Abstract
Increased sugar-sweetened beverage consumption has been linked to higher rates of obesity. Using functional MRI, we assessed brain perfusion responses to drinking two commonly consumed monosaccharides, glucose and fructose, in obese and lean adolescents. Marked differences were observed. In response to drinking glucose, obese adolescents exhibited decreased brain perfusion in brain regions involved in executive function (prefrontal cortex [PFC]) and increased perfusion in homeostatic appetite regions of the brain (hypothalamus). Conversely, in response to drinking glucose, lean adolescents demonstrated increased PFC brain perfusion and no change in perfusion in the hypothalamus. In addition, obese adolescents demonstrated attenuated suppression of serum acyl-ghrelin and increased circulating insulin level after glucose ingestion; furthermore, the change in acyl-ghrelin and insulin levels after both glucose and fructose ingestion was associated with increased hypothalamic, thalamic, and hippocampal blood flow in obese relative to lean adolescents. Additionally, in all subjects there was greater perfusion in the ventral striatum with fructose relative to glucose ingestion. Finally, reduced connectivity between executive, homeostatic, and hedonic brain regions was observed in obese adolescents. These data demonstrate that obese adolescents have impaired prefrontal executive control responses to drinking glucose and fructose, while their homeostatic and hedonic responses appear to be heightened. Thus, obesity-related brain adaptations to glucose and fructose consumption in obese adolescents may contribute to excessive consumption of glucose and fructose, thereby promoting further weight gain.
Introduction
In the U.S., one-third of adolescents are overweight or obese (1) and, thus, at increased risk for developing diabetes and metabolic syndrome (2–5). Increased consumption of sugar-sweetened beverages (SSBs), such as juice, flavored drinks, or soda, is postulated to be a contributor to the obesity epidemic (3). Indeed, adolescents consume the most added sugars, with ∼20% of their caloric intake accounted for by sugar and nearly half of those added-sugar calories coming from SSBs (6). In a recent study, Page et al. (7) reported that glucose, but not fructose, ingestion in lean adults reduced cerebral blood flow (CBF) in brain regions implicated in the regulation of appetite (hypothalamus) and reward-motivation processing (striatum) (7). Although adolescents consume the most added sugar, no previous research has assessed how ingestion of glucose and fructose affects the developing brain or how obesity may affect responses in appetite or higher-order executive brain pathways that influence intake of these monosaccharides. Notably, sugar, in the form of glucose, is the main energy source for the brain, but when consumed in large quantities, especially during periods of critical brain development (8), it may promote untoward, reward-driven eating behaviors (9,10).
It is known that glucose ingestion induces rapid changes in plasma glucose, insulin, and gut-derived hormones, such as the orexigenic hormone ghrelin, sensed by neurons in the hypothalamus (11) and in other brain regions implicated in reward motivation that regulate eating behavior (12). Fructose, on the other hand, is not a major fuel for the brain, and its peripheral metabolism and effects on insulin, as well as gut-derived hormones, are distinctly different from glucose (13,14). Compared with glucose, fructose has diminished capacity to increase circulating insulin as well as suppress ghrelin in lean adults (15,16), both of which modify food intake (17,18). We recently reported that obese adolescents have attenuated suppression of acyl-ghrelin, the active form of ghrelin, after fructose drink consumption (19). Ghrelin plays a role in energy balance, meal initiation, and appetite, and its dopaminergic and cholinergic effects have been implicated in promoting reward behavior (18). Thus, it is possible that acute changes in circulating hormones may influence the activation of reward and motivation-related brain regions after glucose or fructose consumption.
In the current study, we used pulsed arterial spin labeling (PASL) functional MRI (fMRI) to investigate regional CBF (brain perfusion) to assess response to drinking 75 g glucose and 75 g fructose (each dissolved in 300 mL cherry-flavored water, consumed in <5 min) in lean and obese adolescents. Additionally, we examined how time-dependent changes in peripheral hormones (including acyl-ghrelin and insulin) that occurred after drinking these monosaccharides were related to the observed neural responses. We hypothesized that obese adolescents would demonstrate a different perfusion response to drinking glucose and fructose, particularly in cortical (executive function), striatal (hedonic, reward motivation), and hypothalamic (homeostatic, hunger-satiety) regions of the brain, and that obesity-related metabolic responses would influence the observed perfusion responses.
Research Design and Methods
Research Subjects, Anthropometric Measures, and Oral Glucose Tolerance Test
We studied 14 lean (BMI >25th to <75th percentile) and 24 obese (BMI >95th percentile) and otherwise healthy adolescents (13–19 years old), matched for age, sex, ethnicity, and pubertal stage (Tanner stage 4–4.5). The adolescents were not taking any medications that could affect glucose metabolism and were excluded for pregnancy, endocrinopathies, or psychiatric disorders. Evaluation of dietary intake was assessed for each participant using a 3-day food record, which included 2 weekdays and 1 weekend day. The 3-day food diary was analyzed using the Nutrition Data System for Research (version 4.02; University of Minnesota). Twenty-four-hour recall, while retrospective and self-reported, offers the opportunity to quantify intake over multiple meals in a free-living environment. Analysis of the 3-day food record revealed that the percent of added sugar, which was for the most part represented by SSBs, was similar in the lean (17.8%) and obese (16.4%) groups. These data are consistent with the National Health and Nutrition Examination Survey 2007–2008 data of 17.3% in 12- to 17-year-old children (20).
