fig. 4B and Fig. S3A) and also recognized E proteins from several other flaviviruses. It is important to note that levels of total E-specific antibodies from all human flavivirus exposures were significantly reduced compared to levels observed in primary infections (Fig. 5). While maximum E-specific antibody abundance never exceeded the low levels of binding observed for primary YFV and DENV3 exposures, these results suggested that serum levels of anti-E antibodies were predominantly driven by infection histories, and it is conceivable that at least one DENV infection preceded each clinical disease examined with sera from secondary infections.
Given the complexity of the human antibody response from primary ZIKV and secondary DENV infections (Fig. S1C), we attempted both to estimate the probability of previous flavivirus exposures and to identify the likely antecedent virus. We used a supervised machine learning method to classify sera by features of antibody binding to the extended panel of 15 E proteins (Table 1). The support vector machine (SVM) classifier was trained on a positive set of E-specific antibody binding signals from primary flavivirus infections and a negative set of background signals from flavivirus-naive sera. The performance of the SVM was evaluated using a 10-fold cross-validation resampling method, which readily differentiated infected from naive sera and different primary infections, resulting in a total model accuracy of 98.5%. Using a probability cutoff value of ≥0.5, the classifier was used to predict flavivirus exposures that occurred prior to the secondary DENV and ZIKV infections. Four secondary DENV2 sera were predicted to have had a previous DENV4 infection, while high probability for two primary ZIKV sera suggested a previous DENV1 infection (Fig. 4C), which was consistent with clustering based on correlated antibody binding (Fig. S3B). Lower overall probabilities for single virus infections were observed for the remaining secondary DENV and ZIKV samples, and classification to a single group was therefore not possible (Fig. 4C). For example, a secondary DENV3 serum had comparable probability values for DENV2 (0.28) and DENV4 (0.27), suggesting a previous infection with either virus. The inclusion of more-extensive training data sets for primary ZIKV and other viral infections will be important for refining the predictive power of the described SVM method.
DISCUSSION
The coincidence of dengue in areas where there is a Zika virus epidemic limits the reliability of current serological assays and complicates vaccination strategies. The study described here examined the specificity of humoral immune responses to flaviviruses by using microarrays of 11 native viruses and recombinant E proteins from 15 species or lineages of flaviviruses that are pathogenic to humans. Antibodies from the first exposures of nonhuman primates and humans to ZIKV, DENV, WNV, and YFV were predominantly directed toward the E surface antigen from the infecting virus and enabled differentiation of infections. Whereas isolated human monoclonal antibodies that were cross-reactive for E antigens have been described (44), our results with polyclonal antibodies present a global analysis of the composite B-cell response. In contrast to the high specificity observed with E antigens, whole viruses exhibited significant levels of cross-reactivity with serum antibodies from primary ZIKV and DENV infections. Antibodies from human ZIKV or DENV infections that occurred in regions where dengue is endemic recognized heterotypic E antigens and exhibited decreased recognition of the homotypic E protein, consistent with higher levels of IgG from previous flavivirus exposures than from the most recent infection. The high degree of antibody specificity for E protein with sera from primary DENV and ZIKV exposures suggests that the apparent cross-reactivity observed in many assays may result from an overlap in rising and waning antibody responses to independent infections, as modeled in Fig. 6. The interpretation of serological results is further complicated by the lower antibody titers in Zika disease than in dengue, perhaps because serum ZIKV loads are also very low (45).
FIG 6.

Overlap in rising and waning antibody responses to independent infections. The primary infection of a flavivirus-naive individual with dengue virus occurs at day 0 (solid black arrowhead). Levels of virus-specific antibody (gray bars and shading) begin to increase shortly after the acute phase of infection, peak after convalescence, and subside thereafter. A second infection with Zika virus (solid red arrowhead) is followed by an increase in virus-specific antibody (red bars and shading), resulting in detection of a mixture of anti-dengue virus and anti-Zika virus antibodies that will vary with time from infections. The ratio of dengue virus-to-Zika virus antibodies, as shown, will be further increased if the secondary infection results in a less potent activation of serological immune responses.
Clinical management of suspected Zika cases that test negative for viral RNA can be guided by laboratory evidence of ZIKV-specific antibodies (32), particularly to differentiate infections in late convalescence and beyond, as viral RNA is typically no longer detected. However, results from some in vitro assays will be difficult to extrapolate to human cases. Only weak antibody neutralization of ZIKV was reported for sera from DENV-infected patients that exhibited a high degree of cross-reactivity with ZIKV-infected Vero cell lysates (46), while other studies observe enhancement of ZIKV infections in cell culture by anti-DENV antibodies (44). Our results illustrate the application of high-throughput antigen microarrays for the study of antibody responses to ZIKV and other flaviviruses. In addition, printed microarrays provide a high-throughput means for evaluating the performance of many test antigens in the same assay. For example, by including both recombinant proteins and viruses in the same microarray, we determined that E proteins were the most effective probes for detecting serological immune responses. In agreement with previous reports that used antigen preparations from whole virus (9, 31, 46), we observed a high level of antibody cross-reactivity between DENV and ZIKV isolates. Although the precise reason for high cross-reactivity between viruses is unknown, possible mechanisms may include antibodies that interact with additional quaternary and glycosylated epitopes that were not present on the recombinant antigens or other indeterminate factors (14, 47). Further, the results presented here emphasize the value of determining total antibody recognition of E proteins for distinguishing between infections caused by different species of flaviviruses. While virus neutralization assays measure a functional subset of antibodies and provide an important indicator of antiviral immunity, the best correlate of protection against viremia in DENV infection may be total polyclonal antibody titers, rather than neutralizing antibody titers (43). Antibodies that are weakly neutralizing in cell culture assays can contribute to physiologically important non-ADE mechanisms of virus clearance that are facilitated by receptor-mediated uptake and effector cells (48).
