Abstract
The gametocytes of the malaria parasites are obligate for perpetuating the parasite's life cycle through mosquitoes, but the sex-specific biology of gametocytes is poorly understood. We generated a transgenic line in the human malaria parasite Plasmodium falciparum, which allowed us to accurately separate male and female gametocytes by flow cytometry. In-depth analysis of the proteomes by liquid chromatography-tandem mass spectrometry identified 1244 and 1387 proteins in mature male and female gametocytes, respectively. GFP-tagging of nine selected proteins confirmed their sex-partitions to be agreeable with the results from the proteomic analysis. The sex-specific proteomes showed significant differences that are consistent with the divergent functions of the two sexes. Although the male-specific proteome (119 proteins) is enriched in proteins associated with the flagella and genome replication, the female-specific proteome (262 proteins) is more abundant in proteins involved in metabolism, translation and organellar functions. Compared with the Plasmodium berghei sex-specific proteomes, this study revealed both extensive conservation and considerable divergence between these two species, which reflect the disparities between the two species in proteins involved in cytoskeleton, lipid metabolism and protein degradation. Comparison with three sex-specific proteomes allowed us to obtain high-confidence lists of 73 and 89 core male- and female-specific/biased proteins conserved in Plasmodium. The identification of sex-specific/biased proteomes in Plasmodium lays a solid foundation for understanding the molecular mechanisms underlying the unique sex-specific biology in this early-branching eukaryote.
The life cycle of the protozoan malaria parasites encompasses multiple developmental stages and alternates between a human host and an Anopheles mosquito. In human blood, the majority of parasites assumes the asexual replication cycle, starting from merozoite invasion of a red blood cell (RBC) 1 to the maturation of a schizont. Asexual multiplication of the parasites in erythrocytes is responsible for the clinical symptoms of malaria, and thus most antimalarial treatments are directed toward the asexual stages. Meanwhile, a small proportion of the parasites, in response to poorly defined triggers, enters the sexual pathway and develops into male and female gametocytes. Subsequently, circulating gametocytes in the human blood, after ingestion by a vector, develop into gametes in the mosquito midgut. As an essential link to the life stages in mosquitoes and for continued transmission of the parasites, gametocytes have increasingly been recognized as a prime target for interruption of transmission, especially during the malaria elimination phase (1). In this regard, understanding the fundamental biology of sexual development in malaria parasites will provide the knowledge base for designing transmission-blocking drugs and vaccines.
Commitment to sexual development in malaria parasites occurs prior to merozoite formation in the previous erythrocytic cycle, as merozoites from a sexually-committed schizont all develop into either male or female gametocytes, but not both (2, 3). In most Plasmodium species, gametocytes mature within 30 h and they morphologically resemble late trophozoites. In stark contrast, gametocyte development in P. falciparum takes 10–12 days, during which the gametocytes undergo five morphologically distinct stages culminating in the mature crescent form that was observed by Alphonse Laveran in the 1880s (4, 5). This falciform shape transformation is partially driven by the architecture of cytoskeleton, including the inner membrane complex underneath the parasite plasma membrane (6–9). Another distinguishing feature of P. falciparum gametocytes is the sequestration of immature stages in the bone marrow and possibly the spleen (10, 11). The decoding of the parasite genomes and global profiling of gene expression in gametocytes have provided deeper insights into the biology of sexual development (12–20). Investigations into the molecular mechanism of gametocytogenesis led to the recent identification of AP2-G, a member of the AP2 family transcription factors, as the master switch of gametocytogenesis in both P. falciparum and the rodent parasite P. berghei. AP2-G itself, located in a heterochromatin region marked with histone H3 lysine 9 trimethylation and P. falciparum heterochromatin protein 1, is epigenetically silenced (21–23). Stochastic activation of PfAP2-G offers an explanation for the low, baseline production of gametocytes during in vitro culture. In schizonts committed to gametocytogenesis, over 300 genes were induced, including known markers of early gametocyte development (24). Subsequent gametocyte development is accompanied by up- and down-regulation of hundreds of transcripts and proteins (12–16, 25). Another member of AP2 family, AP2-G2, may regulate gametocyte development by repression of specific transcripts for asexual and stages beyond gametocyte development (26). In order to prepare for rapid changes and subsequent development in free-living environment in mosquito, gametocytes adopt several strategies including storage of “maternal” mRNAs by the DOZI and possibly Puf protein complexes that can be mobilized rapidly upon stage transition (27–31), increased expression of protein kinases and phosphatases responsible for signal transduction (32–38), and activation of tricarboxylic acid cycle and lipid metabolism for energy and lipid requirements (39–44).
In P. falciparum sex determination occurs at the same time or soon after commitment to gametocyte development (3, 45), but the underpinning molecular mechanism is unknown. Plasmodium gametocyte population is generally female-biased, but the sex ratio can be modulated by host and environmental factors (46, 47). Once inside the mosquito midgut, male gametocytes (MG) are prepared for rapid DNA replication and mitosis to produce eight motile microgametes, whereas the female gametocytes (FG) are ready for mitosis and subsequent zygote-ookinete development (47, 48). Consistent with this functional division, the MG- and FG-specific proteomes of the rodent parasite P. berghei revealed distinctive sex-specific biology (49). Sex specificity in this parasite is underlined by the identification of 36% of the 650 MG proteome and 19% of the 541 FG proteome as sex-specific. Remarkably, the two sexes only share 69 common proteins. Despite that conservation in gametocyte-specific gene sets across Plasmodium species foretells similarity in sex-specific biology in all malaria parasites, the distinctive features of P. falciparum gametocytes in morphology, development, and sequestration suggest significant differences in the induction, differentiation and development of the two sexes in this parasite (36, 50). Efforts toward dissecting the differences in MG and FG at the molecular level are thwarted by the difficulties in clearly separating the two sexes. An attempt to identify sex-partitioned proteomes of the P. falciparum gametocytes relied heavily on species-species conservation and bioinformatic predictions (51), and thus might have missed important information underlying the true biological differences between these species. Most recently, a comprehensive proteome analysis of separated P. falciparum MG and FG coupled with transcriptome analysis was reported (52). This study identified divergent MG and FG proteomes similar to the sex-specific proteomes in the gametocytes of P. berghei. We report here accurate separation of mature MG from FG in P. falciparum based on differential expression of the green fluorescent protein (GFP) in MG and FG using flow cytometry and in-depth analysis of the proteomes. The sex-specific proteomes of MG and FG exhibited significant difference that is consistent with the functional division of MG and FG. Compared with the P. berghei sex-specific proteomes, the P. falciparum sex-specific proteomes had an overall conservation of features as in P. berghei, while they also exhibited considerable inter-species differences which may underpin the different biology of gametocytogenesis in these species.
EXPERIMENTAL PROCEDURES
Parasite Culture and Gametocyte Induction
Cultures of P. falciparum parasite lines were maintained in complete RPMI 1640 medium supplemented with 10% human serum and hypoxanthine as described previously (53, 54). A modified induction scheme for gametocytogenesis was used to obtain highly synchronous gametocyte cultures (55, 56). Briefly, asexual parasites were synchronized twice in two successive asexual erythrocytic cycles by 5% d-sorbitol treatment of ring-stage parasites. Synchronous cultures at the trophozoite stage were set up at a parasitemia of 2.5–3.2% and a hematocrit of 3%. On the second day (day -2), ring-stage parasitemia typically reaches 8–12%. A half of spent medium was replaced by fresh medium. On the third day (day -1), stressed schizont cultures including spent medium were adjusted with fresh RBCs and medium to a parasitemia of ∼2% and a hematocrit of 3%. From day 1 to 3, 10 units/ml of heparin were added to the culture to block invasions of RBCs from residual contaminating asexual stages (57).
Flow Cytometry and Fluorescence-activated Cell Sorting
A transgenic parasite line, 3D7α−tubII/GFP, where GFP expression is under the control of α-tubulin II promoter (28, 55), was cultured and induced for synchronous gametocytes. On day 12 of induction, stage V gametocytes were purified on a 35%/50% Percoll gradient and immediately sorted on a Beckman Coulter MoFlo Astrios system at 488 nm excitation. Parasites were suspended in a warm “suspended animation buffer”, which inhibits the activation of gametocytes to gametes (58), and injected into the cell sorter under sterile conditions. Forward angle light scatter height and side angle light scatter height and area (used to discriminate doublets) were set at 280 V 1.0 gain and 240 V 1.0 gain photomultiplier tube (PMT) power, respectively. By gating the parasites which were negative for an irrelevant detector (664/22 at 596 V PMT) but positive on the GFP detector (513/26 at 458 V PMT), it was possible to accurately sort the high- and low-GFP parasites into individual tubes.
Examination of Sexual Features of Sorted Gametocytes
Sorted gametocytes were used to make thin smears, stained with Giemsa, and examined by microscopy to confirm the sexual characters of sorted gametocytes with high- and low-GFP signals (29, 59). The five classic criteria are (1) males are larger than females, (2) the ends of the cells are angular in females and round in males, (3) the nucleus is smaller in females than in males, (4) the granules of malaria pigment are centrally located in females and more widely scattered in males, and (5) the cytoplasm stains deep blue in females and pale purple in males (59). Sorted parasites were also used for inducing exflagellation as described previously (29) to further confirm the high-GFP parasites as mature MG.
Protein Extraction and Digestion
The sorted parasites (3.5 × 106) were lysed in SDT-lysis buffer (4% SDS, 100 mm Tris/HCl pH 7.6, 0.1 m dithiothreitol - DTT) for complete dissolution (60). The lysate was separated in a 10% Bis-Tris SDS-PAGE gel. Ten slices were excised and in-gel digestion was performed using a robot (ProGest, DigiLab, Marlborough, MA). Briefly, gel bands were washed using 25 mm ammonium bicarbonate followed by acetonitrile, and then were reduced using 10 mm DTT at 60 °C followed by alkylation with 50 mm iodoacetamide at room temperature. Finally, the gel bands were digested with trypsin (Promega, Madison, WI) at 37 °C for 4 h followed by peptide extraction with formic acid in acetonitrile.
