ABSTRACT
Non-human primate (NHP) studies are often an essential component of antibody development efforts before human trials. Because the efficacy or toxicity of candidate antibodies may depend on their interactions with Fcγ receptors (FcγR) and their resulting ability to induce FcγR-mediated effector functions such as antibody-dependent cell-meditated cytotoxicity and phagocytosis (ADCP), the evaluation of human IgG variants with modulated affinity toward human FcγR is becoming more prevalent in both infectious disease and oncology studies in NHP. Reliable translation of these results necessitates analysis of the cross-reactivity of these human Fc variants with NHP FcγR. We report evaluation of the binding affinities of a panel of human IgG subclasses, Fc amino acid point mutants and Fc glycosylation variants against the common allotypes of human and rhesus macaque FcγR by applying a high-throughput array-based surface plasmon resonance platform. The resulting data indicate that amino acid variation present in rhesus FcγRs can result in disrupted, matched, or even increased affinity of IgG Fc variants compared with human FcγR orthologs. These observations emphasize the importance of evaluating species cross-reactivity and developing an understanding of the potential limitations or suitability of representative in vitro and in vivo models before human clinical studies when either efficacy or toxicity may be associated with FcγR engagement.
KEYWORDS: Antibody, Fc variant, Fcγ receptor, cross-reactivity, rhesus macaque
Introduction
The adaptive and innate immune systems are linked by the interaction between antibodies and Fcγ receptors (FcγR) expressed on a diverse population of immune effector cells. The critical role that FcγR play in driving antibody activity in vivo has been identified in diverse disease settings, including cancer, immunity and infectious disease. As antibody drug products continue to gain predominance in the clinic, understanding of their myriad mechanisms of action has matured into efforts to manipulate and improve upon their properties, including modulating antibody selectivity for individual or sets of FcγR, thereby tuning the effector functions, such as antibody-dependent cell-meditated cytotoxicity (ADCC) and phagocytosis (ADCP), they elicit. Modulation of antibody:FcγR affinity has been accomplished through IgG Fc changes, including subclass selection,1 amino acid sequence engineering2-12 and glycan engineering.13-21 Although rational selection of human IgG subclasses has long been a key aspect of clinical antibody development, Fc amino acid mutations and glycan-optimized antibodies have only made their way into the clinic more recently, with the first US Food and Drug Administration (FDA)-approval of a glycoengineered antibody achieved in 2013,22 and several Fc variants currently in clinical trials.23-25 While many monoclonal IgGs have been shown to be safe and effective in humans, before first-in-human studies, non-human primate (NHP) studies remain a critical step in evaluating drug safety, potential toxicity, and possible efficacy. NHP studies become increasingly important when considering drug development against potential bioterror threats under FDA's ‘animal rule,’ which allows the use of animal model data to show efficacy when human trials are not ethical or logistically feasible.26
Rhesus macaques (macaca mulatta, MM) have been a widely used animal model for preclinical work evaluating monoclonal antibodies for human IgG antigenicity (historically),27 half-life extension,11,12 autoimmunity,28 allograft transplantation,10,29 and efficacy in infectious disease studies, including sepsis,30 cytomegalovirus (CMV),27 chikungunya virus,31 Ebola virus,,32,33 dengue virus (DENV),34,35 respiratory syncytial virus,36 and severe acute respiratory syndrome coronavirus37 infection. MM have extensively contributed to studies of the potential role of antibodies in protection from human immunodeficiency virus (HIV) infection, given their ability to be infected by simian immunodeficiency virus (SIV), and simianized HIV (SHIV).38-44 Because passively administered monoclonal antibodies can lead to protection from infection in challenge experiments,43,44 they have also been used to investigate mechanisms of protection. For example, a pivotal study using IgG-Fc point mutants with abrogated affinity toward Fc receptors in rhesus revealed that broadly neutralizing antibodies also require the engagement of FcγRs for SHIV protection,39,45 though protection was not enhanced when a glycovariant antibody was administered.40
These and other in vivo studies using human Fc-variants in MM expose the potential importance of FcγR engagement for antibody efficacy. However, depending on the particular disease pathogenesis and therapeutic strategy being used, IgG-Fc variants with enhanced or abrogated affinity to human FcγR may be desirable (Table 1). For instance, antibody-dependent enhancement (ADE) of infection has been reported for dengue virus46 due to FcγR engagement;47 therefore ablation of FcγR binding through point mutations has been leveraged in rhesus to prevent ADE.34,35 Efficacy of glycosylation variants has also been assessed in NHP. Administration of nonfucosylated antibodies has produced mixed results; a broadly neutralizing antibody with enhanced affinity to FcγRIIIa and ADCC activity in vitro did not augment protection from a vaginal SHIV challenge,40 while treatment with a nonfucosylated antibody cocktail resulted a higher survival rate compared with a fucosylated antibody cocktail in post-exposure Ebola therapy studies.33 Given the potential utility of such preclinical studies in NHP to determine safety and possible efficacy of FcγR optimized IgG-Fc variants, there is a need to consider the divergence between human and NHP FcγRs in the interpretation and translation of results to the clinic.
Table 1.
Use of human IgG Fc variants in in vivo studies in rhesus macaque.
| Human Fc Variants | Modulation | Results | Reference(s) |
|---|---|---|---|
| IgG2: M428L, T250Q/M428L | Increases FcRn | Clearance rate 1.8x and 2.8x slower than WT | 11 |
| IgG1: M428L/N434S | Increase FcRn | VRC01-LS had 3x longer half-life and enhanced protection from intrarectal SHIV challenge relative to WT | 12 |
| IgG1: K332A, L234A/L235A | KA ablates c1q, LALA ablates c1q/FcγR | LALA variant reduced SHIV protection vs. WT b12 | 39,45 |
| IgG1: afucosylated | Increases FcγRIII | Afucosylated b12 did not improve SHIV protection over WT | 40 |
| IgG1 cocktail: afucosylated | Increases FcγRIII | Afucosylated MB-003 improved post-exposure Ebola survival vs. fucosylated | 33 |
| IgG1: S298A/E333A/K334A | Reduces FcγRIIa/IIb, Increases FcγRIIIa | Limited number of founder viruses during SHIV infection (no WT control) | 8,80 |
| IgG1: APELLGGPS deletion in CH2 | Ablates binding to FcγR | Eliminated ADE of DENV infection in vitro, 100% protection in vivo (no WT control in vivo) | 34,35 |
| IgG1: N297G (aglycosylated) | Ablates binding to FcγR, reduces c1q | Aglycosylated hu5C8 treatment did not significantly extend renal and islet allograft acceptance like WT | 10 |
MM possess genes orthologous to human activating receptors FcγRI, FcγRIIa, and FcγRIIIa, and the inhibitory receptor FcγRIIb, but not FcγRIIc or FcγRIIIb.48 Sequencing efforts have identified several allotypic variants48 that exhibit differences in affinity toward human IgG subclasses relative to their human orthologs,49 likely due to inter-species differences in amino acid residues in contact regions. Cynomolgus macaque FcγR also exhibit differences in affinity to human IgG subclasses.50 Importantly, intra-species FcγR allotypic variation has led to outcome disparities in vivo: B cell depletion efficiency driven by treatment with the anti-CD20 antibody rituximab were associated with rhesus FcγRIIIa allotypes with polymorphisms in the intracellular domain.51 These studies indicate that intra- and inter-species differences in FcγR sequence may manifest significant differences in biologic outcome and point to the potential utility of genotyping animal cohorts.
Beyond sequence disparities leading toward functional differences, the cellular expression profile of FcγR should also be considered. The expression patterns of rhesus FcγRs have been evaluated using antibodies against human FcγR with presumed cross-reactivity to NHP FcγR (Table 2). These studies have resulted in expression profiles generally comparable to those observed in human cell subsets,52-54 with the exception of granulocytes (such as neutrophils or eosinophils). Human granulocytes express FcγRIIIb while MM do not possess an FcγRIIIb, and their granulocytes apparently do not express FcγRIIIa. To the best of our knowledge, FcγRII expression on granulocytes has not been evaluated. A lack of FcγRIII expression on granulocytes has also been observed in other species, including cynomolgus50 and nemestrina.55 This difference may be significant to experimental outcomes considering the abundance of neutrophils in blood and the ability of FcγRIIIb to drive neutrophil extracellular trap release (NETosis),56,57 elicit phagocytosis,58 and inhibit FcγRIIa-driven ADCC59 in human neutrophils.
Table 2.
