ABSTRACT
We examined the prognostic role of immune markers programmed cell death protein-1 (PD-1) and its ligand (PD-L1), CD8+ tumor-infiltrating lymphocytes (TILs), FOXP3+ Tregs and phosphorylated Caspase-8 (T273) in patients with anal squamous cell cancer (ASCC) treated with standard chemoradiotherapy (CRT). The baseline immunohistochemical expression of immune markers was correlated with clinicopathologic characteristics, and cumulative incidence of local failure, disease-free survival (DFS) and overall survival (OS) in 150 patients, also in the context of human papilloma virus 16 (HPV16) DNA load and p16INK4a expression. After a median follow-up of 40 mo (1–205 mo), the 5-y cumulative incidence of local failure and DFS was 19.4% and 67.2%, respectively. Strong immune marker expression was significantly more common in tumors with high HPV16 viral load. In multivariant analysis, high CD8+ and PD-1+ TILs expression predicted for improved local control (p = 0.023 and p = 0.007, respectively) and DFS (p = 0.020 and p = 0.014, respectively). Also, high p16INK4a (p = 0.011) and PD-L1 (p = 0.033) expression predicted for better local control, whereas high FOXP3+ Tregs (p = 0.050) and phosphorylated Caspase-8 (p = 0.031) expression correlated with superior DFS. Female sex and high HPV16 viral load correlated with favorable outcome for all three clinical endpoints. The present data provide, for the first time, robust explanation for the favorable clinical outcome of HPV16-positive ASCC patients harboring strong immune cell infiltration. Our findings are relevant for treatment stratification with immune PD-1/PD-L1 checkpoint inhibitors to complement CRT and should be explored in a clinical trial.
KEYWORDS: Anal cancer, HPV, immune cell infiltration, immunotherapy, PD -1/PD-L1, prognostic
Introduction
Anal squamous cell cancer (ASCC) is a relatively rare malignancy.1 With standard chemoradiotherapy (CRT),2-4 complete and durable remission can be achieved in the majority of patients, but local and/or distant relapse still occur in 15–30%. Randomized trials identified T stage, N stage and sex as independent prognostic factors. Better molecular stratification and new therapies beyond standard CRT are needed for treatment escalation and de-escalation strategies.
ASCC mostly arises from infection with high risk oncogenic human papilloma virus (HPV16,18) via inactivation of tumor suppressor proteins TP53 and pRB by the viral oncoproteins E6 and E7. The prevalence of HPV DNA, and its associated surrogate marker p16INK4a (p16), ranges from 85% to 100% in ASCC.5,6,7 With such high detection rates, a comparison between sole HPV-positive and -negative cancer with respect to oncological outcomes is challenging. In patients with cervical cancer, Kim et al. recently reported that the semi-quantitative measured HPV DNA load was an independent prognostic factor for disease-free survival (DFS) after radical radiotherapy.8 We confirmed the prognostic value of a high HPV16 DNA quantitative load as independent prognostic factor for local control after standard CRT for ASCC patients,9 but the mechanisms behind this phenomenon remain largely unclear.
Programmed cell death protein-1 (PD-1) and its ligand, programmed death ligand 1 (PD-L1) mediate immune tolerance.10,11 PD-1 is expressed on activated T cells, whereas PD-L1 is found on cancer cells, parenchymal and myeloid cells. Activation of the PD-1/PD-L1 axis leads to tumor-infiltrating lymphocytes (TILs) dysfunction,10,11 and occurs in several malignancies via either activated oncogenic signaling (innate resistance), or an inflammatory process (adaptive resistance).10,11,12
Although few studies have investigated the prognostic impact of CD8+ TILs and FOXP3 Tregs in ASCC,13,14 the prognostic role and correlation with PD-1+ TILs and PD-L1+ cells in this disease remains unknown. Also, the prognostic role of PD-1/PD-L1 has not been investigated in ASCC. Here, we assessed CD8+, PD-1, PD-L1, FOXP3, pCasp-8 alone, and also in correlation with HPV16 DNA viral load and p16 in a large patient cohort treated homogeneously with primary CRT.
Results
Immune markers staining characteristics
The results of immune marker scoring, HPV16 viral load, p16 expression in pretreatment biopsies and correlation with clinicopathologic characteristics are shown in Table 1 and Tables S1 and S2. Higher CD8+ TILs expression was more common in patients with early N stage (p = 0.012), high HPV16 viral load (p = 0.043), and also high expression of PD-1 (p = 0.016), PD-L1 (p = 0.006), FOXP3 (p < 0.001) and pCaspase-8 (p = 0.039). High PD-1+ TILs expression correlated with high HPV16 load (p < 0.001), high p16 expression (p = 0.015), high PD-L1 (p < 0.001) and high FOXP3+ Tregs (p = 0.001). PD-L1+ cells and FOXP3+ Tregs were found more often in patients with high CD8+ and PD-1+ TILs expression, whereas only FOXP3 correlated with high HPV16 load (p = 0.006) and high p16 expression (p = 0.035). Representative images of all immune cells and markers with high and low expression is shown in Fig. S1. Additionally, high HPV16 load was more common in females (p = 0.009) and in patients with early T stage (p = 0.017). The expression of immune cell populations and markers was comparable between HIV-positive and HIV-negative patients (Table S3).
Table 1.
Correlation of CD8+, PD-1 and PD-L1 with clinicopathologic parameters in the entire cohort.
