Abstract
Background
To investigate the prognostic value of procalcitonin (PCT), high-sensitivity C-reactive protein (hs-CRP), and pancreatic stone protein (PSP) in children with sepsis.
Material/Methods
A total of 214 patients with sepsis during hospitalization were enrolled. Serum levels of PCT, hs-CRP, and PSP were measured on day 1 of hospitalization and the survival rates of children were recorded after a follow-up of 28 days. Pearson’s correlation analysis was conducted to test the association of PCT, hs-CRP, and PSP with pediatric critical illness score (PCIS). Logistic regression models were used to analyze the risk factors contributing to patients’ death. The AUC was used to determine the value of PCT, hs-CRP, and PSP in the prognosis of patients with sepsis.
Results
The expression of PCT, hs-CRP, and PSP in the dying patients was higher than in the surviving patients (p<0.001). Pearson’s correlation analysis showed that serum PCT, hs-CRP, and PSP levels were negatively correlated with PCIS (p<0.001). Multivariate logistic regression revealed that PCT, hs-CRP, and PSP were independent risk factors for the prognosis of patients with sepsis (p<0.001). ROC analysis showed the AUC values of PCT, hs-CRP, and PSP were 0.83 (95% CI, 0.77–0.88), 0.76 (95% CI, 0.70–0.82), and 0.73 (95% CI, 0.67–0.79), respectively. The combined AUC value of PCT, hs-CRP, and PSP, was 0.92 (95% CI, 0.87–0.95), which was significantly increased compared with PCT, hs-CRP, or PSP (p<0.001).
Conclusions
The combination of serum PCT, hs-CRP, and PSP represents a promising biomarker of risk, and is a useful clinical tool for risk stratification of children with sepsis.
MeSH Keywords: C-Reactive Protein, Pancreatin, Sepsis
Background
Sepsis is the main cause of mortality in pediatric intensive care units (PICUs). It is caused by numerous infectious agents, inducing multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF), and even death [1]. Several studies have demonstrated that the severity of sepsis, as well as early diagnosis and prognosis were directly related to mortality [2,3].
Early diagnosis and prognosis are essential to effectively control sepsis, prevent the incidence of MODS or MOF, and reduce mortality in children with sepsis [4,5]. Currently, the second-generation pediatric index of mortality (PIM-2) or pediatric risk of mortality score (PRISM) are used to assess the severity and prognosis of children with sepsis internationally, while pediatric critical illness score (PCIS) is more commonly used in China [6,7]. PCIS is based on patients’ heart rate, blood pressure, PaO2, pH, Na+, K+, Cr, and Hb. A lower PCIS score indicates higher disease severity [8,9]. In addition, a few non-specific inflammatory markers, such as CD15s, NT-proBNP, soluble urokinase plasminogen activator receptor (suPAR), procalcitonin (PCT), high-sensitivity C-reactive protein (hs-CRP), and pancreatic stone protein (PSP) are established markers for prognostic evaluation of patients with sepsis [10–12]. Baseline procalcitonin levels are linked to severity of pediatric sepsis, while the persistent elevation in procalcitonin despite therapy are associated with increased mortality risk scores [13]. CRP, which is one of the most widely available, most studied, and most used laboratory tests for bacterial infection, has the best diagnostic accuracy when combined with another infection marker during the early phases of sepsis [14]. Recent studies suggest that PSP is a possible biomarker of multiorgan failure and mortality in sepsis [15]. However, its prognostic value in children with sepsis is not entirely clear.
In this study, we conducted a prospective analysis of 214 children with sepsis, to investigate the prognostic value of PCT, hs-CRP, and PSP. We further tested the prognostic value of the combination of the three parameters.
Material and Methods
Patients
We enrolled 214 children with sepsis admitted to intensive care units (ICU) of our hospital between March 2014 and October 2015. Every patient was diagnosed with sepsis according to clinical criteria defined in International Sepsis Definitions Conference in 2001 [16]. Severe sepsis was diagnosed when organ dysfunction, hypoperfusion, or hypotension including lactic acidosis, oliguria, or acute altered mental status occurred in septic patients. Exclusion criteria included: a). obviously irreversible condition; b). cause of death had no definite correlation with sepsis; and c). information was not complete for an earlier death during the study. Patients enrolled included 99 males and 115 females, with an average age of 4.6±1.5 years. We followed patients for up to 28 days. The clinical endpoint was all-cause mortality. All the patients in this study signed informed consent, which was approved by the ethics committee of the First People’s Hospital of Yichang.