Body composition was assessed by DEXA scan, weight, and height as previously described (21). On a day separate from the fMRI sessions, an oral glucose tolerance test (OGTT) was performed to assess glucose tolerance status and insulin sensitivity using the whole-body insulin sensitivity index (WBISI), calculated using the formula WBISI = 10,000/√([fasting plasma glucose × fasting plasma insulin] × [mean OGTT glucose concentration × mean OGTT insulin concentration]) as previously described (19). The study was approved by the Yale Human Investigation Committee. Participants provided assent and parents provided written informed consent to participate in the study.
fMRI Session: Glucose and Fructose Drink Ingestion
All subjects arrived at the Yale Magnetic Resonance Research Center at 7:00 a.m. after an overnight fast. An intravenous catheter was placed, and two baseline (−20 and 0 min) serum samples were obtained for insulin, glucose, leptin, and adiponectin. The scanning procedure was started at 8:00 a.m. (illustrated in Supplementary Fig. 7). A baseline perfusion (CBF) scan was performed in a 3-Tesla Trio scanner with imaging parameters as previously described (22). In a blinded, crossover design, each adolescent drank on two separate random-order days either a 75-g glucose drink or a 75-g fructose drink, dissolved in 300 mL cherry-flavored water with <1 g cherry flavoring made from citric acid and maltodextrin (to control for flavoring) within 5 min. After consuming the glucose or fructose drink, each adolescent underwent a series of perfusion scans for 60 min to measure the temporal brain response to the drink consumed. Throughout each perfusion scan, glucose and insulin were sampled at 10-min intervals. Samples were also obtained for measurement of plasma fructose levels before and at 20, 40, and 60 min after glucose and fructose ingestion. Hunger, satiety, and fullness ratings were assessed using a visual analog scale (higher number on the rating scale indicated stronger feeling) before each of the two drinks and after study completion.
Analytical Methods
Plasma glucose was assessed by glucose analyzer (YSI 2700 STAT Analyzer; Yellow Springs Instruments, Yellow Springs, OH), and plasma fructose levels were determined by use of gas chromatography–tandem mass spectrometry. Measurements of leptin, acyl-ghrelin, and adiponectin were determined with radioimmunoassays from Millipore. Blood samples obtained for acyl-ghrelin were prepared as previously described (19) in EDTA tubes immediately after collection at the bedside. Specifically, the whole blood was promptly treated with Pefabloc SC (Sigma-Aldrich, St. Louis, MO) to a final concentration of 1 mg/mL and then centrifuged for 10 min at 4°C, 4,000 rpm. The plasma obtained was acidified by adding HCl to a final concentration of 0.05 normal and stored at −80°C until analysis using the Millipore kit. All hormone measurements were performed in duplicate, and studies for each individual were analyzed with the same assay to eliminate potential effects from interassay variation.
fMRI Imaging Procedures and Acquisition
Imaging was performed on a 3-Tesla Siemens Trio scanner (Siemens Medical Systems, Erlangen, Germany) using a 12-channel head coil. Each adolescent was positioned in the coil, and head movements were minimized using foam pillows. After a three-plane localizer, a high-resolution whole-brain T1-weighted three-dimensional magnetization-prepared rapid gradient echo volume scan was acquired for multisubject registration. During the baseline condition, an oblique axial T1 image parallel to the anterior commissure–posterior commissure (AC-PC) line was acquired. One 5-min baseline resting state run was then acquired with the exact same prescription as the T1 blood oxygen level–dependent (BOLD) image. An axial T1 image was acquired for perfusion. One 5-min 8-s baseline perfusion run was then acquired with the exact same prescription as the T1 CBF image. The participant was taken out of the scanner and then ingested the drink in <5 min. Immediately after drink ingestion, the participant was placed back in the scanner, and a prescan localizer, a T1 BOLD image, five resting-state runs, a T1 CBF image, and five perfusion runs were acquired. Postdrink resting and perfusion CBF runs were acquired in an alternating fashion. CBF throughout the brain was measured by PASL using the EPISTAR QUIPSS PASL MRI technique as previously described (23). PASL magnetically tags the arterial blood before entering the brain and then examines the transit time for the tagged blood to reach specific tissues, thereby providing a direct measure of CBF (24). Resting state runs were acquired to examine functional connectivity. See Supplementary Data for details of imaging parameters.
Imaging Parameters
The PASL acquisition parameters were as follows: field of view (FOV) 200 × 200 mm2, matrix 128x64y, bandwidth 1,628, slice thickness 4 mm, and interslice spacing 2 mm. The repetition time (TR) was 3,000 ms, the echo time (TE) was 23 ms, and the flip angle (FA) was 90°. For quantification of regional CBF, proton density–weighted images were acquired with the same perfusion sequence, except for the following changes: TR 8,000 ms and TE 23 ms. T1-weighted anatomical scan (TR 300 ms, TE 3.69 ms, bandwidth 300 Hz/pixel, FA 60°, slice thickness 4 mm, FOV 200 × 200 mm, and matrix 256 × 256) was collected with 15 oblique axial slices parallel to the AC-PC. Resting BOLD measurements were acquired with the following parameters: 24 slices, 5 mm thick, 200 × 200 mm2, matrix 64 × 64; bandwidth 2,004, TR 1,500 ms, TE 30 ms, and FA 80°. A T1-weighted anatomical scan (TR 300 ms, TE 2.47 ms, bandwidth 300 Hz/pixel, FA 60°, slice thickness 5 mm, FOV 200 × 200 mm, and matrix 256 × 256) was collected with 24 oblique axial slices parallel to the AC-PC. A high-resolution three-dimensional volume was collected using a magnetization-prepared rapid gradient echo sequence (160 contiguous sagittal slices, TR 1,500 ms, TE 2.83 ms, bandwidth 220 Hz/pixel, FA 15°, slice thickness 1 mm, FOV 256 × 256 mm, and matrix 256 × 256).
All data were converted from Digital Imaging and Communication in Medicine format to Analyze format using XMedCon (xmedcon.sourceforge.net) (25). During the conversion process, six images were discarded to enable the signal to achieve steady-state equilibrium between radio frequency pulsing and relaxation. Perfusion-weighted and the proton density–weighted images were motion corrected using the Statistical Parametric Mapping package (SPM5). Resting state images were slice time–corrected using sinc interpolation and motion corrected using SPM5 (www.fil.ion.ucl.ac.uk/spm/software/spm5) for three translational directions (x, y, or z) and three rotations (pitch, yaw, or roll) (26). Trials with linear motion that had a displacement in excess of 2 mm or rotation in excess of 3° were rejected. Perfusion-weighted images were obtained by pairwise surround subtraction between interleaved label and control pairs (24,27) while the subject was at rest, resulting in a difference perfusion map every 2 TR. A complete perfusion map for each condition was the average of all difference maps for that condition. Details regarding the method and equation for calculating absolute CBF (mL/100 g/min) were previously provided (23).