A more detailed understanding of the interrelationships of antibody responses across flaviviruses is imperative because infections by one species or serotype are known to influence disease susceptibility and severity for infections caused by other related viruses (25–28). New techniques are also needed to guide accurate diagnosis of emerging infections, especially for flavivirus-immune individuals, as antibodies persist at levels that are detectable long after disease resolution (38, 42, 49, 50). Although the length of time from previous exposures may influence detection of responses to new infections, our results demonstrate that antibody recognition patterns from secondary infections can be used to estimate infection histories (Fig. 6). Importantly, since severe dengue is linked to secondary infections with a heterotypic DENV (25–28), it is possible that dengue virus-primed populations are more prone to ZIKV infections, and perhaps the associated severe neurological disorders of Guillain-Barré syndrome (8) and microcephaly (4–6). However, there is currently no evidence of enhanced severity, increased ZIKV loads, or increased incidence of Zika disease in countries with widespread immunity to dengue. Our results indicate that it should be possible to develop protein-based serological assays that are sensitive enough to differentiate flavivirus infections in individuals with preexisting immunity. Based on the assumption that multiple independent antibody binding events were measured for each clinical specimen collected from a region where dengue is endemic, data from primary infections can be used to train machine learning methods for classification of sera from unknown infection histories. The predictive algorithm that we developed for E recognition patterns may find useful applications in disease surveillance for inference of infection histories in both primary and secondary flavivirus encounters. As diagnostic methods by necessity focus only on the current disease, the general approach described here will also be important for addressing any causal relationships between Zika disease and previous infections.
MATERIALS AND METHODS
Viruses.
The ZIKV and DENV presented in Table 1 were propagated and prepared as previously described (34), with some modifications. Briefly, HEK293T cells were maintained in Dulbecco modified Eagle medium (DMEM), supplemented with 10% fetal bovine serum (FBS) at 37°C and 5% CO2. The cells were seeded in T125 flasks to 60% confluence and cultured for 12 h. The cells were infected (2 h) with 5 ml of suspended virus stock diluted 1:25 with fresh culture medium. Infectious suspensions were replaced for virus propagation, and culture supernatants were harvested at early (48-h) and late (144-h) time points to obtain immature and mature virions, respectively, while adding fresh HEK293T cells 72 h postinfection to ensure the presence of enough viable cells for sustained proliferation to generate mature virus. Culture supernatants were filtered using prewashed (Super G blocking buffer [Grace Bio-Labs] followed by sterile phosphate-buffered saline [PBS]) 45-μm syringe filters, and precipitated for 12 h (4°C) in PBS containing 8% polyethylene glycol 8000 (PEG8000) (vol/vol). Precipitates of viruses were pelleted by centrifugation (14,000 × g, 1 h, 4°C), resuspended in 300 μl sterile PBS (∼100-fold concentration by volume), snap-frozen in a dry ice-ethanol bath, and stored at −80°C.
Viral proteins.
