Abstract
Background
Assessing the serum reactivity to HLA is essential for the evaluation of transplant candidates and the follow-up of allograft recipients. In this study, we look for evidence at the clonal level that polyreactive antibodies cross-reactive to apoptotic cells and multiple autoantigens can also react to HLA and contribute to the overall serum reactivity.
Methods
We immortalized B cell clones from the blood of two kidney transplant recipients and characterized their reactivity to self-antigens, apoptotic cells as well as native, denatured and cryptic HLA determinants using ELISA, immunofluorescence, flow cytometry and Luminex assays. We also assessed the reactivity of 300 pre-transplant serum specimens to HLA and apoptotic cells.
Results
We report here 4 distinct B cell clones cross-reactive to self and HLA class I. All 4 clones reacted to numerous HLA class I alleles but did not appear to target canonical “shared” epitopes. In parallel experiments, we observed a strong correlation between IgG reactivity to HLA and apoptotic cells in pre-transplant serum samples collected from 300 kidney transplant recipients. Further analysis revealed that samples with higher reactivity to apoptotic cells displayed significantly higher class I percent PRA compared to samples with low reactivity to apoptotic cells.
Conclusions
We provide here 1) proof of principle at the clonal level that human polyreactive antibodies can cross-react to HLA, multiple self-antigens and apoptotic cells and 2) supportive evidence that polyreactive antibodies contribute to overall HLA reactivity in the serum of patients awaiting kidney transplant.
Introduction
The detection of serum anti-HLA antibodies is essential to assess sensitization of transplant candidates or to follow the development of humoral immunity after transplantation. Over the past years, Luminex based assays, using beads coated with single HLA allelic molecules have become the preferred techniques employed by a majority of transplant centers. These assays offer increased sensitivity when compared to previous methods such as complement dependent cytotoxicity (CDC)1. However, such unprecedented sensitivity has also raised concerns about the clinical relevance of some of the results. In particular, serum HLA reactivity can be detected in healthy donors without evidence of prior immunization2. Likewise, a significant fraction of transplant candidates appears to be sensitized in the absence of any known prior immunizing event such as blood transfusion or previous transplants3, 4. The cause of these spontaneous HLA reactive antibodies is currently unknown. Other cases are kidney allograft recipients who develop antibodies reactive to HLA molecules not expressed by the donor cells after transplantation. These antibodies, called “non-donor specific antibodies” (NDSA) largely contribute to the gradual increase in panel reactive antibodies (PRA) post-transplant5-9. How NDSA develop is still uncertain. The most widely accepted explanation is that NDSA and DSA cross-react to public epitopes shared by multiple HLA6, 10-12. However, in the absence of DSA and when patients do not have history of prior immunizing events, the development of NDSA remains unexplained.
In previous studies, we isolated a B cell clone from a kidney transplant recipient with antibody-mediated rejection (AMR) that secreted a broadly reactive monoclonal antibody that cross-reacted to multiple self-antigens as well as several HLA class I molecules13. This polyreactive antibody also bound apoptotic cells, a known characteristic of “natural antibodies”, which allegedly develop without the need for antigen exposure. This observation provided proof of principle that natural antibodies can cross-react to HLA and therefore contribute to serum reactivity assessed by Luminex. The significance of this contribution, however, was uncertain. It was particularly unclear whether this peculiar clone cross-reactive to HLA and self-antigens was an exception or was representative of an overlooked category of polyreactive B cells. Here we extended our investigation to look for evidence of additional clones displaying the same reactivity patterns. Taking advantage of their capacity to react to apoptotic cells, we also evaluated the contribution of polyreactive antibodies to the serum reactivity to HLA in pre-transplant serum samples collected from 300 kidney transplant recipients treated at Massachusetts General Hospital (MGH) between 1999 and 2007.
Materials and Methods
Patient characteristics and sample collection
The collection of all specimens used in this study was approved by the MGH internal review board. The patient group consisted of 300 non-consecutive kidney transplant recipients treated at MGH between May 1999 and July 2007 and whose pre-transplant serum specimens were available. All serum specimens were collected prior to transplantation as part of the patients’ standard clinical care. The detailed characteristics of the patient population have been reported in a previous publication14. A succinct summary is provided in Table S1.
Isolation and immortalization of B cell clones
B cells clones described in this manuscript were derived either from a cell suspension isolated from multiple fragments of an explanted kidney graft13 (clones 4G4 and 4G10) or from PBMC collected one year post transplant from a kidney transplant recipient (clone 2B4 and 2D9). EBV-transformed B cell clones were generated by incubating immunopurified B cells (CD20 MicroBeads, Miltenyi Biotech, Auburn, CA, USA) with supernatant from EBV-producing B95-8 marmoset cells in presence of the TLR9 agonist CpG 2006 at 2.5 μg/mL (InvivoGen, San Diego, CA). Limiting dilution cloning was performed in 96 well plates by plating serial dilutions of cells on a layer of feeder cells consisting of 50,000 irradiated THP-1 cells or Jurkat cells per well. Once established, clones were maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 4 mM glutamine, 1 mM sodium pyruvate, and 10 mM Hepes and antibiotics. Clonality was confirmed by molecular analysis of rearranged Ig heavy chain genes as described below.
