Abstract
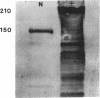
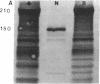
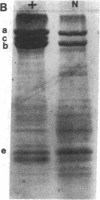
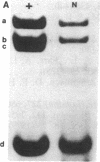
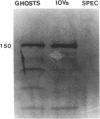
Mice with normoblastosis, nb/nb, have a severe hemolytic anemia. The extreme fragility and shortened lifespan of the mutant erythrocytes result from a defective membrane skeleton. Previous studies in our laboratory indicated a 50% deficiency of spectrin and an absence of normal ankyrin in erythrocyte membranes of nb/nb mice. We now report genetic mapping data that localize both the nb and erythroid ankyrin (Ank-1) loci to the centromeric end of mouse chromosome 8. Using immunological and biochemical methods, we have further characterized the nature of the ankyrin defect in mutant erythrocytes. We do not detect normal sized (210 kDa) erythroid ankyrin by immunoblot analysis in nb/nb reticulocytes. However, nb/nb reticulocytes do contain a 150-kDa ankyrin immunoreactive protein. The 150-kDa protein is present with normal-sized ankyrin in nb/+ reticulocytes but is not found in +/+ reticulocytes. Our genetic and biochemical data indicate that the nb mutation results from a defect in the erythroid ankyrin gene. A human hereditary spherocytosis putatively resulting from an ankyrin defect maps to a segment of human chromosome 8 that is homologous to the nb-ankyrin region of mouse chromosome 8. The linkage data suggest that the mouse and human diseases result from mutations in homologous loci.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey D. W. Recombinant-inbred strains. An aid to finding identity, linkage, and function of histocompatibility and other genes. Transplantation. 1971 Mar;11(3):325–327. doi: 10.1097/00007890-197103000-00013. [DOI] [PubMed] [Google Scholar]
- Bennett V., Branton D. Selective association of spectrin with the cytoplasmic surface of human erythrocyte plasma membranes. Quantitative determination with purified (32P)spectrin. J Biol Chem. 1977 Apr 25;252(8):2753–2763. [PubMed] [Google Scholar]
- Bennett V., Davis J. Erythrocyte ankyrin: immunoreactive analogues are associated with mitotic structures in cultured cells and with microtubules in brain. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7550–7554. doi: 10.1073/pnas.78.12.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1979 Aug 9;280(5722):468–473. doi: 10.1038/280468a0. [DOI] [PubMed] [Google Scholar]
- Bennett V. The membrane skeleton of human erythrocytes and its implications for more complex cells. Annu Rev Biochem. 1985;54:273–304. doi: 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- Bennett V. The spectrin-actin junction of erythrocyte membrane skeletons. Biochim Biophys Acta. 1989 Jan 18;988(1):107–121. doi: 10.1016/0304-4157(89)90006-3. [DOI] [PubMed] [Google Scholar]
- Bernstein S. E. Inherited hemolytic disease in mice: a review and update. Lab Anim Sci. 1980 Apr;30(2 Pt 1):197–205. [PubMed] [Google Scholar]
- Bodine D. M., 4th, Birkenmeier C. S., Barker J. E. Spectrin deficient inherited hemolytic anemias in the mouse: characterization by spectrin synthesis and mRNA activity in reticulocytes. Cell. 1984 Jul;37(3):721–729. doi: 10.1016/0092-8674(84)90408-2. [DOI] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Branton D., Cohen C. M., Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981 Apr;24(1):24–32. doi: 10.1016/0092-8674(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Ceci J. D., Justice M. J., Lock L. F., Jenkins N. A., Copeland N. G. An interspecific backcross linkage map of mouse chromosome 8. Genomics. 1990 Jan;6(1):72–79. doi: 10.1016/0888-7543(90)90449-5. [DOI] [PubMed] [Google Scholar]
- Chilcote R. R., Le Beau M. M., Dampier C., Pergament E., Verlinsky Y., Mohandas N., Frischer H., Rowley J. D. Association of red cell spherocytosis with deletion of the short arm of chromosome 8. Blood. 1987 Jan;69(1):156–159. [PubMed] [Google Scholar]
- Chui D. H., Patterson M., Bayley S. T. Unequal alpha and beta globin mRNA in reticulocytes of normal and mutant f/f fetal mice. Br J Haematol. 1980 Mar;44(3):431–439. doi: 10.1111/j.1365-2141.1980.tb05913.x. [DOI] [PubMed] [Google Scholar]