Previously published rhesus macaque effector cell FcγR expression patterns.
| Cell Type | Confirmed Expression | Confirmed Non-expression | Reference(s) |
|---|---|---|---|
| CD3+ T cells | FcγRII | FcγRI, FcγRIII | 81 |
| CD20+ B cells | FcγRII | FcγRI, FcγRIII | 81 |
| Macrophage/Monocyte | FcγRI, FcγRII, FcγRIII | 82 | |
| Natural killer cell | FcγRIII | FcγRI, FcγRII | 82 |
| Monocyte-derived dendritic cells | FcγRI, FcγRII | FcγRIII | 83 |
| Granulocytes | FcγRIII | 68,70 |
Given the increasing prevalence of FcγR-optimized IgG variants in studies using rhesus macaque, we characterized the affinities of human IgG subclass, glycoform and sequence-engineered variants to the major human and rhesus macaque allotypic FcγR variants. To accomplish this goal, we leveraged a high-throughput surface plasmon resonance (SPR) array and affinities (KD) between species to characterize inter-species IgG-FcγR cross-reactivity. This work expands the knowledge base of cross-reactive Fc-variants available to investigators interested in modulating effector function in preclinical NHP studies, and highlights the use of a high-throughput IgG:FcγR cross-reactivity characterization platform that is extensible to any protein-protein interaction and animal model of interest.
Results
Human IgG subclasses
Human IgG subclasses can variably induce effector functions by differential ability to interact with FcγR, but little is known about how the human IgG subclasses cross-react with MM FcγR. Because this information could contribute to NHP studies, we measured the affinity of each human IgG subclass to both human and rhesus low affinity FcγRs with IgG immobilized on a solid phase and FcγR in solution. Binding of human IgGs to the high affinity rhesus FcγRI was previously evaluated with FcγRI immobilized on a solid surface in a BioLayer Interferometry assay and demonstrated that this receptor retained high affinity for human IgG subclasses in the order of IgG3 = IgG1>IgG4>IgG2.49 Binding of the low affinity human and rhesus FcγR to human serum- and myeloma-derived IgG1, IgG2 and IgG3 and myeloma-derived IgG4 is shown in Fig. 1. Good agreement (between 1–3-fold) was generally observed between serum- and myeloma-derived IgG, with differences potentially due to variance in Fc glycosylation or IgG allotypic variation.
Figure 1.
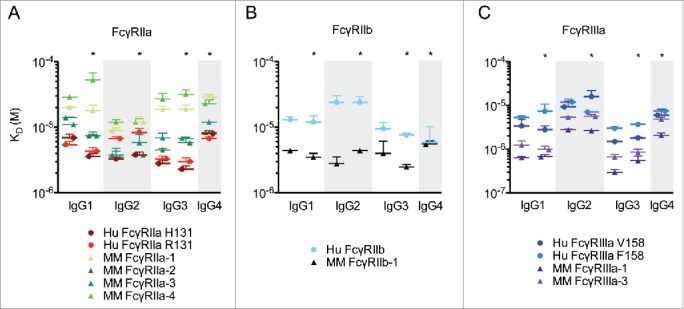
Affinity of human IgG subclasses for human and rhesus FcγR. Equilibrium binding constants (KD) measured by SPR for human and rhesus FcγR allotypes binding to human IgG subclasses from human serum (IgG1, IgG2, IgG3) and human myeloma plasma (IgG1, IgG2, IgG3 and IgG4) for FcγRIIa (A), FcγIIb (B), and FcγRIIIa (C) allotypes. *Indicates human myeloma plasma derived IgG. Data represents the mean of up to 4 replicates and error bars denote the standard deviation. Data are representative of 2 independent experiments.
The affinity of IgG2 was higher for human FcγRIIa H131 compared with the R131 allotypic variant (Fig. 1A), consistent with previous studies.50,60 Interestingly, rhesus FcγRIIa allotypes 2 and 3, which both contain a histidine at this position, displayed similar affinities toward human IgG2 compared with the human H131 allotype. Analogous to the human FcγRIIa-R131 allotype with weaker affinity toward IgG2, rhesus FcγRIIa allotypes 1 and 4 had lower affinity toward IgG2, as well as the other subclasses, possibly due to allotype 4 having a proline at position 131 and allotype 1 having an additional N-glycosylation motif at position 128, a putative contact residue,49 and in close proximity to position 131, a known contact residue in human FcγRIIa.61
The human IgG subclasses demonstrated weaker affinity for human FcγRIIb than either human FcγRIIa or rhesus FcγRIIb-1 (Fig. 1B). Additionally, the affinity of IgG2 relative to the other subclasses was 2–6-fold weaker for human FcγRIIb, while IgG2 demonstrated comparable affinity to the other subclasses for rhesus FcγRIIb-1. Both of these effects may be due in part to rhesus FcγRIIb-1 containing H131 while human FcγRIIb contains R131. This observation is consistent with a previous study evaluating H131 containing cynomolgus FcγRIIb binding to human IgG subclasses.50 The rhesus FcγRIIb-2 allotypic variant was not evaluated in this study because an amino acid substitution of leucine to proline at position 88 severely compromises binding to all human IgG types.49
Human IgG1 and IgG3 exhibited higher affinity than IgG2 and IgG4 for both human and rhesus FcγRIIIa allotypes (Fig. 1C). As expected, the human FcγRIIIa V158 allotype generally displayed higher affinity for human IgG than the F158 allotype.60 The rhesus FcγRIIIa allotypes appeared to have affinities more similar to the higher affinity human FcγRIIIa V158 than the lower affinity F158 allotype. This activity profile is consistent with the presence of valine at this position in rhesus FcγRIIIa-3 and isoleucine, a similar side-chain group, in rhesus FcγRIIIa-1. This position is a known contact residue between human IgG-Fc and human FcγRIIIa.62
Human IgG1 Fv variants
Human IgG1 is the most common subclass used therapeutically due to its long half-life, robust stability, and ability to engage FcγRs. To determine whether the variable region of the monoclonal antibody we evaluated affects the affinity of its interaction with human or rhesus FcγR and to appraise whether the high-throughput SPR assay produces reliable and reproducible results, we printed 4 recombinantly-expressed monoclonal antibodies for affinity assessment. This panel of Fv variants included HIV envelope glycoprotein-specific VRC01 and b12 antibodies, and HER2-specific trastuzumab and pertuzumab antibodies. The affinity of each recombinant human IgG1, which differed only in the variable region, was measured across human and rhesus FcγR allotypes (Fig. 2). As expected, the affinity of human IgG1 to both human and rhesus FcγR was independent of the antibody specificity and variable region sequence.
Figure 2.
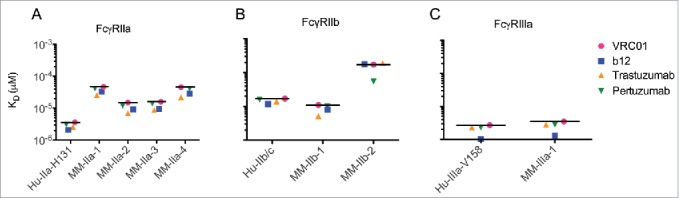
Affinity of recombinant human IgG1 Fv variants for human and rhesus FcγRs. Equilibrium binding constants for the interaction between human VRC01, b12, trastuzumab, and pertuzumab IgG1 antibodies and human and rhesus FcγRIIa (A), FcγIIb (B), and FcγRIIIa (C) allotypes. Bar denotes the mean KD observed across Fv variants.
Human IgG1 Fc variants
As several antibody variants have been identified that can modulate binding to FcγRs, and therefore differentially elicit effector functions such as ADCC or phagocytosis, an evaluation was performed to compare the binding affinity of a subset of these previously reported IgG1 Fc variants to both human and rhesus FcγRs. Fourteen Fc variants (Table 3) of the many that have been previously identified to ablate or improve FcγR affinity and effector function were prepared in the context of the VRC01 Fv, directly printed onto the SPR array surface, and their equilibrium binding constants to both human and rhesus FcγRs subsequently evaluated. This panel of mutants was selected to provide a range of affinity differences (improved and reduced) across all human FcγR and C1q, and variants that had been evaluated simultaneously in in vitro assays with follow up confirmation of relevance in vivo were prioritized. As examples, the VRC01 wild type antibody displayed a typical fast off-rate profile (Fig. 3A), while the afucosylated-Fc and SD/IE/SA variants exhibited much slower off-rates relative to wild type (5x and 300x, respectively) (Fig. 3B,D). The genetically-aglycosylated Fc control (N297Q) had extremely low or undetectable binding to all FcγR, resulting in indeterminate or unreliable KD assessments (Fig. 3C).
