Abstract
The symptoms of attention-deficit/hyperactivity disorder (ADHD) are characterized by inattention and hyperactivity-impulsivity. It is a common childhood neurodevelopmental disorder that often persists into adulthood. Improvements in ADHD symptoms using psychostimulants have been recognized as a paradoxical calming effect. The psychostimulant methylphenidate (MPH) is currently used as the first-line medication for the management of ADHD. Recent studies have drawn attention to altered dopamine-mediated neurotransmission in ADHD, particularly reuptake by the dopamine transporter (DAT). This hypothesis is supported by the observation that DAT knockout mice exhibit marked hyperactivity that is responsive to acute MPH treatment. However, other behaviors relevant to ADHD have not been fully clarified. In the present study, we observed learning impairment in shuttle-box avoidance behavior together with hyperactivity in a novel environment in DAT knockout mice. Methylphenidate normalized these behaviors and enhanced escape activity in the tail suspension test. Interestingly, the effective dose of MPH increased extracellular dopamine in the prefrontal cortex but not striatum, suggesting an important role for changes in prefrontal dopamine in ADHD. Research that uses rodent models such as DAT knockout mice may be useful for elucidating the pathophysiology of ADHD.
Keywords: ADHD, dopamine transporter, frontal cortex, knockout, learning, methylphenidate, norepinephrine transporter
INTRODUCTION
The symptoms of attention-deficit/hyperactivity disorder (ADHD) are characterized by inattention and/or hyperactivity-impulsivity [1]. It is the most prevalent childhood psychiatric disorder. ADHD frequently persists into adult life as either a full-blown condition in approximately 15% of cases or partial remission in approximately 50% of cases. It is associated with significant levels of academic, occupational, and social impairment and high levels of psychiatric comorbidity [2]. Twin, adoption, and segregation studies have estimated 50-90% heritability for ADHD [3]. The genes involved in mesocortical dopaminergic neurotransmission are considered candidates for genetic predisposition to this disorder [4], including the dopamine (DA) transporter (DAT) [5] and D4 receptor genes [6]. Abnormal dopaminergic transmission is therefore implicated in ADHD [7].
Brain DA is involved in motor and reward systems and integrative functions that contribute to adaptive behaviors, such as attention, learning, memory [8, 9], and motivation [10, 11]. Aberrations in these variable brain functions are manifested in individuals with ADHD as three core symptoms: inattention, hyperactivity and impulsivity [12, 13], and deficits in reward and motivation [14-16]. Brain imaging studies of individuals
with ADHD have reported deficient DA neurotrans-mission [12, 13, 17-19] and lower D2/D3 receptor expression and DAT availability only in the nucleus accumbens, midbrain regions, and the caudate [12, 16]. By contrast, decreases of DA synthesis and metabolism were reported in the prefrontal cortex (PFC) in ADHD patients [20].
The evidence mentioned above sheds light on the therapeutic mechanism of action of methylphenidate (MPH), the major pharmacotherapy for ADHD [21]. Methylphenidate is a psychostimulant that inhibits DAT and the norepinephrine transporter (NET) [22]. The efficacy of MPH against ADHD has been empirically recognized for decades. However, much remains to be revealed about how MPH leads to improvements in ADHD symptoms.
Numerous genetically altered rodents, such as DAT knockout (DAT-/-) mice [23] and Adcyap1-deficient mice [24], have a behavioral phenotype that is similar to ADHD. The most distinctive phenotype is marked hyperactivity in a novel environment. Intriguingly, psychostimulants such as amphetamine and MPH suppress hyperactivity, in contrast to their well-known locomotor-stimulatory effects [23, 24]. Especially, DAT-/- mice also exhibit impairments in spatial cognitive function in the radial maze [23] and reversal learning in the water maze [25]. The phenotypic characteristics of DAT-/- mice are similar to those in individuals with ADHD, which is the rationale for proposing DAT-/- mice as an animal model of ADHD. However, little is known about the effect of MPH on learning behavior and the mechanism of action of MPH in DAT-/- mice, including its so-called “paradoxical calming effect”.
The present study investigated the effect of DAT deletion and MPH administration on behaviors regulated by DA, including locomotor activity, escape behavior, and avoidance learning. We also evaluated extracellular DA (DAex) levels in the striatum and prefrontal cortex (PFC) using microdialysis.