| Parameter | No. | CD8 low n (%) | CD8 high n (%) | p value | PD-1 low n (%) | PD-1 high n (%) | p value | PD-L1 low n (%) | PD-L1 high n (%) | p value |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | ||||||||||
| ≤ 59 y | 75 | 48 (48) | 27 (53) | 0.730 | 38 (49) | 37 (51) | 1 | 45 (45) | 30 (60) | 0.119 |
| > 59 y | 75 | 51 (52) | 24 (47) | 39 (51) | 36 (49) | 55 (55) | 20 (40) | |||
| Gender | ||||||||||
| male | 66 | 46 (46) | 20 (39) | 0.488 | 36 (47) | 30 (41) | 0.514 | 45 (45) | 21 (42) | 0.862 |
| female | 84 | 53 ( 54) | 31 (61) | 41 (53) | 43 (59) | 55 (55) | 29 (58) | |||
| T-stage | ||||||||||
| T1/2 | 105 | 66 (66) | 40 (78) | 0.133 | 53 (69) | 51 (71) | 0.859 | 71 (71) | 34 (68) | 0.709 |
| T3/T4 | 45 | 34 (34) | 11 (22) | 24 (31) | 21 (29) | 29 (29) | 16 (32) | |||
| N-stage | ||||||||||
| N0 | 97 | 57 (58) | 40 (78) | 0.012 | 50 (65) | 47 (64) | 1.00 | 64 (64) | 33 (66) | 0.858 |
| N1–3 | 53 | 42 (42) | 11 (22) | 27 (35) | 26 (36) | 36 (36) | 17 (34) | |||
| Grading | ||||||||||
| G1/2 | 114 | 72 (76) | 42 (82) | 0.429 | 60 (79) | 54 (77) | 0.881 | 80 (82) | 34 (71) | .232 |
| G3 | 25 | 19 (20) | 6 (12) | 13 (17) | 12 (17) | 15 (15) | 10 (21) | |||
| Gx | 7 | 4(4) | 3 (6) | 3 (4) | 4 (6) | 3 (3) | 4 (8) | |||
| HPV-16 load | ||||||||||
| HPV-16 low | 67 | 51 (56) | 16 (36) | 0.043 | 47 (68) | 20 (30) | < 0.001 | 50 (55) | 17 (39) | .098 |
| HPV-16 high | 68 | 40 (44) | 28 (64) | 22 (32) | 46 (70) | 41 (45) | 27 (61) | |||
| p16INK4a | ||||||||||
| p16 low | 73 | 54 (55) | 19 (37) | 0.058 | 45 (58) | 28 (38) | 0.015 | 48 (48) | 25 (50) | 0.863 |
| p16 high | 77 | 45 (45) | 32 (63) | 32 (42) | 45 (62) | 52 (52) | 25 (50) | |||
| CD8+ | ||||||||||
| CD8 low | 58 (75) | 41 (56) | 0.016 | 74 (74) | 25 (50) | .006 | ||||
| CD8 high | 19 (25) | 32 (44) | 26 (26) | 25 (50) | ||||||
| PD-1 | ||||||||||
| PD-1 low | 77 | 58 (59) | 19 (37) | 0.016 | 63 (63) | 14 (28) | < 0.001 | |||
| PD-1 high | 73 | 41 (41) | 32 (63) | 37 (37) | 36 (72) | |||||
| PD-L1 | ||||||||||
| PD-L1 low | 100 | 74 (75) | 26 (51) | 0.006 | 63 (82) | 37 (51) | < 0.001 | |||
| PD-L1 high | 50 | 25 (25) | 25 (49) | 14 (18) | 36 (49) | |||||
| FOXP3 | ||||||||||
| FOXP3 low | 77 | 62 (63) | 15 (29) | < 0.001 | 50 (65) | 27 (37) | 0.001 | 62 (62) | 15 (30) | < 0.001 |
| FOXP3 high | 73 | 37 (37) | 36 (71) | 27 (35) | 46 (63) | 38 (38) | 35 (70) | |||
| pCasp-8 | ||||||||||
| pCasp-8 low | 80 | 59 (60) | 21 (41) | 0.039 | 46 (60) | 34 (47) | 0.14 | 53 (53) | 27 (54) | 1.00 |
| pCasp-8 high | 70 | 40 (40) | 30 (59) | 31 (40) | 39 (53) | 47 (47) | 23 (46) |
Abbreviations: HPV, human papilloma virus; pCasp-8: phosphorylated Caspase-8 (pT273).
Significant p values have been marked with bold.
Immune markers and treatment outcome
After a median follow-up of 40 (range, 1–205) mo, locoregional failure occurred in 26 (17.3%) patients, whereas distant metastases were encountered in 17 (11.3%) patients. A total of 36 (24%) patients died during follow-up: 18 (12%) of ASCC, 16 (10.7%) of intercurrent, non-malignant disease and 2 (1.3%) due to treatment-related complications. Complete remission occurred in 123 (82%) patients, 20 (13.3%) had lack of remission. The 5-y and 10-y cumulative incidence of locoregional failure for the entire cohort was 19.4% and 20.9%, respectively. The 5-y and 10-y DFS were 67.2% and 58.3%, respectively. The 5-y and 10-y overall survival (OS) rates amounted to 87.1% and 82.8%, respectively.
In univariant analysis, patients with high total CD8+ TILs expression had a significantly better local control (p = 0.007), DFS (p = 0.008) and OS (p = 0.040) (Fig. 1A; Table 2). Patients with high PD-1+ TILs expression had a significantly better local control (p = 0.003), DFS (p = 0.007) and OS (p = 0.039) (Fig. 1B; Table 2). Similarly, high PD-L1 expression correlated with better local control (p = 0.017) (Fig. 1C; Table 2). Also, high FOXP3+ Tregs and intensity of Caspase-8 phosphorylation predicted for improved local control (p = 0.025 and p = 0.013, respectively) and DFS (p = 0.013 and p = 0.001, respectively) (Fig. S2; Table S2). Female sex and high HPV16 viral load correlated with better outcome for all three clinical endpoints, whereas high p16 expression only predicted for superior local control. Distant metastasis was more common in patients with advanced T stage (p < 0.001). Advanced N stage was associated with worse local control (p = 0.004), DFS (p = 0.010) and OS (p = 0.022).
Figure 1.
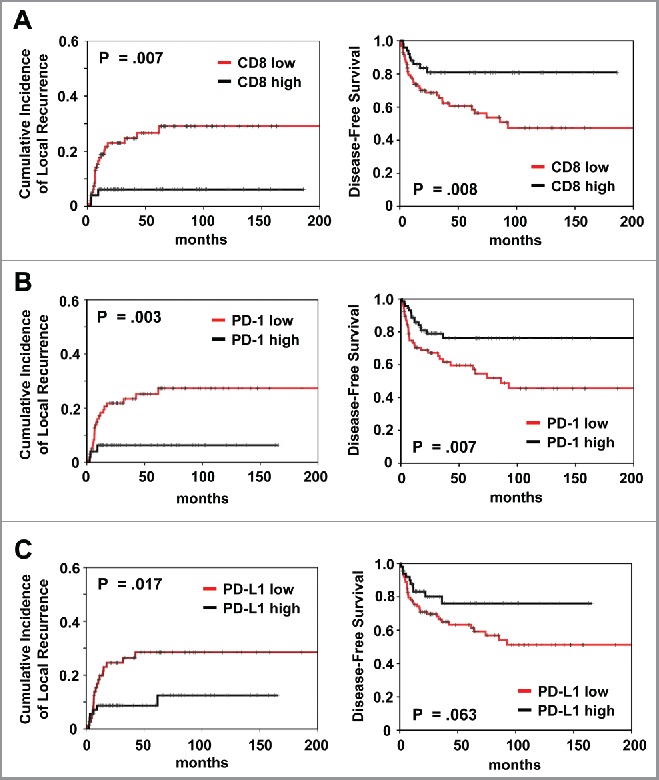
Prognostic impact of (A) CD8+ and (B) PD-1 and (C) PD-L1 expression on cumulative incidence of local recurrence and disease-free survival, as indicated. Analysis was based on the dichotomized total score in patient tumor samples (cut-off according to median value of total score).