Table 3.
Human IgG2 Fc variant panel.
| IgG1 Variant | Abbreviation | Reference |
|---|---|---|
| Wild Type | WT | Wu et al.84 |
| Kifunensine | Kif | Kanda et al.66 |
| S324T | ST | Moore et al.6 |
| H268F/S324T | HFST | |
| S267E/H268F/S324T | SEHFST | |
| S267E/H268F/S324T/I332E/G236A | SEHFSTIEGA | |
| I332E | IE | Richards et al.2 |
| G236A | GA | |
| S239D/I332E | SDIE | |
| I332E/G236A | IEGA | |
| S239D/I332E/G236A | SDIEGA | |
| S239D/I332E/A330L | SDIEAL | Lazar et al.3 |
| S239D/I332E/S298A | SDIESA | Lazar patent US7662925B265 |
| N297Q | NQ | Ferrant et al.10 |
Figure 3.
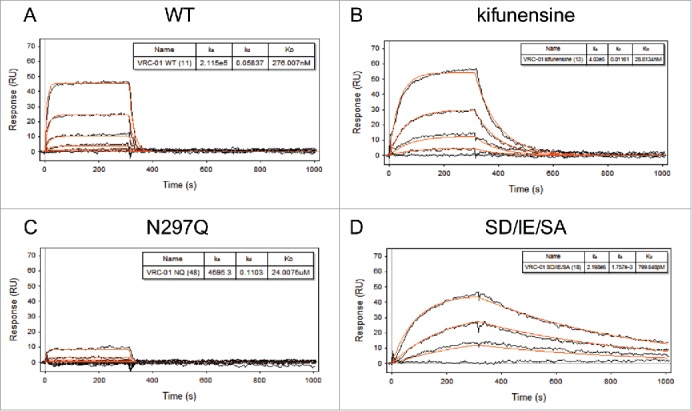
Exemplary SPR response curves and 1:1 stoichiometry kinetic model fits. VRC01 Fc variants were directly printed onto the SPR chip and analyzed for binding to MM FcγRIIIa-3 over the range of 20 μM to 0.2 nM. Raw curves (black) and kinetic fits (red) are shown for wild type (WT, A), oligomannose, afucosylated IgG produced via expression in the presence of kifunensine (B), a genetically aglycosylated Fc variant produced by N297Q point mutation (C), and the Fc-engineered S239D/I332E/S298A point mutant (D).
The equilibrium dissociation constant (KDs) for each individual Fc variant against both human and rhesus macaque FcγRs were determined (Fig. 4). Beyond confirming the effect of these Fc modifications on human FcγR that have been previously reported, for example, the striking effect of afucosylation (kifunensine) on FcγRIII but not FcγRII, this data makes clear that the Fc amino acid substitutions engineered for altering affinity toward human FcγR also often modulated affinity toward rhesus FcγR. However, in many cases the magnitude of this effect was less striking (Fig. 4A).
Figure 4.
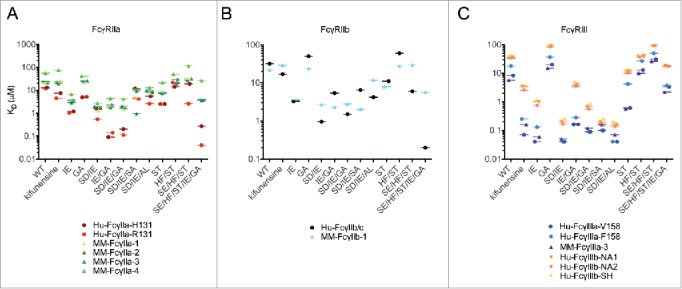
Equilibrium binding constants (KD) of IgG VRC01 Fc variants for human and rhesus FcγR. Equilibrium binding constants for human VRC01 IgG1 Fc variants binding to human and rhesus FcγRIIa (A), FcγIIb (B), and FcγRIIIa (C) allotypes. Data are representative of 2 independent experiments.
The IE and SD/IE substitutions demonstrated improved affinity for FcγRIIa by 10-fold over wild type and the relative changes were within 2-fold between species. The SD/IE/SA mutant has been previously shown to increase affinity to human FcγRIIIa; however, we were surprised to observe a 30-fold improvement in affinity for the rhesus FcγRIIa-2 allotype versus a 1–4-fold improvement for the human and other rhesus FcγRIIa allotypes (Fig. 4A). When comparing amino acid sequence homology, rhesus FcγRIIa-2 has a polymorphism from serine to alanine at position 126 that is in close proximity to proposed contact residues on the FcγR protruding C’ β-strand ridge that sits between the 2 lower hinge regions of the antibody Fc.61 The FcγR ridge makes contact with the antibody N297 residue, proximal to the S298A mutation, indicating the FcγRIIa-2 S126A polymorphism may induce a conformational change in the ridge that results in favorable binding to S298A IgG variant.
IgG Fc variants containing the G236A mutation were originally designed to enhance binding to human FcγRIIa and increase the ratio of binding to the activating FcγRIIa relative to the inhibitory FcγRIIb receptor.2 However, IgG variants containing the GA mutation did not increase binding to rhesus FcγRIIa as much as toward human FcγRIIa (Fig. 4A). For example, the affinity for the GA mutant increased approximately 3-fold for both human allotypes, but did not differ relative to wild type for the rhesus FcγRIIa allotypes. The inability of the GA substitution to result in an affinity increase toward rhesus FcγRIIa is also apparent when analyzing the Fc variants that display concerted effects from multiple mutations. For example, the IE/GA and SD/IE/GA mutants displayed a 65–145-fold increase in affinity for human FcγRIIa, but only a modest 9–15-fold increase for the rhesus FcγRIIa allotypes. Similar, but somewhat less dramatic results were observed in the repeat run. As G236 is a proposed Fc contact residue with Y157 at the tip of the F-G loop of human FcγRIIa,61 disruption of contact with this residue may be caused by nearby changes L159P and F160Y found in all rhesus FcγRIIa allotypes.
The SE/HF/ST and SE/HF/ST/IE/GA mutants are particularly interesting given they contain the S267E mutation. Previous studies have shown this mutation produces a dramatic increase in affinity for R131-containing human FcγRIIa and FcγRIIb receptors, but no change in H131 FcγRIIa affinity.4,5,7 Our results are consistent with these studies, as we observe that human FcγRIIa R131 shows a 7–10-fold increase in affinity for the SE/HF/ST variant, but not the ST or HF/ST variants, while there was no change in binding for any of these mutants toward the human FcγRIIa H131 allotype or toward any of the rhesus allotypes (Figs. 4A, 5A), which also contain H131. Similarly, the SE/HF/ST/IE/GA mutant had a 6-fold greater increase in relative affinity for the human R131 allotype (300-fold) vs. the human H131 allotype (50-fold).
Figure 5.
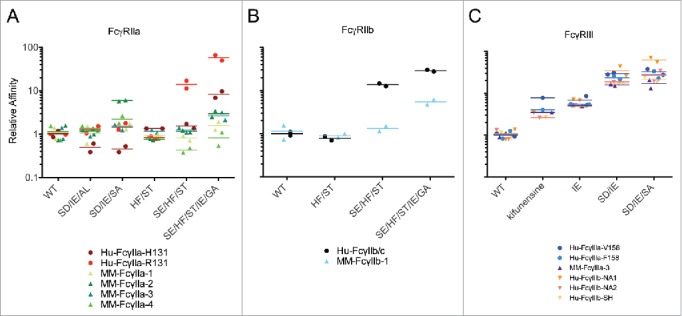
Relative affinity for VRC01 and Trastuzumab Fc variants binding toward human and rhesus macaque FcγR. Relative affinities (WT KD divided by the Fc variant KD) for human and rhesus FcγRIIa (A), FcγIIb (B), and FcγRIIIa (C) allotypes. Symbols represent affinity observed for each mAb and the bar denotes the mean.