MATERIALS AND METHODS
Mice
DAT+/+, DAT+/-, and DAT-/- mouse littermates [26] were obtained by crossing male and female DAT+/- mice on a 129/C57 mixed genetic background. The mice were housed in an animal facility maintained at 22 ± 2ºC and 55 ± 5% relative humidity under a 12/12 h light/dark cycle with lights on at 8:00 AM and off at 8:00 PM. Food and water were available ad libitum. All of the experiments were conducted during the light phase of the light/dark cycle. The mice were brought to the testing room at least 1 h before the experiments began. Twenty- to 30-week-old mice were used in the behavioral tests, and 12- to 50-week-old mice were used in the microdialysis experiment. The experimental procedures and housing conditions were approved by the Institutional Animal Care and Use Committee of the Tokyo Metropolitan Institute of Medical Science, and all of the animals were treated humanely in accordance with our institutional animal experimentation guidelines.
Drugs
Methylphenidate (Sigma, St. Louis, MO, USA) was dissolved in saline. Methylphenidate (30 mg/kg) and saline were injected either intraperitoneally (i.p.) or subcutaneously (s.c.) in a volume of 0.1 ml/10 g of body weight.
Open Field Test
Each mouse was placed in an illuminated chamber (25 cm diameter, 27 cm height) and allowed to freely explore the chamber for 30 min. Methylphenidate was then injected i.p., and observations continued for 180 min. Locomotor activity was measured using Supermex (Muromachi Kikai, Tokyo, Japan) by detecting the radiated body heat of the animal through a sensor mounted above the chamber. Counts were automatically recorded at every 5 min.
Tail Suspension Test
The mice were suspended by the tail on a hook (20 cm from the floor) using adhesive tape (1 cm from tip of tail) in the recording apparatus (Neuroscience, Tokyo, Japan). Body movements of less than 10 Hz were considered escape activity (i.e., running movements, body torsion, and body jerks). Escape activity was monitored for 30 min. Methylphenidate was then injected i.p., and escape activity was further measured for 180 min. Counts were analyzed in 5-min bins.
Conditioned Avoidance Responding Test
The conditioned avoidance responding test was conducted using shuttle boxes equipped with wire grid floors through which electric footshocks were administered (Muromachi Kikai Co., Tokyo, Japan). Each box had two compartments of equal size and shape, separated by a metal barrier with an open doorway, and was placed in a sound-attenuating chamber. A trial began with a 24-s intertrial interval (ITI), followed by presentation of a light and a 2.8-kHz tone (conditioned stimulus [CS]) for 5 s. The CS stopped and the next ITI began when the mouse moved to the other side during CS presentation. An electric footshock (0.1 mA, 1 s; unconditioned stimulus [UCS]) was delivered when the mouse stayed in the same chamber at the end of the CS. The UCS ceased when the mouse jumped or moved to the other compartment, and the next trial began after the ITI. All of the mice underwent 40 trials per session and two sessions per day at intervals of 3 h or longer. The mice were trained without MPH for the first 5 consecutive days and then with MPH for another 5 days. Methylphenidate was injected i.p. immediately before the test. For the analysis, we defined the following parameters: RCS (response to the CS as a recorded movement during the CS), RUCS (response to the UCS as a recorded movement during presentation of the UCS), and E (no response to the CS or UCS). Avoidance responding was calculated using the following equation: (RCS each day / 80) × 100. The sensitivity to footshock was checked by calculating the response rate (RUCS / [RUCS + E]) in the first session.
Microdialysis Procedure
The mice were anesthetized with pentobarbital (50 mg/kg, i.p.) and stereotaxically implanted with microdialysis probes in the striatum (anterior, +0.6 mm; lateral, +1.8 mm; ventral, -4.0 mm from bregma) or PFC (anterior, +2.0 mm; lateral, +0.5 mm; ventral, -3.0 mm from bregma) according to the atlas of Franklin and Paxinos [27]. After 24 h of recovery from implantation, the microdialysis experiments were performed in freely moving mice. Ringer’s solution (145 mM NaCl, 3 mM KCl, 1.26 mM CaCl2, and 1 mM MgCl2, pH 6.5) was perfused at a constant flow rate of 1 µl/min. The perfusates were directly injected into the high-performance liquid chromatography system every 10 min using an autoinjector (EAS-20; Eicom). Dialysate DA was separated with a reverse-phase ODS column (PP-ODS; Eicom) and detected with a graphite electrode (HTEC-500; Eicom) with a detection limit of 0.5 fmol/sample with a signal-to-noise ratio of 2. The mobile phase consisted of 0.1 M phosphate buffer (pH 5.5) that contained 500 mg/L sodium decanesulfonate, 50 mg/L (ethylenedinitrilo)tetraacetic acid, and 1% methanol. Perfusion was initiated 180 min prior to the measurement of DAex. Baseline DAex was first obtained from the average of three consecutive samples. Methylphenidate was injected s.c., and the samples were collected for 180 min. DAex levels are expressed relative to baseline and as the area-under-the-curve (AUC).