Table 2.
Univariate and multivariate analyses of prognostic factors in patients with ASCC.
| Parameter |
Univariate |
|
Multivariate |
|
|---|---|---|---|---|
| |
p value |
HR |
95% CI |
p value |
| Cumulative incidence of local recurrence | ||||
| Age (>/≤ 59 y) | 0.710 | |||
| Sex (female/male) | 0.004 | 0.39 | 0.16 to 0.96 | 0.040 |
| T-category (T3/4 / T1/2) | 0.010 | 1.48 | 0.59 to 3.72 | 0.404 |
| N-category (N1–3/N0) | 0.004 | 2.29 | 0.99 to 5.30 | 0.054 |
| HPV16 load (>/≤ median)* | 0.002 | 0.28 | 0.10 to 0.77 | 0.014 |
| p16 (>/≤ median)* | 0.002 | 0.32 | 0.14 to 0.77 | 0.011 |
| CD8 (>/≤ median)* | 0.007 | 0.25 | 0.07 to 0.83 | 0.023 |
| PD-1 (>/≤ median)* | 0.003 | 0.29 | 0.12 to 0.72 | 0.007 |
| PD-L1 (>/≤ median)* | 0.017 | 0.27 | 0.08 to 0.90 | 0.033 |
| FOXP3 (>/≤median)* | 0.025 | 0.44 | 0.18 to 1.05 | 0.065 |
| pCasp-8 (>/≤ median)* | 0.013 | 0.48 | 0.20 to 1.17 | 0.105 |
| Disease-free survival | ||||
| Age (>/≤ 59 y) | 0.269 | |||
| Sex (female/male) | 0.004 | 0.44 | 0.23 to 0.82 | 0.010 |
| T-category (T3/4 / T1/2) | 0.060 | |||
| N-category (N1–3/N0) | 0.010 | 1.56 | 0.84 to 2.87 | 0.156 |
| HPV16 load (>/≤ median)* | 0.001 | 0.40 | 0.20 to 0.79 | 0.009 |
| p16 (>/≤ median)* | 0.088 | |||
| CD8 (>/≤ median)* | 0.008 | 0.42 | 0.20 to 0.87 | 0.020 |
| PD-1 (>/≤ median)* | 0.007 | 0.46 | 0.25 to 0.86 | 0.014 |
| PD-L1 (>/≤ median)* | 0.063 | |||
| FOXP3 (>/≤median)* | 0.013 | 0.54 | 0.29 to 1.00 | 0.050 |
| pCasp-8 (>/≤ median)* | 0.001 | 0.49 | 0.26 to 0.94 | 0.031 |
| Overall survival | ||||
| Age (>/≤ 59 y) | 0.050 | 2.32 | 1.13 to 4.73 | 0.021 |
| Sex (female/male) | 0.002 | 0.32 | 0.15 to 0.68 | 0.003 |
| T-category (T3/4 / T1/2) | 0.012 | 1.62 | 0.81 to 3.24 | 0.171 |
| N-category (N1–3/N0) | 0.022 | 1.11 | 0.51 to 2.43 | 0.789 |
| HPV16 load (>/≤ median)* | 0.005 | 0.44 | 0.20 to 0.95 | 0.036 |
| p16 (>/≤ median)* | 0.674 | |||
| CD8 (>/≤ median)* | 0.040 | 0.52 | 0.23 to 1.19 | 0.120 |
| PD-1 (>/≤ median)* | 0.039 | 0.54 | 0.26 to 1.10 | 0.090 |
| PD-L1 (>/≤ median)* | 0.250 | |||
| FOXP3 (>/≤median)* | 0.114 | |||
| pCasp-8 (>/≤ median)* | 0.011 | 0.51 | 0.24 to 1.07 | 0.073 |
Due to multicollinearity for HPV16, p16, CD8, PD-1, PD-L1, FOXP3 and caspase-8, and a limited number of events, multivariate analyses were performed separately including only one molecular parameter in conjunction with sex, T- and N-category each time. Only parameters found to be significant in the univariate analysis were included in the multivariate one. HRs and Cis originate from the respective, separate multivariate analysis.
Significant p values have been marked with bold.
Subsequently, we conducted a multivariant analysis by including factors that showed significance in univariant analysis (Table 2). Of note, due to multicollinearity and the low number of local failure events (n = 26), multivariant analysis was performed separately for each of the immune cell markers, always including the standard clinicopathologic parameters (sex, T/N stage and age, if significant in univariant analysis). In the Cox model, female sex and high HPV16 viral load retained their significance for improved outcome for all three clinical endpoints. High CD8+ and PD-1+ TILs expression predicted for improved local control (p = 0.023 and p = 0.007, respectively) and DFS (p = 0.020 and p = 0.014, respectively). Local failure was significantly lower in patients with strong p16 (p = 0.011) and PD-L1 (p = 0.033) expression. Strong FOXP3+ Tregs (p = 0.050) and Caspase-8 phosphorylation (p = 0.031) only correlated with better DFS in multivariant analysis.
Moreover, we assessed the prognostic impact of CD8+ TILs, PD-1+ TILs and FOXP3+ Tregs according to tumor compartment (intraepithelial and stromal; Table S4; Fig. S3). High intraepithelial but not stromal CD8+ and PD-1+ TILs expression predicted for superior local control (p = 0.005 and p = 0.005, respectively), DFS (p = 0.013 and p = 0.002, respectively), and OS (p = 0.041 and p = 0.030, respectively). Improved local control (p = 0.031) and DFS (p = 0.020) was observed for stromal rather than intraepithelial compartment FOXP3+ Tregs expression (Table S4).
We also investigated the prognostic impact of CD8high/PD-L1high vs. CD8low/PD-L1low vs. CD8high/PD-L1low vs. CD8low/PD-L1high)15,16 based on both, the total CD8+ and PD-L1 score (Fig. 2A). Patients with CD8high/PD-L1low expression had both better local control (p = 0.002) and better DFS (p = 0.022) for the comparison between all groups. Similar effects were observed for the combined CD8+/PD-1 expression (Fig. 2B).
Figure 2.