The affinity differences between human and rhesus FcγRIIb were generally relatively subtle (Fig. 4B); however, dramatic differences were seen for Fc variants containing the SE mutation. The relative affinity of human FcγRIIb (R131) was 7–10-fold greater than the rhesus FcγRIIb-1 (H131) allotype for the SE/HF/ST mutant but unchanged for ST and HF/ST (Figs. 4B, 5B), consistent with the FcγRIIa results. This result extends the observation that the S267E mutation improves affinity only to R131-containing FcγRII receptors, but not to any human or macaque FcγRII receptors that contain H131. Given S267 of the Fc participates in a hydrogen bond interaction with H131 in FcγRIIa,63 the mutation of serine to glutamic acid may have a compound effect in that it disrupts the hydrogen bonding with H131 and also creates a salt bridge with human FcγRII receptors containing R131.4
The Fc variants displayed comparable affinities between rhesus and human FcγRIIIa allotypes (Figs. 4C, 5C). Notably, the non-fucosylated/oligomannose antibody variant mediated an increase in affinity for all human FcγRIIIa/b and rhesus FcγRIIIa (10–120-fold) with minimal change toward FcγRIIa or FcγRIIb (1–3-fold), as previously observed for rhesus FcγRIIIa-1 and FcγRIIIa-3, when evaluating a non-fucosylated/complex glycan antibody.40 This observation is consistent with cross-species conservation of a glycosylation motif at residue N162, a glycosylation site previously shown for human FcγRIIIa to contact the N297-linked glycan on the human IgG Fc and stabilize the receptor-antibody complex.64 In addition to the glycovariant, several mutants, including IE, SD/IE, SD/IE/AL, and SD/IE/SA, display improvements in affinity toward FcγRIIIa for both species. It is also noteworthy that the relative affinity for all of the Fc variants toward the 3 FcγRIIIb allotypes track closely with the human FcγRIIIa allotypes, consistent with a previously evaluated IgG Fc variant panel65 and an afucosylated antibody.58
Affinity measurements were made with both VRC01 and trastuzumab IgG for a subset of Fc variants to confirm results, and are displayed as a ratio relative to wildtype to make the differences between wild type and the Fc variants more easily apparent (Fig. 5). Relative to wild type, the change in affinity between FcγRIIa/b allotypes was variable and inconsistent between species (Fig. 5A, B), in contrast to a much more uniform relative profile for the FcγRIIIa variants across species (Fig. 5C). These data indicate aspects of both consistent (FcγRIIIa) and divergent (FcγRIIa/b) recognition profiles between species.
Overall, the observed changes in affinity of the VRC01 IgG1 Fc variants to human FcγRs were consistent with previous studies.2,3,6,10,39,65,66 However, variant-specific differences in affinity were observed for several rhesus receptors. When broadly comparing IgG Fc variant equilibrium affinities across both species and allotypes for each FcγR (Fig. 6), there was a strong correlation between rhesus FcγRIIIa binding and human FcγRIIIa, indicating a high degree of conservation in modes of antibody recognition between rhesus and human FcγRIII (R2 = 0.934 to 0.995, 2-tailed p-value <0.0001 for all). In contrast, the agreement between affinity for human FcγRIIa and rhesus FcγRIIa allotypes was more variant-specific, resulting in correlations that were not as strong as FcγRIII (R2 = 0.123 to 0.428, 2-tailed p-value 0.241 to 0.015). A similarly weak correlation was observed between human and rhesus FcγRIIb (R2 = 0.449 2-tailed p-value 0.012). Collectively, these correlative relationships suggest that Fc modifications are likely to have a similar effect on recognition by FcγRIII for both species, but a less predictable effect on binding to FcγRII.
Figure 6.
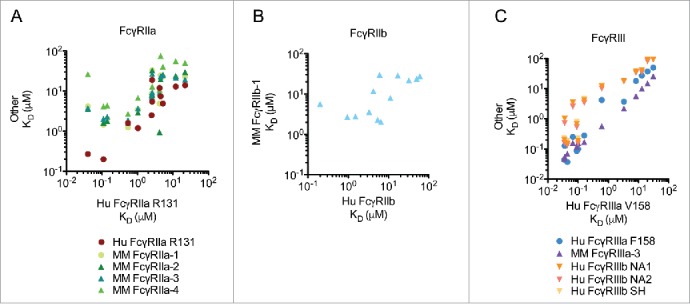
Comparison of human IgG1 VRC01 Fc variant equilibrium constants across species and allotypes for each FcγR. Correlation of binding affinities observed for rhesus and human FcγRIIa vs. human FcγRIIa H131 (A), rhesus FcγRIIb-1 vs. human FcγRIIb (B), and rhesus FcγRIIIa-3 and human FcγRIIIa F158, FcγRIIIb NA1/NA2/SH vs. human FcγRIIIa V158 (C).
Discussion
Employing a high-throughput array-based SPR platform, we efficiently characterized the binding of human IgG subclasses, human IgG1 Fv variants and a panel of Fc-engineered human IgG1 variants to human and rhesus FcγRs. These results indicated that there are differences between species that should be considered in evaluation of antibody Fc variants in rhesus macaque.
As we have shown, rhesus FcγRIIa receptors have a subclass preference similar to that of the human FcγRIIa H131 allotype, and in contrast to the human R131 allotype, consistent with the observation that rhesus allotypes all contain H131. In addition, the affinity of rhesus FcγRIIb-1 for human IgG2 was markedly higher than that of human FcγRIIb, consistent with the fact that rhesus has H131 as opposed to the human receptor's R131. There were also FcγR binding differences between species observed for amino acid engineered IgG Fc variants. For instance, neither the G236A mutation designed to increase the FcγRIIa/FcγRIIb activating to inhibitory (A/I) ratio or the S267E previously shown to enhance binding to R131-containing FcγRII receptors translated to rhesus, likely due to divergence in known contact residues or residues within close proximity. Both mutations have the potential to modulate the A/I ratio, which is a particularly important parameter to consider when designing antibodies with differential binding to FcγR receptors because this ratio can modulate cellular effector functions, as well as cytokine release profiles.67
Unexpectedly, we observed an affinity enhancement for the SD/IE/SA Fc mutant with rhesus FcγRIIa-2. This result highlights the value of screening Fc-engineered variants against the FcγRs of the anticipated animal model because there may be unanticipated changes in affinity. As the Fc variant panel evaluated was not exhaustive, it would be interesting to explore other variants aimed to refine the specificity between the activating and inhibitory FcγRII receptors, such as the P238D/E233D/G237D/H268D/P271G/A330R Fc mutant shown to specifically enhance binding to FcγRIIb.4
In contrast to FcγRII receptors, human subclass and Fc variant binding to rhesus FcγRIIIa closely emulates that of human FcγRIII. This conservation of recognition properties indicates that effector function discrepancies observed between species in vitro or in vivo may more likely be due to differences in cellular expression patterns, such as the lack of rhesus FcγRIIIb or amino acid differences in the FcγRIIIa intracellular domain, as has been observed previously.51 Importantly, there is significant homology between rhesus and cynomolgus macaque FcγRIIIa. Indeed, the only sequence difference is serine 24 in rhesus vs. arginine in cynomolgus,68 a position that is not a proposed contact residue in humans.62 Therefore, the expectation that Fc variants with enhanced human FcγRIIIa affinity should translate to rhesus or cynomolgus is in agreement with in vivo studies in which an anti-CD20 SD/IE Fc antibody mutant targeting B-cell depletion in a cynomolgus model3 and a nonfucosylated MB-003 antibody cocktail for passive immunization against acute Ebola infection in a rhesus model33 displayed greater efficacy over wild type IgG. In contrast, a nonfucosylated monoclonal broadly neutralizing anti-HIV antibody, b12, with enhanced affinity to both rhesus and human FcγRIIIa displayed more potent ADCC in vitro, but did not confer enhanced protection in vivo.40 The nonfucosylated b12 study highlights the importance of continuing to investigate across all of the FcγR interactions and their involvement in antibody efficacy for a particular disease, as well as the potential need to genotype NHPs to balance among study arms. Beyond the variants evaluated, Fc constructs with enhanced binding to FcγRIIIa have become sophisticated by the asymmetric incorporation of Fc point mutations, glycoforms,69 or both,17 and, although our results indicate these constructs would likely cross-react with rhesus FcγRIIIa, confirmation would be required. Lastly, all 3 FcγRIIIb allotypes track with the human FcγRIIIa variants. This finding is important as neutrophils are an abundant FcγRIIIb-expressing cell type in humans, but there is no known FcγRIIIb homolog expressed in rhesus macaque,6870 and therefore rhesus neutrophils may significantly diverge from human neutrophils in aspects of FcγRIIIb-mediated cellular functions such as phagocytosis,58 NETosis,56,57 or the inhibition of FcγRIIa-driven ADCC.59
In this study, we explored the cross-reactivity of human IgG Fc variant binding across human and rhesus macaque FcγRs, but there are a multitude of other Fc receptors that should be evaluated for cross-reactivity to provide a more complete picture when interpreting NHP results. Additionally, while existing sequence data48,49,55,68 suggests they are highly homologous to the rhesus FcγRs, further evaluation of the cynomolgus macaque FcγRs is warranted given their widespread use in safety and toxicity studies. As investigators continue to gain a richer understanding of natural and enhanced antibody activity and effector function in vivo, further experimentation is required to assess the interspecies differences in target and receptor expression level,71 cellular distribution,72 complement binding,73 serum IgG pre-loading of FcγR on effector cells,74 IgG GM allotype variation,75,76 effector function mediated by FcRn,77 IgG transport and clearance via FcRn,78 and FcγR-FcRn modulation design combinations.79
As we have shown, a high-throughput array-based SPR platform can efficiently evaluate interspecies differences between antibody Fc variants and FcγRs with minimal antibody and receptor material requirements. By use of rapid, high-resolution assays to evaluate cross-reactivity between species, researchers evaluating the effector function of Fc-optimized antibodies in NHPs can more confidently anticipate the activity of these variants in humans and can glean additional insight into potential efficacy. As more Fc features such as amino acid mutations and controlled Fc glycoforms continue to improve IgG specificity for individual receptors, understanding of the similarities and differences in NHP and human FcγR biology will remain vital to designing and translating results from this key preclinical model.