Viral RNA for preparation of protein-expressing plasmids was obtained from the following sources: American Type Culture Collection (DENV1 to DENV4), Integrated BioTherapeutics, Inc. (YFV and Japanese encephalitis virus [JEV]); NIAID World Reference Center for Emerging Viruses and Arboviruses (Robert Tesh) (St. Louis encephalitis virus [SLEV], WNV, and Rocio virus [ROCV]). The cDNA templates of E, NS1, and premembrane protein (pM) genes were produced by reverse transcription of full-length viral RNA by using 50 μM oligo(dT)20 primers and the SuperScript III reverse transcriptase first-strand synthesis system (Life Technologies). ZIKV genes (E, NS1, and pM) and E genes from Murray Valley encephalitis virus (MVEV), Powassan virus (POWV), and tick-borne encephalitis virus (TBEV) were synthesized (gBlocks [Integrated DNA Technologies, Inc.] and GeneArt Strings [Life Technologies]), with codon optimization for expression in Escherichia coli. Synthesized genes and cDNA were used as the templates in PCR amplification reactions (50 μl total) with gene-specific primers (final concentration of 2.5 μM) and 2× Phusion high-fidelity PCR master mix with HF buffer (New England BioLabs Inc.). PCR-amplified genes were purified using QIAquick spin column PCR purification kit (Qiagen). NS1 and pM were produced as full-length open reading frames (ORFs), and E genes were truncated to exclude transmembrane domains, as predicted by analysis of amino acid sequences using TMHMM server v.2.0 (Center for Biological Sequence Analysis) (51, 52). Purified DNAs were TOPO cloned into the pENTR/TEV/D-TOPO vector (Gateway Technology, Life Technologies). Sequence-verified entry clones were shuttled into expression vectors by recombination reactions using LR clonase II (Life Technologies). Specifically, the ZIKV-MR766-pM ORF was shuttled into an N-terminal His-labeled maltose-binding protein (HisMBP)-tagged vector (53), while all other flaviviral ORFs were shuttled into the N-terminal 6×His-tagged pDEST17 (Life Technologies). All flavivirus constructs were expressed in E. coli BL21(DE3), propagating bacteria in media containing Luria broth (300 ml) supplemented with 100 μg ml−1 ampicillin and 0.1% glucose. Proteins were induced at mid-log phase with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (EMD Chemicals). Induction conditions were optimized for each protein, and bacteria were grown at either 30°C (2 to 4 h) or 18°C (12 h) prior to harvest by centrifugation. Bacterial pellets were lysed in B-PER reagent (Thermo Scientific) containing EDTA-free 3× Halt protease inhibitor cocktail (Thermo Scientific), 0.2 mg ml−1 lysozyme, 250 U DNase I (Thermo Scientific), and 1 mM IPTG. Protein expression was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Bio-Rad) followed by Coomassie staining, and Western blotting using a mouse anti-His-horseradish peroxidase (HRP)-conjugated polyclonal antibody (Abcam). For purification of insoluble proteins, inclusion body pellets were washed as previously described, with minor modifications (54). Briefly, buffer containing 50 mM Tris-HCl (pH 7.4), 1 M urea, and 1% Triton X-100 was used to wash pellets three times, followed by two washes with Tris-HCl (pH 7.4), with centrifugation at 15,000 × g for 7 min between each wash, and purified pellets were stored at −80°C. Purified inclusion bodies were solubilized in 50 mM HEPES (pH 7.3), 140 mM NaCl, 2 mM dithiothreitol (DTT), and 1% SDS, followed by incubation at 99°C with gentle mixing (5 to 15 min), and centrifugation to remove remaining insoluble protein. Solubilized proteins were analyzed by SDS-PAGE with Coomassie blue staining and by Western blotting using anti-His-HRP-conjugated polyclonal antibody (Abcam). The protein concentration and purity of flavivirus proteins were measured using the Agilent Protein 230 kit and Bioanalyzer 2100 instrument (Agilent Technologies). Purified proteins were stored at −20°C in solubilization buffer, with a final concentration of 25% glycerol.
Microarrays of flavivirus antigens.
Recombinant proteins were diluted to 200 ng μl−1 in microarray printing buffer (50 mM HEPES, 140 mM NaCl, 2 mM DTT [pH 7.3]) with glycerol added to a final concentration of 40%. Flavivirus and control proteins were printed onto microporous nitrocellulose-coated slides (Oncyte SuperNOVA; Grace Bio-Labs, Inc.) in replicates (n = 6) using a noncontact inkjet microarray printer (ArrayJet, Glasgow, United Kingdom). The virus preparations were printed with printing buffer containing 50% glycerol, and preliminary experiments were performed with printed virus to optimize antibody binding signals. Frozen virus stocks were gamma irradiated (6 megarads) for inactivation and visualized by electron microscopy to assess quality and quantity. Concentrated virus and a dilution series of bovine serum albumin (BSA) (Sigma-Aldrich) were subjected to SDS-PAGE and stained with Coomassie blue to determine the relative amounts of viral proteins in each preparation. The final printing parameters were established by comparison of virus gradients printed onto nitrocellulose-coated slides, where deposited material was quantified against both an IgG and a BSA standard gradient by SYPRORuby staining (Thermo Scientific), and by comparing signal strength with a pan-flavivirus polyclonal rabbit antiserum specific to an E-domain II peptide that is highly conserved among flaviviruses. The deposited protein antigens were similarly evaluated using SYPRORuby, an anti-N-terminal 6×His monoclonal antibody (Sigma-Aldrich), and the pan-flavivirus rabbit antisera described above. Printed microarrays were desiccated (12 h) and stored frozen (−20°C) until use.
Microarray assays.
All microarray processing steps were performed at 22°C, protected from light. For IgM detection assays, serum IgG was inactivated using GullSORB (Meridian) prior to performing microarray manipulations. NHP (1:50) and human (1:150) sera, diluted in probe buffer (1× PBS [pH 7.4], 0.1% Tween 20, 1% BSA), were precleared by incubating (1 mg ml−1) with E. coli lysate (Promega) with gentle agitation, followed by centrifugation (17,000 × g, 5 min) to remove the pelleted immunoprecipitates. Microarrays were blocked with Super G blocking buffer (Grace Bio-Labs) for 1.5 h at 22°C and washed three times (for 5 min each time) in wash buffer (1× PBS, 0.2% Tween 20, 1% BSA). The microarrays were incubated for 2 h with E. coli-cleared serum, washed five times for 5 min each time, and incubated for 1 h with either Alexa Fluor 647-conjugated goat anti-human γ-specific IgG (1:1,000) or goat anti-human μ-specific IgM (1:250) secondary antibody (Southern Biotech) diluted in probe buffer. Microarrays were washed three times with wash buffer, rinsed twice with filtered deionized water to remove any residual salts, and dried.
