Abstract
Retinitis pigmentosa (RP) is a genetically heterogeneous retinal disorder. Despite the numerous genes associated with RP already identified, the genetic basis remains unknown in a substantial number of patients and families. In this study, we performed whole exome sequencing to investigate the molecular basis of a syndromic RP case which cannot be solved by mutations in known disease-causing genes. After applying a series of variant filtering strategies, we identified an apparently homozygous frameshift mutation, c.31delC (p.Q11Rfs*24) in the ADIPOR1 gene. The reported phenotypes of Adipor1-null mice contain retinal dystrophy, obesity and behavioral abnormalities, which highly mimic those in the syndromic RP patient. We further confirmed ADIPOR1 retina expression by immunohistochemistry. Our results established ADIPOR1 as a novel disease-causing gene for syndromic RP and highlight the importance of fatty acid transport in the retina.
Keywords: Next-generation sequencing, ADIPOR1, retina, obesity, fatty acid
Docosahexaenoic acid (DHA, 22:6ω3), one of the omega-3 polyunsaturated fatty acid (PUFA) species, is an essential PUFA retained in the central nervous system (Bazan, et al., 2011). In the retina, DHAs are highly enriched in the disc membranes of photoreceptor outer segments (OS) as a main component of phospholipids (SanGiovanni and Chew, 2005). DHAs are transported initially to retinal pigment epithelial (RPE) cells from the choriocapillaris. Then, through the interphotoreceptor matrix, DHAs are retained in the photoreceptor inner segments (IS), where they are acylated to phospholipids and transported to OS (SanGiovanni and Chew, 2005). Furthermore, a correlation between reduced DHA concentration and progressive retinal deterioration has long been noticed (Anderson, et al., 1991; Hoffman and Birch, 1995). These observations suggest the potential importance of DHA for proper function of photoreceptor cells.
ADIPOR1 (MIM# 607945; RefSeq NM_015999.5) encodes a transmembrane protein whose role has been known mainly in glucose metabolism and various signaling transduction pathways (Bjursell, et al., 2007; Yamauchi, et al., 2007). However, a recent study revealed that ADIPOR1 localizes in both the photoreceptors and RPE to facilitate the uptake and retention of DHA in the retina (Rice, et al., 2015). Genetic ablation of Adipor1 in two independent mouse lines results in reduced DHA uptake, degeneration of photoreceptors/RPE, and a compromised retinal function, highly mimicking the phenotype of human retinal degenerative diseases (Rice, et al., 2015). This study revealed the essential role of ADIPOR1 in photoreceptor/RPE survival and suggested that mutations in human ADIPOR1 may lead to a retinal dystrophy.
Among the most common retinal dystrophies is retinitis pigmentosa (RP; MIM# 268000), with a prevalence from 1/7,000 to 1/3,000 (Ferrari, et al., 2011). RP has a highly complex genetic etiology, with at least 79 disease-causing genes identified to date (RetNet, the Retinal Information Network). However, only about 60% of RP cases and families can be explained by mutations in these known RP-causing genes, suggesting that novel loci are yet to be identified (Wang, et al., 2014). In this study, we identified an apparently homozygous frameshift ADIPOR1 mutation in a patient diagnosed with syndromic RP, providing the first link between ADIPOR1 deficiency and a human genetic disorder.
The subject in this study was diagnosed in Cullen Eye Center, Baylor College of Medicine, Houston, United States. Informed written consent was obtained from the patient through an active protocol approved by the Institutional Review Board for Human Subject Research. This study adhered to the tenets of Declaration of Helsinki. The subject is a 27-year-old male from India of South Asian descent born from a consanguineous marriage between first cousins (Table1, Figure 1A). He had been the first and only pregnancy of his then 19-year-old mother and 21-year-old father. The patient's first ophthalmic examination occurred at age 7 ¾ years. He presented with a compound myopic refractive error (-6.00 sph + 4.00 cyl × 090 O.U.) and advanced pigmentary retinopathy at that time. By age 17 years, his visual acuity had dropped to 20/70 O.D. and 20/50 O.S. At age 27, his best corrected vision was about 20/200 O.U.. He had no functional visual field or peripheral vision. Bio-microscopy revealed early posterior cortical pre-capsular cataracts in each eye, considerable vitreous syneresis with strands, cells, and pigment, and the retinal examination showed diffuse vascular attenuation, advanced waxy optic nerve pallor, and extensive pigmentary retinopathy in all four quadrants of each eye (Table 1).
Table 1.