Data collection
Age, sex, body height, weight, body mass index (BMI), blood pressure, and surgical history were recorded by specialists in the ICU. PCIS was evaluated by two specialists. If the two scores differed by more than 5 points, another ICU physician was invited to perform the final assessment.
Serum collection and testing
Fasting blood samples were drawn from each subject and used for biochemical analysis. Supernatants were obtained after centrifugation (4°C, 3,000 rev/minutes, 10 minutes) and stored at −80°C until further analysis. The serum levels of PCT and hs-CRP were tested by microparticle enzyme immunoassay (MEIA). PSP was measured using the DakoCytomation immunoturbidimetric assay.
Statistical analysis
SPSS, MedCalc, and GraphPad Prism were used to perform statistical analysis. Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were displayed as counts or percentages. Student’s t-test was used for the analysis of continuous variables and χ2-test for categorical variables; p<0.05 was considered significant.
Spearman correlation was used to analyze the relationship between PCT, hs-CRP, PSP, and PCIS. Multivariate logistic regression was used to analyze the risk factors for 28-day mortality in patients with sepsis. Receiver operating characteristic (ROC) analysis was used to compare the prognostic value of PCT, hs-CRP, and PSP in children with sepsis. Furthermore, standard indices of validity, such as Youden index, sensitivity, and specificity were calculated based on the ROC results.
Results
Baseline patient demographics
A total of 214 patients were enrolled in this study, with an average age of 4.6±1.5 years, and including 99 males and 115 females. After a follow-up of 28 days, 79 patients were dead, accounting for 36.92% of all the study children. No significant differences in patients’ age, sex, or weight were found between the dying and surviving groups of patients. PCIS scores in the dying patients were lower than in the surviving group (p<0.001). Further, the serum PCT, hs-CRP, and PSP levels were higher in the dying group than in the surviving group of patients (p<0.001; Table 1).
Table 1.
Baseline characteristics of patients with sepsis.
| Total (n=214) | Survival (n=135) | Death (n=79) | P-value | |
|---|---|---|---|---|
| Age(years) | 4.6 (1.5–11.9) | 4.8 (1.5–12.7) | 4.6 (1.7–12.7) | 0.213 |
| Sex (%) | ||||
| Male | 99 (46.3%) | 47 (47.5%) | 52 (52.5%) | 0.410 |
| Infection sites | ||||
| Chest | 86 (40.2%) | 51 (37.8%) | 35 (44.3%) | 0.325 |
| Abdomen | 57 (26.6%) | 37 (27.4%) | 20 (25.3%) | 0.217 |
| Combined* | 38 (17.8%) | 23 (17.0%) | 15 (19.0%) | 0.146 |
| Other | 33 (15.4%) | 24 (17.8%) | 9 (11.4%) | 0.217 |
| Etiology | ||||
| Gram stain positive | 110 (51.4%) | 68 (50.4%) | 42 (53.2%) | 0.136 |
| Gram stain negative | 76 (35.5%) | 50 (38.5%) | 26 (32.9%) | 0.107 |
| Fungus | 21 (9.8%) | 14 (10.3%) | 7 (8.9%) | 0.114 |
| Other | 7 (3.3%) | 3 (2.2%) | 4 (5.1%) | 0.211 |
| PCIS | 57.9±16.2 | 67.7±15.9 | 47.6±11.3 | <0.001 |
| PCT (ng/mL) | 51.7±24.8 | 43.9±18.4 | 70.6±23.3 | <0.001 |
| hs-CRP (mg/mL) | 74.3±22.3 | 67.1±17.5 | 88.5±22.9 | <0.001 |
| PSP (ng/L) | 272.8±79.5 | 241.5±67.5 | 324.7±80.5 | <0.001 |
PCIS – pediatric critical illness score; PCT – procalcitonin; hs-CRP – high-sensitivity c-reactive protein; PSP – pancreatic stone protein; Combined* – combine chest and abdomen.