Statistical Analysis
The statistical analyses were conducted using StatView 5.0 software (SAS Institute). Locomotor activity in the open field test and escape activity in the tail suspension test were analyzed using ANOVA and Scheffe’s post hoc test. In the conditioned avoidance responding test, the effects of training on avoidance responding were subjected to mixed-design ANOVA or one-way repeated-measures ANOVA for each genotype. One-way ANOVA was used for response rate (i.e., avoidance responding on days 5 and 10), followed by Scheffe’s post hoc test. Avoidance responding on day 6 was compared with day 5 for each genotype using paired t-tests. In the microdialysis experiments, AUC values were analyzed using two-way ANOVA followed by Fisher’s PLSD post hoc test. Values of P < 0.05 were considered statistically significant.
RESULTS
Locomotor Activity
Locomotor activity was significantly different in DAT+/+, DAT+/-, and DAT-/- mice (main effect of genotype, F(2,32) = 3.720, P= 0.035, one-way analysis of variance [ANOVA]; Fig. 1A), and DAT-/- mice were hyperactive compared with DAT+/+ mice (P = 0.044,
Fig. (1).
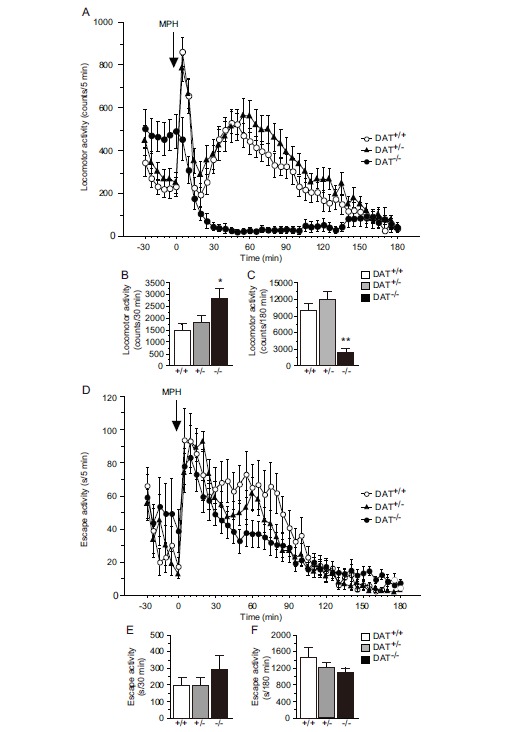
Distinct responses to MPH in DAT-/- mice. In the open field test, (A, B) DAT-/- mice exhibited hyperactivity in a novel environment. (A, C) Methylphenidate (30 mg/kg, i.p.) inhibited locomotor activity in DAT-/- mice (n = 9-16 per group). (D, E) DAT-/- mice exhibited comparable escape activity when assessed in the tail suspension test. (D, F) The response to MPH (30 mg/kg, i.p.) was equal among the three genotypes (n = 8-14). *P < 0.05, **P < 0.01, vs DAT+/+ mice (Scheffe’s test). The data are expressed as mean ± SEM.
Scheffe’s test; Fig. 1B). After MPH injections, a difference in locomotor activity was observed in DAT+/+, DAT+/-, and DAT-/- mice (main effect of genotype, F(2,32) = 12.218, P < 0.001, one-way ANOVA; Fig. 1C), although in a distinct manner. DAT-/- mice exhibited a remarkable decrease in locomotor activity compared with DAT+/+ mice (P = 0.006, Scheffe’s test; Fig. 1C).
Tail Suspension Test
Escape activity was similar in magnitude in DAT+/+, DAT+/-, and DAT-/- mice before MPH treatment (main effect of genotype, F(2,28) = 0.839, P = 0.443, one-way ANOVA; Fig. 1D, E). In contrast to locomotor activity, MPH enhanced escape activity equally in DAT+/+, DAT+/-, and DAT-/- mice (main effect of genotype, F(2,28) = 0.969, P = 0.392, one-way ANOVA; Fig. 1D, F).