Serum IgG purification
Serum IgG were purified from patient specimens using the Melon Gel IgG Purification Kit (Thermo Scientific, Rockford, IL) according to the manufacturer’s instructions. Briefly, serum samples were diluted 1:10 in a dilution buffer and incubated with a resin that retains non-IgG immunoglobulin as well as other abundant serum proteins. Purified IgG was used to assess its reactivity to apoptotic Jurkat cells.
Flow Cytometry
We used flow cytometry to assess the reactivity of cell culture supernatants and purified IgG from 300 patients to apoptotic Jurkat cells. Jurkat cells were exposed to UV light (240×10−3 J) to induce apoptosis using a UV stratalinker 2400 (Stratagene, Santa Clara, CA). Then 1×106 apoptotic Jurkat cells were incubated for 30 minutes at 37°C with 80μl cell culture supernatants or 60μl purified IgG samples further diluted 1:2 in PBS (final dilution 1:20). After two washes in 3ml PBS at 4°C, samples were incubated with FITC-conjugated anti-IgM or anti-IgG F(ab’)2 secondary antibodies, respectively (Invitrogen, Camarillo, CA) for 30 minutes at 4°C. After two additional washes in 3ml PBS at 4°C, cells were acquired using an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA) after gating on apoptotic cells. To avoid inter-experiment variations, all samples were assessed at the same time in the same experiment using the same instrument settings. Results are reported as log2 values of the mean fluorescence intensity (MFI) of positive cells.
Detection of reactivity to HLA and MICA molecules
The reactivity of B cells clone supernatants as well as patient’s serum to HLA molecules was assessed using beads coated with either mixed HLA molecules (LABScreen Mixed, One Lambda, Los Angeles, CA) or a single HLA molecules (LABScreen Single Antigen HLA Class I and Class II, One Lambda). In addition, variations of the single antigen beads like the iBEADS, with reduced levels of denatured molecules, and acid-treated beads which have only denatured HLA class I molecules were used. To reveal cryptic HLA epitopes, SAB were treated with ImmunoPure IgG elution buffer (Pierce, Rockford, IL) as previously described12. Bound antibodies were detected with an anti-IgM (Invitrogen, Carlsbad, CA) or anti-IgG (One Lambda) PE-conjugated secondary antibody on a Luminex 200 apparatus (Luminex, Austin, TX).
ELISA assays
The immunoglobulin concentration of B cell culture supernatants was quantified using an IgM and IgG ELISA quantification kit (Bethyl Laboratories, Montgomery, TX) according to the manufacturer’s instructions. Samples were diluted 1:100 in PBS. ELISA for the detection of cell culture supernatants to lipopolysaccharide (LPS), double-stranded DNA (dsDNA) and insulin were performed as described previously15. Briefly, polystyrene plates (Corning Incorporated, Corning, NY) were coated overnight at 4°C with 10 μg/mL LPS, dsDNA or recombinant human insulin (Sigma Aldrich, St. Louis, MO). After five washes in PBS with 0.05% Tween 20, cell culture supernatants were added and incubated for 3h at room temperature. Plates were then washed in PBS with 0.05% Tween 20, incubated for 1h with horse radish peroxidase (HRP)-conjugated goat anti-human IgM (Invitrogen, Camarillo, CA), washed again, and developed using 3, 3’, 5, 5’-tetramethylbenzidine (eBioscience, San Diego, CA). Optical density was measured at 450 nm. ELISA for the detection of reactivity to whole protein extract from human embryonic kidney cell line (HEK-293) was performed as described previously15. Briefly, proteins were extracted in radioimmunoprecipitation assay buffer (Boston Bioproducts Inc, Worcester, MA) supplemented with protease inhibitor (Roche Diagnostics, Indianapolis, IN) and phenylmethanesulfonyl fluoride (Sigma-Aldrich). The protein extract was used to coat polystyrene plates (Corning Incorporated, Corning, NY) at the equivalent of 10,000 cells per well. The coated plates were used following the same ELISA procedure as the one described for LPS, dsDNA and insulin.
Immunofluorescence assays
Hep-2 cell coated slides (Bion Enterprises Ltd, Des Plaines, IL) were incubated at room temperature with cell culture supernatants 30 min, washed in PBS, stained with fluorescein isothiocyanate-conjugated anti-human IgM (Invitrogen, Camarillo, CA), washed with PBS, and visualized by fluorescence microscopy.