Correlation of PCT, hs-CRP, and PSP with PCIS
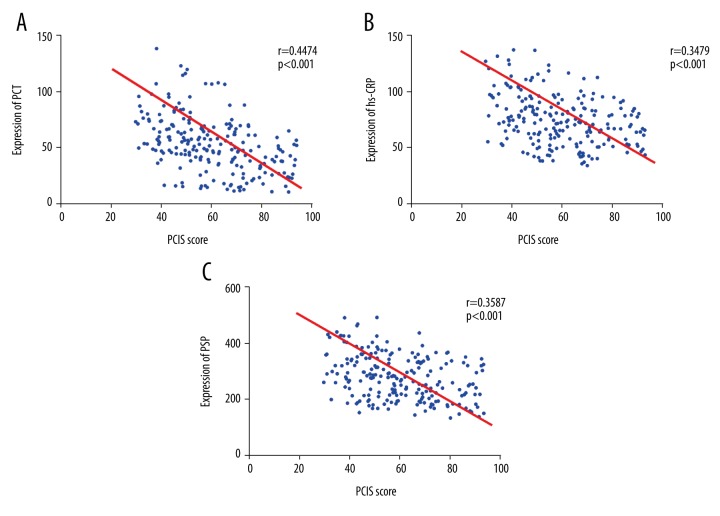
As shown in Figure 1, PCT was negatively correlated with PCIS, r=−0.4474 (p<0.001; Figure 1A); hs-CRP was negatively correlated with PCIS, significantly (r=−0.3479, p<0.001; Figure 1B); and PSP showed a distinctly negative correlation with PCIS (Figure 1C). The results indicated that the levels of PCT, hs-CRP, and PSP were correlated with disease severity.
Figure 1.
Correlation analysis: procalcitonin, high-sensitivity C-reactive protein and pancreatic stone protein with Pediatric Critical Illness Score. (A) PCT showed a negative correlation with PCIS; (B) hs-CRP was negatively correlated with PCIS; (C) PSP was negatively correlated with PCIS. PCIS – pediatric critical illness score; PCT – procalcitonin; hs-CRP – high-sensitivity C-reactive protein; PSP – pancreatic stone protein.
Analysis of risk factors for 28-day mortality
As shown in Table 2, multivariate logistic regression analysis revealed PCIS as a protective factor in the 28-day mortality of children with sepsis (OR=0.79; 95% CI=0.67–0.89). Conversely, PCT, hs-CRP, and PSP were risk factors. Furthermore, PSP (OR=2.38, 95% CI=1.46–5.76) was more sensitive than PCT (OR=1.34, 95% CI=1.02–2.25), p=0.0031. The relationship between PSP and hs-CRP was not significant (p=0.114).
Table 2.
Multivariable Logistic regression analysis for patients with sepsis.
| OR | 95% CI | P-value | |
|---|---|---|---|
| Age (years) | 1.31 | 0.82–1.89 | 0.36 |
| Male | 1.15 | 1.07–1.29 | 0.351 |
| Weight (kg) | 1.04 | 1.01–1.11 | 0.017 |
| PCIS | 0.79 | 0.67–0.89 | 0.011 |
| PCT (ng/mL) | 1.34 | 1.02–2.25 | 0.004 |
| hs-CRP (mg/mL) | 1.79 | 1.31–2.42 | 0.001 |
| PSP (ng/L) | 2.38 | 1.46–5.76 | 0.001 |
PCIS – pediatric critical illness score; PCT – procalcitonin; hs-CRP – high-sensitivity c-reactive protein; PSP – pancreatic stone protein.