Data acquisition and analysis.
Microarray slides were scanned at 635 nm using a confocal laser scanner (GenePix 4400A scanner; Molecular Devices) using settings below signal saturation. Background-subtracted pixel counts were determined with GenePix Pro 7 software, and outliers among data replicates, identified using a modified Z-score (median absolute deviation of >3.5), were removed. Pixel counts from replicate spots were averaged to obtain mean fluorescence intensity (MFI) and used for subsequent analyses. Relative binding was calculated as RB = (x/xi)(100) where x is the MFI originating from microarrayed antigens and i is the infecting virus species. Relative binding signals were used in hierarchical clustering analyses (average-linkage Pearson correlation) performed using MeV v4.8.1 within the TM4 software suite (55). Student's t tests, polynomial curve fitting, principal-component analyses, and one-way analysis of variance (ANOVA) with Tukey's posthoc honestly significant difference (HSD) test were performed using OriginPro v9.0 (Origin Lab Corporation).
Machine learning.
The support vector machine (SVM) method LIBSVM (https://www.csie.ntu.edu.tw/~cjlin/libsvm/), available in the R package e1071 (56), was used for predictions of infection histories with quantile normalized microarray data. An optimal separating hyperplane between data classes was determined with the SVM by maximizing the margin between the closest points and minimizing the classification error. All binary subclassifiers were fitted to the model, and the correct class was identified by a voting mechanism (i.e., the class with the highest probability). We used a radial basis function (RBF) as the kernel function, which is defined by K(u,v) = exp(−γ||u − v||2), where u and v are two data vectors and γ (set at 0.001) is a training parameter that makes the decision boundary smoother as the value becomes smaller. The regularization factor C, set at 100, controls the trade-off between a low training error and a large margin. A grid search was used for selection of C (1 to 1,000) and γ (0.0001 to 1) using 10-fold cross-validation of the training data set and the built-in “tune” function of e1071. The final SVM model was generated using the optimal parameters with complete training data sets. To evaluate the performance of the model, a 10-fold cross-validation was implemented on the training data set, which consisted of a positive set of E-specific antibody binding signals from primary flavivirus infections (n = 32 [human and NHP]) and a negative set of background signals from flavivirus-naive sera (n = 34 [human and NHP]). Based on one-way ANOVA followed by Tukey's range test, the overall antibody binding patterns of DENV2-challenged NHPs and humans were not statistically significantly different (P > 0.05). Therefore, the positive training set consisted of data from both rDEN2Δ30-challenged humans and NHPs. The training data set was randomly divided into 10 equal parts, and each run of cross-validation was comprised of 1/10 as the independent test data set and the remaining 9/10 as the training data set. The performance of the model was calculated as accuracy = (TP + TN)/(TP + FP + TN + FN).
E-protein molecular phylogeny.
A phylogenetic tree was generated based on E-protein amino acid sequences (Asian-YAP/2007 and African-MR-766/1947 lineage ZIKV selected as representative strains). CLUSTAL W2 (57) was used to generate three multiple-sequence alignments (MSAs), each with a different gap opening penalty (5, 10, 25), Blosum62 as the protein weight matrix, and all other options left as default. T-Coffee Combine (58, 59) was then used to generate a single alignment that had the best agreement of all three MSAs. gBlocks (60, 61) with relaxed settings (small blocks allowed, gap positions allowed within final blocks, and less-strict flanking positions) was used to eliminate poorly aligned positions and divergent regions in the combined alignment, and 202 conserved columns within the alignment were retained. A molecular phylogeny was generated using the maximum likelihood method implemented in the PhyML program (v3.0 a LRT) (62). The Blosum62 substitution model and four gamma-distributed rate categories were selected to account for rate heterogeneity across sites. The gamma shape parameter was estimated directly from the data (γ = 1.564). Tree topology and branch length were optimized for the starting tree and subtree pruning and regrafting selected for tree improvement.
Animal and human sera. (i) Animal use statements.
All macaque monkeys used in this study were cared for by the staff at the Wisconsin National Primate Research Center (WNPRC) in accordance with the regulations and guidelines outlined in the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals (63), and the recommendations of the Weatherall report (64). This study was approved by the University of Wisconsin—Madison Graduate School Institutional Animal Care and Use Committee (Animal Care and Use Protocol G005401). For all procedures (i.e., physical examinations, virus inoculations, ultrasound examinations, blood and swab collection), animals were anesthetized with an intramuscular dose of ketamine (10 ml kg of body weight−1). Blood samples were obtained using a Vacutainer system or needle and syringe from the femoral or saphenous vein.
(ii) Human use statements.
Research on human subjects was conducted in full compliance with the U.S. Department of Defense (DoD), National Institutes of Health (NIH), federal, and state statutes and regulations relating to the protection of human subjects and adheres to principles identified in the Belmont Report (65). All specimens, data, and human subject research were gathered and conducted for this publication under institutional review board (IRB)-approved protocols.