LC-MS/MS Analysis
The extracted peptides were analyzed by nano LC/MS/MS with a Waters NanoAcquity HPLC system interfaced to a Q Exactive™ Hybrid Quadrupole-Orbitrap Mass Spectrometer (Thermo Scientific). Peptides were loaded on a trapping column and eluted over a 75 μm analytical column at 350 nL/min. MS and MS/MS were performed at 70,000 FWHM and 17,500 FWHM resolution, respectively. The 15 most abundant ions were selected for MS/MS and Mascot Generic Files (MGF) from RAW files were extracted by Proteome Discoverer v1.4 (Thermo Scientific). Parasite proteins were identified by searching the Uniprot P. falciparum protein database (v01/2014, 5369 entries) concatenated with the SWISS-PROT Human database (20,160 entries). The combined database (25,529 entries) was reversed and appended back to the forward database (51,058 entries total). All peak list files (MGFs) were analyzed using the Mascot (Matrix Science; London, UK; version 2.5.1). Search parameters included trypsin digestion (C-terminal K and R cleavage) full cleavage with 2 missed sites, fixed modification: +57 on C (carbamidomethyl), variable modifications: −17 on n (Gln->pyro-Glu), +1 on NQ (deamidated), +16 on M (oxidation), +42 on n (acetyl), fragment ion mass tolerance of 0.02 Da, peptide mass tolerance of 10 ppm. Data were filtered at 1% spectra, 1% protein, 0.2% peptide false discovery rate (FDR), and at least two unique peptides per protein. FDR was calculated by counting the number of reverse entries and dividing that by the total number of forward and reverse entries. Peptides of interest were independently manually verified. Mascot DAT files were parsed into the Scaffold software for validation and filtering to create a nonredundant list per sample. Normalization of protein abundance using normalized spectral abundance factors (NSAFs) was performed based on protein sizes (61–63). The relative abundance of identified proteins was also calculated by spectral counts (62). The raw data and peptide information of proteomes were deposited in the ProteomeXchange via the PRIDE database (data set identifier PXD003556).
Validation of Sex-specific Protein Expression
To validate the sex-specific expression of nine proteins identified from the proteomic analysis, GFP was tagged to these endogenous proteins. For each gene, ∼ 1 kb fragment from the C terminus end (without the stop codon) was amplified and subcloned to fuse with the GFP and 3′ UTR region of P. berghei dhfr-ts gene, and cloned into the transfection vector pHD22Y with the human DHFR cassette (supplemental Fig. S1A, supplemental Table S1A) (64, 65). Parasite transfection, drug selection and cloning were performed as described (29, 65). Positive clones were verified by integration-specific PCR and detection of GFP expression by fluorescence microscopy (supplemental Fig. S1B, supplemental Table S1B). Gametocytes from these tagged parasite lines were examined by microscopy (29). Because bright-field microscopy does not always clearly separate MG from FG by morphology, sex-specific expression of the GFP-fusion proteins was analyzed by sorting the GFP-positive and -negative gametocytes, or high- and low-GFP gametocytes, which were stained with Giemsa and subjected to sex identification using the sex-specific morphological characters described above.
Experimental Design and Statistical Rationale
Three sorting experiments were performed for the separation of MG and FG from three different gametocyte cultures, which represent three biological replicates for this study. The separated gametocytes (sample number n = 3 for low-GFP gametocytes and n = 3 for high-GFP gametocytes) were used for LC-MS/MS analysis. Spearman correlation was used to assess the correlation between different proteome profiles. The numbers of proteins and their abundance in the MG and FG proteomes were compared by Pearson's chi-square test. To compare the MG and FG proteomes in different functional groups, one-sided two-sample t test was applied. To compare the differences between shared MG and FG proteins, we analyzed the protein abundance using DESeq implemented in the R/Bioconductor package (66), which test the difference between spectral counts of shared MG and FG proteins based on the negative binomial distribution, with mean and variance linked by local regression, resulting in the corresponding marginal p value.
RESULTS
Separation of P. falciparum Male and Female Gametocytes
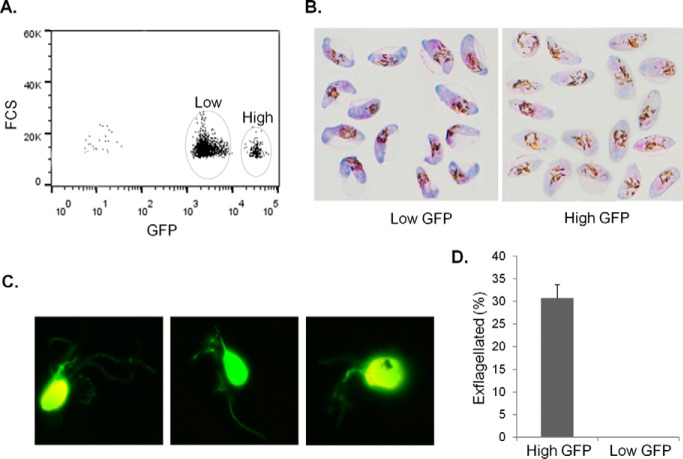
To separate P. falciparum MG from FG, we generated a transgenic 3D7 parasite line (3D7α−tubII/GFP), where GFP expression is under the control of the α-tubulin II promoter (28, 55). The GFP expression cassette was integrated into the parasite genome and a single clone was selected to ensure homogeneous expression of GFP (55). No noticeable GFP expression was found in asexual stages of the 3D7α−tubII/GFP, whereas all mature gametocytes showed GFP expression with two distinct populations that differed in GFP fluorescence intensity by >10 folds (Fig. 1A). This expression pattern is consistent with a significantly higher level of expression of α-tubulin II in mature MG than in FG (67). Giemsa staining of the gametocytes separated by fluorescence-activated cell sorting revealed that the low- and high-GFP populations were consistent with the classic morphological characteristics of FG and MG, respectively (Fig. 1B) (29, 59). In agreement with a female-biased sex ratio, stage V 3D7α−tubII/GFP gametocytes had a MG:FG ratio of ∼1:5. Activation of sorted parasites resulted in exflagellation of the gametocytes in the high-GFP population (Fig. 1C,D), further corroborating this gametocyte population as MG. Based on the difference in GFP fluorescence, we sorted equal numbers of stage-V MG and FG and used them for in-depth proteomic studies. In total, three independent experiments with a fixed number (3.5 × 106) of sorted MG and FG each were performed for proteome analysis. Because there was no GFP signal in asexual stage parasites and no or a very low number of asexual parasites existed in long-term gametocyte cultures, contamination with asexual parasites in the sorted gametocytes was rare.
Fig. 1.
Separation of P. falciparum stage V MG and FG by flow cytometry based on differential expression of the GFP reporter under the control of α-tubulin II promoter. A, Representative plot of GFP fluorescence intensities from 3D7α−tubII/GFP stage V gametocytes shows the separation of low- and high-GFP populations (circled). Stage V gametocytes were first isolated by Percoll and then analyzed by flow cytometry. FCS: Forward scattered light. B, Sorted gametocytes from low- and high-GFP populations shown in (A) stained with Giemsa show the typical characteristics of mature FG (left) and MG (right). MG appear pink with rounded cell ends and more widely scattered pigments, whereas FG appear blue with angular cell ends and more centralized pigment. C, Pictures of exflagellating gametocytes from the high-GFP population. D, Percentage of exflagellated gametocytes from the high- and low-GFP populations. Data are shown as mean ± s.d. from three replicates.
Proteomes of Mature Male and Female Gametocytes
We determined the proteomes of MG and FG by high-accuracy LC-MS/MS (Table I, supplemental Table S2). The proteome data from three biological replicates showed high reproducibility, with strong correlations among the MG (r = 0.68–0.86) and FG (r = 0.81–0.91) proteome profiles (supplemental Table S2A). By using a threshold of 1% protein, 0.2% peptide FDR and at least two unique peptides per protein, we identified a total of 25524 unique peptides, corresponding to 1506 proteins from the sorted stage-V gametocytes. Among them, 1244 and 1387 proteins were from MG and FG, respectively (Table I, supplemental Table S2H and S2I). The MS/MS raw date and peptides information, including peptide sequence, number of unique peptide and spectrum count, percentage sequence coverage and Mascot scores, were deposited in the ProteomeXchange via the PRIDE database (data set identifier PXD003556) for open access.
Table I. Summary of mass spectrometry data of mature male and female gametocytes in P. falciparum.
| MS analysis | Replicate 1 |
Replicate 2 |
Replicate 3 |
Total |
||||
|---|---|---|---|---|---|---|---|---|
| MG | FG | MG | FG | MG | FG | MG | FG | |
| # of total spectra count | 34,058 | 43,636 | 14,960 | 27,691 | 27,185 | 34,461 | 73,397 | 105,415 |
| # of exclusive spectra count | 33,474 | 43039 | 14,748 | 27,453 | 26,834 | 33,994 | 70,334 | 98,819 |
| # of unique spectra count | 17,519 | 22,949 | 7,897 | 16,135 | 12,891 | 18,159 | 22,576 | 30,290 |
| # of unique peptide | 13,959 | 17,364 | 6,726 | 12,166 | 10,033 | 13,343 | 17,240 | 21,805 |
| Unique proteins identified | 1,117 | 1,269 | 807 | 1,020 | 908 | 1,058 | 1,244 | 1,387 |
Compared with earlier proteomic studies in P. falciparum, our combined FG and MG proteome data set showed the strongest correlation (r = 0.71–0.80) with two recently published mature gametocyte proteomes (18, 51) (supplemental Table S3A). The present gametocyte proteome data set showed 1099 proteins shared with earlier data sets and 97 new gametocyte proteins (supplemental Fig. S2A, supplemental Table S3B). Further comparison of the mature gametocyte proteomes with a combination of published asexual-stage proteomes of 2380 proteins led to the classification of 348 proteins as gametocyte specific (supplemental Table S3C), among which 44 were newly found in this study (supplemental Table S3D). These gametocyte-specific proteins contain many known gametocyte proteins such as Pfs25, Pfs230p, Pfs47, ABCG2, dyneins, PfNEK4, and LCCL domain-containing proteins (supplemental Table S3C) (68–71).