Prognostic value of PCT, hs-CRP and PSP: ROC curves
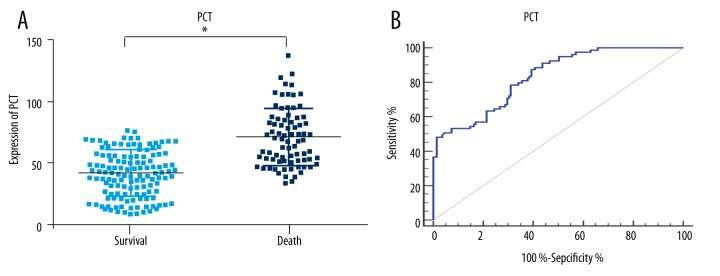
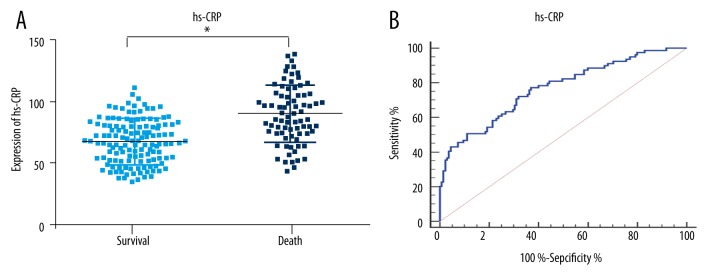
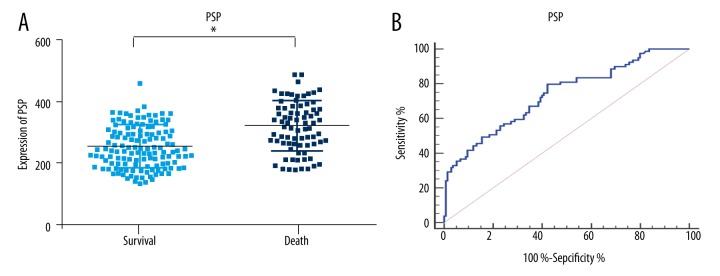
The serum concentrations of PCT, hs-CRP, and PSP were higher in the dying group of patients (p<0.01; Figures 2–4). To further determine the prognostic value of the three markers in children with sepsis, receiver operating characteristic (ROC) curves were used to evaluate the predictive power. The results indicated that area under the curve (AUC) values of PCT, hs-CRP, and PSP were 0.83 (95% CI, 0.77–0.88), 0.76 (95% CI, 0.70–0.82) and 0.73 (95% CI, 0.67–0.79), respectively. As illustrated in Table 3, the sensitivity and specificity of cutoff values were calculated according to ROC curve analysis. Subsequently, the ROC curve comparison revealed a higher prognostic value of PCT compared with hs-CRP and PSP (p<0.001) as shown in Figure 5. Multivariate logistic regression was conducted to calculate the coefficients of these biomarkers when used in predicting mortality in patients with sepsis (PCT & hs-CRP & PSP) = −12.3125 + 0.068404*PCT + 0.058065*hs-CRP + 0.012057*PSP. The results of ROC analysis of (PCT & hs-CRP & PSP) are shown in Table 3. The prognostic value of the combined PCT, hs-CRP, and PSP levels in children with sepsis was higher than the individual levels (p<0.001; Figure 5).
Figure 2.
Procalcitonin expression and ROC analysis of 28-day mortality in patients with sepsis. (A) PCT expression in the two groups. (B) ROC analysis of PCT in 28-day mortality of patients with sepsis. Cutoff value for PCT is 47 ng/mL. PCT – procalcitonin; * p<0.01, compared with survival.
Figure 3.
High-sensitivity C-reactive protein expression and ROC analysis in 28-day mortality. (A) Expression of hs-CRP in the two groups. (B) ROC analysis of hs-CRP in 28-day mortality. Cutoff value for hs-CRP is 76.1 mg/mL. hs-CRP – high-sensitivity C-reactive protein; * p<0.01, compared with survival.
Figure 4.
Pancreatic stone protein expression and ROC analysis in 28-day mortality. (A) PSP expression in the two groups; (B) ROC analysis of PSP in 28-day mortality. Cutoff value for PSP is 256 ng/L. PSP – pancreatic stone protein; * p<0.01, compared with survival.
Table 3.
ROC analysis of Procalcitonin, High-sensitivity C-reactive protein and Pancreatic Stone Protein on 28-days mortality of patients with sepsis.
| Values | auROC | 95%CI | P | Youden | Cut-off | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| PCT | 0.83 | 0.77–0.88 | <0.001 | 0.48 | 47 | 87.3% | 60.7% |
| hs-CRP | 0.76 | 0.70–0.82 | <0.001 | 0.40 | 76.1 | 72.1% | 68.1% |
| PSP | 0.73 | 0.67–0.79 | <0.001 | 0.37 | 256 | 79.7% | 57.7% |
| PCT &hs-CRP & PSP | 0.92 | 0.87–0.95 | <0.001 | 0.67 | 0.14 | 73.4% | 93.3% |
PCT – procalcitonin; hs-CRP – high-sensitivity c-reactive protein; PSP – pancreatic stone protein. Value of (PCT &hs-CRP & PSP) = −12.3125 + 0.068404*PCT + 0.058065*hs-CRP + 0.012057*PSP
Figure 5.