ZIKV.
Three groups of Indian origin Macaca mulatta (three individuals per group) were challenged subcutaneously with a different dose (106, 105, or 104 PFU) of either an Asian (study identification [ID] ZIKV001 and ZIKV004) or African (study ID ZIKV002) lineage ZIKV (Table 1; see Fig. S1A in the supplemental material). Sera were collected prior to ZIKV challenge (day 0) and daily for 10 days (all cohorts), followed by two to three times a week from 11 to 28 days postinfection (dpi) (ZIKV001 and ZIKV002 only) (33) (Zika Open-Research Portal [https://zika.labkey.com]). Human sera from ZIKV infections were collected from four female patients from the Dominican Republic that developed symptoms of ZIKV (fever, joint pain, headache, conjunctivitis, rash, and muscle pain) in January 2016 (Fig. S1C). Three patients were PCR confirmed for ZIKV infection by the CDC within the first 2 weeks of symptom onset, whereas the remaining patient tested positive for the presence of anti-ZIKV IgG by a microplate enzyme-linked immunosorbent assay (ELISA) (Euroimmun, Inc.), as performed by BocaBiolistics (Pompano Beach, FL), and sera were collected 12 to 31 days after the onset of symptoms.
DENV.
Sixteen healthy, flavivirus naive rhesus macaques (M. mulatta) were subcutaneously injected with 105 PFU of DENV1 (West Pac 74), DENV2 (S16803), DENV3 (CH53489), or DENV4 (341750) (n = 4 per challenge group; Fig. S1A and 4), derived from low-passage, near-wild-type virus isolates. Sera were collected prior to infection and 30 dpi (37, 43). Sera from human primary DENV2 infections (n = 10; Fig. S1B) were collected as part of a DENV human challenge model originally developed by the Laboratory of Infectious Diseases at the U.S. National Institutes of Health. Sera from participants that had no history or serological evidence of flavivirus infection were collected prior to challenge and 28 days postchallenge with 103 PFU of rDEN2Δ30. rDEN2Δ30 induced viremia in all participants by 5 dpi (39, 40). Two individuals exhibited elevated binding to flavivirus E prior to challenge with rDEN2Δ30 (Fig. S4) and were further excluded from our analysis. Convalescent DENV sera (n = 7) were collected in Peru by the U.S. Naval Medical Research Unit No. 6 (NAMRU-6) between February 2011 and November 2013. Subjects had febrile illness for 5 days or less and were confirmed to have DENV infections (DENV2, n = 5; DENV3, n = 2; Fig. S1C) by PCR during the acute phase of infection. Sera were collected 14 to 24 days after confirmation of acute infection.
YFV.
Early immune yellow fever virus antisera from three NHPs (Fig. S1A; NR-29335, NR-29337, and NR-29338; BEI Resources, NIAID, NIH), immunized by subcutaneous injection of 0.5 ml of live, attenuated YFV vaccine (strain 17D [38]), were collected 30 days after vaccination. Human sera from 17D-vaccinated individuals (seven primary and six boosted [Fig. S1D]), collected 14 to 118 days after vaccination, were obtained from the Department of Defense Serum Repository (Silver Spring, MD).
WNV.
Confirmed WNV-infected human sera (n = 20; Fig. S1D) were collected between 2009 and 2011 at FDA-approved blood donor locations within the United States in accordance with a surveillance protocol performed by the National Heart, Lung, and Blood Institute (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) (66). Sera were identified as WNV positive by nucleic acid testing, indicating a current WNV infection at the time of blood donation. WNV-positive donors were then contacted for study enrollment, at which point subjects completed symptom questionnaires and provided subsequent blood samples at several weekly and monthly visits after the initial donation. Each specimen tested positive for the presence of WNV-specific IgM and IgG antibodies (67).
Control sera.
Sera collected by SeraCare Life Sciences, Inc., from healthy U.S. donors (n = 5) were used for negative controls. These sera were selected based on no detected antibodies to human immunodeficiency virus type 1 and type 2, hepatitis A and B viruses, and all flaviviruses used in the microarray.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge grant support from NIAID to R.G.U. (R01AI096215) and D.H.O. (R01AI116382-01A1). This research was supported in part by the appointment of S.L.K. and C.L.P. to the Postgraduate Research Participation Program administered by the Oak Ridge Institute for Science and Education through an interagency agreement with the U.S. Department of Energy.
We thank Andrew Haddow, USAMRIID, for supplying many of the ZIKV strains used in this study, Julia S. Ampuero, Carolina Guevara, NAMRU-6, Lima, Peru, and the staff of DIRESA Loreto for collection and analysis of human disease sera and also for veterinary and animal care scientific protocol implementation, and the Pathology staff at the Wisconsin National Primate Research Center (WNPRC) for their contributions to this study.
The opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by any branch of the U.S. government.
We declare that we have no competing interests.