Materials and methods
Protein expression and preparation
Construction and expression of VRC01 Fc variants
The construction and expression of VRC01 Fc variants was recently described in detail.65 Briefly, CMV/R mammalian expression vectors for the VRC01 IgG1 light and heavy chains were obtained from the National Institutes of Health AIDS Reagent Program. Fc domain amino acid point mutations (Table 3) were incorporated via quickchange PCR (Stratagene), and expression in the presence 20 μM kifunensine (Tocris) was used to produce afucosylated, oligomannose Fc glycans as described previously.13 For expression, sequence-verified DNA was isolated via maxi-prep (Qiagen), used to transfect suspension cultures of human embryonic kidney (HEK) 293F cells grown in Freestyle media (Invitrogen), and transfected using a mixture of DNA and 25 kD branched PEI (PolySciences). Antibodies were transiently expressed for 7 d at 37°C, 8% CO2.
Construction and expression of human and rhesus macaque FcγR
The construction and expression of FcγRs was recently described in detail.49,65 Briefly, the extracellular domains (ECDs) of human FcγRs, including FcγRI, FcγRIIa, FcγRIIb, FcγRIIIa, and FcγRIIIb were PCR amplified using a forward primer incorporating the CMV/R leader sequence and reverse primer including a sortase A recognition site (amino acid sequence: LPETG) and 6-his tag to enable affinity purification, site-specific conjugation and complementary restriction sites to for ligation into the CMV/R vector. Allotypic variants were generated by single or multiple point mutations using the quickchange strategy (Stratagene). Rhesus FcγR were constructed similarly using the following Genbank identifiers: AFD32558.1, AFD32559.1 and AFD32560.2 for FcγRI; AFD32561.1, AFD32562.1, AFD32563.1 and AFD32564.2 for FcγRIIa; AFD32565.2 and AFD32566.2 for FcγRIIb; AFD32568.1, AFD32569.1 and AFD32570.1 for FcγRIIIa with the substitution of an avitag rather than sortase A site upstream of the 6-his tag. After clone selection and sequence verification, receptors were transiently expressed in HEK293-F cells as described above.
Antibody and FcγR purification
The preparation of VRC01 variants and human, as well as MM FcγRs has been described in detail previously.49,65 Briefly, after centrifugation and 0.2 μm membrane filtration (Steritop Express, EMD-Millipore), VRC01 production supernatants were loaded onto a Mabselect Protein A column (GE), washed with phosphate-buffered saline (PBS; Teknova) and eluted with 100 mM glycine pH 3.0. The peaks were neutralized with 1 M Tris-Cl at pH 10.5, then filtered (Steriflip, EMD-Millipore), concentrated with a 10 kD membrane (Amicon Ultra-15, EMD-Millipore) and loaded over a size-exclusion chromatography (SEC) column, HiPrep 16/60 Sephacryl S-200 HR (GE), operating under the manufacturer's recommendations with PBS to remove aggregates. IgG1, IgG2, IgG4 (Athens Research) and IgG3 (Sigma Aldrich) from myeloma plasma and serum IgG1, IgG2 and IgG3 from healthy donors (Athens Research) were also purified using a Sephacryl S-200 (GE) SEC column to remove aggregates. FcγRs were purified via immobilized metal affinity chromatography. Filtered supernatants were fortified to 500 mM NaCl, 20 mM sodium phosphate and 20 mM imidazole using buffer concentrates, loaded onto nickel-charged Sepharose 4 Fast Flow column (GE), washed with 500 mM NaCl, 20 mM sodium phosphate, then washed with 20 mM imidazole pH 7.5 followed by isocratic elution with 250 mM imidazole. The eluted fraction was filtered (Steriflip, EMD-Millipore), concentrated with a 10 kD (Amicon Ultra-15, EMD-Millipore) and loaded onto a 120 ml Superdex 75 SEC column (GE) operating under the manufacturer's recommendations with PBS to isolate the monomeric fraction. Appropriate molecular weight and purity of all recombinant protein was confirmed by SDS-PAGE.
Kinetic measurements
SPR was used to measure equilibrium binding affinities. A Continuous Flow Microspotter (CFM) (Wasatch Microfluidics) was used to print up to 96 individual regions of interests (ROI) on a single gold prism surface functionalized with carboxymethyldextran substrate (200M Xantec Bioanalytics). CFM fluid paths were primed with 25 mM sodium acetate pH 5.0 + 0.01% Tween 20 before activation. The substrate of each ROI was activated for 5–7 min with 100 μL of 1.2 mM N-hydroxysulfosuccinimide (NHS) (Pierce) and 0.3 mM 1-ethyl-3-[3 dimethlyaminopropyl]carbodiimide-HCl (EDC) (Pierce) in deionized water under flow at 45 μL/min. Antibodies prepared at 50, 25, 12.5 and 6.15 μg/ml in 25 mM sodium acetate pH 4.5–5.0 and printed on the activated ROI at the 4 resulting ligand (IgG) densities. The image-based array reader (MX96, IBIS Technologies) was primed with 25 mM sodium acetate pH 5.0 + 0.01% Tween 20 and the prism loaded and quenched with 120 μL of 0.5 M ethanolamine (Sigma Aldrich), before priming, conditioning and analyte injections. FcγRs were diluted in running buffer (PBS + 0.01% BSA + 0.005% Tween 20) and injected over an 8-part, 3-fold serial dilution series, generally starting between 10 μM to 50 μM, and consisting of the following steps: 0.5 min baseline, 5 min association, 5 min dissociation, 0.5 min baseline. The prism was regenerated with 0.5 min 100 mM glycine pH 3.0, and 0.5 min baseline between each analyte (FcγR) dilution tested. ROI signal was double referenced using signal from blank injections and signal from uncoupled interspots to account for nonspecific binding. Data was processed in Scrubber 2 (Biologic Software Ltd) by kinetic analysis applying global analysis to determine the equilibrium dissociation constant KD. A panel of serum derived IgGs, mAbs and Fc variants were used as ligands and a genetically aglycosylated human IgG1 (N297Q) was used as a negative control. Excellent relative agreement was observed between these results and published affinities for human FcγRs,2,6 although an absolute affinity offset was apparent, and could be attributed to differing chip coupling strategies. Given these different experimental setups and protein sources/preparations, relative but not absolute affinities observed here are comparable to those observed in other studies.2,6,55,60 Experiments were repeated 2 to 12 times with different conjugation densities and print pH conditions. Data from 2 separate experiments, with multiple print spots for each IgG sample, are presented.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery under grant OPP1114729; and the National Institute of Allergy and Infectious Disease under grants R01AI102691 and P01AI120758.