Conditioned Avoidance Responding Test
The sensitivity to footshock, expressed as response rate, was comparable between genotypes (main effect of genotype, F(2,54) = 2.291, P = 0.111, one-way ANOVA; Fig. 2A). Prior to MPH treatment, a difference in avoidance responding was observed between genotypes (main effect of genotype, F(2,216) = 41.661, P < 0.001, mixed-design ANOVA). Training also influenced avoidance responding (main effect of training, F(4,216) = 91.312, P < 0.001, mixed-design ANOVA). Unequal temporal changes in avoidance responding were observed on day 1-5 (genotype × training, F(8,216) = 25.358, P < 0.001, mixed-design ANOVA). DAT+/+ and DAT+/- mice acquired the task (main effect of training; DAT+/+, F(4,88) = 60.991, P < 0.001; DAT+/-, F(4,64) = 57.988, P < 0.001; one-way repeated-measures ANOVA), but DAT-/- mice did not. A trend toward a change in avoidance responding (main effect of training; DAT-/-, F(4,64) = 2.494, P = 0.052, one-way repeated-measures ANOVA) was likely attributable to a decline on day 2-5 (Fig. 2B). On day 5, avoidance responding was comparable between DAT+/+ mice (77%) and DAT+/- mice (74%) and higher than in DAT-/- mice (5%; main effect of genotype, F(2,54) = 66.917, P < 0.0001, one-way ANOVA; DAT+/-, P = 0.914; DAT-/-, P < 0.0001, vs DAT+/+ mice; DAT+/-, P < 0.001, vs DAT-/- mice, Scheffe’s test).
Fig. (2).
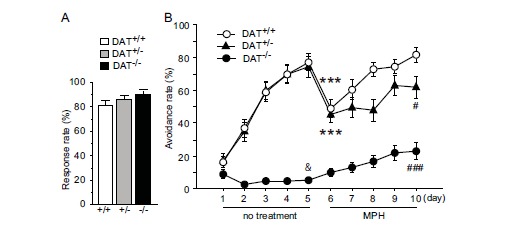
Effects of training and MPH on avoidance responding. (A) The sensitivity to footshock, expressed as response rate, was comparable in DAT+/+, DAT+/-, and DAT-/- mice (n = 17-23 per group). (B) Training alone was effective in DAT+/+ and DAT+/- mice but not in DAT-/- mice. Methylphenidate had beneficial effects on acquisition in this task in DAT-/- mice. The reduction of avoidance responding on day 6 in DAT+/+ and DAT+/- mice was transient and recovered by training. &P < 0.05, vs DAT+/+ mice; ***P < 0.001, vs day 5 (paired t-test); #P < 0.05, vs DAT+/+ mice; ###P < 0.001, vs DAT+/- mice (Scheffe’s test). The data are expressed as mean ± SEM.
When training was continued with MPH administration, a drop in avoidance responding was observed on day 6 in DAT+/+ and DAT+/- mice (DAT+/+, t(22) = 4.836, P < 0.001; DAT+/-, t(16) = 4.979, P < 0.001, vs day 5; paired t-test) but not in DAT-/- mice (t(16) = -1.736, P = 0.102, paired t-test; Fig. 2A, B). Avoidance responding differed across all genotypes and training, with a significant genotype × training interaction (main effect of genotype, F(2,216) = 36.453, P < 0.001, mixed-design ANOVA; main effect of training, F(4,216) = 23.062, P < 0.001, mixed-design ANOVA; genotype × training, F(8,216) = 2.689, P = 0.008, mixed-design ANOVA). An increase in avoidance responding was observed in DAT+/+, DAT+/-, and DAT-/- mice (main effect of training; DAT+/+, F(4,88) = 23.564, P < 0.001; DAT+/-, F(4,64) = 3.963, P = 0.006; DAT-/-, F(4,64) = 6.506, P < 0.001; one-way repeated-measures ANOVA; Fig. 2B). In contrast to day 5, avoidance responding was higher in DAT+/+ mice (82%) than in DAT+/- (62%) and DAT-/- (23%) mice on day 10 (main effect of genotype, F(2,54) = 30.784, P < 0.001, one-way ANOVA; P = 0.035, DAT+/+ vs DAT+/-; P < 0.001, DAT+/+ vs DAT-/-; P < 0.001, DAT+/- vs DAT-/-; Scheffe’s test; Fig. 2B).
Extracellular Dopamine in the Striatum and PFC
In the striatum, DAex levels were highest 40-60 min after MPH administration, whereas no temporal changes were noted in DAT-/- mice (Fig. 3A). Methylphenidate markedly elevated the AUC of DAex in DAT+/+ mice (P < 0.001, Fisher’s protected least significant difference [PLSD] test) but not in DAT-/- mice (Fig. 3B). In the PFC, an increase in DAex levels was observed in both DAT+/+ and DAT-/- mice treated with MPH, reaching a plateau at 40-60 min (Fig. 3C). Methylphenidate significantly augmented the DAex AUC in DAT+/+ and DAT-/- mice (main effect of treatment, F(3,340) = 8.039, P = 0.001, two-way ANOVA; Fig. 3D).
Fig. (3).