R.G.U., C.L.P., S.L.K., J.L.S., and S.M.R.J. designed the study. D.M.D. and A.P.D. collected and organized sera. C.L.P., J.L.S., and S.M.R.J. performed the experiments. S.L.K., C.L.P., J.L.S., S.M.R.J., D.H.O., R.D.H, and R.G.U. analyzed the data. S.L.K., C.L.P., J.L.S., S.M.R.J., and R.G.U. wrote the manuscript. All authors reviewed and edited the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/CVI.00036-17.
REFERENCES
- 1.Ferreira-de-Brito A, Ribeiro IP, Miranda RM, Fernandes RS, Campos SS, Silva KA, Castro MG, Bonaldo MC, Brasil P, Lourenco-de-Oliveira R. 2016. First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Mem Inst Oswaldo Cruz 111:655–658. doi: 10.1590/0074-02760160332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasilakis N, Weaver SC. 2016. Flavivirus transmission focusing on Zika. Curr Opin Virol 22:30–35. doi: 10.1016/j.coviro.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. 2015. Potential sexual transmission of Zika virus. Emerg Infect Dis 21:359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, Salje H, Van Kerkhove MD, Abadie V, Garel C, Fontanet A, Mallet HP. 2016. Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study. Lancet 387:2125–2132. doi: 10.1016/S0140-6736(16)00651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, Doriqui MJ, Neri JI, Neto JM, Wanderley HY, Cernach M, El-Husny AS, Pone MV, Serao CL, Sanseverino MT, Brazilian Medical Genetics Society-Zika Embryopathy Task Force. 2016. Possible association between Zika virus infection and microcephaly - Brazil, 2015. MMWR Morb Mortal Wkly Rep 65:59–62. doi: 10.15585/mmwr.mm6503e2. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. 2016. Zika virus and birth defects–reviewing the evidence for causality. N Engl J Med 374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 7.Lazear HM, Diamond MS. 2016. Zika virus: new clinical syndromes and its emergence in the Western Hemisphere. J Virol 90:4864–4875. doi: 10.1128/JVI.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial AL, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra JC, Despres P, Fournier E, Mallet HP, Musso D, Fontanet A, Neil J, Ghawche F. 2016. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. 2009. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 10.Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M. 2014. Current Zika virus epidemiology and recent epidemics. Med Mal Infect 44:302–307. doi: 10.1016/j.medmal.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Musso D, Gubler DJ. 2016. Zika virus. Clin Microbiol Rev 29:487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kindhauser MK, Allen T, Frank V, Santhana RS, Dye C. 2016. Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ 94:675C–686C. doi: 10.2471/BLT.16.171082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kostyuchenko VA, Lim EX, Zhang S, Fibriansah G, Ng TS, Ooi JS, Shi J, Lok SM. 2016. Structure of the thermally stable Zika virus. Nature 533:425–428. doi: 10.1038/nature17994. [DOI] [PubMed] [Google Scholar]
- 14.Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, Kuhn RJ. 2016. The 3.8 A resolution cryo-EM structure of Zika virus. Science 352:467–470. doi: 10.1126/science.aaf5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Ge P, Yu X, Brannan JM, Bi G, Zhang Q, Schein S, Zhou ZH. 2013. Cryo-EM structure of the mature dengue virus at 3.5-A resolution. Nat Struct Mol Biol 20:105–110. doi: 10.1038/nsmb.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukhopadhyay S, Kim BS, Chipman PR, Rossmann MG, Kuhn RJ. 2003. Structure of West Nile virus. Science 302:248. doi: 10.1126/science.1089316. [DOI] [PubMed] [Google Scholar]
- 17.Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. 1995. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 18.Luca VC, AbiMansour J, Nelson CA, Fremont DH. 2012. Crystal structure of the Japanese encephalitis virus envelope protein. J Virol 86:2337–2346. doi: 10.1128/JVI.06072-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lok SM. 2016. The interplay of dengue virus morphological diversity and human antibodies. Trends Microbiol 24:284–293. doi: 10.1016/j.tim.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Dai L, Song J, Lu X, Deng YQ, Musyoki AM, Cheng H, Zhang Y, Yuan Y, Song H, Haywood J, Xiao H, Yan J, Shi Y, Qin CF, Qi J, Gao GF. 2016. Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe 19:696–704. doi: 10.1016/j.chom.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Aliota MT, Dudley DM, Newman CM, Mohr EL, Gellerup DD, Breitbach ME, Buechler CR, Rasheed MN, Mohns MS, Weiler AM, Barry GL, Weisgrau KL, Eudailey JA, Rakasz EG, Vosler LJ, Post J, Capuano S III, Golos TG, Permar SR, Osorio JE, Friedrich TC, O'Connor SL, O'Connor DH. 2016. Heterologous protection against Asian Zika virus challenge in rhesus macaques. PLoS Negl Trop Dis 10:e0005168. doi: 10.1371/journal.pntd.0005168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, Foglierini M, Pedotti M, Simonelli L, Dowall S, Atkinson B, Percivalle E, Simmons CP, Varani L, Blum J, Baldanti F, Cameroni E, Hewson R, Harris E, Lanzavecchia A, Sallusto F, Corti D. 2016. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 353:823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 23.Song H, Qi J, Haywood J, Shi Y, Gao GF. 2016. Zika virus NS1 structure reveals diversity of electrostatic surfaces among flaviviruses. Nat Struct Mol Biol 23:456–458. doi: 10.1038/nsmb.3213. [DOI] [PubMed] [Google Scholar]