Clinical features of the syndromic RP patient and genetic evidence supporting ADIPOR1 as the disease-causing gene.
| Patient age, sex and ethnicity | 27-year-old male; South Asian |
| Ocular phenotypes | Extensive pigmentary retinopathy; tunnel vision; cataracts; advanced waxy optic nerve pallor |
| Neurological phenotypes | Intellectual disability; speech delay |
| Other phenotypes | Truncal obesity; flat feet |
| Number of known RD (including BBS) genes screened | 226 (Supp. Table S1) |
| Control databases for variant frequency filtering | ExAC; CHARGE consortium; NHLBI-ESP-6500; 1K Genome Project (See Supplementary Material for details) |
| ADIPOR1 variant information |
NM_015999.5: c.31delC (p.Q11Rfs*24) (Homozygous) Absent in control databases |
| AOH region covering the ADIPOR1 mutation | Chr1: 202544307 – 204103714 (1.56 Mb) |
RD, retinal disease; BBS, Bardet-Biedl syndrome; AOH, absence of heterozygosity. ExAC, exome aggregation consortium; CHARGE, Cohorts for Heart and Aging Research in Genomic Epidemiology; NHLBI-ESP, National Heart, Lung, and Blood Institute Grand Opportunities Exome Sequencing Project. Genome coordinates were based on human hg19 genome. Nucleotide numbering uses +1 as the A of the ATG translation initiation codon in the reference sequence, with the initiation codon as codon 1.
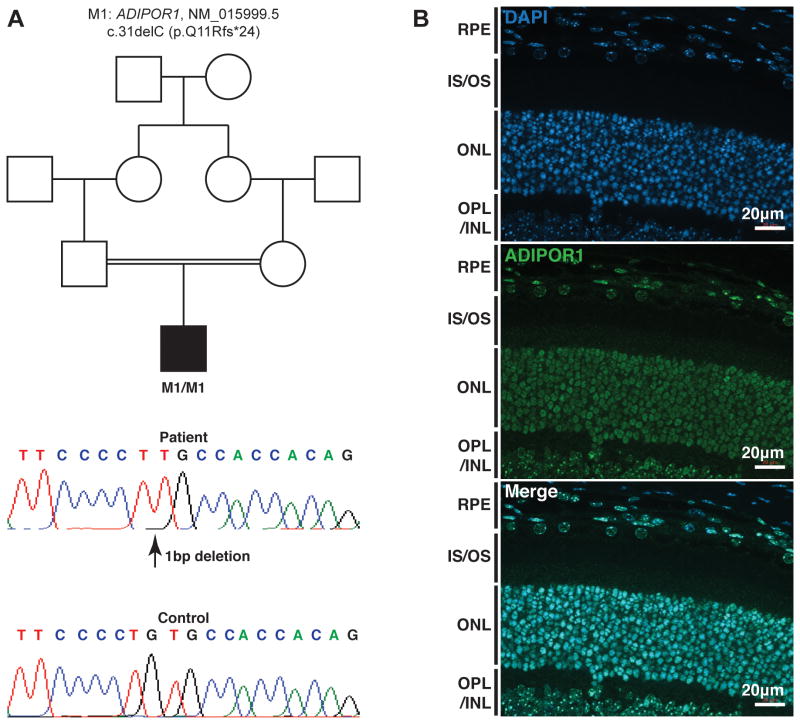
Figure 1. Genetic findings in the syndromic RP patient and ADIPOR1 immunohistochemistry.
A. A homozygous frameshift variant c.31delC (p.Q11Rfs*24) in ADIPOR1 (NM_015999.5) was identified and Sanger sequencing confirmed its identity. Nucleotide numbering uses +1 as the A of the ATG translation initiation codon in the reference sequence, with the initiation codon as codon 1.
B. Immunohistochemistry showed ADIPOR1 expresses in photoreceptor and RPE cells. ONL, outer nuclear layer; OS, outer segments; IS, inner segments; OPL, outer plexiform layer; INL, inner nuclear layer. Scale bar, 20μm.
In addition to the ocular phenotypes, the patient presented with a history of intellectual disability manifested in part by profound speech delay, speaking his first recognizable words at age 3½ years. He was noted to have a limited attention span, considerable developmental delay, and speech pathology that required special education. He was also noted to have flat feet and mostly truncal obesity (Table 1). Some of his clinical features simulate Bardet-Biedl Syndrome, but he has no polydactyly and no history of renal disease or other constitutional complications and is therefore diagnosed with syndromic RP. No other family history of developmental delay, obesity, retinal disease, or blindness was reported.