References
- 1.Salfeld JG. Isotype selection in antibody engineering. Nat Biotechnol 2007; 25:1369-72; PMID:18066027; http://dx.doi.org/ 10.1038/nbt1207-1369 [DOI] [PubMed] [Google Scholar]
- 2.Richards JO, Karki S, Lazar GA, Chen H, Dang W, Desjarlais JR. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Ther 2008; 7:2517-27; PMID:18723496; http://dx.doi.org/ 10.1158/1535-7163.MCT-08-0201 [DOI] [PubMed] [Google Scholar]
- 3.Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, et al.. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A 2006; 103:4005-10; PMID:16537476; http://dx.doi.org/ 10.1073/pnas.0508123103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mimoto F, Katada H, Kadono S, Igawa T, Kuramochi T, Muraoka M, Wada Y, Haraya K, Miyazaki T, Hattori K. Engineered antibody Fc variant with selectively enhanced FcgammaRIIb binding over both FcgammaRIIa(R131) and FcgammaRIIa(H131). Protein Eng Des Sel 2013; 26:589-98; PMID:23744091; http://dx.doi.org/ 10.1093/protein/gzt022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu SY, Vostiar I, Karki S, Moore GL, Lazar GA, Pong E, Joyce PF, Szymkowski DE, Desjarlais JR. Inhibition of B cell receptor-mediated activation of primary human B cells by coengagement of CD19 and FcgammaRIIb with Fc-engineered antibodies. Mol Immunol 2008; 45:3926-33; PMID:18691763; http://dx.doi.org/ 10.1016/j.molimm.2008.06.027 [DOI] [PubMed] [Google Scholar]
- 6.Moore GL, Chen H, Karki S, Lazar GA. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. MAbs 2010; 2:181-9; PMID:20150767; http://dx.doi.org/ 10.4161/mabs.2.2.11158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith P, DiLillo DJ, Bournazos S, Li F, Ravetch JV. Mouse model recapitulating human Fcgamma receptor structural and functional diversity. Proc Natl Acad Sci U S A 2012; 109:6181-6; PMID:22474370; http://dx.doi.org/ 10.1073/pnas.1203954109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, et al.. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem 2001; 276:6591-604; PMID:11096108; http://dx.doi.org/ 10.1074/jbc.M009483200 [DOI] [PubMed] [Google Scholar]
- 9.Kelton W, Mehta N, Charab W, Lee J, Lee CH, Kojima T, Kang TH, Georgiou G. IgGA: a “cross-isotype” engineered human Fc antibody domain that displays both IgG-like and IgA-like effector functions. Chem Biol 2014; 21:1603-9; PMID:25500223; http://dx.doi.org/ 10.1016/j.chembiol.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 10.Ferrant JL, Benjamin CD, Cutler AH, Kalled SL, Hsu YM, Garber EA, Hess DM, Shapiro RI, Kenyon NS, Harlan DM, et al.. The contribution of Fc effector mechanisms in the efficacy of anti-CD154 immunotherapy depends on the nature of the immune challenge. Int Immunol 2004; 16:1583-94; PMID:15466914; http://dx.doi.org/ 10.1093/intimm/dxh162 [DOI] [PubMed] [Google Scholar]
- 11.Hinton PR, Johlfs MG, Xiong JM, Hanestad K, Ong KC, Bullock C, Keller S, Tang MT, Tso JY, Vasquez M, et al.. Engineered human IgG antibodies with longer serum half-lives in primates. J Biol Chem 2004; 279:6213-6; PMID:14699147; http://dx.doi.org/ 10.1074/jbc.C300470200 [DOI] [PubMed] [Google Scholar]
- 12.Li Q, Zeng M, Duan L, Voss JE, Smith AJ, Pambuccian S, Shang L, Wietgrefe S, Southern PJ, Reilly CS, et al.. Live simian immunodeficiency virus vaccine correlate of protection: local antibody production and concentration on the path of virus entry. J Immunol 2014; 193:3113-25; http://dx.doi.org/ 10.4049/jimmunol.1400820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baruah K, Bowden TA, Krishna BA, Dwek RA, Crispin M, Scanlan CN. Selective deactivation of serum IgG: a general strategy for the enhancement of monoclonal antibody receptor interactions. J Mol Biol 2012; 420:1-7; PMID:22484364; http://dx.doi.org/ 10.1016/j.jmb.2012.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolton GR, Ackerman ME, Boesch AW. Separation of nonfucosylated antibodies with immobilized FcgammaRIII receptors. Biotechnol Prog 2013; 29:825-8; PMID:23554380; http://dx.doi.org/ 10.1002/btpr.1717 [DOI] [PubMed] [Google Scholar]
- 15.Hodoniczky J, Zheng YZ, James DC. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol Prog 2005; 21:1644-52; PMID:16321047; http://dx.doi.org/ 10.1021/bp050228w [DOI] [PubMed] [Google Scholar]
- 16.Imai-Nishiya H, Mori K, Inoue M, Wakitani M, Iida S, Shitara K, Satoh M. Double knockdown of alpha1,6-fucosyltransferase (FUT8) and GDP-mannose 4,6-dehydratase (GMD) in antibody-producing cells: a new strategy for generating fully non-fucosylated therapeutic antibodies with enhanced ADCC. BMC Biotechnol 2007; 7:84; PMID:18047682; http://dx.doi.org/ 10.1186/1472-6750-7-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mimoto F, Igawa T, Kuramochi T, Katada H, Kadono S, Kamikawa T, Shida-Kawazoe M, Hattori K. Novel asymmetrically engineered antibody Fc variant with superior FcgammaR binding affinity and specificity compared with afucosylated Fc variant. MAbs 2013; 5:229-36; PMID:23406628; http://dx.doi.org/ 10.4161/mabs.23452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niwa R, Natsume A, Uehara A, Wakitani M, Iida S, Uchida K, Satoh M, Shitara K. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J Immunol Methods 2005; 306:151-60; PMID:16219319; http://dx.doi.org/ 10.1016/j.jim.2005.08.009 [DOI] [PubMed] [Google Scholar]
- 19.Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem 2002; 277:26733-40; PMID:11986321; http://dx.doi.org/ 10.1074/jbc.M202069200 [DOI] [PubMed] [Google Scholar]
- 20.Umana P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol 1999; 17:176-80; PMID:10052355; http://dx.doi.org/ 10.1038/6179 [DOI] [PubMed] [Google Scholar]
- 21.Zou G, Ochiai H, Huang W, Yang Q, Li C, Wang LX. Chemoenzymatic synthesis and Fcgamma receptor binding of homogeneous glycoforms of antibody Fc domain. Presence of a bisecting sugar moiety enhances the affinity of Fc to FcgammaIIIa receptor. J Am Chem Soc 2011; 133:18975-91; http://dx.doi.org/ 10.1021/ja208390n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullard A. 2013 FDA drug approvals. Nat Rev Drug Discov 2014; 13:85-9; http://dx.doi.org/ 10.1038/nrd4239 [DOI] [PubMed] [Google Scholar]
- 23.Forero-Torres A, de Vos S, Pohlman BL, Pashkevich M, Cronier DM, Dang NH, Carpenter SP, Allan BW, Nelson JG, Slapak CA, et al.. Results of a phase 1 study of AME-133v (LY2469298), an Fc-engineered humanized monoclonal anti-CD20 antibody, in FcgammaRIIIa-genotyped patients with previously treated follicular lymphoma. Clin Cancer Res 2012; 18:1395-403; PMID:22223529; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-0850 [DOI] [PubMed] [Google Scholar]
- 24.Tobinai K, Ogura M, Kobayashi Y, Uchida T, Watanabe T, Oyama T, Maruyama D, Suzuki T, Mori M, Kasai M, et al.. Phase I study of LY2469298, an Fc-engineered humanized anti-CD20 antibody, in patients with relapsed or refractory follicular lymphoma. Cancer Sci 2011; 102:432-8; PMID:21205069; http://dx.doi.org/ 10.1111/j.1349-7006.2010.01809.x [DOI] [PubMed] [Google Scholar]
- 25.Strohl WR. Optimization of Fc-mediated effector functions of monoclonal antibodies. Curr Opin Biotechnol 2009; 20:685-91; PMID:19896358; http://dx.doi.org/ 10.1016/j.copbio.2009.10.011 [DOI] [PubMed] [Google Scholar]
- 26.Sullivan NJ, Martin JE, Graham BS, Nabel GJ. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat Rev Microbiol 2009; 7:393-400; PMID:19369954; http://dx.doi.org/ 10.1038/nrmicro2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehrlich PH, Harfeldt KE, Justice JC, Moustafa ZA, Ostberg L. Rhesus monkey responses to multiple injections of human monoclonal antibodies. Hybridoma 1987; 6:151-60; PMID:3494660; http://dx.doi.org/ 10.1089/hyb.1987.6.151 [DOI] [PubMed] [Google Scholar]