Sex-specific Features of Mature Gametocyte Proteomes
Despite that abundance of each protein in MG and FG showed a high level of correlation, the total spectral counts obtained from FG were 1.4 times higher than those from MG (p > 0.05) (Table I, Fig. 2A). This is consistent with the ratio between the amounts of total proteins extracted from the same number of sorted FG and MG (supplemental Table S3E). The MG and FG protein profiles discovered in this study clearly illustrate the sex-specific biology in P. falciparum. Cluster analysis by the GO terms showed that the number of proteins in each of the 13 clusters was not significantly different between FG and MG with the exception of proteins associated with the axonemes and flagella (p < 0.01) (supplemental Fig. S2B, supplemental Table S4). However, the relative abundance of certain clusters clearly differed between the FG and MG proteomes (Fig. 2B, supplemental Table S4). The FG proteome was more abundant in apicoplast (FG 4111: MG 2781), mitochondrial (FG 5921: MG 4111) (p < 0.05), and ribosomal proteins (FG 5170: MG 2405) (p < 0.001) (supplemental Table S4). FG biases were also identified in functional groups of metabolic enzymes, proteases, chaperones, proteins associated energy generation and utility, and proteasome- and redox-associated proteins. Noteworthy, the proteases identified in both sexes include several falcipains and plasmepsins, some of which might be important for the egress of the parasite from the erythrocyte during gametogenesis (72, 73). In contrast, all proteins associated with DNA replication were enriched in the MG proteome (p < 0.001) (supplemental Table S4). Although the majority of proteins associated with axonemes were found in MG only or enriched in MG, the overall protein abundance was even slightly higher in FG because of a particular dynein (PF3D7_0729900), which was highly abundant in the FG proteome (supplemental Table S4).
Fig. 2.
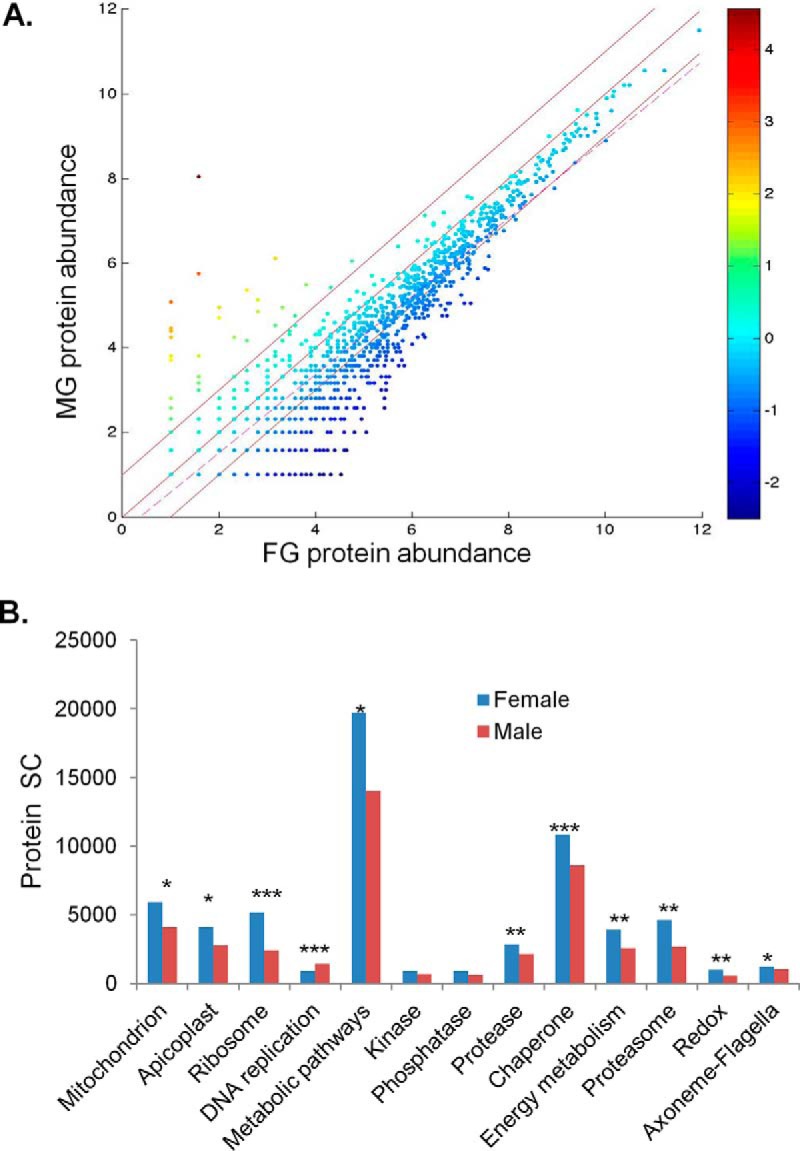
Comparison between the P. falciparum MG and FG proteomes. A, Correlation of protein abundance (log2 transformed spectral counts) between MG and FG. The regression line (dashed line) indicates the degree of similarity in the abundance of individual proteins between MG and FG. Two paralleling lines above and below the diagonal line mark the boundaries of 2 fold difference in protein abundance in MG and FG, respectively. The color scale indicates enrichment in MG (positive values) and FG (negative values), respectively. B, Relative abundance of proteins in each GO category in the MG and FG proteomes. Protein abundance is shown in spectral count (SC). *, **, and *** indicate significant differences between MG and FG at p = 0.05, 0.01, and 0.001, respectively.
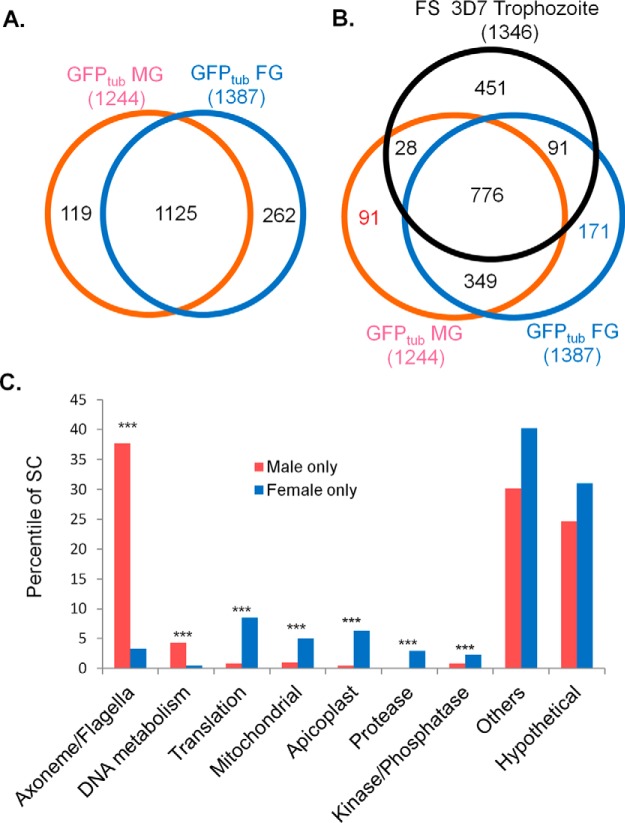
To further dissect the sex dichotomy in protein expression, detailed comparison was performed on MG- and FG-specific proteins (proteins identified only in one sex). Comparison of the MG and FG proteomes identified 119 (10%) and 262 (19%) proteins as MG- and FG-specific, respectively, and 1125 as shared proteins between the two sexes (Fig. 3A, supplemental Table S5A–S5C). After filtering out proteins also expressed in asexual stages by comparing the gametocyte proteomes with the proteome from synchronous trophozoites of the gametocyte-less parasite clone F12 (18), 91 (7.3%) and 171 (12%) proteins were left as gametocyte-specific that are expressed in MG and FG only, and 349 as shared gametocyte-specific proteins between the two sexes (Fig. 3B, supplemental Table S5D–S5F).
Fig. 3.
P. falciparum sex-specific proteomes of mature gametocytes. A, Comparison of the P. falciparum sex-specific proteomes from mature MG (GFPtub MG) and FG (GFPtub FG). B, Comparison of the P. falciparum sex-specific proteomes from mature MG and FG and asexual proteome from synchronous trophozoites of the gametocyte-less clone F12 (FS 3D7 Trophozoite) (Silvestrini et al. 2010, ref. 3). C, Functional differences between MG- and FG-specific proteins in protein abundance shown as percentiles in spectral count (SC). *** indicates significant differences between MG and FG at p = 0.001.
In agreement with the biology of MG in the preparation for rapid DNA replication and formation of motile gametes, the MG-specific proteome (119 proteins) contains significantly more proteins associated with DNA replication and the axonemes (p < 0.001). Specifically, nine proteins are potentially involved in DNA replication, whereas ten proteins are associated with motility. In comparison, the FG-specific proteome (262 proteins) contains significantly more proteases and proteins involved in translation in both rankings of protein numbers and their spectral counts (p < 0.05, Fig. 3C and supplemental Fig. S2C), which are in accordance with higher abundance of ribosomes in FG. Although the numbers of protein kinases and phosphatases were similar between the two sex-specific proteomes, their relative abundance in spectral count was significantly higher in FG (p < 0.001). In addition, significantly more mitochondrial and apicoplast proteins were identified in the FG-specific proteome (p < 0.05) (Fig. 3C, supplemental Table S5A and S5B). Gametocyte-specific, MG- and FG-specific proteins (Fig. 3C) exhibited similar sex-specific features as shown above (supplemental Table S5D and S5E).
We also compared the MG and FG proteomes to obtain a more conserved data set of proteins that showed biased expression in one sex. The relative abundance of individual protein levels was determined by fold change in spectral counts between the two sexes (supplemental Table S5C). We used 2-fold cutoff as it corresponded to ∼96.5 percentile of the MG/FG ratio. Fifteen proteins associated with DNA replication were among the top 40 proteins with > 2-fold higher abundance in MG than in FG. In comparison, 392 proteins showed > 2-fold higher abundance in FG than in MG, which include proteins associated with translation, transcription, proteasome, redox system, and enzymes in the metabolic pathways (supplemental Table S5C). Interestingly, the RNA-binding protein PfPuf1, all components of DOZI complex found in P. berghei, two epigenetic regulators Sir2A and protein arginine methyltransferase 1 (PRMT1) were also enriched in FG (supplemental Table S5C and S5G). Altogether, 159 MG-specific/biased proteins and 654 FG-specific/biased proteins were identified in this study.