First we performed target capture sequencing to screen mutations in 226 known retinal disease-causing genes, including all genes associated with Bardet-Biedl syndrome (Supp. Table S1). More details on next-generation sequencing, data analysis and additional experiments were described in online supporting information (Supp. Table S2 and Supp. Methods). No causative mutation was identified, suggesting that the disease might be caused by mutations in a novel gene. Hence we performed WES and obtained the sequencing data with a mean coverage of 49× for exonic regions. More than 137,000 variants were called initially. Through a series of filtering and annotation procedures, 342 rare protein-altering variants remained. Among them, an apparently homozygous frameshift variant in ADIPOR1 appeared to be the top candidate (Table 1) based on the pedigree and the phenotypic concordance with existing mouse models (Bjursell, et al., 2007; Rice, et al., 2015). In Adipor1-null mice, the retina thickness and photo-response show anomalies from 4 weeks of age (Rice, et al., 2015). Obesity and behavioral problems were also reported in Adipor1-null mice (Bjursell, et al., 2007). The ADIPOR1 frameshift variant, c.31delC (p.Q11Rfs*24), occurs at the first exon of this gene, thus likely resulting in complete loss of ADIPOR1 function due to the abolishment of its transmembrane domains (Tanabe, et al., 2015). This variant is absent in control databases, suggesting it is extremely rare (Table 1). We performed Sanger sequencing and confirmed the homozygosity of this variant (Figure 1A). Since the frameshift occurs relatively early during protein translation, to exclude the possibility that protein translation may re-initiate downstream of the variant, we predicted the translation initiation sites (TIS) of ADIPOR1 mRNA using weakAUG (Tikole and Sankararamakrishnan, 2008) and Net Start 1.0 (Pedersen and Nielsen, 1997). The canonical TIS is the only one considered positive by both algorithms, strongly suggesting this variant as a null allele. This variant was submitted to Leiden open variation database (LOVD: http://www.lovd.nl/ADIPOR1). We screened additional 308 RP patients which have not been solved by target capture sequencing. However, we did not identify any patient samples with biallelic ADIPOR1 mutations, suggesting that mutations in ADIPOR1 a rare cause of human syndromic retinal dystrophy.
Previous studies have detected ADIPOR1 expression in the RPE cells in human retina section (Lin, et al., 2013). Here, we performed ADIPOR1 immunostaining in adult albino mouse retina. The result showed ADIPOR1 expression in mouse RPE and photoreceptor cells (Figure 1B), consistent with the model that ADIPOR1 function in these two cell types (Rice, et al., 2015).
Adipor1-null mice are grossly normal (Bjursell, et al., 2007; Yamauchi, et al., 2007) but recently reported to have retinal dystrophy phenotype (Rice, et al., 2015). Given the specific enrichment of DHA in photoreceptors (SanGiovanni and Chew, 2005), the reduction of DHA and dysregulated lipidome are likely to be the cause for compromised photoreceptor survival and function. Recently, the crystal structure of human ADIPOR1 was reported (Tanabe, et al., 2015). This may offer a mechanism on how ADIPOR1 facilitates the uptake and retention of DHA in the retina.
ADIPOR1 defects also probably lead to obesity in this patient due to its well-established role in glucose metabolism regulation (Bjursell, et al., 2007; Yamauchi, et al., 2007). The cognate ligand for ADIPOR1, adiponectin, is a metabolic hormone that increases insulin sensitivity and contributes to weight loss in mice (Fruebis, et al., 2001; Qi, et al., 2004; Yamauchi, et al., 2002; Yamauchi, et al., 2001). Adult male Adipor1-null mice show increased adiposity (Bjursell, et al., 2007). In addition, a negative correlation between ADIPOR1 adipose tissue mRNA level and body mass index has been observed in human individuals (Rasmussen, et al., 2006). These studies support our contention that a genetic defect in ADIPOR1 may underlie human obesity. Since the defect of adiponectin itself in mice does not show retinal abnormalities (Rice, et al., 2015), ADIPOR1 probably acts in adipose tissue and the retina with two independent functions.
Adipor1-null mice show decreased spontaneous locomotion and increased time spent in corner of cages (Bjursell, et al., 2007), which might be associated with neurological phenotypes in our patient, though it remains unknown whether ADIPOR1 plays an essential role in the brain. Due to the consanguineous origin of our subject, we cannot exclude the possibility that the genetic burden in other regions results in the phenotypes other than retinal dystrophy and obesity. Additional reports of patients with ADIPOR1 mutations will affirm the phenotypes with which ADIPOR1 truly associates.
In summary, by identifying a homozygous ADIPOR1 null allele in a syndromic RP patient, we established ADIPOR1 as a novel disease-causing gene for autosomal recessive syndromic RP. Our finding consolidates the notion that defects in the retinal lipidome can be a pathological mechanism for human retinal dystrophies. Future WES-based studies may identify more disease-causing genes related to retinal FA metabolism and transport. Importantly, therapeutic approaches for DHA delivery which detour through non-canonical paths might pave the way for the treatment of RP patients with ADIPOR1 mutations.