- 24.Calisher CH, Karabatsos N, Dalrymple JM, Shope RE, Porterfield JS, Westaway EG, Brandt WE. 1989. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol 70:37–43. [DOI] [PubMed] [Google Scholar]
- 25.Anderson KB, Gibbons RV, Thomas SJ, Rothman AL, Nisalak A, Berkelman RL, Libraty DH, Endy TP. 2011. Preexisting Japanese encephalitis virus neutralizing antibodies and increased symptomatic dengue illness in a school-based cohort in Thailand. PLoS Negl Trop Dis 5:e1311. doi: 10.1371/journal.pntd.0001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reisen WK, Lothrop HD, Wheeler SS, Kennsington M, Gutierrez A, Fang Y, Garcia S, Lothrop B. 2008. Persistent West Nile virus transmission and the apparent displacement St. Louis encephalitis virus in southeastern California, 2003-2006. J Med Entomol 45:494–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. 1988. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg 38:411–419. [DOI] [PubMed] [Google Scholar]
- 28.Halstead SB, Marchette NJ, Sung Chow JS, Lolekha S. 1976. Dengue virus replication enhancement in peripheral blood leukocytes from immune human beings. Proc Soc Exp Biol Med 151:136–139. doi: 10.3181/00379727-151-39160. [DOI] [PubMed] [Google Scholar]
- 29.Halstead SB, Rojanasuphot S, Sangkawibha N. 1983. Original antigenic sin in dengue. Am J Trop Med Hyg 32:154–156. [DOI] [PubMed] [Google Scholar]
- 30.Russell PK, Udomsakdi S, Halstead SB. 1967. Antibody response in dengue and dengue hemorrhagic fever. Jpn J Med Sci Biol 20(Suppl):103–108. [PubMed] [Google Scholar]
- 31.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. 7 February 2016, posting date Revised diagnostic testing for Zika, chikungunya, and dengue viruses in US public health laboratories. Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 33.Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, Mohns MS, Breitbach ME, Rasheed MN, Newman CM, Gellerup DD, Moncla LH, Post J, Schultz-Darken N, Schotzko ML, Hayes JM, Eudailey JA, Moody MA, Permar SR, O'Connor SL, Rakasz EG, Simmons HA, Capuano S, Golos TG, Osorio JE, Friedrich TC, O'Connor DH. 2016. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun 7:12204. doi: 10.1038/ncomms12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan JL, Lok SM. 2014. Dengue virus purification and sample preparation for cryo-electron microscopy. Methods Mol Biol 1138:41–52. doi: 10.1007/978-1-4939-0348-1_4. [DOI] [PubMed] [Google Scholar]
- 35.Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, Guzman H, Tesh RB, Weaver SC. 2012. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis 6:e1477. doi: 10.1371/journal.pntd.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dowd KA, DeMaso CR, Pelc RS, Speer SD, Smith AR, Goo L, Platt DJ, Mascola JR, Graham BS, Mulligan MJ, Diamond MS, Ledgerwood JE, Pierson TC. 2016. Broadly neutralizing activity of Zika virus-immune sera identifies a single viral serotype. Cell Rep 16:1485–1491. doi: 10.1016/j.celrep.2016.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez S, Cisney ED, Tikhonov AP, Schweitzer B, Putnak RJ, Simmons M, Ulrich RG. 2011. Antibody recognition of the dengue virus proteome and implications for development of vaccines. Clin Vaccine Immunol 18:523–532. doi: 10.1128/CVI.00016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durieux C. 1956. Mass yellow fever vaccination in French Africa south of the Sahara, p 115–121. In Smithburn KC, Durieux C, Koerber R, Panna HA, Dic GWA, Courtois G, de Sousa Manso C, Stuart G, Bonnel PH (ed), Yellow fever vaccination. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 39.Larsen CP, Whitehead SS, Durbin AP. 2015. Dengue human infection models to advance dengue vaccine development. Vaccine 33:7075–7082. doi: 10.1016/j.vaccine.2015.09.052. [DOI] [PubMed] [Google Scholar]
- 40.Kirkpatrick BD, Whitehead SS, Pierce KK, Tibery CM, Grier PL, Hynes NA, Larsson CJ, Sabundayo BP, Talaat KR, Janiak A, Carmolli MP, Luke CJ, Diehl SA, Durbin AP. 2016. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci Transl Med 8:330ra336. doi: 10.1126/scitranslmed.aaf1517. [DOI] [PubMed] [Google Scholar]
- 41.VanBlargan LA, Mukherjee S, Dowd KA, Durbin AP, Whitehead SS, Pierson TC. 2013. The type-specific neutralizing antibody response elicited by a dengue vaccine candidate is focused on two amino acids of the envelope protein. PLoS Pathog 9:e1003761. doi: 10.1371/journal.ppat.1003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monath TCM, Teuwen DE. 2008. Yellow fever vaccine, p 959–1055. In Plotkin SA, Orenstein W, Offit PA (ed), Vaccines, 5th ed Saunders Elsevier, Philadelphia, PA. [Google Scholar]