Confirmation of Expression of Selected Proteins by GFP Tagging
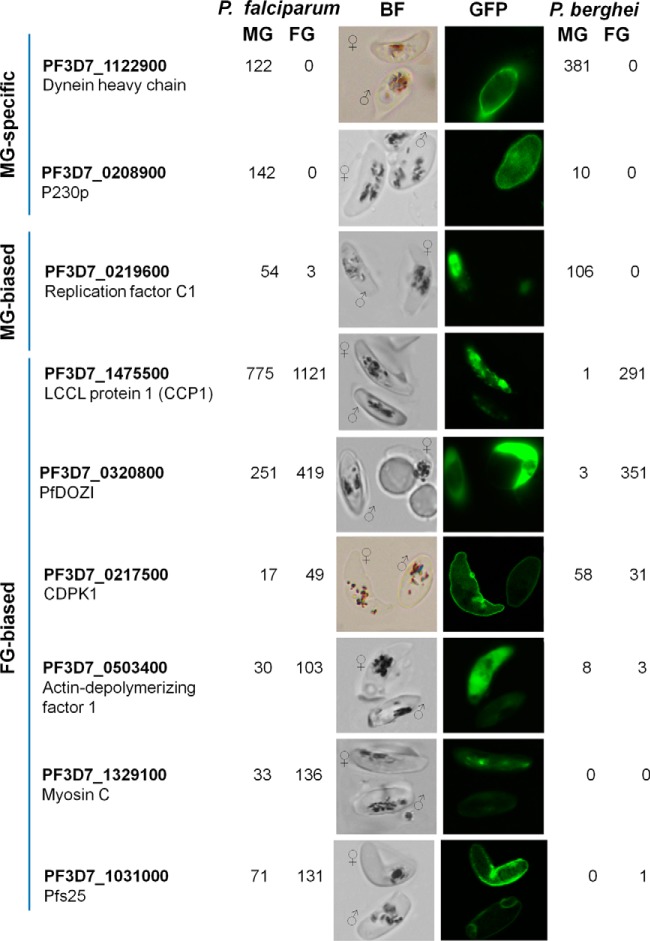
Based on the results of this study and comparison with the P. berghei gametocyte proteomes, we selected three MG-specific/biased and six FG-biased genes based on their spectral counts for experimental validation. The criteria used for the selection include (1) male-specific proteins identified here not yet validated by GFP tagging before, (2) female-biased proteins shown as female-specific proteins in the P. berghei proteome data set, (3) female-biased proteins shown as male-specific proteins in the P. berghei proteome data set, and (4) proteins newly identified only in our data set as compared with the P. berghei data set. Using a single-crossover strategy, we tagged each of the nine selected proteins with GFP at the C terminus (Fig. 4). Stable integration of the plasmids at endogenous loci of the respective genes was confirmed by integration-specific PCR (supplemental Fig. S1, supplemental Table S1). GFP-positive and -negative mature gametocytes or gametocytes with high and low GFP intensities from these tagged parasite lines were sorted in order to identify their sex by Giemsa staining (supplemental Fig. S3). Consistent with the proteomic data, PF3D7_1122900 (dynein heavy chain) and PF3D7_0208900 (P230p) were only detected in MG, whereas PF3D7_0219600 (replication factor C subunit 1) was predominantly expressed in MG (Fig. 4). The expression patterns of these three MG-specific or -biased proteins were consistent with those of their orthologs in P. berghei (49, 51). Among the six FG-biased proteins selected, only DOZI was consistently enriched in the FG proteomes of both parasite species, whereas others showed incompatible expression patterns between the two species (Fig. 4). GFP-tagged PfCCP1 (PF3D7_1475500), a LCCL domain-containing protein, showed 1.5-fold enrichment in FG in P. falciparum (Fig. 4), whereas its ortholog was only detected in the P. berghei FG (49). Recent re-analysis of the P. berghei sex-specific proteomes revealed over 100-fold enrichment of this protein in FG (51). The punctuate GFP foci of PfCCP1-GFP associated with the surface of mature gametocytes were concordant with the pattern previously described by using the anti-PfCCP1 antibodies (74, 75). Similarly, PF3D7_0217500 (calcium-dependent protein kinase 1, CDPK1) and PF3D7_0503400 (actin-depolymerizing factor 1) displayed 2.9- and 3.4-fold enrichment in FG in P. falciparum, respectively (Fig. 4), whereas their P. berghei orthologs were identified as weakly MG-biased in the P. berghei proteomes (49, 51). Myosin C (PF3D7_1329100) showed 4.1-fold enrichment in FG (Fig. 4), but its ortholog was not identified in the P. berghei proteome. Given that the present and earlier proteomic studies detected the major ookinete surface protein Pfs25 in gametocytes (25, 51), we evaluated its expression by GFP-tagging, and confirmed that Pfs25 protein is enriched in FG (Fig. 4), whereas re-analysis of the P. berghei sex-specific proteomes identified one peptide of P25 in FG. Collectively, GFP-tagging of endogenous genes confirmed the accuracy and specificity of our P. falciparum gametocyte-specific proteomes.
Fig. 4.
Experimental evaluation of sex-specific/-biased protein expression of nine selected genes in mature MG and FG. Three MG-specific or biased proteins and six FG-biased proteins were tagged at their C termini with GFP. Numbers are the spectral counts of the respective proteins identified in P. falciparum (left) and P. berghei (right) re-analyzed MG and FG proteomes (Khan et al. 2005, ref. 49; Tao et al., 2014, ref. 51). BF, bright field.
Species-specific Features of Gametocyte Proteomes
A previous study has determined the sex-specific proteomes from P. berghei (49). Re-analysis of the P. berghei sex-specific proteomes using updated genome annotation and an advanced MS search engine has expanded the list of identified proteins from 779 to 1546, of which 1180 and 1128 proteins were from FG and MG, respectively (51). Surprisingly, this re-analysis resulted in the assignments of 366 and 418 proteins as MG- and FG-specific, respectively, with the switching of 100 and 85 originally identified MG- and FG-specific proteins to shared proteins, respectively (51) (Fig. 5A). In addition, 762 (49.3%) were shared proteins between the two sexes in P. berghei as compared with 1125 (74.7%) in P. falciparum. As such, many original sex-specific proteins were changed to sex-enriched. Of the 10 sex-specific proteins originally defined as male-specific (six) and female-specific (four), re-analysis of the P. berghei data classified them as male- and female-enriched, respectively (51). Interestingly, our P. falciparum proteomes revealed that four proteins are in agreement with the re-analyzed P. berghei data as shared proteins and four proteins are consistent with the original assignment as sex-specific in the P. berghei data (supplemental Table S6A). Re-analysis of the P. berghei sex-specific proteomes also drastically changed the assignment of nine sex-specific proteins from one sex to the opposite sex (51). The assignment of seven of these proteins is consistent with that in our P. falciparum proteomes (supplemental Table S6B).
Fig. 5.
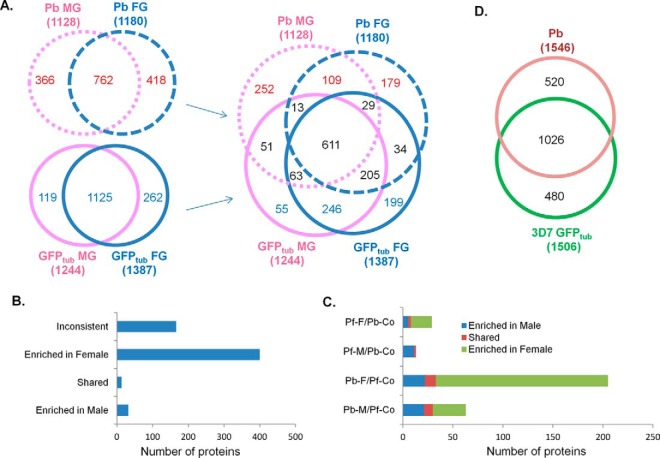
Comparison of MG and FG proteomes between P. falciparum and P. berghei. A, Comparison of the MG and FG proteomes between P. falciparum and P. berghei. B, Partitioning of the 611 proteins present in both species and in both sexes. Proteins showing opposite sex-partitioning in P. falciparum and P. berghei are labeled as “inconsistent”. C, Sex-specific proteins in one species but shared proteins in the other species. Proteins were grouped as MG- and FG-specific in P. falciparum but shared in both sexes of P. berghei (Pf-M/Pb-Co and Pf-F/Pb-Co, respectively), as well as MG- and FG-specific in P. berghei but shared in both sexes of P. falciparum (Pb-M/Pf-Co and Pb-F/Pf-Co, respectively). Different colors indicate relative protein abundance in different sexes of P. berghei (Pb-Co) or P. falciparum (Pf-Co). D, Comparison of gametocyte proteomes between P. falciparum and P. berghei.
To identify the functional similarity as well as evolutionary divergence between the two species in their sex-specific proteomes, we performed a comprehensive comparison of the MG and FG proteomes between P. berghei and P. falciparum (Fig. 5A). MG-specific proteins in P. falciparum (119 proteins) and P. berghei (366 proteins) can be divided into three groups: shared (51 proteins), species-specific (55 in P. falciparum and 252 in P. berghei) and MG-specific in one species but not in the other (13 from P. falciparum and 63 from P. berghei) (Fig. 5A, supplemental Table S7A–S7E). Likewise, FG-specific proteins in P. falciparum (262 proteins) and P. berghei (418 proteins) can be divided into three groups: shared (34 proteins), specie-specific (199 in P. falciparum and 179 in P. berghei), and FG-specific in one species but not in the other (29 from P. falciparum and 205 from P. berghei) (Fig. 5A, supplemental Table S7F–S7J). Comparison of the shared proteins of both sexes between P. falciparum (1125) and P. berghei (762) identified 611 common proteins present in both species and in both sexes, as well as 246 and 109 species-specific proteins shared between the two sexes in P. falciparum and P. berghei, respectively (Fig. 5A, supplemental Table S7K–S7M). Further details of the analysis are as follows.
I. Core Sex-Specific Proteins in Both Species
As shown in Fig. 5A, the two species share only 51 and 34 proteins as MG- and FG-specific proteins. The shared MG-specific proteins contain a number of proteins known as male-specific such as P230p and mitogen-activated protein kinase 2 (MAPK2), and those functioning in motility (six dyneins, six myosins, one kinesin, and one actin) and DNA replication (supplemental Table S7A). The shared FG-specific proteins contain two ribosome-associated proteins, two transcription-associated proteins, three cytoskeleton-associated proteins (two dyneins and one myosin), and six mitochondrion/aplicoplast associated proteins (supplemental Table S7F). The rest of the core sex-specific proteins largely contain hypothetical proteins with unknown function, which may play important roles in sex-specific biology. These shared MG- and FG-specific proteins should represent the core sex-specific proteins in both species.