To take the individual subject data into a common reference space, we calculated three registrations within the Yale BioImage Suite software package (http://www.bioimagesuite.org/ [28,29]). The first registration performed a linear registration between the individual subject’s raw functional image and that subject's two-dimensional anatomical image. The two-dimensional anatomical image was then linearly registered to the individual's three-dimensional anatomical image. Finally, a nonlinear registration was computed between the individual three-dimensional anatomical image and a commonly used three-dimensional reference image (the Colin Brain [30]) in Montreal Neurological Institute (MNI) space (31). All three registrations were applied sequentially to the individual maps to bring all data into the common reference space. A standard whole-brain template (MNI 1 mm), as used previously in adolescents (32–34), was used for spatial normalization of the individual data.
Selection of End Points/Outcome Measures
The primary outcome was relative change in CBF in response to acute glucose and fructose ingestion. Secondary outcomes included 1) use of ANCOVA to assess the effect of dynamic peripheral metabolic hormone changes on CBF response, 2) whole-brain correlations to assess the impact of fasting hormone levels on relative increases or decreases in regional CBF, and 3) functional connectivity analysis to investigate brain regions with MRI signal responses that were correlated with the neural responses in other brain regions.
fMRI Data Analyses and CBF Group Analyses
First-level analyses of CBF data were performed by assessing postdrink relative to precedent baseline for each of the glucose and fructose drink conditions. Second-level group analyses were conducted using whole-brain repeated-measures general linear model analyses to examine main effect of group (lean/obese) and drink (glucose/fructose) and the interaction between group and drink. These were followed by paired t test analyses to explain significant main effects and interactions and to assess responses to each drink in lean and obese groups. In addition, secondary ANCOVA analyses also examined the role of fasting leptin, glucose, insulin, and acyl-ghrelin as well as hunger ratings on CBF responses to group, drink, and their interactions. The significance threshold was set at P < 0.05, family-wise error (FWE) whole-brain corrected for each of the above comparisons. The AlphaSim script in Analysis of Functional NeuroImages (AFNI) (http://afni.nimh.nih.gov [35]) was used to perform the FWE correction (using a smoothing kernel of 6 mm) for multiple comparisons for the CBF data (http://afni.nimh.nih.gov) (35). Finally, whole-brain voxel-based correlation analyses were also conducted with fasting leptin, insulin, and acyl-ghrelin and change with drink, implemented via BioImageSuite with the application of AlphaSim in AFNI to perform FWE correction for multiple comparisons. All analyses were done in AFNI.
Connectivity
Baseline and postdrink connectivity maps for glucose and fructose were analyzed in the Yale BioImage Suite software. Data were converted to AFNI format (http://afni.nimh.nih.gov [11]) for group analysis. Within-group connectivity maps were then analyzed by applying the linear mixed effects model using the linear mixed effects modeling program 3dLME from AFNI (http://afni.nimh.nih.gov/sscc/gangc/lme.html). Results were converted back into Analyze format for viewing in BioImage Suite. AFNI AlphaSim script (http://afni.nimh.nih.gov [11]) was used to perform the FWE correction for multiple comparisons.
Individual seed-based connectivity analyses were performed to assess brain regions that are temporally and thus functionally related using the Yale BioImage Suite software package (http://www.bioimagesuite.org/) (28,29). Two seeds were defined on the MNI reference brain from the obese CBF glucose ingestion minus baseline map at P < 0.05, FWE whole-brain corrected: 1) bilateral hypothalamus and 2) the left prefrontal cortex (Brodmann area [BA]10). The resulting images were spatially smoothed with a 6-mm Gaussian kernel to account for variations in the location of activation across subjects.
Given that head motion can influence voxel-wise measures of connectivity (36), motion-based exclusion criteria were applied to the mean frame-to-frame displacement resulting in these analyses being conducted only in a subgroup of obese (n = 17) and lean (n = 8) adolescents who were not different in terms of head motion (37). The mean frame-to-frame displacement was calculated for both lean and obese groups. Runs having a mean frame-to-frame displacement between 0.07 and 0.15 mm were included for further analysis. As a result, the mean frame-to-frame displacement for the lean group was 0.09 mm (two-tailed t test between glucose postdrink and baseline P = 0.15) and for the obese group was 0.11 mm (two-tailed t test between glucose postdrink and baseline P = 0.65).
Results
As shown in Table 1, sex, age, and race were similar in both groups. As expected, all indices of body fat and lean body mass were increased in the obese (vs. the lean) group. The obese group also had increased fasting leptin (P < 0.0001), insulin (P < 0.0001), and glucose (P = 0.05) levels and reduced insulin sensitivity (WBISI) (P = 0.004), fasting acyl-ghrelin (P = 0.02), and adiponectin (P = 0.02) levels (Table 1).
Table 1.
Demographic and metabolic measures
| Lean | Obese | Significance | |
|---|---|---|---|
| N | 14 | 24 | |
| Sex, % m (m/f) | 71 (10/4) | 46 (11/13) | 0.181‡ |
| Race (B/W/L) | 4/5/5 | 8/8/8 | 0.955‡‡ |
| Age (years) | 15.8 ± 1.6 | 15.3 ± 1.8 | 0.401 |
| Height (cm) | 169 ± 10.5 | 166 ± 11.2 | 0.466 |
| Weight (kg) | 61.7 ± 8.7 | 93.4 ± 13.7 | <0.0001 |
| BMI (kg/m2) | 21.8 ± 2.3 | 34.4 ± 4.7 | <0.0001 |
| BMI Z score | 0.3 ± 0.6 | 2.2 ± 0.3 | <0.0001 |
| Body fat (%) | 22.2 ± 8.2 | 40.9 ± 6.9 | <0.0001 |
| Fat mass (kg) | 14.0 ± 5.3 | 39.1 ± 8.9 | <0.0001 |
| Lean body mass (kg) | 49.3 ± 10.0 | 56.4 ± 10.1 | 0.043 |
| Metabolic profile | |||
| Fasting glucose (mg/dL) | 88 ± 5.0 | 91.3 ± 5.4 | 0.050 |
| Fasting insulin (µU/mL) | 14.6 ± 6.2 | 33.5 ± 16.2 | <0.0001 |
| HOMA-IR | 3.9 ± 3.4 | 9.6 ± 11.7 | 0.086 |
| WBISI (L2/mg × µU) | 3.7 ± 1.6 | 2.1 ± 1.4 | 0.004 |
| Leptin (ng/mL) | 8.3 ± 9.4 | 37.1 ± 17.8 | <0.0001 |
| Adiponectin (µg/mL) | 10.9 ± 3.4 | 8.2 ± 3.1 | 0.020 |
| Fasting acyl-ghrelin (pg/mL) | 126.5 ± 41.8 | 96.9 ± 37.2 | 0.038 |
Data are means ± SD unless otherwise indicated. Independent samples t test. Glucose and insulin results are means of −20 and 0 min samples from both study days. WBISI assessed by OGTT. B/W/L, black/white/Latino; f, female; HOMA-IR, HOMA of insulin resistance; m, male.