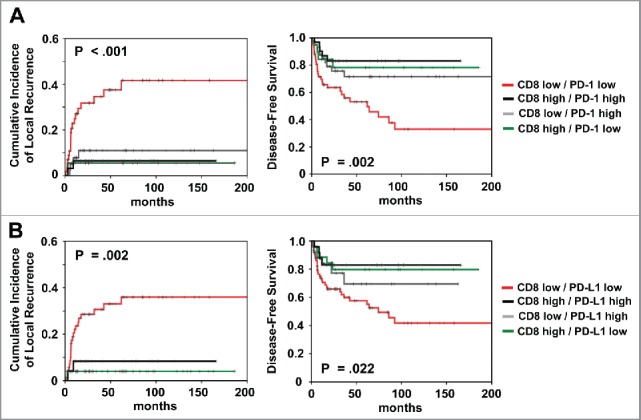
Prognostic impact of (A) combined total CD8+/PD-1 and (B) combined CD8+/PD-L1 expression on cumulative incidence of local recurrence and disease-free survival, as indicated. Analysis was based on the dichotomized total score in patient tumor samples (cut-off according to median value of total score).
The correlation of immune markers with HPV
Patients with high HPV16 viral load presented a significantly improved outcome compared with patients with low load in univariant analysis (cumulative incidence of local recurrence, p = 0.002; DFS, p = 0.001 and OS, p = 0.005) (Fig. 3; Table 2). High p16 expression correlated significantly with better local control (p = 0.002) but not DFS (p = 0.088).
Figure 3.
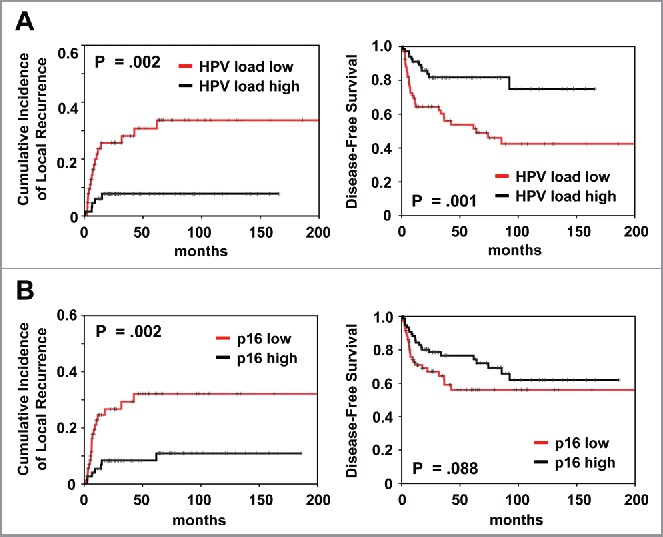
Prognostic impact of (A) HPV viral load and (B) p16 expression on cumulative incidence of local recurrence and disease-free survival, as indicated. Analysis was based on the dichotomized total score in patient tumor samples (cut-off according to median value).
Because HPV is directly linked with tumor immunogenicity, we analyzed the prognostic impact of CD8+ combined with HPV16 viral load and p16 (Fig. 4; Fig. S4). Patients with HPV16high/CD8high and p16high /CD8high tumors had superior local control (p = 0.003 and p = 0.002, respectively). Regarding DFS, patients with high HPV viral load and p16 expression presented a favorable outcome (p = 0.001 and p = 0.029, respectively), both in case of low and high CD8+ TILs expression. Similar data were observed for the combination of HPV16 and p16 with PD-1, PD-L1 and FOXP3 expression (Fig. 4; Fig. S4).
Figure 4.
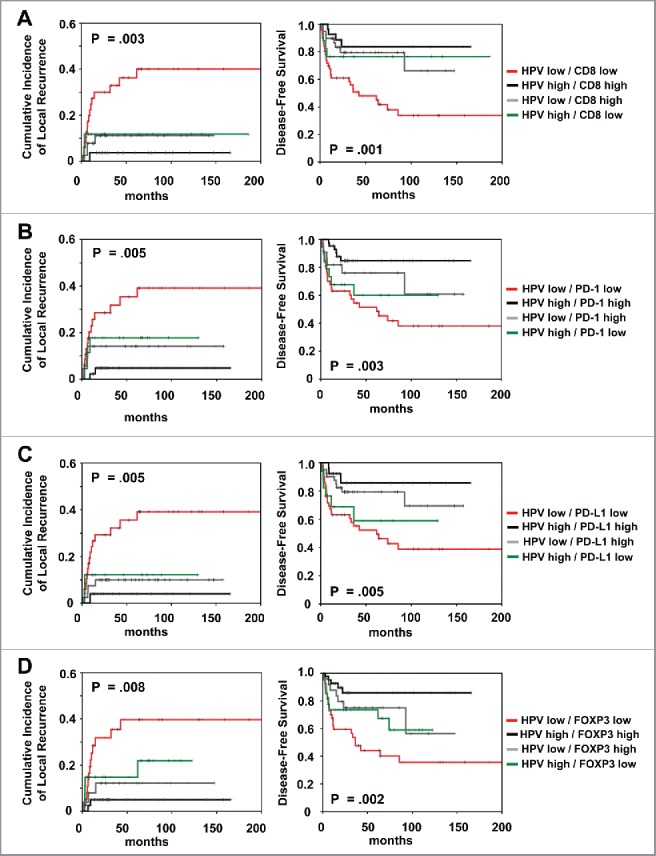
Prognostic impact of (A) combined HPV viral load/CD8+ expression and (B) combined HPV viral load/PD-1 expression and (C) combined HPV viral load/PD-L1 expression and (D) combined HPV viral load/FOXP3 expression on cumulative incidence of local recurrence and disease-free survival, as indicated. Analysis was based on the dichotomized total score in patient tumor samples (cut-off according to median value of total score).
Discussion
Patients with HPV16/p16 positive ASCC have a more favorable outcome with better response to CRT compared with HPV16/p16-negative patients.17,18 Several mechanisms, including impaired repair of DNA damage, cell cycle arrest and upregulation of TP53 have been proposed to explain the better outcome after CRT.19 The classical “5 Rs” of radiobiology20 cannot explain, why ASCC often show a delayed response over a period of months after completion of CRT.4 It becomes increasingly recognized that the higher immunogenicity of HPV-positive tumors might determine the improved response. Also, radiotherapy has profound immunomodulatory effects and can, via induction of an immunogenic cancer cell death activate cytotoxic T cells.21 Therefore, the slow regression often observed in ASCC might represent an activated immune response that unfolds progressively over a period of weeks after completion of CRT.