Supplementary Material
Acknowledgments
We thank the patient for participating in our study. We thank the Exome Aggregation Consortium that provided exome variant data. We thank Mr. Justin Branch for revising the manuscript. This work was supported by NIH Shared Instrument Grant 1S10RR026550, grant from National Eye Institute R01EY022356 and R01EY018571, grant from Foundation Fighting Blindness BR-GE-0613-0618-BCM to RC. RAL is supported in part by unrestricted funds from Research to Prevent Blindness, N.Y. to his primary department and by private donors.
Grant Sponsor: NIH (R01EY022356 and R01EY018571)
Footnotes
Competing Interests Statement: The authors declare no competing interests
Contributorship Statement: MX and RC designed the study. MX, FW and LZ analyzed WES data. AE performed IHC experiments and analyzed data. JW, JL, XW, NX, YL and LCW performed NGS experiments and analyzed data. RAL collected clinical data. MX, LCW, RAL and RC drafted the manuscript. All authors critically revised the manuscript, approved the published version and agreed to be accountable for all aspects of the work.
References
- Online Mendelian Inheritance in Man, OMIM®. McKusick-Nathans Institute of Genetic Medicine. Johns Hopkins University; Baltimore, MD: [Google Scholar]
- Anderson RE, Maude MB, Alvarez RA, Acland GM, Aguirre GD. Plasma lipid abnormalities in the miniature poodle with progressive rod-cone degeneration. Exp Eye Res. 1991;52(3):349–55. doi: 10.1016/0014-4835(91)90100-s. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Molina MF, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer's, and other neurodegenerative diseases. Annu Rev Nutr. 2011;31:321–51. doi: 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjursell M, Ahnmark A, Bohlooly YM, William-Olsson L, Rhedin M, Peng XR, Ploj K, Gerdin AK, Arnerup G, Elmgren A, Berg AL, Oscarsson J, et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56(3):583–93. doi: 10.2337/db06-1432. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Di Iorio E, Barbaro V, Ponzin D, Sorrentino FS, Parmeggiani F. Retinitis pigmentosa: genes and disease mechanisms. Curr Genomics. 2011;12(4):238–49. doi: 10.2174/138920211795860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98(4):2005–10. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DR, Birch DG. Docosahexaenoic acid in red blood cells of patients with X-linked retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1995;36(6):1009–18. [PubMed] [Google Scholar]
- Lin T, Qiu Y, Liu Y, Mohan R, Li Q, Lei B. Expression of adiponectin and its receptors in type 1 diabetes mellitus in human and mouse retinas. Mol Vis. 2013;19:1769–78. [PMC free article] [PubMed] [Google Scholar]
- Pedersen AG, Nielsen H. Neural network prediction of translation initiation sites in eukaryotes: perspectives for EST and genome analysis. Proc Int Conf Intell Syst Mol Biol. 1997;5:226–33. [PubMed] [Google Scholar]
- Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10(5):524–9. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- Rasmussen MS, Lihn AS, Pedersen SB, Bruun JM, Rasmussen M, Richelsen B. Adiponectin receptors in human adipose tissue: effects of obesity, weight loss, and fat depots. Obesity (Silver Spring) 2006;14(1):28–35. doi: 10.1038/oby.2006.5. [DOI] [PubMed] [Google Scholar]
- Rice DS, Calandria JM, Gordon WC, Jun B, Zhou Y, Gelfman CM, Li S, Jin M, Knott EJ, Chang B, Abuin A, Issa T, et al. Adiponectin receptor 1 conserves docosahexaenoic acid and promotes photoreceptor cell survival. Nat Commun. 2015;6:6228. doi: 10.1038/ncomms7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24(1):87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Tanabe H, Fujii Y, Okada-Iwabu M, Iwabu M, Nakamura Y, Hosaka T, Motoyama K, Ikeda M, Wakiyama M, Terada T, Ohsawa N, Hato M, et al. Crystal structures of the human adiponectin receptors. Nature. 2015 doi: 10.1038/nature14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikole S, Sankararamakrishnan R. Prediction of translation initiation sites in human mRNA sequences with AUG start codon in weak Kozak context: A neural network approach. Biochem Biophys Res Commun. 2008;369(4):1166–8. doi: 10.1016/j.bbrc.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Wang F, Wang H, Tuan HF, Nguyen DH, Sun V, Keser V, Bowne SJ, Sullivan LS, Luo H, Zhao L, Wang X, Zaneveld JE, et al. Next generation sequencing-based molecular diagnosis of retinitis pigmentosa: identification of a novel genotype-phenotype correlation and clinical refinements. Hum Genet. 2014;133(3):331–45. doi: 10.1007/s00439-013-1381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.