‡Fisher test.
‡‡χ2.
Metabolic Response to Glucose and Fructose Ingestion
After glucose ingestion, plasma glucose rose similarly in both groups (P = not significant), whereas insulin was higher in obese compared with lean participants (group: F[1,36] = 8.76, P = 0.005; effect size: f = 0.48) (Supplementary Fig. 1). Additionally, in obese relative to lean adolescents the percent suppression of acyl-ghrelin was attenuated after glucose consumption (group: F[1,33] = 6.25, P = 0.02, f = 0.41) (Supplementary Fig. 2). After fructose consumption, a similar, but much smaller, increase in plasma glucose (P = not significant) occurred in both groups and plasma fructose also rose similarly (data not shown). The percent suppression of acyl-ghrelin was also attenuated after fructose ingestion (F[1,35] = 12.82, P = 0.001, f = 0.58), whereas insulin levels in the obese group were higher (F[1,36] = 11.81, P = 0.002, f = 0.57) (Supplementary Fig. 1).
There were no significant differences between the two groups in predrink baseline hunger, satiety, or fullness ratings. However, after both glucose and fructose ingestion, hunger increased significantly in the obese adolescents (P < 0.001 and P < 0.002, respectively), whereas it did not change in lean adolescents (Supplementary Fig. 3). Neither group reported changes in satiety or fullness after drinking glucose. However, lean adolescents reported increased fullness after drinking fructose (P = 0.034), which was not reported by the obese group.
Brain Response to Glucose and Fructose Ingestion
ANOVA/ANCOVA Analyses
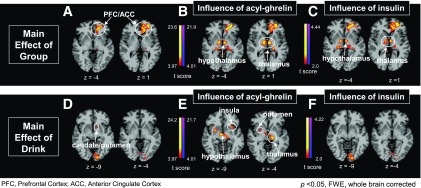
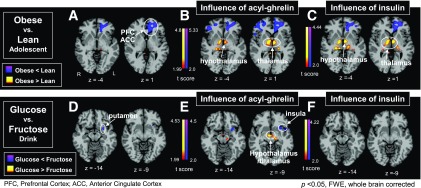
ANOVA and ANCOVA results for group and drink main effect and a group × drink interaction were analyzed. A significant group main effect in the ANOVA revealed reduced perfusion in the dorsolateral prefrontal cortex (PFC) and medial anterior cingulate cortex (ACC) (Fig. 1 and Supplementary Table 1) that was not related to the acyl-ghrelin or insulin response. We then conducted ANCOVA to discern whether hormonal responses to glucose and fructose consumption influenced the observed differences in cerebral perfusion. Covarying (ANCOVA) for acyl-ghrelin or insulin resulted in a significant group main effect that showed increased perfusion in the hypothalamus, thalamus, and hippocampus in the obese group relative to the lean group (Figs. 1 and 2). Neither acyl-ghrelin nor insulin responses to drink influenced the reduced perfusion in the PFC and ACC seen in the obese versus the lean group. A main effect of drink was observed in the ventral and dorsal striatum and hypothalamus (Fig. 1 and Supplementary Table 1), with greater perfusion in the ventral striatum with fructose relative to glucose (Fig. 2). Covarying main effect of drink for acyl-ghrelin response revealed additional increased perfusion in the putamen, thalamus, insula, and hypothalamus while covarying for insulin increased perfusion in visual regions (Fig. 2 and Supplementary Table 1). Covarying for leptin in main effect of drink did not show any significant CBF effects in ANCOVA models. Additionally, we examined main effect of sex and found activation in the fusiform gyrus but no significant sex × group or sex × drink interactions. Finally, there were no significant group × drink interactions in the ANOVA or ANCOVA described above.
Figure 1.
CBF main effect of group and main effect of drink. The main effect of group (ANOVA) (A) and the main effect of group covaried (ANCOVA) for change in acyl-ghrelin (B) and insulin (C) levels after drink consumption. D: The main effect of drink and the main effect of drink covaried (ANCOVA) for change in acyl-ghrelin (E) and insulin (F) levels after drink consumption. There was no significant group-by-drink interaction.
Figure 2.
CBF in obese vs. lean adolescents and glucose vs. fructose drink. A: A significant group main effect in obese vs. lean (drink vs. baseline) was observed with reduced blood flow (in blue) in obese relative to lean adolescents in the medial PFC and ACC. Additionally, drink-related changes in acyl-ghrelin (B) and insulin (C) levels promote increased CBF in the hypothalamus and thalamus in obese vs. lean adolescents (in yellow-red). D: Comparing response to glucose vs. fructose drink in all adolescents demonstrated a significant CBF main effect of drink with increased ventral striatum (putamen) activity in response to fructose (in blue) relative to glucose. E: Accounting for the drink-related acyl-ghrelin changes led to significantly greater hypothalamus and thalamus CBF with glucose relative to fructose drink (in yellow-red), while greater changes with fructose relative to glucose drink were demonstrated in the left insula and striatum (in blue). F: This effect was not observed in relation to change in insulin levels.