- 28.Jonker M, van Lambalgen R, Mitchell DJ, Durham SK, Steinman L. Successful treatment of EAE in rhesus monkeys with MHC class II specific monoclonal antibodies. J Autoimmun 1988; 1:399-414; PMID:3254183; http://dx.doi.org/ 10.1016/0896-8411(88)90064-9 [DOI] [PubMed] [Google Scholar]
- 29.Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchanan K, JH Fechner Jr., Germond RL, Kampen RL, Patterson NB, et al.. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med 1999; 5:686-93; PMID:10371508; http://dx.doi.org/ 10.1038/9536 [DOI] [PubMed] [Google Scholar]
- 30.Fiedler VB, Loof I, Sander E, Voehringer V, Galanos C, Fournel MA. Monoclonal antibody to tumor necrosis factor–alpha prevents lethal endotoxin sepsis in adult rhesus monkeys. J Lab Clin Med 1992; 120:574-88; PMID:1402333 [PubMed] [Google Scholar]
- 31.Pal P, Fox JM, Hawman DW, Huang YJ, Messaoudi I, Kreklywich C, Denton M, Legasse AW, Smith PP, Johnson S, et al.. Chikungunya viruses that escape monoclonal antibody therapy are clinically attenuated, stable, and not purified in mosquitoes. J Virol 2014; 88:8213-26; PMID:24829346; http://dx.doi.org/ 10.1128/JVI.01032-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oswald WB, Geisbert TW, Davis KJ, Geisbert JB, Sullivan NJ, Jahrling PB, Parren PW, Burton DR. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLoS Pathog 2007; 3:e9; PMID:17238286; http://dx.doi.org/ 10.1371/journal.ppat.0030009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.GG Olinger Jr., Pettitt J, Kim D, Working C, Bohorov O, Bratcher B, Hiatt E, Hume SD, Johnson AK, Morton J, et al.. Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proc Natl Acad Sci U S A 2012; 109:18030-5; PMID:23071322; http://dx.doi.org/ 10.1073/pnas.1213709109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai CJ. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc Natl Acad Sci U S A 2007; 104:9422-7; PMID:17517625; http://dx.doi.org/ 10.1073/pnas.0703498104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai CJ, Goncalvez AP, Men R, Wernly C, Donau O, Engle RE, Purcell RH. Epitope determinants of a chimpanzee dengue virus type 4 (DENV-4)-neutralizing antibody and protection against DENV-4 challenge in mice and rhesus monkeys by passively transferred humanized antibody. J Virol 2007; 81:12766-74; PMID:17881450; http://dx.doi.org/ 10.1128/JVI.01420-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weltzin R, Traina-Dorge V, Soike K, Zhang JY, Mack P, Soman G, Drabik G, Monath TP. Intranasal monoclonal IgA antibody to respiratory syncytial virus protects rhesus monkeys against upper and lower respiratory tract infection. J Infect Dis 1996; 174:256-61; PMID:8699052; http://dx.doi.org/ 10.1093/infdis/174.2.256 [DOI] [PubMed] [Google Scholar]
- 37.Miyoshi-Akiyama T, Ishida I, Fukushi M, Yamaguchi K, Matsuoka Y, Ishihara T, Tsukahara M, Hatakeyama S, Itoh N, Morisawa A, et al.. Fully human monoclonal antibody directed to proteolytic cleavage site in severe acute respiratory syndrome (SARS) coronavirus S protein neutralizes the virus in a rhesus macaque SARS model. J Infect Dis 2011; 203:1574-81; PMID:21592986; http://dx.doi.org/ 10.1093/infdis/jir084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, et al.. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med 2000; 6:200-6; PMID:10655110; http://dx.doi.org/ 10.1038/72309 [DOI] [PubMed] [Google Scholar]
- 39.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, et al.. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 2007; 449:101-4; PMID:17805298; http://dx.doi.org/ 10.1038/nature06106 [DOI] [PubMed] [Google Scholar]
- 40.Moldt B, Shibata-Koyama M, Rakasz EG, Schultz N, Kanda Y, Dunlop DC, Finstad SL, Jin C, Landucci G, Alpert MD, et al.. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol 2012; 86:6189-96; PMID:22457527; http://dx.doi.org/ 10.1128/JVI.00491-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, et al.. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 2000; 6:207-10; PMID:10655111; http://dx.doi.org/ 10.1038/72318 [DOI] [PubMed] [Google Scholar]
- 42.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, et al.. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med 2003; 9:343-6; PMID:12579198; http://dx.doi.org/ 10.1038/nm833 [DOI] [PubMed] [Google Scholar]
- 43.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol 2001; 75:8340-7; PMID:11483779; http://dx.doi.org/ 10.1128/JVI.75.17.8340-8347.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, et al.. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A 2012; 109:18921-5; PMID:23100539; http://dx.doi.org/ 10.1073/pnas.1214785109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 2009; 15:951-4; PMID:19525965; http://dx.doi.org/ 10.1038/nm.1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science 1988; 239:476-81; PMID:3277268; http://dx.doi.org/ 10.1126/science.3277268 [DOI] [PubMed] [Google Scholar]
- 47.Kontny U, Kurane I, Ennis FA. Gamma interferon augments Fc gamma receptor-mediated dengue virus infection of human monocytic cells. J Virol 1988; 62:3928-33; PMID:2459406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen DC, Scinicariello F, Attanasio R. Characterization and allelic polymorphisms of rhesus macaque (Macaca mulatta) IgG Fc receptor genes. Immunogenetics 2011; 63:351-62; PMID:21327607; http://dx.doi.org/ 10.1007/s00251-011-0514-z [DOI] [PubMed] [Google Scholar]
- 49.Chan YN, Boesch AW, Osei-Owusu NY, Emileh A, Crowley AR, Cocklin SL, Finstad SL, Linde CH, Howell RA, Zentner I, et al.. IgG binding characteristics of rhesus macaque FcgammaR. J Immunol 2016; 197:2936-47; http://dx.doi.org/ 10.4049/jimmunol.1502252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warncke M, Calzascia T, Coulot M, Balke N, Touil R, Kolbinger F, Heusser C. Different adaptations of IgG effector function in human and nonhuman primates and implications for therapeutic antibody treatment. J Immunol 2012; 188:4405-11; http://dx.doi.org/ 10.4049/jimmunol.1200090 [DOI] [PubMed] [Google Scholar]
- 51.Miller CJ, Genesca M, Abel K, Montefiori D, Forthal D, Bost K, Li J, Favre D, McCune JM. Antiviral antibodies are necessary for control of simian immunodeficiency virus replication. J Virol 2007; 81:5024-35; PMID:17329327; http://dx.doi.org/ 10.1128/JVI.02444-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandilands GP, MacPherson SA, Burnett ER, Russell AJ, Downie I, MacSween RN. Differential expression of CD32 isoforms following alloactivation of human T cells. Immunology 1997; 91:204-11; PMID:9227318; http://dx.doi.org/ 10.1046/j.1365-2567.1997.00241.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fanger NA, Wardwell K, Shen L, Tedder TF, Guyre PM. Type I (CD64) and type II (CD32) Fc gamma receptor-mediated phagocytosis by human blood dendritic cells. J Immunol 1996; 157:541-8. [PubMed] [Google Scholar]
- 54.Hogarth PM, Pietersz GA. Fc receptor-targeted therapies for the treatment of inflammation, cancer and beyond. Nat Rev Drug Discov 2012; 11:311-31; PMID:22460124; http://dx.doi.org/ 10.1038/nrd2909 [DOI] [PubMed] [Google Scholar]
- 55.Trist HM, Tan PS, Wines BD, Ramsland PA, Orlowski E, Stubbs J, Gardiner EE, Pietersz GA, Kent SJ, Stratov I, et al.. Polymorphisms and interspecies differences of the activating and inhibitory FcgammaRII of Macaca nemestrina influence the binding of human IgG subclasses. J Immunol 2014; 192:792-803; http://dx.doi.org/ 10.4049/jimmunol.1301554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen K, Nishi H, Travers R, Tsuboi N, Martinod K, Wagner DD, Stan R, Croce K, Mayadas TN. Endocytosis of soluble immune complexes leads to their clearance by FcgammaRIIIB but induces neutrophil extracellular traps via FcgammaRIIA in vivo. Blood 2012; 120:4421-31; PMID:22955924; http://dx.doi.org/ 10.1182/blood-2011-12-401133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Behnen M, Leschczyk C, Moller S, Batel T, Klinger M, Solbach W, Laskay T. Immobilized immune complexes induce neutrophil extracellular trap release by human neutrophil granulocytes via FcgammaRIIIB and Mac-1. J Immunol 2014; 193:1954-65 ; http://dx.doi.org/ 10.4049/jimmunol.1400478 [DOI] [PubMed] [Google Scholar]