- 43.Simmons M, Burgess T, Lynch J, Putnak R. 2010. Protection against dengue virus by non-replicating and live attenuated vaccines used together in a prime boost vaccination strategy. Virology 396:280–288. doi: 10.1016/j.virol.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 44.Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, Mongkolsapaya J, Screaton GR. 2016. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with Zika virus. Nat Immunol 17:1102–1108. doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.St George K, Sohi IS, Dufort EM, Dean AB, White JL, Limberger R, Sommer JN, Ostrowski S, Wong SJ, Backenson PB, Kuhles D, Blog D, Taylor J, Hutton B, Zucker HA. 2017. Zika virus testing considerations: lessons learned from the first 80 real-time reverse transcription-PCR-positive cases diagnosed in New York State. J Clin Microbiol 55:535–544. doi: 10.1128/JCM.01232-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Priyamvada L, Cho A, Onlamoon N, Zheng NY, Huang M, Kovalenkov Y, Chokephaibulkit K, Angkasekwinai N, Pattanapanyasat K, Ahmed R, Wilson PC, Wrammert J. 2016. B cell responses during secondary dengue virus infection are dominated by highly cross-reactive, memory-derived plasmablasts. J Virol 90:5574–5585. doi: 10.1128/JVI.03203-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinski A, Jumnainsong A, Edwards C, Quyen NT, Duangchinda T, Grimes JM, Tsai WY, Lai CY, Wang WK, Malasit P, Farrar J, Simmons CP, Zhou ZH, Rey FA, Mongkolsapaya J, Screaton GR. 2015. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol 16:170–177. doi: 10.1038/ni.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klasse PJ. 2014. Neutralization of virus infectivity by antibodies: old problems in new perspectives. Adv Biol 2014:157895. doi: 10.1155/2014/157895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prince HE, Tobler LH, Yeh C, Gefter N, Custer B, Busch MP. 2007. Persistence of West Nile virus-specific antibodies in viremic blood donors. Clin Vaccine Immunol 14:1228–1230. doi: 10.1128/CVI.00233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roehrig JT, Nash D, Maldin B, Labowitz A, Martin DA, Lanciotti RS, Campbell GL. 2003. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg Infect Dis 9:376–379. doi: 10.3201/eid0903.020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 52.Sonnhammer EL, von Heijne G, Krogh A. 1998. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc Int Conf Intell Syst Mol Biol 6:175–182. [PubMed] [Google Scholar]
- 53.Nallamsetty S, Austin BP, Penrose KJ, Waugh DS. 2005. Gateway vectors for the production of combinatorially-tagged His6-MBP fusion proteins in the cytoplasm and periplasm of Escherichia coli. Protein Sci 14:2964–2971. doi: 10.1110/ps.051718605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmer I, Wingfield PT. 2012. Preparation and extraction of insoluble (inclusion-body) proteins from Escherichia coli. Curr Protoc Protein Sci Chapter 6:Unit 6.3. doi: 10.1002/0471140864.ps0603s70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. 2003. TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34:374–378. [DOI] [PubMed] [Google Scholar]
- 56.Meyer D, Dimitriadou E, Hornik K, Weingessel A, Leisch F, Chang C, Lin C. 5 August 2015. Support vector machines, misc functions of the Department of Statistics, Probability Theory Group (Formerly: E1071), Technische Universität (TU) Wien, Vienna, Germany. [Google Scholar]
- 57.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 58.Di Tommaso P, Moretti S, Xenarios I, Orobitg M, Montanyola A, Chang JM, Taly JF, Notredame C. 2011. T-Coffee: a web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res 39:W13–W17. doi: 10.1093/nar/gkr245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Notredame C, Higgins DG, Heringa J. 2000. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J Mol Biol 302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- 60.Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- 61.Talavera G, Castresana J. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol 56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 62.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 63.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 64.Weatherall D, Goodfellow P, Harris J, Hinde R, Johnson L, Morris R, Ross N, Skehel J, Tickell C. 2006. The use of non-human primates in research. A working group report chaired by Sir David Weatherall FRS FMedSci. Academy of Medical Science, London, United Kingdom. [Google Scholar]
- 65.US Department of Health, Education, and Welfare. 1979. The Belmont report. Ethical principles and guidelines for the protection of human subjects of research. Office of the Secretary, The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, US Department of Health and Human Services, Washington, DC. [PubMed] [Google Scholar]
- 66.National Institutes of Health. 18 June 2012, posting date WNV Study. Viral and immune parameters of dengue and WNV in donors: blood safety implications. WNV Manual of Procedures. National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. [Google Scholar]
- 67.Ramos HJ, Lanteri MC, Blahnik G, Negash A, Suthar MS, Brassil MM, Sodhi K, Treuting PM, Busch MP, Norris PJ, Gale M Jr. 2012. IL-1beta signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog 8:e1003039. doi: 10.1371/journal.ppat.1003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.