Molecular analysis of rearranged immunoglobulin heavy chain genes
Total RNA was extracted from immortalized B cell clones using PureLink RNA Mini Kit (Invitrogen, Carlsbad, CA). Superscript III reverse transcriptase kit (Invitrogen) was used to generate cDNA. Variable regions of the Ig heavy chain were then amplified by PCR using six family-specific forward primers (VH1-VH6) and a consensus JH reverse primer. Forward primers used for the PCR amplification of the 6 Ig VH families as well as the consensus JH have been described in a previous study13. The PCR conditions were as follows: 95°C/5′, (95°C/30′; 56°C/30′; 72°C/30′) ×35 cycles; 72°C/10′. PCR products were sequenced using the corresponding VH primer to confirm the clonality of the B cells by the identification of a unique rearranged CDR3 sequence. Sequencing was done at the Massachusetts General Hospital DNA core facility.
Serum adsorption on apoptotic cells
Serum IgG were purified from patients specimens using the Melon Gel IgG Purification Kit (Thermo Scientific, Rockford, IL) according to the manufacturer’s instructions. Purified IgG were adsorbed by incubation for 1 hour at 37°C with 10.106 apoptotic Jurkat cells followed by centrifugation to remove the cells. Adsorbed IgG were then assessed for their reactivity to HLA molecules by Luminex (Labscreen and SAB).
Statistical analysis
Mann-Whitney tests were performed to compare IgG reactivity to apoptotic cells between patients with high reactivity to HLA and patients with low reactivity to HLA, to compare the percentage of HLA antigens recognized by serum showing high reactivity to apoptotic cells with that recognized by serum showing low reactivity to apoptotic cells. We used Spearman correlation tests to determine the association between IgG reactivity to apoptotic cells and IgG reactivity to HLA class I, class II and MICA, respectively. All tests were two-sided and a P-value of < 0.05 was considered to be statistically significant. General data analysis was conducted using GraphPad Prism (GraphPad Software 5.0, San Diego, CA).
Results
B cells clones reactivity to HLA class I alleles
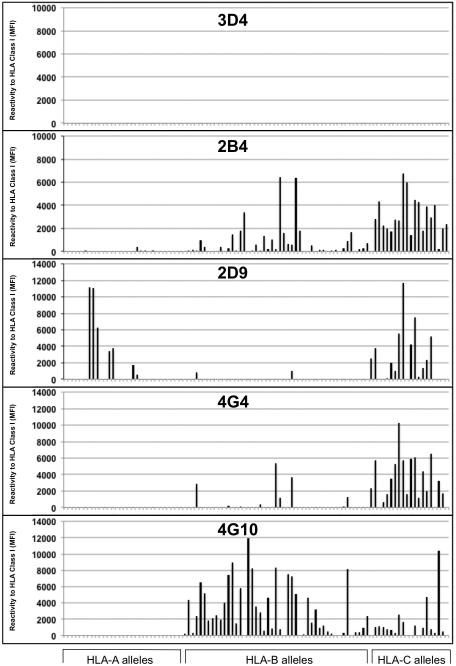
One hundred seven and 91 clones were generated and propagated in vitro from two different kidney transplant recipients respectively. All B cell clones secreted monoclonal antibodies in culture that were used to determine the reactivity of the corresponding B cells. HLA and MICA reactivity of culture supernatants from all clones were assessed by Luminex using beads coated with mixed HLA molecules (SAB). Two HLA or MICA reactive clones, 4G4 and 4G10, were identified in the first series of 107 B cell clones and their partial reactivity profiles have been described in a previous report13. Two additional HLA class I reactive clones, 2B4 and 2D9, were identified within the 91 clones generated from another kidney transplant recipient, suggesting that B cells displaying such reactivity are not exceptional. None of the remaining clones reacted to HLA beads. A comparison of the complete reactivity patterns to HLA class I molecules for the four clones is presented in Figure 1. A non-HLA-reactive clone (3D4) was used as control. As depicted in the figure, clone 2B4, 2D9, 4G4 and 4G10 bound to multiple HLA class I molecules, whereas clone 3D4 did not. A complete reactivity profile for the 5 clones is reported in supplementary Figures S1-S5. Remarkably, all four clones cross-reacted to at least one donor HLA antigen. 4G10 and 4G4 were isolated from a patient with low level DSA as previously reported13, whereas 2B4 and 2D9 were derived from the blood of a kidney transplant recipient without DSA.
Figure 1. Reactivity of B cell clones’ supernatants to HLA class I molecules.
The reactivity of non-polyreactive antibody 3D4 and polyreactive antibodies 2B4, 2D9, 4G4 and 4G10 to HLA class I antigens was tested by Luminex using single antigen beads. Each bar represents reactivity to one corresponding class I molecule. Antigens are grouped according to their loci (A, B and C).