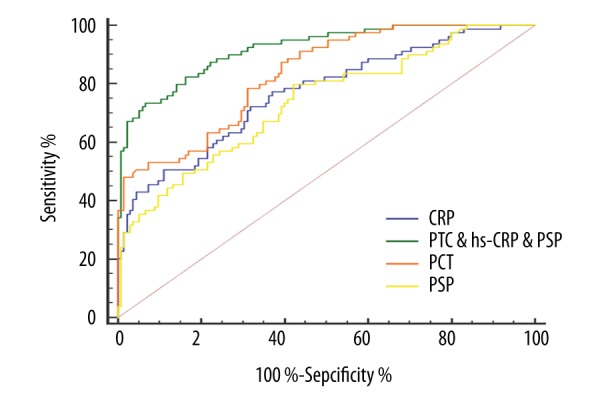
ROC analysis of serum procalcitonin, high-sensitivity C-reactive protein and pancreatic stone protein in 28-day mortality of patients with sepsis. PCT – procalcitonin; hs-CRP – high-sensitivity C-reactive protein; PSP – pancreatic stone protein.
Discussion
Sepsis is routinely observed in pediatric intensive care units (PICU). It develops into severe sepsis or septic shock, resulting in MODS, MOF, or even death. Interestingly, recent studies suggest that nonspecific inflammation and abnormal expression of inflammatory cytokines rather than microbial infection leads to organ damage [17]. Early diagnosis and intervention has been shown to reduce the risk of sepsis-related mortality [2,18]. In our study, the overall mortality was relatively high, which might be due to the high percentage (40.5%) of post-surgery patients.
Procalcitonin (PCT) is rarely released into vascular circulation under normal circumstances. Its diagnostic and predictive value in patients with sepsis has been confirmed in several studies [19]. Steinwald et al. found that serum PCT was an early systemic marker of sepsis, which was correlated closely with mortality and inversely with serum calcium in bacterial peritonitis of hamster [20]. Miglietta et al. found that serum PCT increased sharply during early sepsis, and that the persistent levels decreased only after effective antibiotic treatment [21]. In addition, Nakamura et al. found that PCT level was a good indicator of the severity of infection, with prognostic value in 393 adult patients with sepsis [22]. Similarly, Zurek et al. reported that serum levels of PCT were positively correlated with prognosis of pediatric sepsis [23]. Our results showed that PCT levels were significantly higher in the dying patients than in the surviving group, and were negatively correlated with PCIS, reminding us that PCT was related to the severity of sepsis. Furthermore, the results of ROC curve analysis indicated that an AUC value of 0.83 (95% CI, 0.77–0.88, supported the prognostic value of PCT in children with sepsis.
High-sensitivity C-reactive protein (hs-CRP) is a key inflammatory cytokine, which is present in small amounts under normal conditions. Plasma hs-CRP is an early marker of sepsis severity and poor prognosis, and is one of the risk factors for cardiovascular disease [24,25]. Pancreatic stone protein (PSP) belongs to the family of lectin-binding proteins, and is constitutively secreted by pancreatic acinar cells into pancreatic juice along with zymogens. PSP is a relatively novel marker and research related to it is sparse [26,27]. Palmiere et al. reported that PSP and PCT were positively correlated with mortality in patients with or without sepsis, and that the PSP levels were distinctly higher than in sepsis [28]. Peng et al. observed a significant positive correlation between PSP and WBC, as well as serum PCT levels [7]. Schlapbach et al. also used combined testing of PSP and PCT to diagnose early-onset sepsis [27]. The levels of PSP were closely related to the degree of infection. Dynamic monitoring of PSP was used to evaluate a patient’s condition and assess their risk of death. Our study showed that serum hs-CRP and PSP levels increased in dying patients and were negatively correlated with PCIS. These results indicated that serum hs-CRP and PSP levels were correlated with the severity of sepsis. Furthermore, multivariate logistic regression analysis revealed that both hs-CRP and PSP were independent risk factors for pediatric sepsis, with HRs of 1.79 (95% CI, 1.31–2.42) and 2.38 (95% CI, 1.46–5.76). In addition, ROC analysis showed that both hs-CRP and PSP levels were appropriate for clinical application.