II. Shared Proteins between the Two Sexes and Also in Both Species
Totally 611 proteins, probably with house-keeping importance, are shared between both sexes and are also common in both species (Fig. 5A, supplemental Table S7K). Using spectral counts as the proxy for protein abundance, we further divided them into three groups as MG-enriched, FG-enriched and equally shared proteins. Of these proteins, 445 (72.8%) show the same sex partitioning in both species (Fig. 5B, supplemental Table S7K), among which 32 (7.2%), 13 (2.9%) and 400 (89.9%) are MG-enriched, equally shared and FG-enriched proteins, respectively. In addition, 21 of the 32 MG-enriched proteins in both species are associated with DNA replication and chromosome regulation, whereas 276 from 400 FG-enriched proteins in both species are associated with translation, transcription, proteasome, redox system, and enzymes in metabolic pathways. The osmiophilic body protein (G377), protein arginine methyltransferase 1 (PRMT1) and 8 of the 11 components of DOZI complex are also in this FG-enriched proteins list (supplemental Table S7K). Such sex-enrichment reflects a similar sex partition in both species.
III. Sex-Specific Proteins Present Only in One Species
13 proteins from P. falciparum and 63 proteins from P. berghei are MG-specific only in one species but are shared proteins in the other (Fig. 5A, supplemental Table S7D and E). Likewise, 29 and 205 proteins are FG-specific only in one species (Fig. 5A, supplemental Table S7I and J). Detailed analysis revealed that 11 of 13 MG-specific proteins in P. falciparum are MG-enriched proteins in P. berghei. Likewise, 21 of 63 MG-specific proteins in P. berghei are MG-enriched proteins in P. falciparum, whereas 21 of 29 FG-specific proteins in P. falciparum are FG-enriched proteins in P. berghei, and 172 of 205 FG-specific proteins in P. berghei are FG-enriched proteins in P. falciparum (Fig. 5C).
IV. Species-specific Proteins
Despite the high degree of conservation in sex-specific proteomes shown above, comparison between the re-analyzed P. berghei gametocyte proteomes of 1546 proteins and the P. falciparum mature gametocyte proteomes of 1506 proteins revealed 480 proteins from P. falciparum and 520 proteins from P. berghei as species-specific proteins (Fig. 5D, supplemental Table S8). Seventy eight from 480 in P. falciparum and 64 from 520 in P. berghei were identified as unique proteins in one species with no syntenic orthologs in the other species (Fig. 5D, supplemental Table S8A and S8B). Many of these unique proteins were exported proteins such as the nine PfGXPs (P. falciparum gametocyte-exported proteins), eight PHIST in P. falciparum, and four BIR proteins and seven Plasmodium exported proteins in P. berghei. Thirty one of 64 P. berghei- and 37 of 78 P. falciparum-unique proteins are encoded by genes located in the subtelomeric regions, consistent with the fact that the majority of the differences between the two species lies in the subtelomeric regions (20). Other unique proteins include Pfs27, Pfs16, Pf11–1in P. falciparum, and intra-erythrocytic stage-induced structure protein 1, tryptophan-rich antigen in P. berghei.
Classification of the above species-specific proteins by sex specificity resulted in different proportions of proteins in each species that are classified as MG- and FG-specific, suggesting that the two species have considerable divergence in the sex-specific proteomes (Fig. 5A). A detailed comparison identified several biological groups representing clear divergence between these two species. A striking difference between the two species lies in the detection of 13 cytoskeletal proteins (myosins and inner membrane complex proteins) in mature P. falciparum gametocytes but not in P. berghei gametocytes, which likely represent the building components of the subpellicular membrane complex responsible for the unique crescent shape of the P. falciparum gametocyte (Table II) (6–9). Other substantial differences in species-specific proteomes between these two species were identified in biological processes such as protein degradation, lipid metabolism and transcriptional regulators (supplemental Table S8D). P. falciparum species-specific proteomes contains a greater number (twelve) of unique proteins associated with ubiquitin-related degradation as compared with three in P. berghei (supplemental Table S8D). Interestingly, the P. falciparum species-specific proteome identified plasmepsin I-III, FP2a and FP3, subtilisin-like protease 2 (SUB2) and dipeptidyl aminopeptidase 2 (DPAP2), whereas the P. berghei species-specific proteomes revealed SUB1 and SERA1–4 (supplemental Table S8D). Previous studies showed that SUB1, SERAs, plasmepsin II, DPAP 3 and FP2 were involved in parasite egress at the schizont stage (73), and exflagellation of activated P. berghei and P. falciparum gametocytes can be blocked by the cysteine/serine protease inhibitors (72). These different proteases in both species suggest that there might be different degradation pathways in mature gametocytes for egress. P. falciparum species-specific proteome contains a significantly greater number (twenty-four) of proteins associated with lipid metabolism than in P. berghei (seven) (supplemental Table S8D). These include PfABCG2, two acyl-CoA synthetases, proteins associated with de novo fatty acid synthesis, and enzymes associated with signaling lipid metabolism, which is in agreement with the finding that in P. falciparum there is a high demand for lipids at the gametocyte stage (43, 44) and a need of de novo fatty acid synthesis for subsequent development in the free-living environment in a mosquito (42). Comparison with the recent P. falciparum sex-specific/biased proteomes largely confirmed these species-specific differences (supplemental Table S8D) (52).
Table II. Unique cytoskeleton-associated proteins in P. falciparum gametocyte proteome.
| Gene ID | Male SCa | Female SC | Protein Name |
|---|---|---|---|
| PF3D7_1342600 | 18 | 14 | myosin A (MyoA) |
| PF3D7_1329100 | 33 | 136 | myosin C (MyoC) |
| PF3D7_1110700 | 25 | 47 | actin-like protein, putative (ALP1) |
| PF3D7_0522600 | 5 | 2 | inner membrane complex protein |
| PF3D7_1323700 | 23 | 59 | glideosome associated protein with multiple membrane spans 1 (GAPM1) |
| PF3D7_1460600 | 2 | 5 | inner membrane complex sub-compartment protein 3, putative (ISP3) |
| PF3D7_1222700 | 49 | 60 | glideosome-associated protein 45 (GAP45) |
| PF3D7_0708000 | 16 | 38 | cytoskeleton associated protein, putative |
| PF3D7_1453100 | 5 | dynactin subunit 4, putative | |
| PF3D7_0319400 | 2 | kinesin-8, putative | |
| PF3D7_0514000 | 10 | tubulin–tyrosine ligase, putative (TTL) | |
| PF3D7_0906910 | 3 | tubulin-specific chaperone, putative | |
| PF3D7_1115900 | 2 | palmitoyltransferase, putative (DHHC9) |
a SC: spectral count.
Although both parasite species contain a similar number of species-specific transcriptional regulators, their expression profiles differ significantly (supplemental Table S8D). Specifically, P. falciparum-specific proteome contains three AP2 proteins in relative higher abundance than the only one AP2 in P. berghei. Of the six LCCL domain-containing proteins, three (PbCCp2, PbCCp4 and PbFNPA) were not identified in P. berghei, whereas all PfCCps except PfCCp4 were identified in P. falciparum (supplemental Table S8D). This is in agreement with previous studies, which showed translational repression of PbCCps in P. berghei but not in P. falciaprum (76, 77). Taken together, these results highlighted considerable differences in the gametocyte proteomes between these two parasite species.
An earlier effort, based on subtraction between the proteomes of normal and male-defective P. falciparum gametocytes, and P. berghei sex-specific proteomes under the assumption of a high-degree conservation between the P. falciparum and P. berghei sex-specific proteomes, predicted 258 FG- and 174 MG-specific proteins in P. falciparum gametocytes (supplemental Fig. S4A and S4B) (51). However, of these proteins, only 21 and 38 are found in the 262 FG- and 119 MG-specific proteins in P. falciparum, respectively (supplemental Fig. S4A and S4B, supplemental Table S9A and S9B). Interestingly, these common proteins are all found in the 51 and 34 shared sex-specific proteins between P. berghei and P. falciparum (Fig. 5A), indicating that the earlier deduction-based approach only captured the conserved, putative core sex-specific proteins in the malaria parasites. However, 189 FG- and 56 MG-specific proteins deduced in the earlier approach are found in the shared proteins between MG and FG proteomes in our analysis (supplemental Fig. S4C). Additionally, 41 FG- and 39 MG-specific proteins from this earlier prediction are in different sex partitions in our proteomes, whereas 148 FG- and 17 MG-specific proteins are in the same sex-specific partitions (supplemental Fig. S4D, supplemental Table S9A and S9B).
Sex-specific/biased Proteins in Plasmodium - Lasonder et al
recently separated mature MG and FG based on marker expression under the male-specific protein dynein heavy chain (PF3D7_1023100) and the female-specific protein Pfs47 and identified 1960 proteins (at least two unique peptides per protein) (52). This combined FG and MG proteome data set showed a strong correlation (r = 0.77) with our combined proteome. The FG proteome showed even stronger correlation with our FG proteome (r = 0.81), however, correlation was relatively low between these two MG proteomes (r = 0.56). This protein data set and our present MG and FG data set shared 1338 proteins (supplemental Fig. S5A). Together with the re-analyzed P. berghei gametocyte proteomes (51), the three data sets shared 986 proteins. Here we used these three gametocyte proteome data sets based on sorted MG and FG to derive high-confidence lists of sex-specific/biased proteins. Among these shared proteins, our data set and the P. berghei data set had 99 and 219 proteins showing ≥ 2-fold higher expression in MG, and 321 and 577 proteins showing ≥ 2-fold higher expression in FG. Using proteins showing biased expression in the three data sets, we obtained a high-confidence list of 73 MG-specific/biased and 89 FG-specific/biased proteins, which represent the core MG- and FG-specific/biased proteins in Plasmodium (supplemental Fig. S5B and C, supplemental Table S9C and S9D).
These core MG- and FG-biased protein lists clearly show the sex-specific biology of Plasmodium. In the 73 core MG-specific/biased proteome, 33 and 26% of proteins are involved in DNA replication and motility/cytoskeleton, respectively, whereas in the 89 core FG-specific/biased proteome, there is a significant enrichment of proteins associated with translation, proteasome, mitochondrion/apicoplast, transport and redox (supplemental Fig. S5D and S5E).