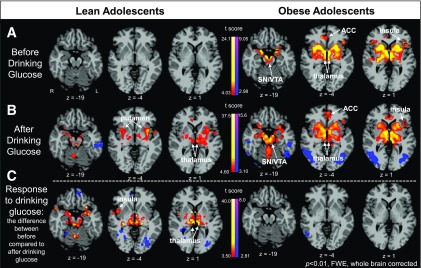
Pairwise post hoc analyses of within-group effects in each drink were conducted secondarily to explore whether specific effects of each drink were due to responses in obese or lean groups. In response to glucose ingestion (vs. baseline), lean adolescents demonstrated increased CBF in the PFC (BA10), ACC, insula, and caudate but not the hypothalamus. In contrast, the obese adolescents demonstrated decreased CBF in the PFC and ACC and increased CBF in the hypothalamus, caudate, putamen, insula, and thalamus. In response to fructose ingestion (vs. baseline), lean adolescents demonstrated decreased CBF in the parahippocampus, whereas obese adolescents once again demonstrated decreased CBF in the PFC and increased CBF in the ventral striatum (nucleus accumbens [NAcc], putamen) and thalamus (Supplementary Fig. 4 and Supplementary Table 2).
Whole-Brain Correlation Analyses
Whole-brain correlation analyses to assess the relationship between fasting leptin, as a reflection of fat mass, and CBF response to oral glucose and fructose ingestion were also performed. Correlation of fasting leptin was significantly inversely correlated with perfusion in the PFC in obese but not lean adolescents (Supplementary Fig. 5).
Exploratory Functional Brain Connectivity Analyses
To assess the different pattern of perfusion responses in lean and obese individuals, we conducted follow-up exploratory functional connectivity analyses with functional seeds in the bilateral hypothalamus region and in the left PFC (in BA10) for glucose and in the NAcc for fructose. These seeds were defined on the MNI reference brain by the differential perfusion response between the lean and obese adolescents (38) for the glucose ingestion versus baseline brain image map and for fructose ingestion versus baseline brain image maps at P < 0.05, FWE whole-brain corrected. Given that head motion can influence voxel-wise measures of connectivity (36), motion-based exclusion criteria were applied to the mean frame-to-frame displacement resulting in these analyses being conducted only in a subgroup of obese and lean adolescents who did not differ in head motion (37).
Within-Group Functional Brain Connectivity in Response to Oral Glucose
Within-group connectivity analysis in lean adolescents revealed no significantly increased connectivity with the hypothalamic seed before drink consumption, but in response to glucose ingestion relative to the baseline condition, greater connectivity was observed between the hypothalamus seed and the striatum and amygdala (P < 0.01, FWE) (Fig. 3C). Conversely, obese adolescents showed significant connectivity in the hypothalamic-corticolimbic-striatal network (ACC, insula, thalamus, putamen, caudate) before drink but no subsequent change/strengthening of connectivity in response to glucose ingestion relative to the baseline condition (P < 0.01, FWE) (Fig. 3C).
Figure 3.
Within-group functional connectivity with the hypothalamus as the seed region in response to drinking glucose. A: At baseline (before drink ingestion), lean adolescents demonstrated no increased connectivity, whereas obese adolescents demonstrated increased corticolimbic-striatal connectivity with the hypothalamic seed. B: After drink ingestion, obese adolescents continued to demonstrate a pattern of connectivity similar to what they demonstrated at baseline, whereas now lean adolescents also exhibited increased connectivity with striatal and limbic regions. C: Interestingly, in examination of the difference between before drink consumption and after drink consumption, lean adolescents exhibited relatively strengthened connectivity in response to drinking glucose, whereas obese adolescents did not have a relative change in connectivity relative to their state before drinking glucose. SN/VTA, substantia nigra/ventral tegmental area.
Functional connectivity with the BA10 seed in the PFC region revealed that in lean adolescents, glucose ingestion strengthened BA10-positive connectivity with the PFC and ACC and also strengthened BA10-negative connectivity to the hypothalamus (P < 0.05, FWE). In contrast, no such increased positive or negative connectivity from the BA10 seed to other PFC regions or the hypothalamus was observed in obese adolescents after glucose ingestion (Supplementary Fig. 6).
Within-Group Functional Brain Connectivity in Response to Oral Fructose
Within-group connectivity analysis using the NAcc as a seed was conducted before and after fructose ingestion. The analyses revealed that prior to fructose ingestion, lean adolescents demonstrated increased connectivity with the NAcc seed with the caudate, putamen, insula, and ACC, while in response to drinking fructose they demonstrated increased connectivity with the caudate and ACC. The change in connectivity after ingestion of fructose relative to baseline was increased in the dorsal ACC (BA25) and decreased in the medial temporal gyrus. Before fructose drink ingestion, the obese adolescents exhibited increased connectivity with the caudate, hypothalamus, lateral orbitofrontal cortex (OFC), ACC, and thalamus. In response to fructose drink ingestion, obese adolescents demonstrated increased connectivity in the caudate, putamen, thalamus, and ACC. The change in connectivity after ingestion of fructose relative to baseline was increased in the posterior insula and putamen.
Discussion
The rise in adolescent obesity has paralleled increases in added-sugar consumption; thus, a better understanding of the mechanisms contributing to this phenomenon may create opportunities for intervention. In this study, we investigated brain responses to drinking two of the most widely consumed simple sugars, glucose and fructose, in obese and lean adolescents. We found strikingly different brain responses between obese and lean adolescents. While lean adolescents exhibited increased perfusion in regions of the brain implicated in executive function, behavioral choices, and decision making (the PFC) and no change in a region vital for homeostatic appetite processes (the hypothalamus), the obese adolescents exhibited decreased perfusion in the PFC and increased perfusion in the hypothalamus and striatum when their altered acyl-ghrelin and insulin responses to drinking glucose were accounted for. Furthermore, the perfusion response to fructose ingestion also differed in the two groups. In obese, but not lean, adolescents, perfusion was decreased in the PFC and increased in the striatum, a brain region implicated in reward and craving as well as increased neurotransmission in response to food (39,40). Taken together, these findings suggest that obese adolescents may have attenuated executive control response to ingestion of these two simple sugars and diminished capacity to downregulate homeostatic and hedonic regions of the brain, potentially contributing to continued food consumption.