It is unlikely that any single biomarker is a predictor of outcomes in the pediatric population. The complexity of interactions driving host immune response and genetic variation suggests that multiple biomarkers are involved in predicting outcomes. Stratification of pediatric patients based on their genome expression is a reasonable approach to identify high- and low-risk groups [2,4]. In our study, we used multivariate logistic regression to calculate each index before using the three markers: (PCT & hs-CRP & PSP) = −12.3125 + 0.068404*PCT + 0.058065*hs-CRP + 0.012057*PSP. The ROC curve analysis yielded an AUC value (PCT & hs-CRP & PSP) up to 0.92, which was clearly superior to PCT, hs-CRP, or PSP alone. Technological advances facilitate testing of multiple inflammatory cytokines rapidly and sensitively using small amounts of serum sample. Therefore, the joint detection of PCT, hs-CRP, and PSP clearly improves the sensitivity and specificity of prognosis in children with sepsis, and represents a useful serum biomarker.
Conclusions
Our study found that serum PCT, hs-CRP, and PSP levels were promising biomarkers of risk and useful clinical tools for risk stratification of pediatric sepsis. Furthermore, the combination of serum PCT, hs-CRP, and PSP is potentially a very useful biomarker with high sensitivity and specificity, and remarkable prognostic value in pediatric sepsis.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (No.832305823) and the Science Research and Development Project of Yichang (No. 2013 A13301-18)
Competing interest statement
All authors: no conflicts.
References
- 1.Samransamruajkit R, Uppala R, Pongsanon K, et al. Clinical outcomes after utilizing surviving sepsis campaign in children with septic shock and prognostic value of initial plasma NT-proBNP. Indian J Crit Care Med. 2014;18(2):70–76. doi: 10.4103/0972-5229.126075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briassoulis G, Galani A. Prognostic markers of pediatric meningococcal sepsis. Expert Rev Anti Infect Ther. 2014;2(9):1017–20. doi: 10.1586/14787210.2014.945431. [DOI] [PubMed] [Google Scholar]
- 3.Kyr M, Fedora M, Elbl L, et al. Modeling effect of the septic condition and trauma on C-reactive protein levels in children with sepsis: A retrospective study. Crit Care. 2007;11(3):R70. doi: 10.1186/cc5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong HR, Weiss SL, Giuliano JS, Jr, et al. Testing the prognostic accuracy of the updated pediatric sepsis biomarker risk model. PLoS One. 2014;9(1):e86242. doi: 10.1371/journal.pone.0086242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oren H, Cingoz I, Duman M, et al. Disseminated intravascular coagulation in pediatric patients: clinical and laboratory features and prognostic factors influencing the survival[J] Pediatr Hematol Oncol. 2005;22(8):679–88. doi: 10.1080/08880010500278749. [DOI] [PubMed] [Google Scholar]
- 6.Qi YZ, Muzhaper D. Levels and prognostic significance of serum procalcitonin and D-dimer in children with systemic inflammatory response syndrome. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16(4):384–88. [PubMed] [Google Scholar]
- 7.Qi YZ. Prognostic values of serum procalcitonin level and pediatric critical illness score in children with sepsis. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16(2):190–93. [PubMed] [Google Scholar]
- 8.Qian SY, Liu J. Relationship between serum albumin level and prognosis in children with sepsis, severe sepsis or septic shock. Zhonghua Er Ke Za Zhi. 2012;50(3):184–87. [PubMed] [Google Scholar]
- 9.Xiong Y, Wang J, Wei D, et al. Prognostic values of Th17 cells level in bronchoalveolar lavage fluid in children of sepsis with acute lung injury. Zhongguo Dang Dai Er Ke Za Zhi. 2015;17(9):942–45. [PubMed] [Google Scholar]
- 10.Jincharadze N, Abelashvili D, McHedlishvili M, Kacharava M. Diagnostic value of C-reactive protein test at early-onset sepsis in preterm infants. Georgian Med News. 2006;(130):87–91. [PubMed] [Google Scholar]