The role of the immune system in ASCC, however, remains largely unexplored.22 In that context, we demonstrated a strong association between high HPV16 viral load and high CD8+ and PD-1+ TILs expression in our series, supporting the notion that HPV can render tumors more immunogenic.22,23 Also, phosphorylated Caspase-8, a marker of apoptosis23 that is closely linked to immunogenic cell death,24 correlated linearly with HPV16 viral load and CD8+ TILs infiltration.
Although few studies have examined the impact of TILs and Tregs in human ASCC,13,14 the prognostic value of PD-1 and PD-L1, and their association with TILs and Tregs remain unknown. Here, patients with strong CD8+ TILs and PD-1+ TILs infiltration had a significantly superior local control and DFS compared with patients with low expression. Similar findings were observed for PD-L1 positivity and FOXP3+ Tregs for local recurrence and DFS, respectively. Accumulating evidence highlights the importance of TILs in mediating response to RT/CRT.25 CD8+ binds to the major histocompatibility complex class I molecule together with the T-cell receptor to elicit the cytotoxic effect of TILs on cancer cells.26,27 In agreement with our findings, numerous reports have revealed superior clinical outcomes in patients with high CD8+ TILs expression in esophageal, colorectal, head and neck, breast, ovarian, renal, pancreatic and lung cancer,28,29 whereas only two studies have confirmed the favorable prognostic role of TILs in ASCC.13,30
Strong intratumoral FOXP3+ Tregs infiltration was associated with HPV16-positivity and better local control in our work. Tregs can promote immune evasion but also exert anti-inflammatory effects that limit tumor progression.31 A meta-analysis in over 15,000 patients showed that high FOXP3+ Tregs expression predicted for worse survival in breast, cervical, melanoma and renal cancers, but better outcome in colorectal, esophageal and oropharyngeal cancers.32 Several groups have found higher Tregs infiltration in HPV-positive oropharyngeal cancer,33-35 whereas reports on the correlation of Tregs and HPV in ASCC are lacking. A plausible explanation for the unexpected positive role of Tregs in our analysis may also rely on the co-infiltration with effector T cells.
Regarding PD-1+ TILs, our data are in agreement with previous work demonstrating a positive prognostic impact for these cells in head and neck, ovarian cancer, pancreatic and colorectal cancer, among others.36-41 Albeit surprising due to its immunosuppressive function, several mechanisms could explain the paradox of better outcome in patients with PD-1+ TILs. Antigen-specific immune activation following T-cell receptor stimulation can lead to upregulation of PD-1 on TILs.42 Badoual et al. observed upregulation of the immune activation markers HLA-DR and CD38 in PD-1+ TILs as compared with PD-1- TILs. Thus, PD-1+ TILs could represent a previous endogenous antitumor immune response that decelerated tumor growth, albeit it failed to induce regression due to TILs dysfunction.11
The prognostic impact of CD8+ and PD-1+ TILs is tumor compartment-dependent.15 Indeed, high intratumoral but not stromal compartment CD8+ and TILs and PD-1+ TILs predicted for better local control and DFS in our cohort. Mixed findings have been reported regarding the clinical impact of TILs according to the tumor compartment in different malignancies that could be attributed to the heterogeneous patient cohorts and treatments.43-47
This analysis also provides more insight into other aspects of ASCC. In accordance to the literature,4 HIV-positivity was more common in young males, whereas we failed to detect a difference in immune marker expression according to HIV status. The comparable response and survival between HIV-positive and HIV-negative patients with ASCC indicates that antiretroviral treatment facilitates adequate immune response in this group.4 Also, consistent with numerous reports, female sex predicted for superior outcome in our series. Interestingly, female sex was strongly correlated with a higher HPV-16 viral load that could explain the well-known clinical observation of favorable prognosis in female patients with ASCC, at least in part.
A four group classification according to the combined TILs/PD-L1 status has been proposed in melanoma to guide future immunotherapy decisions.15,16,48 These included type I (TILshigh/PD-L1high mediating adaptive immune resistance), type II (TILslow/PD-L1low mediating immunologic ignorance), type III (TILslow/PD-L1high mediating intrinsic induction) and type IV (TILshigh/PD-L1low mediating tolerance).15,16 In our cohort, type I ASCC (CD8high/PD-L1high) showed better local control and DFS compared with the other groups. Similar findings were observed for CD8high/PD-1high tumors. Examination of the immuno-genomic properties in the whole Cancer Genome Atlas (n = 9677 cases) revealed a strong association between type I tumors and high mutational burden23 that correlates with better response to immunotherapies.49 Indeed, melanoma responders to immune checkpoint inhibitors had higher baseline expression of CD8+ and PD-1+ TILs and PD-L1 upregulation due to release of IFNγ by PD-1+ TILs.11 Such adaptive immune resistance have also been reported in breast cancer50 and Merkel cell carcinoma.51 The efficacy of immune checkpoint inhibitors appears to be higher in patients with pre-existing immunity suppressed by the PD-1/PD-L1 pathway that can be reinvigorated with these drugs.38 Our analysis thus provides an important framework for future combination strategies with CRT in ASCC since type I malignancies are most likely to benefit from immune checkpoint blockade.
Our work has limitations. First, the retrospective design of this analysis could have resulted in selection bias. Second, the number of events observed was small that limited multivariant analyses. Third, the median follow-up was relatively short. Clearly, our findings warrant validation in other data sets and possibly in a prospective cohort.
In conclusion, we here showed that high tumor HPV16 viral load and associated infiltration with CD8+ and PD-1+ TILs may, in conjunction with PD-L1, identify patients with favorable prognosis after standard CRT for ASCC. The response to CRT can potentially be further enhanced by immune checkpoint inhibitors as these agents appear to be more effective in patients with immunogenic tumors, and high CD8+ and PD-1+ TILs expression in baseline. In that context, nivolumab a monoclonal antibody against PD-1, demonstrated impressive response rates in 37 heavily pre-treated patients with PD-L1+ metastatic ASCC.52 Our data provide a strong rationale for testing the efficacy of immune checkpoint inhibitors or other forms of immunotherapy with CRT to improve the outcome in patients with ASCC.
Patients and methods
Patient and treatment characteristics
In total, 150 patients were treated with primary CRT for ASCC at the Departments of Radiotherapy at the University Hospital of Frankfurt/Main and at the University Medical Center of Göttingen. Written consent and approval from the institutional review board had been previously obtained, also in accordance with the Helsinki Declaration of 1975. The eligibility criteria for this analysis were histological confirmation of anal SCC, curative intent of 5-fluorouracil (5-FU)/mitomycin C-based CRT and lack of previous malignancies. As part of disease staging, patients received clinical examination, CT/MRI of the abdomen and pelvis, chest X-ray, proctoscopy with biopsy, complete blood count and serum chemistry.