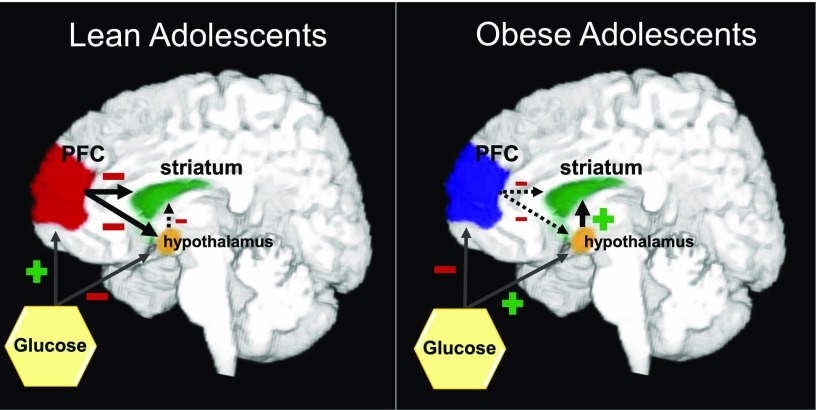
To investigate the impact of obesity on the change in the cerebral perfusion response to glucose ingestion in adolescents, we used ANOVA models and found a significant main effect of group in the PFC. This effect was in large part derived from the obese group, who demonstrated decreased perfusion in the PFC. This finding is intriguing, as the PFC exerts higher-order inhibitory control over homeostatic processes, thereby regulating energy, food intake, and reward signals via neural projections to the hypothalamus and striatal-limbic regions of the brain (41,42). As ingestion of glucose increased PFC perfusion in lean adolescents but decreased PFC perfusion in obese adolescents in conjunction with increased hypothalamic and striatal perfusion related to altered acyl-ghrelin and insulin responses, we speculate that decreased PFC perfusion in obesity may confer decreased cognitive control of monosaccharide intake, promoting hypothalamic activity and, in turn, a striatal motivation-reward pathway response, thereby contributing to the overconsumption of glucose (Fig. 4). This posit is supported by a positron emission tomography–fluorodeoxyglucose study in adults demonstrating that higher BMI is associated with lower baseline prefrontal glucose metabolism (43) as well as previous reports of thinner OFCs (part of the PFC) and diminished performance on various cognitive tasks (visual working memory tasks and digital vigilance tasks) in obese adolescents (44). In keeping with these observations, it has been reported that obese adolescents (mean age 17 years) have higher ratings of disinhibition on the Three Factor Eating Questionnaire, lower performance on the cognitive tests (Stroop color-word score), and lower OFC volume (assessed by MRI) and that disinhibition correlated with BMI, Stroop color-word score, and OFC volume (45).
Figure 4.
Schematic diagram to illustrate altered regional CBF response to glucose in lean and obese adolescents. When lean adolescents drink glucose they demonstrate increase CBF in the PFC, which may exert inhibitory signals to the hypothalamus and striatum, decreasing CBF in these regions. On the other hand, when obese adolescents drink glucose they exhibit decreased CBF in the PFC, which then may exert less inhibitory control on the striatum and hypothalamus, perhaps increasing striatal perfusion.
It is also noteworthy that animal studies have demonstrated that intermittent sugar exposure results in the binge-drinking of sugar, craving during periods of sugar abstinence, and cross-sensitization (consuming other substances when sugar is not available) (46). Thus, intermittent excessive intake of sugar may induce neurochemical adaptations in the brain that are akin to those observed in response to other rewarding substances such as psychoactive drugs (46). Indeed, SSB-associated cues and reward responses result in learning-related brain changes via processes such as conditioning, to alter motivation pathways that could facilitate greater consumption of SSBs. While we do not have direct evidence that the neural differences we observed in obese adolescents following glucose and fructose ingestion parallel the rodent studies, it is intriguing that animal data also show sugar-related adaptations in the reward and motivation brain circuits. Interestingly, in our study, fructose appeared to induce a greater perfusion increase in a brain reward region (ventral striatum) in obese adolescents, a finding not seen in the lean adolescents. This response included the NAcc, a key striatal region implicated in pleasure, reinforcement, and food reward (40). This change was associated with decreased perfusion in the PFC. Taken together, these data suggest that fructose ingestion in obese adolescents generates a reduction in PFC perfusion similar to that with glucose and, additionally, increases perfusion in the pleasure-reward region (the NAcc), which may enhance the desire to consume fructose. In contrast, lean adolescents appear to be less sensitive to such fructose-related changes in striatal reward regions.
We also observed that circulating serum insulin and acyl-ghrelin (hormones that are transported across the blood-brain barrier [47,48]) significantly affected the brain perfusion response to drinking glucose and fructose. Covarying (ANCOVA) for insulin or acyl-ghrelin resulted in increased perfusion in homeostatic regions (hypothalamus) as well as the limbic (thalamus and hippocampus) regions of the brain in the obese versus the lean group (significant main effect of group) (Figs. 1B and C and 2B and C), implying that these hormonal differences between groups in response to each drink might influence the altered CBF response observed in the obese state. Insulin is ubiquitous in the central nervous system, particularly in the hypothalamus (49), and insulin receptors are widely distributed throughout the brain including in the hippocampus, thalamus, striatum, amygdala (50), ventral tegmental area (51), and cerebral cortex (including PFC) (50), as well as the hypothalamus (49). Ghrelin, a gut-derived orexigenic hormone, is modified posttranslationally to the active acylated form of ghrelin, which is able to bind to its receptor, the growth hormone secretagogue receptor (GHS-R1a) (52,53). Ghrelin receptors are also expressed in many brain regions including the hippocampus, hypothalamus (particularly the arcuate nucleus), cortex, and amygdala (54). Preclinical studies have shown that ghrelin is expressed in the hypothalamus (55) and that a subset of neurons coexpress receptors for ghrelin and dopamine (DRD1), which may form heterodimers in hypothalamic neurons that regulate appetite (54). Although the group difference in perfusion in PFC regions in the obese and lean groups could not be attributed to either of these hormonal covariates, the exaggerated rise in insulin as well as the blunted suppression of acyl-ghrelin in obese participants influenced greater hippocampal, hypothalamic, and thalamic responses seen in obese relative to lean adolescents. Thus, it is interesting to speculate that altered insulin and ghrelin response to both glucose and fructose ingestion in obese adolescents may be acting synergistically in the brain to affect motivation for food and eating behavior.