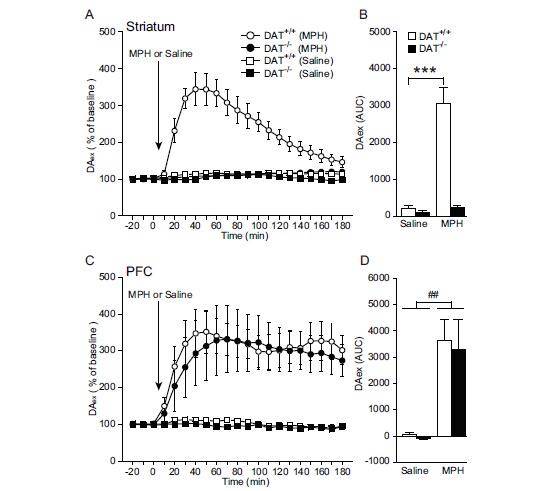
DAex response to MPH in the striatum and PFC. (A, B) In the striatum, DAT+/+ mice exhibited a marked increase in DAex in response to MPH but DAT-/- mice did not (n = 4-5 per group; basal level, mean ± SEM: DAT+/+ mice, 57.35 ± 8.87 fmol/10 min; DAT-/- mice, 546.97 ± 94.26 fmol/10 min). (C, D) In contrast, MPH elevated DAex in the PFC in DAT+/+ and DAT-/- mice (n = 4-8 per group; basal level, mean ± SEM: DAT+/+ mice, 2.32 ± 0.25 fmol/10 min; DAT-/- mice, 2.27 ± 0.48 fmol/10 min). Notice the absence of temporal changes in DAex by saline in the striatum and PFC in DAT+/+ and DAT-/- mice. ***P < 0.001, vs saline (Fisher’s PLSD test); ##P < 0.01, vs saline (two-way ANOVA). The data are expressed as mean ± SEM.
DISCUSSION
In the present study, abnormal behaviors relevant to ADHD were observed in DAT-/- mice. Locomotor activity was elevated when the mice were exposed to a novel environment, consistent with a previous report [23]. DAT-/- mice had difficulty avoiding the footshock in the conditioned avoidance responding test, which requires cognitive behaviors [28, 29]. Escape activity remained unchanged in the mutant mice, which was inconsistent with the literature [30]. This may have been attributable to the measurements because we recorded body movements of less than 10 Hz. Moreover, MPH, the medication most frequently used for individuals with ADHD, ameliorated the behavioral phenotypes in DAT-/- mice. Notably, locomotor activity was markedly suppressed by MPH, whereas escape activity was stimulated, similar to DAT+/+ and DAT+/- mice. The latter finding is consistent with previous studies of rodents treated with antidepressants [31] and in contrast to the notion that MPH possesses “a paradoxical calming effect” [23]. Additionally, DAT-/- mice exhibited a substantial improvement in the conditioned avoidance responding test with MPH treatment. Therefore, MPH appeared to exert a cognitive-enhancing effect rather than a sedative effect.
Systemically administered MPH elevated DAex levels in the PFC but not in the striatum in DAT-/- mice. The distinct distribution patterns of DAT and NET might explain this result. Neurons in the PFC have high levels of NET, whereas DAT expression is low [32-34]. In the striatum, the levels of DAT expression are high, and NET expression is low [34]. Importantly, NET serves an uptake function for both DA and norepinephrine [35, 36]. The inhibition of NET results in the accumulation of DA in the PFC in animals [37, 38], including DAT-/- mice [39]. A PFC-specific increase in DAex parallels these previous studies and may modulate the effects of MPH in DAT-/- mice. Another possibility is that the increase in extracellular norepinephrine (NEex) caused by MPH-induced NET inhibition [40] may underlie the behavioral effects of MPH in DAT-/- mice. This possibility is supported by the recent approval of guanfacine, a selective α2A-adrenergic receptor agonist, for the treatment of ADHD by the Food and Drug Administration (FDA). However, the limited efficacy of guanfacine compared with MPH suggests that the increase in DAex in the PFC may be more involved in the effects of MPH on ADHD-like behaviors.
When discussing the relationship between behavior and brain DA in DAT-/- mice, one consideration is that the mutant animals have persistent hyperdopaminergic tone in the striatum [41]. This may give rise to striatum dysfunction, leading to hyperactivity and learning deficits. Consistent with this possibility, MPH loses its locomotor-stimulating effects when selective dopaminergic lesions are made using 6-hydroxydopamine in the striatum [42] but not in the PFC [43]. A combined double lesion in the nucleus accumbens and striatum resulted in a severe deficit in the conditioned avoidance responding test [44]. The crucial role of a transient increase in PFC DAex is stressed for avoidance behavior [45, 46]. In the present study, the effects of MPH in DAT-/- mice were associated with elevated levels of DAex in the PFC. Thus, the PFC is suggested to play an important role in correcting pathologic behaviors in DAT-/- mice, even with striatum dysfunction caused by a constitutive hyperdopaminergic state.