Radiotherapy was applied using linear accelerators (Elekta, Crowley, UK; Varian, Palo Alto, USA) with either 3-D conformal RT or intensity-modulated RT with a median dose of 53.4 Gy (range 46.8–64.8 Gy, including the boost) using daily fractions of 1.8–2 Gy. Two patients received a brachytherapy-boost of 10 Gy. An external boost to the primary tumor and/or enlarged lymph nodes was applied in 71 patients using a median dose of 7.2 Gy (range 3.6–19.8 Gy). Chemotherapy consisted of two cycles of 5-fluorouracil (1.000 mg/m2/24 h) either as 4- or 5-d continuous infusion in the first and fifth week of RT, whereas mitomycin C (10 mg/m2) was applied as intravenous bolus on day one of each cycle.
Follow-up examination
Patients were initially assessed 8–10 weeks after completion of therapy and thereafter every 3 mo for the first 2 y followed by 6-mo intervals. Follow-up examination included rectal-digital examination, proctoscopy (with biopsies taken in case of suspicious residual tumor), and pelvic CT/MRI-scan.
Immunohistochemical staining and scoring of immune cell populations and markers
Slides from 150 patients were subjected to an automatic staining procedure with standardized DAKO EnVision™ FLEX Peroxidase Blocking reagent (K8000, DAKO, Hamburg, Germany) on a DAKO Autostainer Link 48 (DAKO). Antigen retrieval was performed via pretreatment of the paraffin sections (SuperFrost Plus, Thermo Scientific) using either an Epitope Retrieval Solution (Trilog, Cell Marque, Rocklin, CA) or Citrate Buffer 6.0 (Abcam) for 20 min. Slides were stained with the primary antibodies for either CD8+ (1:100, clone C8/144B; Dako M7103), FOXP3 (1:300, clone 236A/E7; Abcam AB20034), PD-1 (1:100, clone NAT105; Abcam AB52587), PD-L1 (1:50, clone E1L3N(R); Cell Signaling Technology) and T273 phosphorylated Caspase-8 antibodies23 for 120 min at room temperature. Following this, dextran polymer conjugated horseradish peroxidase and 3,3′-diamino-benzidine (DAB) chromogen or LSAB Detection system (PD-1, K5005, Dako) was used for visualization of the epitope–antibody reaction product and hematoxylin solution (Gill 3, Sigma Aldrich, Munich, Germany) for counterstaining. Negative control slides in the absence of primary antibodies were included. The expression of CD8+ TILs, PD-1+ TILs, PD-L1 tumor (and myeloid cells) and FOXP3+ Tregs was scored semi-quantitatively via measurement of cell density as described before.43 Scoring was as follows: (1) no, or sporadic cells; (2) moderate numbers of cells; (3) abundant occurrence of cells and (4) highly abundant occurrence of cells. Cell density was assessed in both the intra-epithelial compartment and stromal compartment. The total score was calculated by adding the separate scores from both compartments (range, 2–8). The median score was used as cut-off to classify patients into two groups: low or high CD8+, PD-1+, PD-L1 and FOXP3+ cells expression. We did not score PD-L1 separately in the intra-epithelial and stromal compartment. Analysis of intensity Caspase-8 T273 phosphorylation as well as PCR-based HPV16 viral load detection and histochemical p16INK4a (CINtec histology Kit, Roche) expression were reported in detail before.9,53 Images were acquired with the AxioImager Z1 microscope using the Axiovision 4.6 software (Zeiss, Germany). To minimize interobserver variability, two investigators (PB and FR) without knowledge of the clinicopathologic data performed scoring. In cases of discrepancy, a final decision was made after additional examination of the specimens.
Statistical analysis
The Spearman's coefficient assessed the correlation between the different parameters. The cumulative incidence of locoregional failure was calculated from the beginning of CRT to non-complete response at restaging or locoregional tumor detection after initial complete response. Data from patients, who were alive and free of recurrences or who died without having a recurrence were censored for these endpoints. DFS was measured from the beginning of CRT to the day of locoregional failure or distant recurrence, or death from any cause. OS was calculated from the beginning of CRT to death for any reasons or to cancer-related death, or the day of the last follow-up. The Kaplan–Meier method was used to plot the clinical endpoints. Univariable and multivariant analyses were conducted using the log-rank test and the Cox proportional hazard model, respectively. Due to multicollinearity for HPV16, p16, CD8+, PD-1, PD-L1, FOXP3 and pCaspase-8, and a limited number of events, multivariable analyses were performed separately for each immune marker, including only one each time, in conjunction with sex, T- and N-stage. Only parameters found to be significant in the univariant analysis were included in the multivariable one. A p < 0.05 was considered statistically significant. Statistical analyses were performed using the IBM SPSS Version 21.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors gratefully acknowledge the excellent technical assistance of Mrs Yvonne Michel, Senckenberg Institute of Pathology, Goethe-University, Frankfurt am Main, Mrs Nabila Ristow and Mrs Monika Junk, National Reference Center for Papilloma- and Polyomaviruses, University of Cologne, and Mr Julius Oppermann, Department of Radiotherapy and Oncology, Goethe-University, Frankfurt am Main.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2016; 66:7-30; PMID:28055103; http://dx.doi.org/18430910 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB 3rd, Thomas CR Jr., Mayer RJ, Haddock MG, Rich TA, Willett C. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. Jama 2008; 299:1914-21; PMID:18430910; http://dx.doi.org/ 10.1001/jama.299.16.1914 [DOI] [PubMed] [Google Scholar]
- 3.James RD, Glynne-Jones R, Meadows HM, Cunningham D, Myint AS, Saunders MP, Maughan T, McDonald A, Essapen S, Leslie M et al.. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol 2013; 14:516-24; PMID:23578724; http://dx.doi.org/ 10.1016/S1470-2045(13)70086-X [DOI] [PubMed] [Google Scholar]
- 4.Glynne-Jones R, Nilsson PJ, Aschele C, Goh V, Peiffert D, Cervantes A, Arnold D, ESMO; ESSO; ESTRO . Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother Oncol 2014; 111:330-9; PMID:24947004; http://dx.doi.org/ 10.1016/j.radonc.2014.04.013 [DOI] [PubMed] [Google Scholar]