DISCUSSION
Malaria parasites with the same set of haploid genome can differentiate into two drastically different sexual forms that are needed for continued transmission through mosquitoes. A thorough understanding of the differences between the two sexes in the human malaria parasite P. falciparum is hindered by the difficulties in accurately separating the two sexes and thus relied heavily on the only known sex-specific proteomes of P. berghei gametocytes. Here, by using a parasite line 3D7α−tubII/GFP that produces two distinct populations of gametocytes differing in GFP fluorescence intensity, we were able to clearly separate P. falciparum mature MG and FG by flow cytometry. With three highly reproducible replicates of ∼3.5 × 106 sorted MG and FG each and using highly stringent protein identification criteria after LC-MS/MS analysis, we identified 1506 proteins from 178,812 spectra, which account for ∼28% of the predicted proteins encoded by the parasite genome and constitute the first comprehensive sex-specific proteomes of accurately separated mature gametocytes in P. falciparum. This sex-specific P. falciparum gametocyte proteome, with about 2-fold higher total spectral counts and unique peptides than the re-analyzed P. berghei sex-specific proteomes (51), provides much increased depth and accuracy of protein identification and a better understanding of the sex-specific biology in this parasite. Whereas this analysis identified significant differences between the two sexes that are consistent with their functional divisions, it also identified substantial sharing of proteins in both sexes with 1125 proteins (74.7% of all P. falciparum gametocyte proteins) being identified in the 1244 MG proteome and the 1387 FG proteome. By excluding proteins that are also expressed in asexual stages, 91, 171, and 349 gametocyte proteins are MG-specific, FG-specific and shared.
It is intriguing that we detected proteins such as liver-specific protein 2, merozoite surface protein 1, and cytoadherence linked asexual protein 8 in our sex-specific proteomes, which are unlikely expressed in gametocytes. Similarly, such type of proteins were also identified in almost all earlier gametocyte proteome work (14, 15, 18), including the recent P. falciparum sex-specific proteome identified by Lasonder et al. (52). Whereas these proteins might be resulted from contaminating asexual stages, it should be rare or impossible in the present and recent studies (52) because both studies used sorted MG and FG that were cultured for an extended period and were supposedly devoid of asexual stages. Thus, future work is needed to verify whether these proteins are also expressed at very low levels in gametocytes.
Like in P. berghei, the sex-specific proteomes are consistent with the major morphological distinctions of MG and FG in the malaria parasites, highlighting conceivable conservation among different parasite species. As MG are prepared for three rounds of rapid DNA replication to produce eight motile flagella-like gametes during gametogenesis, the MG proteome is specially enriched in proteins involved in these processes. In fact, 9/119 MG-specific proteins are involved DNA replication, whereas 10/119 are predicted to be associated with flagella formation. Intriguingly, a specific dynein (PF3D7_0729900) was extremely enriched in the FG-specific proteome. Because FG have been shown to also produce long nanotubes during gametogenesis (78), it would be interesting to determine whether this dynein is involved in the formation of the nanotubes or in other transport processes. Ultrastructurally, FG are highly enriched in ribosomes, indicating that FG are prepared for subsequent protein synthesis. Consistently, FG are enriched in proteins involved in translation, metabolic pathways, energy generation and utility, protein degradation and redox system. In addition, the distributions of proteins in the two organelles mitochondrion and apicoplast are also FG-biased. A similar sex-partition was found in P. berghei for mitochondrion only, whereas apicoplast-associated proteins are equally present in both sexes (49). The facts that apicoplast and mitochondrion are closely associated with critical functions in gametocytes and apicoplast is lost only during microgametogenesis but not macrogametogenesis indicate that the apicoplast presumably plays a more pivotal role in FG (42, 79).
The original study of the P. berghei gametocyte proteomes identified very limited sharing of proteins between the two sexes (49), whereas reanalysis of the spectral data increased the number of shared proteins from 371 to 762, making up 67% of the 1128 MG proteome and 64% of the 1180 FG proteome, respectively (51). This reanalysis changed 185 originally defined MG- or FG-specific proteins to the shared category. The present study, with dramatically increased depth of protein discovery, revealed more extensive sharing of proteins between the MG (91%) and FG (80%) proteomes. We speculate that many proteins that are very differentially expressed in the two sexes were identified previously in one sex only, and with enhanced sensitivity of protein identification, they were identified in both sexes albeit with a strong sex bias. As such, a large number of FG-biased proteins function in translation and a variety of metabolic pathways such as TCA, glycolysis, protein degradation, energy and redox processes. In contrast, a number of MG-enriched proteins function in motility and DNA replication. Such sex-biased proteomes were further corroborated by the recent analysis of sorted P. falciparum MG and FG proteomes (52). Furthermore, we confirmed several of these new predictions of sex partitioning by tagging the endogenous genes with GFP. Nevertheless, several sex-specific proteins have been identified and confirmed in this study, which may serve as markers for quantification of MG or FG in samples. In addition, comparison of the three sex-specific proteome studies led to the identification of 73 core MG-specific/biased and 89 core FG-specific/biased proteins, which may play conserved roles in sex-specific biology in all Plasmodium species. Among them, new candidates can be identified for future functional studies of sexual differentiation in malaria parasites.
This study identified a Puf-family RNA-binding protein PfPuf1 and all 11 components of the DOZI complex (27) as FG-enriched proteins. In the P. berghei gametocyte proteome, PbPuf1 was a FG-specific protein and 9 of DOZI complex as FG-enriched (8 proteins) or FG-specific (1 protein), suggesting that RNA-binding proteins may play conserved roles in translational regulation between these parasite species.
The comparison of sex-specific proteomes on P. falciparum mature gametocytes between this study and the recent study by Lasonder et al. revealed a core MG- and FG-specific/biased proteomes shared by the two data sets, although some discrepancies existed between the two studies, possibly because of different markers used to separate the two sexes and study methods (52). The predicted P. falciparum sex-specific proteomes based on a subtraction method (49), while differing vastly from those in the present study, contain such core sex-specific proteomes. Further comparison with the sex-specific proteomes of gametocytes between P. berghei and P. falciparum reveals core sex-specific/biased proteomes that are likely conserved among most malaria species. In addition to the overall conservation in sex-specific proteomes between the two species, the present study also identified considerable divergence between the two species, which could underpin their differences in gametocyte biology (36, 48, 50). Whereas MG-specific proteins such as those involved in motility and genome replication as well as FG-specific proteins in translation, mitochondrion and many metabolic pathways are agreeable between the two species (49, 51), species-specific proteomes that diverged substantially are involved in cytoskeleton, lipid metabolism and signal transduction, protein degradation, DNA replication and transcription. More cytoskeleton proteins discovered in this study is in concordance with the presence of the subpellicular membrane structure needed for the transformation of P. falciparum gametocytes to a crescent shape (6–9). More proteins associated lipid metabolism is in agreement with a significant increase and compositional change of lipids in gametocytes (43, 44). Of them, three signal lipid-associated proteins (phosphoethanolamine methyltransferase, cytidine diphosphate-diacylglycerol synthase and calponin homology domain-containing protein) have been studied, showing that the disruption of these genes resulted in the arrest of gametocyte development (37, 80). The mechanisms underlining these biological disparities between these Plasmodium species remain to be further elucidated.
Supplementary Material
Footnotes
Author contributions: J.M. and L.C. designed, executed, analyzed experiments, and wrote the paper. Z.C., Z.W. and R.L. provided statistical support. X.L. and S.S. helped with parasite culture.
* This study was supported by funding from the National Institute of Allergy and Infectious Diseases, NIH (R21AI098058, R01AI104946 and U19AI089672). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
 This article contains supplemental material.
This article contains supplemental material.
All authors declare no conflicts of interests.
1 The abbreviations used are:
- RBC
- red blood cell
- MG
- Male gametocytes
- FG
- Female gametocytes
- GFP
- Green fluorescent protein
- FDR
- False discovery rate
- HPLC
- High-performance liquid chromatography
- LC-MS/MS
- Liquid chromatography coupled with tandem mass spectrometry
- SDS-PAGE
- Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- PCR
- Polymerase chain reaction.
REFERENCES
- 1. Alonso P. L., Brown G., Arevalo-Herrera M., Binka F., Chitnis C., Collins F., Doumbo O. K., Greenwood B., Hall B. F., Levine M. M., Mendis K., Newman R. D., Plowe C. V., Rodriguez M. H., Sinden R., Slutsker L., and Tanner M. (2011) A research agenda to underpin malaria eradication. PLoS Med. 8, e1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bruce M. C., Alano P., Duthie S., and Carter R. (1990) Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology 100, 191–200 [DOI] [PubMed] [Google Scholar]
- 3. Silvestrini F., Alano P., and Williams J. L. (2000) Commitment to the production of male and female gametocytes in the human malaria parasite Plasmodium falciparum. Parasitology 121, 465–471 [DOI] [PubMed] [Google Scholar]
- 4. Carter R., and Graves P. M. (1988) Gametocytes. In: Wernsdorfer W. H., and Sir McGregor I., eds. Malaria: Principles and Practice of Malariology, pp. 253–305, Churchill Livingstone, London [Google Scholar]