Also interesting is the response of DAT+/+ and DAT+/- mice to MPH that was associated with increased DA levels in both the PFC and striatum. Escape activity was enhanced similarly across all genotypes, implicating the greater involvement of the PFC than the striatum. Locomotor stimulation and worsened avoidance responding may reflect the influence of elevated DA in the striatum that surmounted the elevation of DA in the PFC. Avoidance responding on day 10 was highest in DAT+/+ mice, followed by DAT+/- and DAT-/- mice, suggesting that haploinsufficiency of the DAT gene disturbs striatum function to a certain extent.
The present findings in DAT mutant mice will expand our understanding of the pathophysiology of ADHD in humans. Individuals with ADHD have lower DAT availability in the striatum [12, 16] and impaired inhibition of prepotent motor responses that depend on the integrity of both the PFC and striatum [47-49]. Frontostriatal circuitry is one of the most prominent brain pathways involved in the control of locomotion, affect, impulsivity, attention, and emotion [50, 51]. Functional magnetic resonance imaging studies revealed that subjects with ADHD exhibited underactivation in the PFC, which was reversed by MPH [52, 53]. These results are consistent with the present study, in which MPH improved hyperactivity and learning deficits in DAT-/- mice by augmenting DA levels in the PFC. Cognitive function that involves the frontal cortex should be further investigated with regard to ADHD using animal models, including DAT-/- mice.
CONCLUSION
We observed behavioral abnormalities relevant to ADHD in DAT-/- mice. Methylphenidate ameliorated these behavioral deficits in association with increased DAex in the PFC. The present findings suggest that the increase in PFC DAex by MPH reversed the constitutive hyperdopaminergic state in the striatum and consequent abnormal behaviors. Future studies on the PFC in DAT-/- mice will deepen our understanding of ADHD, particularly with regard to frontal cortical function and DA.
ACKNOWLEDGEMENTS
We acknowledge Dr. Hiroaki Niki for supporting this study, Ms. Junko Hasegawa, Ms. Satomi Soma, Ms. Etsuko Kamegaya, and Ms. Yurie Nakamoto for technical assistance, and Mr. Michael Arends for assistance with editing the manuscript. This research was supported by grants from the Ministry of Health, Labour and Welfare (MHLW) of Japan (H21-3jigan-ippan-011, H22-Iyaku-015, and H25-Iyaku-020), JSPS KAKENHI Grant Numbers 20602020, 20390162, 22790518, 23390377, 24659490, 24659549, MEXT KAKENHI Grant Number 25116532, Smoking Research Foundation, and Astellas Foundation for Research on Metabolic Disorders.
ABBREVIATIONS
- ADHD
Attention-deficit/hyperactivity disorder
- AUC
Area-under-the-curve
- CS
Conditioned stimulus
- DA
Dopamine
- DAex
Extracellular dopamine
- DAT
Dopamine transporter
- i.p.
Intraperitoneally
- ITI
Intertidal interval
- MPH
Methylphenidate
- NEex
Extracellular norepinephrine
- NET
Norepinephrine transporter
- PFC
Prefrontal cortex
- s.c.
Subcutaneously
- UCS
Unconditioned stimulus
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. pp. 59–66. [Google Scholar]
- 2.Faraone S.V., Biederman J., Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol. Med. 2006;36(2):159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- 3.Solanto M.V. Attention-deficit/hyperactivity disorder: clinical features. In: Solanto M.V., Arnsten A.F., Castellanos F.X., editors. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. New York: Oxford University Press; 2001. pp. 3–30. [Google Scholar]
- 4.Solanto M.V. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behav. Brain Res. 2002;130(1-2):65–71. doi: 10.1016/s0166-4328(01)00431-4. [DOI] [PubMed] [Google Scholar]
- 5.Cook E.H., Jr, Stein M.A., Krasowski M.D., et al. Association of attention-deficit disorder and the dopamine transporter gene. Am. J. Hum. Genet. 1995;56(4):993–998. [PMC free article] [PubMed] [Google Scholar]
- 6.LaHoste G.J., Swanson J.M., Wigal S.B., et al. Dopamine D4 receptor gene polymorphism is associated with attention deficit hyperactivity disorder. Mol. Psychiatry. 1996;1(2):121–124. [PubMed] [Google Scholar]
- 7.Swanson J., Castellanos F.X., Murias M., LaHoste G., Kennedy J. Cognitive neuroscience of attention deficit hyperactivity disorder and hyperkinetic disorder. Curr. Opin. Neurobiol. 1998;8(2):263–271. doi: 10.1016/s0959-4388(98)80150-5. [DOI] [PubMed] [Google Scholar]