Whole-brain correlations were used to examine the potential relationships between fasting leptin levels, reflective of fat mass, on the observed differences in PFC perfusion. Higher fasting leptin levels correlated with reductions in perfusion in the medial PFC of obese adolescents (Supplementary Fig. 5), suggesting that altered (or dysfunctional) leptin signaling may contribute to lower PFC perfusion in these individuals. In keeping with a potential role for leptin in PFC function, leptin replacement in leptin-deficient adults has been reported to increase activation in the PFC (56). Thus, obesity-related leptin resistance might contribute to the diminished PFC inhibitory control over the hypothalamic response to glucose ingestion. Future studies will be needed to address this possibility directly.
The PFC-striatal-hypothalamic circuit was further explored using within-group functional connectivity analysis seeding the hypothalamus and BA10 region of the PFC. Obese adolescents demonstrated heightened connectivity of the hypothalamus with the corticolimbic-striatal network even prior to glucose ingestion but subsequently had negligible change in connectivity in response to glucose ingestion compared with baseline (P < 0.05, FWE) (Fig. 3). In striking contrast, lean adolescents before drink consumption demonstrated no evidence of significant hypothalamic connectivity with other brain regions, but in response to drinking glucose lean adolescents exhibited hypothalamic connectivity with striatal-limbic regions, as has been previously reported in adults (7). It is intriguing to speculate that lean adolescents are able to appropriately sense ingestion of glucose by the hypothalamus and strengthen connectivity to brain reward and motivation regions, whereas due to chronic tonic striatal-limbic connectivity at baseline, glucose ingestion failed to provide an adequate reward stimulus in obese adolescents. The increased hunger ratings found in the obese group are consistent with such an explanation. Differences in connectivity before and after glucose ingestion were also observed in the lean and obese adolescents using the PFC (BA10) as the seed region. In lean adolescents, the PFC region showed strengthened negative or inhibitory connectivity with the hypothalamus; however, no such increase in inhibitory connectivity between the PFC and hypothalamus was seen in obese adolescents (Supplementary Fig. 6). These findings suggest that the functional capacity of the PFC in the adolescent brain to exert an anticorrelated inhibitory connection with the hypothalamus is diminished in obese adolescents, which in turn may contribute to dysregulated glucose intake.
A limitation of this study is that fMRI does not provide a direct measure of neuronal activity; rather, it provides information regarding neuronal activity as it relates to local brain perfusion. Additionally, the application of stringent head motion criteria to minimize contamination of the functional connectivity analyses rendered the functional connectivity results exploratory with need for replication. The study data are also cross-sectional, and eating behavior was not directly measured in this study. Given the insignificant differences in the sex distribution between the groups, we examined main effect of sex and found differences in activation in the fusiform gyrus but no other significant changes in brain CBF relating to sex by group or by drink interactions. Thus, it is unlikely that the observed differences in the fMRI response to glucose and fructose ingestion seen in obese and lean adolescents are sex related. Finally, glucose and fructose (the components of sucrose) are usually not consumed in isolation, and therefore future studies will be required to assess the brain response to ingestion of sucrose.
Nevertheless, these data have important clinical implications. The results suggest that brain CBF response to ingested monosaccharides is altered in obese adolescents, resulting in a lack of adequate (appropriate) neural regulation of glucose and fructose consumption, particularly by executive control areas of the brain. This change may at least in part be influenced by dynamic changes in peripheral hormone levels. Interestingly, the decreased PFC response in obesity is associated with heightened tonic connectivity between homeostatic (hypothalamus) and hedonic (striatum) brain regions at baseline, but no significant change in connectivity after ingestion of glucose, raising the possibility that the diminished CBF response in brain executive control regions to ingested glucose in obese adolescents is linked to a deficient reward response to glucose ingestion. In adolescent obesity, these alterations in brain responses to ingestion of both glucose and fructose might contribute to dysfunctional control of consumption of simple sugars and potentially drive further weight gain.
Article Information
Funding. This work was made possible by National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH), grants 1R01-DK-085577-01 (principal investigator [PI]: S.C.), Diabetes Research Center P30-DK-045735, R37-DK-20495 (PI: R.S.S.), R01-DK-099039 (PI: R.S.), and 5K12-DK-094714; Endocrine Fellows Foundation Development research grant 1K23-DK-101694 (PI: A.M.J.); the NIH Roadmap for Medical Research Common Fund grants UL1-DE019586, UL1-RR024139, and PL1-DA024859; and Clinical and Translational Science Award UL1 TR000142 from the National Center for Advancing Translational Sciences, a component of the NIH.
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.M.J., R.S., J.A., M.A.V.N., and R.T.C. conducted data analysis. A.M.J., R.S., J.A., M.A.V.N., R.T.C., R.S.S., and S.C. contributed to the interpretation of data. A.M.J., R.S., J.A., M.A.V.N., R.T.C., R.S.S., and S.C. wrote the manuscript. R.S., R.S.S., and S.C. were responsible for the study design and funding. R.S., C.G., J.K., S.M., N.S., M.S., E.J.D., B.P., G.C., and S.C. were responsible for data collection. S.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01808846, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1216/-/DC1.
See accompanying article, p. 1797.