- Coetzer T. L., Lawler J., Liu S. C., Prchal J. T., Gualtieri R. J., Brain M. C., Dacie J. V., Palek J. Partial ankyrin and spectrin deficiency in severe, atypical hereditary spherocytosis. N Engl J Med. 1988 Jan 28;318(4):230–234. doi: 10.1056/NEJM198801283180407. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Ruddle F. H. Glutamate oxalate transaminase (GOT) genetics in Mus musculus: linkage, polymorphism, and phenotypes of the Got-2 and Got-1 loci. Biochem Genet. 1970 Apr;4(2):259–273. doi: 10.1007/BF00485777. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Frankel W. N., Stoye J. P., Taylor B. A., Coffin J. M. Genetic analysis of endogenous xenotropic murine leukemia viruses: association with two common mouse mutations and the viral restriction locus Fv-1. J Virol. 1989 Apr;63(4):1763–1774. doi: 10.1128/jvi.63.4.1763-1774.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Litman D., Hsu D. J., Marchesi V. T. Evidence that spectrin binds to macromolecular complexes on the inner surface of the red cell membrane. J Cell Sci. 1980 Apr;42:1–22. doi: 10.1242/jcs.42.1.1. [DOI] [PubMed] [Google Scholar]
- Lux S. E., Pease B., Tomaselli M. B., John K. M., Bernstein S. E. Hemolytic anemias associated with deficient or dysfunctional spectrin. Prog Clin Biol Res. 1979;30:463–469. [PubMed] [Google Scholar]
- Marchesi V. T. Stabilizing infrastructure of cell membranes. Annu Rev Cell Biol. 1985;1:531–561. doi: 10.1146/annurev.cb.01.110185.002531. [DOI] [PubMed] [Google Scholar]
- Nadeau J. H. Maps of linkage and synteny homologies between mouse and man. Trends Genet. 1989 Mar;5(3):82–86. doi: 10.1016/0168-9525(89)90031-0. [DOI] [PubMed] [Google Scholar]
- Nichols E. A., Ruddle F. H. Polymorphism and linkage of glutathione reductase in Mus musculus. Biochem Genet. 1975 Jun;13(5-6):323–329. doi: 10.1007/BF00485817. [DOI] [PubMed] [Google Scholar]
- Ouellette A. J., Pravtcheva D., Ruddle F. H., James M. Localization of the cryptdin locus on mouse chromosome 8. Genomics. 1989 Aug;5(2):233–239. doi: 10.1016/0888-7543(89)90051-7. [DOI] [PubMed] [Google Scholar]
- Palek J. Hereditary elliptocytosis, spherocytosis and related disorders: consequences of a deficiency or a mutation of membrane skeletal proteins. Blood Rev. 1987 Sep;1(3):147–168. doi: 10.1016/0268-960x(87)90031-2. [DOI] [PubMed] [Google Scholar]
- Rajput B., Marshall A., Killary A. M., Lalley P. A., Naylor S. L., Belin D., Rickles R. J., Strickland S. Chromosomal assignments of genes for tissue plasminogen activator and urokinase in mouse. Somat Cell Mol Genet. 1987 Sep;13(5):581–586. doi: 10.1007/BF01534500. [DOI] [PubMed] [Google Scholar]
- Rickles R. J., Darrow A. L., Strickland S. Molecular cloning of complementary DNA to mouse tissue plasminogen activator mRNA and its expression during F9 teratocarcinoma cell differentiation. J Biol Chem. 1988 Jan 25;263(3):1563–1569. [PubMed] [Google Scholar]
- Silver J. Confidence limits for estimates of gene linkage based on analysis of recombinant inbred strains. J Hered. 1985 Nov-Dec;76(6):436–440. doi: 10.1093/oxfordjournals.jhered.a110140. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Kronenberg M., Heinzmann C., Daher K. A., Klisak I., Ganz T., Mohandas T. Assignment of defensin gene(s) to human chromosome 8p23. Genomics. 1989 Aug;5(2):240–244. doi: 10.1016/0888-7543(89)90052-9. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Hargreaves W. R., Branton D. Purification of two spectrin-binding proteins: biochemical and electron microscopic evidence for site-specific reassociation between spectrin and bands 2.1 and 4.1. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5192–5196. doi: 10.1073/pnas.76.10.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Feng T. L., Opdenakker G., Volckaert G., Francke U. Human tissue-type plasminogen activator gene located near chromosomal breakpoint in myeloproliferative disorder. Am J Hum Genet. 1986 Jul;39(1):79–87. [PMC free article] [PubMed] [Google Scholar]
- de la Chapelle A., Icen A., Aula P., Leisti J., Turleau C., de Grouchy J. Mapping of the gene for glutathione reductase on chromosome 8. Ann Genet. 1976 Dec;19(4):253–256. [PubMed] [Google Scholar]