- 8.Nieoullon A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002;67(1):53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 9.Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci. Biobehav. Rev. 2006;30(1):1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Tye K.M., Mirzabekov J.J., Warden M.R., et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493(7433):537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lammel S., Lim B.K., Ran C., et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491(7423):212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volkow N.D., Wang G.J., Newcorn J., et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2007;64(8):932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- 13.Rosa Neto P., Lou H., Cumming P., Pryds O., Gjedde A. Methylphenidate-evoked potentiation of extracellular dopamine in the brain of adolescents with premature birth: correlation with attentional deficit. Ann. N. Y. Acad. Sci. 2002;965:434–439. doi: 10.1111/j.1749-6632.2002.tb04184.x. [DOI] [PubMed] [Google Scholar]
- 14.Luman M., Oosterlaan J., Sergeant J.A. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin. Psychol. Rev. 2005;25(2):183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Johansen E.B., Killeen P.R., Russell V.A., et al. Origins of altered reinforcement effects in ADHD. Behav. Brain Funct. 2009;5:7. doi: 10.1186/1744-9081-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volkow N.D., Wang G.J., Kollins S.H., et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302(10):1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernst M., Zametkin A.J., Matochik J.A., Pascualvaca D., Jons P.H., Cohen R.M. High midbrain [18F]DOPA accumulation in children with attention deficit hyperactivity disorder. Am. J. Psychiatry. 1999;156(8):1209–1215. doi: 10.1176/ajp.156.8.1209. [DOI] [PubMed] [Google Scholar]
- 18.Volkow N.D., Wang G.J., Newcorn J., et al. Brain dopamine transporter levels in treatment and drug naive adults with ADHD. Neuroimage. 2007;34(3):1182–1190. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Lou H.C., Rosa P., Pryds O., et al. ADHD: increased dopamine receptor availability linked to attention deficit and low neonatal cerebral blood flow. Dev. Med. Child Neurol. 2004;46(3):179–183. doi: 10.1017/s0012162204000313. [DOI] [PubMed] [Google Scholar]
- 20.Ernst M., Zametkin A.J., Matochik J.A., Jons P.H., Cohen R.M. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J. Neurosci. 1998;18(15):5901–5907. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenhill L.L., Pliszka S., Dulcan M.K., et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41(2) Suppl.:26S–49S. doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- 22.Han D.D., Gu H.H. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gainetdinov R.R., Wetsel W.C., Jones S.R., Levin E.D., Jaber M., Caron M.G. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283(5400):397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka K., Shintani N., Hashimoto H., et al. Psychostimulant-induced attenuation of hyperactivity and prepulse inhibition deficits in Adcyap1-deficient mice. J. Neurosci. 2006;26(19):5091–5097. doi: 10.1523/JNEUROSCI.4376-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morice E., Billard J.M., Denis C., et al. Parallel loss of hippocampal LTD and cognitive flexibility in a genetic model of hyperdopaminergia. Neuropsychopharmacology. 2007;32(10):2108–2116. doi: 10.1038/sj.npp.1301354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sora I., Wichems C., Takahashi N., et al. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc. Natl. Acad. Sci. USA. 1998;95(13):7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franklin K.B., Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 28.Bovet D., Bovet-Nitti F., Oliverio A. Genetic aspects of learning and memory in mice. Science. 1969;163(3863):139–149. doi: 10.1126/science.163.3863.139. [DOI] [PubMed] [Google Scholar]
- 29.Stark H., Bischof A., Wagner T., Scheich H. Stages of avoidance strategy formation in gerbils are correlated with dopaminergic transmission activity. Eur. J. Pharmacol. 2000;405(1-3):263–275. doi: 10.1016/s0014-2999(00)00558-6. [DOI] [PubMed] [Google Scholar]
- 30.Perona M.T., Waters S., Hall F.S., et al. Animal models of depression in dopamine, serotonin, and norepinephrine transporter knockout mice: prominent effects of dopamine transporter deletions. Behav. Pharmacol. 2008;19(5-6):566–574. doi: 10.1097/FBP.0b013e32830cd80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teste J.F., Pelsy-Johann I., Decelle T., Boulu R.G. Anti-immobility activity of different antidepressant drugs using the tail suspension test in normal or reserpinized mice. Fundam. Clin. Pharmacol. 1993;7(5):219–226. doi: 10.1111/j.1472-8206.1993.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 32.Ciliax B.J., Heilman C., Demchyshyn L.L., et al. The dopamine transporter: immunochemical characterization and localization in brain. J. Neurosci. 1995;15(3 Pt 1):1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sesack S.R., Hawrylak V.A., Matus C., Guido M.A., Levey A.I. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J. Neurosci. 1998;18(7):2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moll G.H., Mehnert C., Wicker M., et al. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Res. Dev. Brain Res. 2000;119(2):251–257. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- 35.Horn A.S. Structure-activity relations for the inhibition of catecholamine uptake into synaptosomes from noradrenaline and dopaminergic neurones in rat brain homogenates. Br. J. Pharmacol. 1973;47(2):332–338. doi: 10.1111/j.1476-5381.1973.tb08331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raiteri M., Del Carmine R., Bertollini A., Levi G. Effect of sympathomimetic amines on the synaptosomal transport of noradrenaline, dopamine and 5-hydroxytryptamine. Eur. J. Pharmacol. 1977;41(2):133–143. doi: 10.1016/0014-2999(77)90202-3. [DOI] [PubMed] [Google Scholar]
- 37.Carboni E., Tanda G.L., Frau R., Di Chiara G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J. Neurochem. 1990;55(3):1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto B.K., Novotney S. Regulation of extracellular dopamine by the norepinephrine transporter. J. Neurochem. 1998;71(1):274–280. doi: 10.1046/j.1471-4159.1998.71010274.x. [DOI] [PubMed] [Google Scholar]
- 39.Moron J.A., Brockington A., Wise R.A., Rocha B.A., Hope B.T. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J. Neurosci. 2002;22(2):389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koda K., Ago Y., Cong Y., Kita Y., Takuma K., Matsuda T. Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J. Neurochem. 2010;114(1):259–270. doi: 10.1111/j.1471-4159.2010.06750.x. [DOI] [PubMed] [Google Scholar]
- 41.Shen H.W., Hagino Y., Kobayashi H., et al. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29(10):1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- 42.Claussen C.M., Chong S.L., Dafny N. Selective bilateral lesion to caudate nucleus modulates the acute and chronic methylphenidate effects. Pharmacol. Biochem. Behav. 2012;101(2):208–216. doi: 10.1016/j.pbb.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wanchoo S.J., Lee M.J., Swann A.C., Dafny N. Bilateral six-hydroxydopamine administration to PFC prevents the expression of behavioral sensitization to methylphenidate. Brain Res. 2010;1312:89–100. doi: 10.1016/j.brainres.2009.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koob G.F., Simon H., Herman J.P., Le Moal M. Neuroleptic-like disruption of the conditioned avoidance response requires destruction of both the mesolimbic and nigrostriatal dopamine systems. Brain Res. 1984;303(2):319–329. doi: 10.1016/0006-8993(84)91218-6. [DOI] [PubMed] [Google Scholar]
- 45.Stark H., Bischof A., Scheich H. Increase of extracellular dopamine in prefrontal cortex of gerbils during acquisition of the avoidance strategy in the shuttle-box. Neurosci. Lett. 1999;264(1-3):77–80. doi: 10.1016/s0304-3940(99)00174-3. [DOI] [PubMed] [Google Scholar]
- 46.Stark H., Rothe T., Wagner T., Scheich H. Learning a new behavioral strategy in the shuttle-box increases prefrontal dopamine. Neuroscience. 2004;126(1):21–29. doi: 10.1016/j.neuroscience.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 47.Godefroy O., Lhullier C., Rousseaux M. Non-spatial attention disorders in patients with frontal or posterior brain damage. Brain. 1996;119(Pt 1):191–202. doi: 10.1093/brain/119.1.191. [DOI] [PubMed] [Google Scholar]
- 48.Leimkuhler M.E., Mesulam M.M. Reversible go-no go deficits in a case of frontal lobe tumor. Ann. Neurol. 1985;18(5):617–619. doi: 10.1002/ana.410180518. [DOI] [PubMed] [Google Scholar]
- 49.Trommer B.L., Hoeppner J.A., Zecker S.G. The go-no go test in attention deficit disorder is sensitive to methylphenidate. J. Child Neurol. 1991;6(Suppl.):S128–S131. doi: 10.1177/0883073891006001s13. [DOI] [PubMed] [Google Scholar]
- 50.Carlsson M., Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia--implications for schizophrenia and Parkinson's disease. Trends Neurosci. 1990;13(7):272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- 51.Robbins T.W. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp. Brain Res. 2000;133(1):130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- 52.Rubia K., Halari R., Cubillo A., et al. Methylphenidate normalizes fronto-striatal underactivation during interference inhibition in medication-naive boys with attention-deficit hyperactivity disorder. Neuropsychopharmacology. 2011;36(8):1575–1586. doi: 10.1038/npp.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubia K., Halari R., Mohammad A.M., Taylor E., Brammer M. Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2011;70(3):255–262. doi: 10.1016/j.biopsych.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