- 5.De Vuyst H, Clifford G, Li N, Franceschi S. HPV infection in Europe. Euro J Cancer 2009; 45:2632-9; PMID:19709878; http://dx.doi.org/ 10.1016/j.ejca.2009.07.019 [DOI] [PubMed] [Google Scholar]
- 6.Bernardi MP, Ngan SY, Michael M, Lynch AC, Heriot AG, Ramsay RG, Phillips WA. Molecular biology of anal squamous cell carcinoma: implications for future research and clinical intervention. Lancet Oncol 2015; 16:e611-21; PMID:26678214; http://dx.doi.org/ 10.1016/S1470-2045(15)00292-2 [DOI] [PubMed] [Google Scholar]
- 7.Serup-Hansen E, Linnemann D, Skovrider-Ruminski W, Hogdall E, Geertsen PF, Havsteen H. Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American Joint Committee on cancer stages I to III carcinoma of the anal canal. J Clin Oncol 2014; 32:1812-7; PMID:24821878; http://dx.doi.org/ 10.1200/JCO.2013.52.3464 [DOI] [PubMed] [Google Scholar]
- 8.Kim JY, Park S, Nam BH, Roh JW, Lee CH, Kim YH, Shin HJ, Lee SK, Kong SY, Seong MW et al.. Low initial human papilloma viral load implicates worse prognosis in patients with uterine cervical cancer treated with radiotherapy. J Clin Oncol 2009; 27:5088-93; PMID:19770372; http://dx.doi.org/ 10.1200/JCO.2009.22.4659 [DOI] [PubMed] [Google Scholar]
- 9.Rödel F, Wieland U, Fraunholz I, Kitz J, Rave-Frank M, Wolff HA, Weiss C, Wirtz R, Balermpas P, Fokas E et al.. Human papillomavirus DNA load and p16INK4a expression predict for local control in patients with anal squamous cell carcinoma treated with chemoradiotherapy. Int J Cancer 2015; 136:278-88; PMID:24839133; http://dx.doi.org/ 10.1002/ijc.28979 [DOI] [PubMed] [Google Scholar]
- 10.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27:450-61; PMID:25858804; http://dx.doi.org/ 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016; 16:275-87; PMID:27079802; http://dx.doi.org/ 10.1038/nrc.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callahan MK, Postow MA, Wolchok JD. Targeting T cell co-receptors for cancer therapy. Immunity 2016; 44:1069-78; PMID:27192570; http://dx.doi.org/ 10.1016/j.immuni.2016.04.023 [DOI] [PubMed] [Google Scholar]
- 13.Gilbert DC, Serup-Hansen E, Linnemann D, Hogdall E, Bailey C, Summers J, Havsteen H, Thomas GJ. Tumour-infiltrating lymphocyte scores effectively stratify outcomes over and above p16 post chemo-radiotherapy in anal cancer. Br J Cancer 2016; 114:134-7; PMID:26730577; http://dx.doi.org/ 10.1038/bjc.2015.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabenbauer GG, Lahmer G, Distel L, Niedobitek G. Tumor-infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res 2006; 12:3355-60; PMID:16740757; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-2434 [DOI] [PubMed] [Google Scholar]
- 15.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res 2015; 75:2139-45; PMID:25977340; http://dx.doi.org/ 10.1158/0008-5472.CAN-15-0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL et al.. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127ra37; PMID:22461641; http://dx.doi.org/20530316 10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C et al.. Human papillomavirus and survival of patients with oropharyngeal cancer. N Eng J Med 2010; 363:24-35; PMID:20530316; http://dx.doi.org/ 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rischin D, Young RJ, Fisher R, Fox SB, Le QT, Peters LJ, Solomon B, Choi J, O'Sullivan B, Kenny LM et al.. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol 2010; 28:4142-8; PMID:20697079; http://dx.doi.org/ 10.1200/JCO.2010.29.2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blitzer GC, Smith MA, Harris SL, Kimple RJ. Review of the clinical and biologic aspects of human papillomavirus-positive squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys 2014; 88:761-70; PMID:24606845; http://dx.doi.org/ 10.1016/j.ijrobp.2013.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steel GG, McMillan TJ, Peacock JH. The 5Rs of radiobiology. Int J Radiat Biol 1989; 56:1045-8; PMID:2574214; http://dx.doi.org/ 10.1080/09553008914552491 [DOI] [PubMed] [Google Scholar]
- 21.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015; 16:e498-509; PMID:26433823; http://dx.doi.org/ 10.1016/S1470-2045(15)00007-8 [DOI] [PubMed] [Google Scholar]
- 22.Andersen AS, Koldjaer Solling AS, Ovesen T, Rusan M. The interplay between HPV and host immunity in head and neck squamous cell carcinoma. Int J Cancer 2014; 134:2755-63; PMID:23913554; http://dx.doi.org/ 10.1002/ijc.28411 [DOI] [PubMed] [Google Scholar]
- 23.Ock CY, Keam B, Kim S, Lee JS, Kim M, Kim TM, Jeon YK, Kim DW, Chung DH, Heo DS. Pan-cancer immunogenomic perspective on the tumor microenvironment based on PD-L1 and CD8 T-cell infiltration. Clin Cancer Res 2016; 22:2261-70; PMID:26819449; http://dx.doi.org/ 10.1158/1078-0432.CCR-15-2834 [DOI] [PubMed] [Google Scholar]
- 24.Galluzzi L, Lopez-Soto A, Kumar S, Kroemer G. Caspases connect cell-death signaling to organismal homeostasis. Immunity 2016; 44:221-31; PMID:26885855; http://dx.doi.org/ 10.1016/j.immuni.2016.01.020 [DOI] [PubMed] [Google Scholar]
- 25.Demaria S, Formenti SC. Role of T lymphocytes in tumor response to radiotherapy. Front Oncol 2012; 2:95; PMID:22937524; http://dx.doi.org/ 10.3389/fonc.2012.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298-306; PMID:22419253; http://dx.doi.org/ 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 27.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565-70; PMID:21436444; http://dx.doi.org/ 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 28.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011; 105:93-103; PMID:21629244; http://dx.doi.org/ 10.1038/bjc.2011.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lieberman J, Shankar P, Manjunath N, Andersson J. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood 2001; 98:1667-77; PMID:11535496; http://dx.doi.org/ 10.1182/blood.V98.6.1667 [DOI] [PubMed] [Google Scholar]
- 30.Hu WH, Miyai K, Cajas-Monson LC, Luo L, Liu L, Ramamoorthy SL. Tumor-infiltrating CD8(+) T lymphocytes associated with clinical outcome in anal squamous cell carcinoma. J Surg Oncol 2015; 112:421-6; PMID:26287957; http://dx.doi.org/ 10.1002/jso.23998 [DOI] [PubMed] [Google Scholar]