- 5. Sinden R. E. (1998) Gametocytes and sexual development. In: Sherman I. W., ed. Malaria: Parasite Biology, Pathogenesis and Protection, pp. 25–48, ASM Press, Washington, DC [Google Scholar]
- 6. Dixon M. W., Dearnley M. K., Hanssen E., Gilberger T., and Tilley L. (2012) Shape-shifting gametocytes: how and why does P. falciparum go banana-shaped? Trends Parasitol. 28, 471–478 [DOI] [PubMed] [Google Scholar]
- 7. Dearnley M. K., Yeoman J. A., Hanssen E., Kenny S., Turnbull L., Whitchurch C. B., Tilley L., and Dixon M. W. (2011) Origin, composition, organization and function of the inner membrane complex of Plasmodium falciparum gametocytes. J. Cell Sci. 125, 2053–2063 [DOI] [PubMed] [Google Scholar]
- 8. Kono M., Herrmann S., Loughran N. B., Cabrera A., Engelberg K., Lehmann C., Sinha D., Prinz B., Ruch U., Heussler V., Spielmann T., Parkinson J., and Gilberger T. W. (2012) Evolution and architecture of the inner membrane complex in asexual and sexual stages of the malaria parasite. Mol. Biol. Evol. 29, 2113–2132 [DOI] [PubMed] [Google Scholar]
- 9. Hliscs M., Millet C., Dixon M. W., Siden-Kiamos I., McMillan P., and Tilley L. (2015) Organization and function of an actin cytoskeleton in Plasmodium falciparum gametocytes. Cell Microbiol. 17, 207–225 [DOI] [PubMed] [Google Scholar]
- 10. Farfour E., Charlotte F., Settegrana C., Miyara M., and Buffet P. (2012) The extravascular compartment of the bone marrow: a niche for Plasmodium falciparum gametocyte maturation? Malar. J. 11, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joice R., Nilsson S. K., Montgomery J., Dankwa S., Egan E., Morahan B., Seydel K. B., Bertuccini L., Alano P., Williamson K. C., Duraisingh M. T., Taylor T. E., Milner D. A., and Marti M. (2014) Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci. Transl. Med. 6, 244re245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le Roch K. G., Zhou Y., Blair P. L., Grainger M., Moch J. K., Haynes J. D., De La Vega P., Holder A. A., Batalov S., Carucci D. J., and Winzeler E. A. (2003) Discovery of gene function by expression profiling of the malaria parasite life cycle. Science 301, 1503–1508 [DOI] [PubMed] [Google Scholar]
- 13. Le Roch K. G., Johnson J. R., Florens L., Zhou Y., Santrosyan A., Grainger M., Yan S. F., Williamson K. C., Holder A. A., Carucci D. J., Yates J. R. 3rd, Winzeler E. A. (2004) Global analysis of transcript and protein levels across the Plasmodium falciparum life cycle. Genome Res. 14, 2308–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lasonder E., Ishihama Y., Andersen J. S., Vermunt A. M., Pain A., Sauerwein R. W., Eling W. M., Hall N., Waters A. P., Stunnenberg H. G., and Mann M. (2002) Analysis of the Plasmodium falciparum proteome by high-accuracy mass spectrometry. Nature 419, 537–542 [DOI] [PubMed] [Google Scholar]
- 15. Florens L., Washburn M. P., Raine J. D., Anthony R. M., Grainger M., Haynes J. D., Moch J. K., Muster N., Sacci J. B., Tabb D. L., Witney A. A., Wolters D., Wu Y., Gardner M. J., Holder A. A., Sinden R. E., Yates J. R., and Carucci D. J. (2002) A proteomic view of the Plasmodium falciparum life cycle. Nature 419, 520–526 [DOI] [PubMed] [Google Scholar]
- 16. Young J. A., Fivelman Q. L., Blair P. L., de la Vega P., Le Roch K. G., Zhou Y., Carucci D. J., Baker D. A., and Winzeler E. A. (2005) The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol. Biochem. Parasitol. 143, 67–79 [DOI] [PubMed] [Google Scholar]
- 17. Lopez-Barragan M. J., Lemieux J., Quinones M., Williamson K. C., Molina-Cruz A., Cui K., Barillas-Mury C., Zhao K., and Su X. Z. (2011) Directional gene expression and antisense transcripts in sexual and asexual stages of Plasmodium falciparum. BMC Genomics 12, 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silvestrini F., Lasonder E., Olivieri A., Camarda G., van Schaijk B., Sanchez M., Younis Younis S., Sauerwein R., and Alano P. (2010) Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol. Cell Proteomics 9, 1437–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gardner M. J., Hall N., Fung E., White O., Berriman M., Hyman R. W., Carlton J. M., Pain A., Nelson K. E., Bowman S., Paulsen I. T., James K., Eisen J. A., Rutherford K., Salzberg S. L., Craig A., Kyes S., Chan M. S., Nene V., Shallom S. J., Suh B., Peterson J., Angiuoli S., Pertea M., Allen J., Selengut J., Haft D., Mather M. W., Vaidya A. B., Martin D. M., Fairlamb A. H., Fraunholz M. J., Roos D. S., Ralph S. A., McFadden G. I., Cummings L. M., Subramanian G. M., Mungall C., Venter J. C., Carucci D. J., Hoffman S. L., Newbold C., Davis R. W., Fraser C. M., and Barrell B. (2002) Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419, 498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hall N., Karras M., Raine J. D., Carlton J. M., Kooij T. W., Berriman M., Florens L., Janssen C. S., Pain A., Christophides G. K., James K., Rutherford K., Harris B., Harris D., Churcher C., Quail M. A., Ormond D., Doggett J., Trueman H. E., Mendoza J., Bidwell S. L., Rajandream M. A., Carucci D. J., Yates JR 3rd, Kafatos F. C., Janse C. J., Barrell B., Turner C. M., Waters A. P., and Sinden R. E. (2005) A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science 307, 82–86 [DOI] [PubMed] [Google Scholar]
- 21. Coleman B. I., Skillman K. M., Jiang R. H., Childs L. M., Altenhofen L. M., Ganter M., Leung Y., Goldowitz I., Kafsack B. F., Marti M., Llinas M., Buckee C. O., and Duraisingh M. T. (2014) A Plasmodium falciparum histone deacetylase regulates antigenic variation and gametocyte conversion. Cell Host Microbe 16, 177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flueck C., Bartfai R., Volz J., Niederwieser I., Salcedo-Amaya A. M., Alako B. T., Ehlgen F., Ralph S. A., Cowman A. F., Bozdech Z., Stunnenberg H. G., and Voss T. S. (2009) Plasmodium falciparum heterochromatin protein 1 marks genomic loci linked to phenotypic variation of exported virulence factors. PLoS Pathog. 5, e1000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brancucci N. M., Bertschi N. L., Zhu L., Niederwieser I., Chin W. H., Wampfler R., Freymond C., Rottmann M., Felger I., Bozdech Z., and Voss T. S. (2014) Heterochromatin protein 1 secures survival and transmission of malaria parasites. Cell Host Microbe 16, 165–176 [DOI] [PubMed] [Google Scholar]
- 24. Pelle K. G., Oh K., Buchholz K., Narasimhan V., Joice R., Milner D. A., Brancucci N. M., Ma S., Voss T. S., Ketman K., Seydel K. B., Taylor T. E., Barteneva N. S., Huttenhower C., and Marti M. (2015) Transcriptional profiling defines dynamics of parasite tissue sequestration during malaria infection. Genome Med. 7, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silvestrini F., Bozdech Z., Lanfrancotti A., Di Giulio E., Bultrini E., Picci L., Derisi J. L., Pizzi E., and Alano P. (2005) Genome-wide identification of genes upregulated at the onset of gametocytogenesis in Plasmodium falciparum. Mol. Biochem. Parasitol. 143, 100–110 [DOI] [PubMed] [Google Scholar]
- 26. Yuda M., Iwanaga S., Kaneko I., and Kato T. (2015) Global transcriptional repression: An initial and essential step for Plasmodium sexual development. Proc. Natl. Acad. Sci. U.S.A. 112, 12824–12829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cui L., Lindner S., and Miao J. (2015) Translational regulation during stage transitions in malaria parasites. Ann. N.Y. Acad. Sci. 1342, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miao J., Fan Q., Parker D., Li X., Li J., and Cui L. (2013) Puf mediates translation repression of transmission-blocking vaccine candidates in malaria parasites. PLoS Pathog. 9, e1003268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miao J., Li J., Fan Q., Li X., and Cui L. (2010) The Puf-family RNA-binding protein PfPuf2 regulates sexual development and sex differentiation in the malaria parasite Plasmodium falciparum. J. Cell Sci. 123, 1039–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muller K., Matuschewski K., and Silvie O. (2011) The Puf-family RNA-binding protein Puf2 controls sporozoite conversion to liver stages in the malaria parasite. PLoS ONE 6, e19860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gomes-Santos C. S., Braks J., Prudencio M., Carret C., Gomes A. R., Pain A., Feltwell T., Khan S., Waters A., Janse C., Mair G. R., and Mota M. M. (2011) Transition of Plasmodium sporozoites into liver stage-like forms is regulated by the RNA binding protein Pumilio. PLoS Pathog. 7, e1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sebastian S., Brochet M., Collins M. O., Schwach F., Jones M. L., Goulding D., Rayner J. C., Choudhary J. S., and Billker O. (2012) A Plasmodium calcium-dependent protein kinase controls zygote development and transmission by translationally activating repressed mRNAs. Cell Host Microbe 12, 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hopp C. S., Bowyer P. W., and Baker D. A. (2012) The role of cGMP signalling in regulating life cycle progression of Plasmodium. Microbes Infect. 14, 831–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morahan B., and Garcia-Bustos J. (2014) Kinase signalling in Plasmodium sexual stages and interventions to stop malaria transmission. Mol. Biochem. Parasitol. 193, 23–32 [DOI] [PubMed] [Google Scholar]