- 11.El-Mekkawy MS, Saleh NY, Sonbol AA. Soluble urokinase plasminogen activator receptor: A new biomarker in the pediatric intensive care unit. Indian J Pediatr. 2016;83(7):661–69. doi: 10.1007/s12098-016-2063-9. [DOI] [PubMed] [Google Scholar]
- 12.Markic J, Jeroncic A, Polancec D, et al. CD15s is a potential biomarker of serious bacterial infection in infants admitted to hospital. Eur J Pediatr. 2013;172(10):1363–69. doi: 10.1007/s00431-013-2047-y. [DOI] [PubMed] [Google Scholar]
- 13.Hatherill M, Tibby SM, Turner C, et al. Procalcitonin and cytokine levels: relationship to organ failure and mortality in pediatric septic shock. Crit Care Med. 2000;28(7):2591–94. doi: 10.1097/00003246-200007000-00068. [DOI] [PubMed] [Google Scholar]
- 14.Hofer N, Zacharias E, Muller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: Current insights and new tasks. Neonatology. 2012;102(1):25–36. doi: 10.1159/000336629. [DOI] [PubMed] [Google Scholar]
- 15.Jiri Z, Kyr M, Vavrina M, Fedora M. Pancreatic stone protein – a possible biomarker of multiorgan failure and mortality in children sepsis. Cytokine. 2014;66(2):106–11. doi: 10.1016/j.cyto.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–56. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 17.Xu XJ, Tang YM, Song H, et al. A multiplex cytokine score for the prediction of disease severity in pediatric hematology/oncology patients with septic shock. Cytokine. 2013;64(2):590–96. doi: 10.1016/j.cyto.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Rousseaux J, Grandbastien B, Dorkenoo A, et al. Prognostic value of shock index in children with septic shock. Pediatr Emerg Care. 2013;29(10):1055–59. doi: 10.1097/PEC.0b013e3182a5c99c. [DOI] [PubMed] [Google Scholar]
- 19.Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta-analysis. Lancet Infect Dis. 2013;13(5):426–35. doi: 10.1016/S1473-3099(12)70323-7. [DOI] [PubMed] [Google Scholar]
- 20.Steinwald PM, Whang KT, Becker KL, et al. Elevated calcitonin precursor levels are related to mortality in an animal model of sepsis. Crit Care. 1999;3(1):11–16. doi: 10.1186/cc300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miglietta F, Faneschi ML, Lobreglio G, et al. Procalcitonin, C-reactive protein and serum lactate dehydrogenase in the diagnosis of bacterial sepsis, SIRS and systemic candidiasis. Infez Med. 2015;23(3):230–37. [PubMed] [Google Scholar]
- 22.Nakamura Y, Murai A, Mizunuma M, et al. Potential use of procalcitonin as biomarker for bacterial sepsis in patients with or without acute kidney injury. J Infect Chemother. 2015;21(4):257–63. doi: 10.1016/j.jiac.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Zurek J, Vavrina M. Procalcitonin biomarker kinetics to predict multiorgan dysfunction syndrome in children with sepsis and systemic inflammatory response syndrome. Iran J Pediatr. 2015;25(1):e324. doi: 10.5812/ijp.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding H, Cao XY, Ma XG, Zhou WJ. Endothelial cell injury with inflammatory cytokine and coagulation in patients with sepsis. World J Emerg Med. 2013;4(4):285–89. doi: 10.5847/wjem.j.issn.issn.1920-8642.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adly AA, Ismail EA, Andrawes NG, El-Saadany MA. Circulating soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) as diagnostic and prognostic marker in neonatal sepsis. Cytokine. 2014;65(2):184–91. doi: 10.1016/j.cyto.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Schlapbach LJ, Giannoni E, Wellmann S, et al. Normal values for pancreatic stone protein in different age groups. BMC Anesthesiol. 2015;15:168. doi: 10.1186/s12871-015-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlapbach LJ, Graf R, Woerner A, et al. Pancreatic stone protein as a novel marker for neonatal sepsis. Intensive Care Med. 2013;39(4):754–63. doi: 10.1007/s00134-012-2798-3. [DOI] [PubMed] [Google Scholar]
- 28.Palmiere C, Augsburger M. Pancreatic stone protein as a postmortem biochemical marker for the diagnosis of sepsis. Leg Med (Tokyo) 2015;17(1):9–13. doi: 10.1016/j.legalmed.2014.08.003. [DOI] [PubMed] [Google Scholar]