- 31.Whiteside TL. The role of regulatory T cells in cancer immunology. Immunotargets Ther 2015; 4:159-71; PMID:27471721; http://dx.doi.org/ 10.2147/ITT.S55415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep 2015; 5:15179; PMID:26462617; http://dx.doi.org/ 10.1038/srep15179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Punt S, Dronkers EA, Welters MJ, Goedemans R, Koljenovic S, Bloemena E, Snijders PJ, Gorter A, van der Burg SH, Baatenburg de Jong RJ et al.. A beneficial tumor microenvironment in oropharyngeal squamous cell carcinoma is characterized by a high T cell and low IL-17(+) cell frequency. Cancer Immunol Immunother 2016; 65:393-403; PMID:26899388; http://dx.doi.org/ 10.1007/s00262-016-1805-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nasman A, Romanitan M, Nordfors C, Grun N, Johansson H, Hammarstedt L, Marklund L, Munck-Wikland E, Dalianis T, Ramqvist T. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PloS One 2012; 7:e38711; PMID:22701698; http://dx.doi.org/ 10.1371/journal.pone.0038711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heusinkveld M, Welters MJ, van Poelgeest MI, van der Hulst JM, Melief CJ, Fleuren GJ, Kenter GG, van der Burg SH. The detection of circulating human papillomavirus-specific T cells is associated with improved survival of patients with deeply infiltrating tumors. Int J Cancer 2011; 128:379-89; PMID:20473854; http://dx.doi.org/ 10.1002/ijc.25361 [DOI] [PubMed] [Google Scholar]
- 36.Darb-Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S, Lindner J, Budczies J, Bockmayr M, Dietel M, Denkert C et al.. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget 2016; 7:1486-99; PMID:26625204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webb JR, Milne K, Nelson BH. PD-1 and CD103 are widely coexpressed on prognostically favorable intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol Res 2015; 3:926-35; PMID:25957117; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0239 [DOI] [PubMed] [Google Scholar]
- 38.Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N et al.. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 2013; 73:128-38; PMID:23135914; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-2606 [DOI] [PubMed] [Google Scholar]
- 39.Chapon M, Randriamampita C, Maubec E, Badoual C, Fouquet S, Wang SF, Marinho E, Farhi D, Garcette M, Jacobelli S et al.. Progressive upregulation of PD-1 in primary and metastatic melanomas associated with blunted TCR signaling in infiltrating T lymphocytes. J Invest Dermatol 2011; 131:1300-7; PMID:21346771; http://dx.doi.org/ 10.1038/jid.2011.30 [DOI] [PubMed] [Google Scholar]
- 40.Carreras J, Lopez-Guillermo A, Roncador G, Villamor N, Colomo L, Martinez A, Hamoudi R, Howat WJ, Montserrat E, Campo E. High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol 2009; 27:1470-6; PMID:19224853; http://dx.doi.org/ 10.1200/JCO.2008.18.0513 [DOI] [PubMed] [Google Scholar]
- 41.Mlecnik B, Tosolini M, Charoentong P, Kirilovsky A, Bindea G, Berger A, Camus M, Gillard M, Bruneval P, Fridman WH et al.. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology 2010; 138:1429-40; PMID:19909745; http://dx.doi.org/ 10.1053/j.gastro.2009.10.057 [DOI] [PubMed] [Google Scholar]
- 42.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007; 27:670-84; PMID:17950003; http://dx.doi.org/ 10.1016/j.immuni.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 43.Dahlin AM, Henriksson ML, Van Guelpen B, Stenling R, Oberg A, Rutegard J, Palmqvist R. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol 2011; 24:671-82; PMID:21240258; http://dx.doi.org/ 10.1038/modpathol.2010.234 [DOI] [PubMed] [Google Scholar]
- 44.Nedergaard BS, Ladekarl M, Thomsen HF, Nyengaard JR, Nielsen K. Low density of CD3+, CD4+ and CD8+ cells is associated with increased risk of relapse in squamous cell cervical cancer. Br J Cancer 2007; 97:1135-8; PMID:17940503; http://dx.doi.org/ 10.1038/sj.bjc.6604001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C et al.. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010; 28:105-13; PMID:19917869; http://dx.doi.org/ 10.1200/JCO.2009.23.7370 [DOI] [PubMed] [Google Scholar]
- 46.Balermpas P, Michel Y, Wagenblast J, Seitz O, Weiss C, Rödel F, Rödel C, Fokas E. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer 2014; 110:501-9; PMID:24129245; http://dx.doi.org/ 10.1038/bjc.2013.640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diana A, Wang LM, D'Costa Z, Allen P, Azad A, Silva MA, Soonawalla Z, Liu S, McKenna WG, Muschel RJ et al.. Prognostic value, localization and correlation of PD-1/PD-L1, CD8 and FOXP3 with the desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget 2016; 7:40992-1004; PMID:27637082; http://dx.doi.org/25838375 10.18632/oncotarget.12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, Tjulandin SA, Gladkov OA, Holcombe RF, Korn R et al.. Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin Cancer Res 2016; 22:2848-54; PMID:26813359; http://dx.doi.org/25838375 10.1158/1078-0432.CCR-15-2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348:69-74; PMID:25838375; http://dx.doi.org/ 10.1126/science.aaa4971 [DOI] [PubMed] [Google Scholar]
- 50.Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y, Meeker A, Xu H, Sharma R, Lecksell K, Cornish TC et al.. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol 2016; 47:52-63; PMID:26527522; http://dx.doi.org/ 10.1016/j.humpath.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, Wang H, Xu H, Nayar SK, Wang TS, Sidransky D et al.. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res 2013; 1:54-63; PMID:24416729; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris VK, Ciombor KK, Salem ME, Nimeiri HS, Iqbal S, Singh PP, Polite BN, Deming DA, Chan E, Wade JL et al.. NCI9673: A multi-institutional eETCTN phase II study of nivolumab in refractory metastatic squamous cell carcinoma of the anal canal (SCCA). J Clin Oncol 34, 2016. (suppl; abstr 3503) [Google Scholar]
- 53.Helmke C, Raab M, Rödel F, Matthess Y, Oellerich T, Mandal R, Sanhaji M, Urlaub H, Rödel C, Becker S et al.. Ligand stimulation of CD95 induces activation of Plk3 followed by phosphorylation of caspase-8. Cell Res 2016; 26:914-34; PMID:27325299; http://dx.doi.org/ 10.1038/cr.2016.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