- 35. Tewari R., Straschil U., Bateman A., Bohme U., Cherevach I., Gong P., Pain A., and Billker O. (2010) The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe 8, 377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sinden R. E. (2015) The cell biology of malaria infection of mosquito: advances and opportunities. Cell Microbiol. 17, 451–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bobenchik A. M., Witola W. H., Augagneur Y., Nic Lochlainn L., Garg A., Pachikara N., Choi J. Y., Zhao Y. O., Usmani-Brown S., Lee A., Adjalley S. H., Samanta S., Fidock D. A., Voelker D. R., Fikrig E., and Ben Mamoun C. (2013) Plasmodium falciparum phosphoethanolamine methyltransferase is essential for malaria transmission. Proc. Natl. Acad. Sci. U.S.A. 110, 18262–18267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McNamara C. W., Lee M. C., Lim C. S., Lim S. H., Roland J., Nagle A., Simon O., Yeung B. K., Chatterjee A. K., McCormack S. L., Manary M. J., Zeeman A. M., Dechering K. J., Kumar T. R., Henrich P. P., Gagaring K., Ibanez M., Kato N., Kuhen K. L., Fischli C., Rottmann M., Plouffe D. M., Bursulaya B., Meister S., Rameh L., Trappe J., Haasen D., Timmerman M., Sauerwein R. W., Suwanarusk R., Russell B., Renia L., Nosten F., Tully D. C., Kocken C. H., Glynne R. J., Bodenreider C., Fidock D. A., Diagana T. T., and Winzeler E. A. (2013) Targeting Plasmodium PI(4)K to eliminate malaria. Nature 504, 248–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ke H., Lewis I. A., Morrisey J. M., McLean K. J., Ganesan S. M., Painter H. J., Mather M. W., Jacobs-Lorena M., Llinas M., and Vaidya A. B. (2015) Genetic investigation of tricarboxylic acid metabolism during the Plasmodium falciparum life cycle. Cell Rep. 11, 164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. MacRae J. I., Dixon M. W., Dearnley M. K., Chua H. H., Chambers J. M., Kenny S., Bottova I., Tilley L., and McConville M. J. (2013) Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol. 11, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lamour S. D., Straschil U., Saric J., and Delves M. J. (2014) Changes in metabolic phenotypes of Plasmodium falciparum in vitro cultures during gametocyte development. Malar. J. 13, 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Schaijk B. C., Kumar T. R., Vos M. W., Richman A., van Gemert G. J., Li T., Eappen A. G., Williamson K. C., Morahan B. J., Fishbaugher M., Kennedy M., Camargo N., Khan S. M., Janse C. J., Sim K. L., Hoffman S. L., Kappe S. H., Sauerwein R. W., Fidock D. A., and Vaughan A. M. (2014) Type II fatty acid biosynthesis is essential for Plasmodium falciparum sporozoite development in the midgut of Anopheles mosquitoes. Eukaryot. Cell 13, 550–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tran P. N., Brown S. H., Rug M., Ridgway M. C., Mitchell T. W., and Maier A. G. (2016) Changes in lipid composition during sexual development of the malaria parasite Plasmodium falciparum. Malar. J. 15, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gulati S., Ekland E. H., Ruggles K. V., Chan R. B., Jayabalasingham B., Zhou B., Mantel P. Y., Lee M. C., Spottiswoode N., Coburn-Flynn O., Hjelmqvist D., Worgall T. S., Marti M., Di Paolo G., and Fidock D. A. (2015) Profiling the Essential Nature of Lipid Metabolism in Asexual Blood and Gametocyte Stages of Plasmodium falciparum. Cell Host Microbe 18, 371–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith T. G., Lourenco P., Carter R., Walliker D., and Ranford-Cartwright L. C. (2000) Commitment to sexual differentiation in the human malaria parasite, Plasmodium falciparum. Parasitology 121, 127–133 [DOI] [PubMed] [Google Scholar]
- 46. Dixon M. W., Thompson J., Gardiner D. L., and Trenholme K. R. (2008) Sex in Plasmodium: a sign of commitment. Trends Parasitol. 24, 168–175 [DOI] [PubMed] [Google Scholar]
- 47. Alano P. (2007) Plasmodium falciparum gametocytes: still many secrets of a hidden life. Mol. Microbiol. 66, 291–302 [DOI] [PubMed] [Google Scholar]
- 48. Janse C., and Waters A. P. (2004) Sexual development of malaria Parasites In: Waters AP, Janse CJ, editors. Malaria parasites: genomes and molecular biology. The Netherlands Caister Academic Press; pp. 445–474 [Google Scholar]
- 49. Khan S. M., Franke-Fayard B., Mair G. R., Lasonder E., Janse C. J., Mann M., and Waters A. P. (2005) Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell 121, 675–687 [DOI] [PubMed] [Google Scholar]
- 50. Sinden R. E., Carter R., Drakeley C., and Leroy D. (2012) The biology of sexual development of Plasmodium: the design and implementation of transmission-blocking strategies. Malar. J. 11, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tao D., Ubaida-Mohien C., Mathias D. K., King J. G., Pastrana-Mena R., Tripathi A., Goldowitz I., Graham D. R., Moss E., Marti M., and Dinglasan R. R. (2014) Sex-partitioning of the Plasmodium falciparum stage V gametocyte proteome provides insight into falciparum-specific cell biology. Mol. Cell Proteomics 13, 2705–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lasonder E., Rijpma S. R., van Schaijk B. C., Hoeijmakers W. A., Kensche P. R., Gresnigt M. S., Italiaander A., Vos M. W., Woestenenk R., Bousema T., Mair G. R., Khan S. M., Janse C. J., Bartfai R., and Sauerwein R. W. (2016) Integrated transcriptomic and proteomic analyses of P. falciparum gametocytes: molecular insight into sex-specific processes and translational repression. Nucleic Acids Res. 44, 6087–6101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Trager W., and Jensen J. B. (1976) Human malaria parasites in continuous culture. Science 193, 673–675 [DOI] [PubMed] [Google Scholar]
- 54. Ponnudurai T., Meuwissen J. H., Leeuwenberg A. D., Verhave J. P., and Lensen A. H. (1982) The production of mature gametocytes of Plasmodium falciparum in continuous cultures of different isolates infective to mosquitoes. Trans. R Soc. Trop. Med. Hyg. 76, 242–250 [DOI] [PubMed] [Google Scholar]
- 55. Wang Z., Liu M., Liang X., Siriwat S., Li X., Chen X., Parker D. M., Miao J., and Cui L. (2014) A flow cytometry-based quantitative drug sensitivity assay for all Plasmodium falciparum gametocyte stages. PLoS ONE 9, e93825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fivelman Q. L., McRobert L., Sharp S., Taylor C. J., Saeed M., Swales C. A., Sutherland C. J., and Baker D. A. (2007) Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol. Biochem. Parasitol. 154, 119–123 [DOI] [PubMed] [Google Scholar]
- 57. Miao J., Wang Z., Liu M., Parker D., Li X., Chen X., and Cui L. (2013) Plasmodium falciparum: generation of pure gametocyte culture by heparin treatment. Exp. Parasitol. 135, 541–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ogwan'g R., Mwangi J., Gachihi G., Nwachukwu A., Roberts C. R., and Martin S. K. (1993) Use of pharmacological agents to implicate a role for phosphoinositide hydrolysis products in malaria gamete formation. Biochem. Pharmacol. 46, 1601–1606 [DOI] [PubMed] [Google Scholar]
- 59. Talman A. M., Paul R. E., Sokhna C. S., Domarle O., Ariey F., Trape J. F., and Robert V. (2004) Influence of chemotherapy on the Plasmodium gametocyte sex ratio of mice and humans. Am. J. Trop. Med. Hyg. 71, 739–744 [PubMed] [Google Scholar]
- 60. Liebler D. C., and Ham A. J. (2009) Spin filter-based sample preparation for shotgun proteomics. Nat. Methods 6, 785; author reply 785–786 [DOI] [PubMed] [Google Scholar]
- 61. Pavelka N., Fournier M. L., Swanson S. K., Pelizzola M., Ricciardi-Castagnoli P., Florens L., and Washburn M. P. (2008) Statistical similarities between transcriptomics and quantitative shotgun proteomics data. Mol. Cell Proteomics 7, 631–644 [DOI] [PubMed] [Google Scholar]
- 62. Lundgren D. H., Hwang S. I., Wu L., and Han D. K. (2010) Role of spectral counting in quantitative proteomics. Expert Rev. Proteomics 7, 39–53 [DOI] [PubMed] [Google Scholar]
- 63. Zybailov B. L., Florens L., and Washburn M. P. (2007) Quantitative shotgun proteomics using a protease with broad specificity and normalized spectral abundance factors. Mol. Biosyst. 3, 354–360 [DOI] [PubMed] [Google Scholar]
- 64. Fidock D. A., and Wellems T. E. (1997) Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc. Natl. Acad. Sci. U.S.A. 94, 10931–10936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Miao J., Fan Q., Cui L., Li X., Wang H., Ning G., Reese J. C., and Cui L. (2010) The MYST family histone acetyltransferase regulates gene expression and cell cycle in malaria parasite Plasmodium falciparum. Mol. Microbiol. 78, 883–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Anders S., and Huber W. (2010) Differential expression analysis for sequence count data. Genome Biol. 11, R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schwank S., Sutherland C. J., and Drakeley C. J. (2010) Promiscuous expression of alpha-tubulin II in maturing male and female Plasmodium falciparum gametocytes. PLoS ONE 5, e14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. van Schaijk B. C., van Dijk M. R., van de Vegte-Bolmer M., van Gemert G. J., van Dooren M. W., Eksi S., Roeffen W. F., Janse C. J., Waters A. P., and Sauerwein R. W. (2006) Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Mol. Biochem. Parasitol. 149, 216–222 [DOI] [PubMed] [Google Scholar]
- 69. Eksi S., and Williamson K. C. (2002) Male-specific expression of the paralog of malaria transmission-blocking target antigen Pfs230, PfB0400w. Mol. Biochem. Parasitol. 122, 127–130 [DOI] [PubMed] [Google Scholar]
- 70. Tran P. N., Brown S. H., Mitchell T. W., Matuschewski K., McMillan P. J., Kirk K., Dixon M. W., and Maier A. G. (2014) A female gametocyte-specific ABC transporter plays a role in lipid metabolism in the malaria parasite. Nat. Commun. 5, 4773. [DOI] [PubMed] [Google Scholar]
- 71. Reininger L., Garcia M., Tomlins A., Muller S., and Doerig C. (2012) The Plasmodium falciparum, Nima-related kinase Pfnek-4: a marker for asexual parasites committed to sexual differentiation. Malar. J. 11, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sologub L., Kuehn A., Kern S., Przyborski J., Schillig R., and Pradel G. (2011) Malaria proteases mediate inside-out egress of gametocytes from red blood cells following parasite transmission to the mosquito. Cell Microbiol. 13, 897–912 [DOI] [PubMed] [Google Scholar]
- 73. Wirth C. C., and Pradel G. (2012) Molecular mechanisms of host cell egress by malaria parasites. Int. J. Med. Microbiol. 302, 172–178 [DOI] [PubMed] [Google Scholar]
- 74. Pradel G., Hayton K., Aravind L., Iyer L. M., Abrahamsen M. S., Bonawitz A., Mejia C., and Templeton T. J. (2004) A multidomain adhesion protein family expressed in Plasmodium falciparum is essential for transmission to the mosquito. J. Exp. Med. 199, 1533–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Scholz S. M., Simon N., Lavazec C., Dude M. A., Templeton T. J., and Pradel G. (2008) PfCCp proteins of Plasmodium falciparum: gametocyte-specific expression and role in complement-mediated inhibition of exflagellation. Int. J. Parasitol. 38, 327–340 [DOI] [PubMed] [Google Scholar]
- 76. Saeed S., Carter V., Tremp A. Z., and Dessens J. T. (2013) Translational repression controls temporal expression of the Plasmodium berghei LCCL protein complex. Mol. Biochem. Parasitol. 189, 38–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Simon N., Scholz S. M., Moreira C. K., Templeton T. J., Kuehn A., Dude M. A., and Pradel G. (2009) Sexual stage adhesion proteins form multi-protein complexes in the malaria parasite Plasmodium falciparum. J. Biol. Chem. 284, 14537–14546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rupp I., Sologub L., Williamson K. C., Scheuermayer M., Reininger L., Doerig C., Eksi S., Kombila D. U., Frank M., and Pradel G. (2011) Malaria parasites form filamentous cell-to-cell connections during reproduction in the mosquito midgut. Cell Res. 21, 683–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Okamoto N., Spurck T. P., Goodman C. D., and McFadden G. I. (2009) Apicoplast and mitochondrion in gametocytogenesis of Plasmodium falciparum. Eukaryot. Cell 8, 128–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ikadai H., Shaw Saliba K., Kanzok S. M., McLean K. J., Tanaka T. Q., Cao J., Williamson K. C., and Jacobs-Lorena M. (2013) Transposon mutagenesis identifies genes essential for Plasmodium falciparum gametocytogenesis. Proc. Natl. Acad. Sci. U.S.A. 110, E1676–E1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.