- 58.Shibata-Koyama M, Iida S, Misaka H, Mori K, Yano K, Shitara K, Satoh M. Nonfucosylated rituximab potentiates human neutrophil phagocytosis through its high binding for FcgammaRIIIb and MHC class II expression on the phagocytotic neutrophils. Exp Hematol 2009; 37:309-21; PMID:19218011; http://dx.doi.org/ 10.1016/j.exphem.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 59.Derer S, Glorius P, Schlaeth M, Lohse S, Klausz K, Muchhal U, Desjarlais JR, Humpe A, Valerius T, Peipp M. Increasing FcgammaRIIa affinity of an FcgammaRIII-optimized anti-EGFR antibody restores neutrophil-mediated cytotoxicity. MAbs 2014; 6:409-21; PMID:24492248; http://dx.doi.org/ 10.4161/mabs.27457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009; 113:3716-25; PMID:19018092; http://dx.doi.org/ 10.1182/blood-2008-09-179754 [DOI] [PubMed] [Google Scholar]
- 61.Ramsland PA, Farrugia W, Bradford TM, Sardjono CT, Esparon S, Trist HM, Powell MS, Tan PS, Cendron AC, Wines BD, et al.. Structural basis for Fc gammaRIIa recognition of human IgG and formation of inflammatory signaling complexes. J Immunol 2011; 187:3208-17; http://dx.doi.org/ 10.4049/jimmunol.1101467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature 2000; 406:267-73; PMID:10917521; http://dx.doi.org/ 10.1038/35018508 [DOI] [PubMed] [Google Scholar]
- 63.Ackerman ME, Nimmerjahn F. Antibody Fc: linking adaptive and innate immunity. Amsterdam, Burlington: Elsevier/Academic Press, 2014. [Google Scholar]
- 64.Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, et al.. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A 2011; 108:12669-74; PMID:21768335; http://dx.doi.org/ 10.1073/pnas.1108455108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boesch AW, Brown EP, Cheng HD, Ofori MO, Normandin E, Nigrovic PA, Alter G, Ackerman ME. Highly parallel characterization of IgG Fc binding interactions. MAbs 2014; 6:915-27; PMID:24927273; http://dx.doi.org/ 10.4161/mabs.28808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kanda Y, Yamada T, Mori K, Okazaki A, Inoue M, Kitajima-Miyama K, Kuni-Kamochi R, Nakano R, Yano K, Kakita S, et al.. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types. Glycobiology 2007; 17:104-18; PMID:17012310; http://dx.doi.org/ 10.1093/glycob/cwl057 [DOI] [PubMed] [Google Scholar]
- 67.Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest 2005; 115:2914-23; PMID:16167082; http://dx.doi.org/ 10.1172/JCI24772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rogers KA, Scinicariello F, Attanasio R. IgG Fc receptor III homologues in nonhuman primate species: genetic characterization and ligand interactions. Journal of immunology 2006; 177:3848-56; http://dx.doi.org/ 10.4049/jimmunol.177.6.3848 [DOI] [PubMed] [Google Scholar]
- 69.Shatz W, Chung S, Li B, Marshall B, Tejada M, Phung W, Sandoval W, Kelley RF, Scheer JM. Knobs-into-holes antibody production in mammalian cell lines reveals that asymmetric afucosylation is sufficient for full antibody-dependent cellular cytotoxicity. MAbs 2013; 5:872-81; PMID:23995614; http://dx.doi.org/ 10.4161/mabs.26307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi EI, Wang R, Peterson L, Letvin NL, Reimann KA. Use of an anti-CD16 antibody for in vivo depletion of natural killer cells in rhesus macaques. Immunology 2008; 124:215-22; PMID:18201184; http://dx.doi.org/ 10.1111/j.1365-2567.2007.02757.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Furness AJ, Vargas FA, Peggs KS, Quezada SA. Impact of tumour microenvironment and Fc receptors on the activity of immunomodulatory antibodies. Trends Immunol 2014; 35:290-8; PMID:24953012; http://dx.doi.org/ 10.1016/j.it.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 72.Eastwood D, Findlay L, Poole S, Bird C, Wadhwa M, Moore M, Burns C, Thorpe R, Stebbings R. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br J Pharmacol 2010; 161:512-26; PMID:20880392; http://dx.doi.org/ 10.1111/j.1476-5381.2010.00922.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJ, et al.. Complement is activated by IgG hexamers assembled at the cell surface. Science 2014; 343:1260-3; PMID:24626930; http://dx.doi.org/ 10.1126/science.1248943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Poel CE, Spaapen RM, van de Winkel JG, Leusen JH. Functional characteristics of the high affinity IgG receptor, FcgammaRI. J Immunol 2011; 186:2699-704; http://dx.doi.org/ 10.4049/jimmunol.1003526 [DOI] [PubMed] [Google Scholar]
- 75.de Lange GG. Polymorphisms of human immunoglobulins: Gm, Am, Em and Km allotypes. Exp Clin Immunogenet 1989; 6:7-17; PMID:2698222 [PubMed] [Google Scholar]
- 76.Kumpel BM, Wiener E, Urbaniak SJ, Bradley BA. Human monoclonal anti-D antibodies. II. The relationship between IgG subclass, Gm allotype and Fc mediated function. Br J Haematol 1989; 71:415-20; http://dx.doi.org/ 10.1111/j.1365-2141.1989.tb04300.x [DOI] [PubMed] [Google Scholar]
- 77.Vidarsson G, Stemerding AM, Stapleton NM, Spliethoff SE, Janssen H, Rebers FE, de Haas M, van de Winkel JG. FcRn: an IgG receptor on phagocytes with a novel role in phagocytosis. Blood 2006; 108:3573-9; PMID:16849638; http://dx.doi.org/ 10.1182/blood-2006-05-024539 [DOI] [PubMed] [Google Scholar]
- 78.Neuber T, Frese K, Jaehrling J, Jager S, Daubert D, Felderer K, Linnemann M, Hohne A, Kaden S, Kolln J, et al.. Characterization and screening of IgG binding to the neonatal Fc receptor. MAbs 2014; 6:928-42; PMID:24802048; http://dx.doi.org/ 10.4161/mabs.28744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Monnet C, Jorieux S, Souyris N, Zaki O, Jacquet A, Fournier N, Crozet F, de Romeuf C, Bouayadi K, Urbain R, et al.. Combined glyco- and protein-Fc engineering simultaneously enhance cytotoxicity and half-life of a therapeutic antibody. MAbs 2014; 6:422-36; PMID:24492301; http://dx.doi.org/ 10.4161/mabs.27854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Santra S, Tomaras GD, Warrier R, Nicely NI, Liao HX, Pollara J, Liu P, Alam SM, Zhang R, Cocklin SL, et al.. Human non-neutralizing HIV-1 envelope monoclonal antibodies limit the number of founder viruses during SHIV mucosal infection in rhesus macaques. PLoS Pathog 2015; 11:e1005042; PMID:26237403; http://dx.doi.org/ 10.1371/journal.ppat.1005042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sestak K, Scheiners C, Wu XW, Hollemweguer E. Identification of anti-human CD antibodies reactive with rhesus macaque peripheral blood cells. Vet Immunol Immunopathol 2007; 119:21-6; PMID:17681612; http://dx.doi.org/ 10.1016/j.vetimm.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 82.Carter DL, Shieh TM, Blosser RL, Chadwick KR, Margolick JB, Hildreth JE, Clements JE, Zink MC. CD56 identifies monocytes and not natural killer cells in rhesus macaques. Cytometry 1999; 37:41-50; PMID:10451505; http://dx.doi.org/ 10.1002/(SICI)1097-0320(19990901)37:1%3c41::AID-CYTO5%3e3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- 83.O'Doherty U, Ignatius R, Bhardwaj N, Pope M. Generation of monocyte-derived dendritic cells from precursors in rhesus macaque blood. J Immunol Methods 1997; 207:185-94; PMID:9368645; http://dx.doi.org/ 10.1016/S0022-1759(97)00119-1 [DOI] [PubMed] [Google Scholar]
- 84.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, et al.. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 2010; 329:856-61; PMID:20616233; http://dx.doi.org/ 10.1126/science.1187659 [DOI] [PMC free article] [PubMed] [Google Scholar]