References
- 1.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA 2010;303:242–249 [DOI] [PubMed] [Google Scholar]
- 2.Popkin BM, Nielsen SJ. The sweetening of the world’s diet. Obes Res 2003;11:1325–1332 [DOI] [PubMed] [Google Scholar]
- 3.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet 2001;357:505–508 [DOI] [PubMed] [Google Scholar]
- 4.Weiss R, Dziura J, Burgert TS, et al. . Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–2374 [DOI] [PubMed] [Google Scholar]
- 5.Williams DE, Cadwell BL, Cheng YJ, et al. . Prevalence of impaired fasting glucose and its relationship with cardiovascular disease risk factors in US adolescents, 1999-2000. Pediatrics 2005;116:1122–1126 [DOI] [PubMed] [Google Scholar]
- 6.Guthrie JF, Morton JF. Food sources of added sweeteners in the diets of Americans. J Am Diet Assoc 2000;100:43–51; quiz 49–50 [DOI] [PubMed] [Google Scholar]
- 7.Page KA, Chan O, Arora J, et al. . Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA 2013;309:63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 2000;24:417–463 [DOI] [PubMed] [Google Scholar]
- 9.Avena NM, Bocarsly ME, Hoebel BG. Animal models of sugar and fat bingeing: relationship to food addiction and increased body weight. Methods Mol Biol 2012;829:351–365 [DOI] [PubMed] [Google Scholar]
- 10.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neuroscience Biobehav Rev 2008;32:20–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661–671 [DOI] [PubMed] [Google Scholar]
- 12.Avena NM, Gold JA, Kroll C, Gold MS. Further developments in the neurobiology of food and addiction: update on the state of the science. Nutrition 2012;28:341–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curry DL. Effects of mannose and fructose on the synthesis and secretion of insulin. Pancreas 1989;4:2–9 [DOI] [PubMed] [Google Scholar]
- 14.Lindqvist A, Baelemans A, Erlanson-Albertsson C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul Pept 2008;150:26–32 [DOI] [PubMed] [Google Scholar]
- 15.Prodam F, Me E, Riganti F, et al. . The nutritional control of ghrelin secretion in humans: the effects of enteral vs. parenteral nutrition. Eur J Nutr 2006;45:399–405 [DOI] [PubMed] [Google Scholar]
- 16.Teff KL, Elliott SS, Tschöp M, et al. . Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab 2004;89:2963–2972 [DOI] [PubMed] [Google Scholar]
- 17.Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol 2011;340:80–87 [DOI] [PubMed] [Google Scholar]
- 18.Woods SC. The control of food intake: behavioral versus molecular perspectives. Cell Metab 2009;9:489–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Name M, Giannini C, Santoro N, et al. . Blunted suppression of acyl-ghrelin in response to fructose ingestion in obese adolescents: the role of insulin resistance. Obesity (Silver Spring) 2015;23:653–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsh JA, Sharma AJ, Grellinger L, Vos MB. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr 2011;94:726–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss R, Dufour S, Taksali SE, et al. . Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 2003;362:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page KA, Seo D, Belfort-DeAguiar R, et al. . Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest 2011;121:4161–4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page KA, Arora J, Qiu M, Relwani R, Constable RT, Sherwin RS. Small decrements in systemic glucose provoke increases in hypothalamic blood flow prior to the release of counterregulatory hormones. Diabetes 2009;58:448–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguirre GK, Detre JA, Zarahn E, Alsop DC. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. Neuroimage 2002;15:488–500 [DOI] [PubMed] [Google Scholar]
- 25.Nolf E. XMedCon - an open-source medical image conversion toolkit. Eur J Nucl Med 2003;30:S246 [Google Scholar]
- 26.Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med 1996;35:346–355 [DOI] [PubMed]
- 27.Wong EC, Buxton RB, Frank LR. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR Biomed 1997;10:237–249 [DOI] [PubMed] [Google Scholar]
- 28.Duncan JS, Papademetris X, Yang J, Jackowski M, Zeng X, Staib LH. Geometric strategies for neuroanatomic analysis from MRI. Neuroimage 2004;23(Suppl. 1):S34–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papademetris X, Jackowski M, Rajeevan N, Constable R, Staib L. BioImage Suite. New Haven, CT, Yale School of Medicine. Available from http://www.bioimagesuite.org. Accessed 28 July 2011
- 30.Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 1998;22:324–333 [DOI] [PubMed] [Google Scholar]
- 31.Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM. 3D statistical neuroanatomical models from 305 MRI volumes. Late-breaking abstract presented at the Nuclear Science Symposium and Medical Imaging Conference, San Francisco, CA, 31 October–6 November 1993 [Google Scholar]
- 32.Hommer RE, Seo D, Lacadie CM, et al. . Neural correlates of stress and favorite-food cue exposure in adolescents: a functional magnetic resonance imaging study. Hum Brain Mapp 2013;34:2561–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lubsen J, Vohr B, Myers E, et al. . Microstructural and functional connectivity in the developing preterm brain. Semin Perinatol 2011;35:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers EH, Hampson M, Vohr B, et al. . Functional connectivity to a right hemisphere language center in prematurely born adolescents. Neuroimage 2010;51:1445–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162–173 [DOI] [PubMed] [Google Scholar]
- 36.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 2012;59:431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finn ES, Shen X, Holahan JM, et al. Disruption of functional networks in dyslexia: a whole-brain, data-driven analysis of connectivity. Biol Psychiatry 2014;76:397–404 [DOI] [PMC free article] [PubMed]
- 38.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 2009;19:72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci 2004;24:2825–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 2005;86:773–795 [DOI] [PubMed] [Google Scholar]
- 41.Ongür D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol 1998;401:480–505 [PubMed] [Google Scholar]
- 42.Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 2000;10:206–219 [DOI] [PubMed] [Google Scholar]
- 43.Volkow ND, Wang GJ, Telang F, et al. . Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity (Silver Spring) 2009;17:60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross N, Yau PL, Convit A. Obesity, fitness, and brain integrity in adolescence. Appetite 2015;93:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maayan L, Hoogendoorn C, Sweat V, Convit A. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity (Silver Spring) 2011;19:1382–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev 2008;32:20–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther 2002;302:822–827 [DOI] [PubMed] [Google Scholar]
- 48.Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther 2012;136:82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D Jr. Insulin in the brain: a hormonal regulator of energy balance. Endocr Rev 1992;13:387–414 [DOI] [PubMed] [Google Scholar]
- 50.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature 1978;272:827–829 [DOI] [PubMed] [Google Scholar]
- 51.Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res 2003;964:107–115 [DOI] [PubMed] [Google Scholar]
- 52.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev 2005;85:495–522 [DOI] [PubMed] [Google Scholar]
- 53.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 2008;132:387–396 [DOI] [PubMed] [Google Scholar]
- 54.Kern A, Grande C, Smith RG. Apo-Ghrelin Receptor (apo-GHSR1a) Regulates Dopamine Signaling in the Brain. Front Endocrinol (Lausanne) 2014;5:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cowley MA, Smith RG, Diano S, et al. . The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003;37:649–661 [DOI] [PubMed] [Google Scholar]
- 56.Baicy K, London ED, Monterosso J, et al. . Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci U S A 2007;104:18276–18279 [DOI] [PMC free article] [PubMed] [Google Scholar]