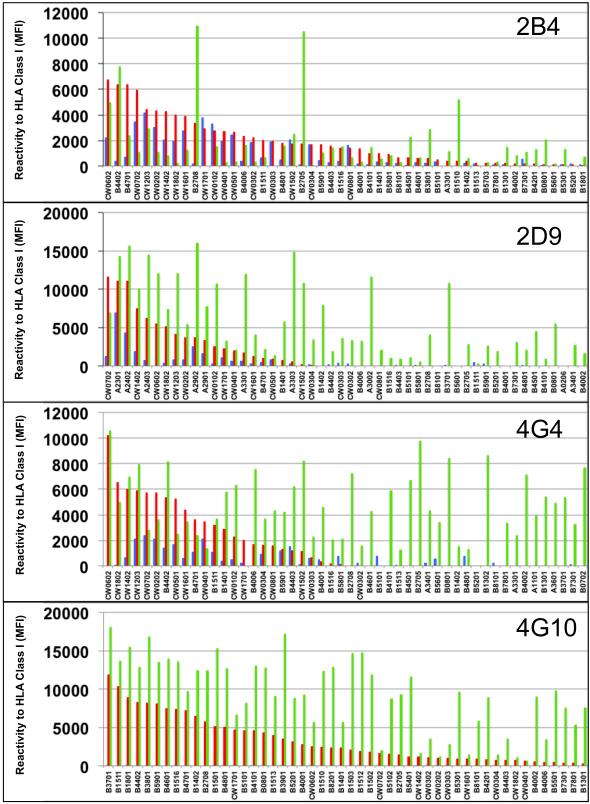
Regular SAB are coated with a combination of denatured and native antigens, whereas iBEADS are only coated with native antigens. It has also been reported that SAB treatment with acid reveals additional denatured epitopes often called cryptic12. To investigate whether the clones supernatants were reactive to native, cryptic or denatured epitopes on the HLA class I molecules, we tested their capacity to bind to iBEADS as well as regular SAB treated with acid. The 50 antigens towards which the clones reacted the most are reported in Figure 2. The complete reactivity profiles are included in supplementary Figures S1-S5. Overall, all four monoclonal antibodies reacted primarily to denatured, i.e. non-native antigens. However, each appeared to display a unique pattern of reactivity profile that also included the recognition of cryptic and native antigens:
Figure 2. Reactivity of B cell clones’ supernatants to HLA class I native, denatured and cryptic epitopes.
The reactivity of non-polyreactive antibody 3D4 and polyreactive antibodies 2B4, 2D9, 4G4 and 4G10 to HLA class I antigens was tested by Luminex using regular single antigen beads (orange bars), iBEADS (blue bars) and regular single antigen beads treated with acid (green bars). Each bar represents reactivity to one corresponding class I molecule labeled below. Only the 50 antigens towards which the supernatants reacted the most are depicted for each clone. Antigens are sorted based on the supernatant reactivity to regular single antigen beads. Complete reactivity profiles are reported in supplementary Figure S1-S5.
2B4
The 2B4 antibody reacted mainly with HLA-B and C antigens on all three types of beads. The strongest signal was seen with acid treated B*27:05 and B*27:08 beads. These two antigens share the cryptic epitope 501112, 16. However, all other antigens recognized by this clone (reactivity > 1000 MFI) do not share the same epitope. Clone 2B4 also reacted to most Cw antigens on iBEADS. These antigens share an Arginine at position 69 (69R).
2D9
Clone 2D9 reacted mainly with HLA-A and C locus antigens on regular SAB. No epitope is shared by all antigens recognized by 2D9. This clone displayed the strongest reactivity with A23, 24 and 29 on iBEADS but these antigens do not share an exclusive epitope. Analysis of the 2D9 reactivity to acid treated beads showed weak to strong binding to many A, B and C-locus antigens, which do not share a cryptic epitope.
4G4
The strongest reactivity was observed with C*06:02 on regular SAB although additional B and C antigens were also recognized. No known epitope is shared by all antigens that clone 4G4 reacts to. Using iBEADS, this clone reacted weakly to several antigens, including A69, B44, Cw2, 4, 5, 7 and 12. These antigens do not share an exclusive epitope. Moreover, 4G4 appeared to react to multiple cryptic epitopes on A, B, and C locus antigens as revealed by the use of acid treated beads. No exclusive epitope could be defined.
4G10
Unlike the other three clones, clone 4G10 showed no reactivity to iBEADS but reacted to a majority of B and most C locus antigens with standard SAB and acid treated beads. Epitope search did not reveal any exclusive cryptic epitope shared between all the positive antigens, suggesting a very broad reactivity pattern.
In parallel assays we also tested the reactivity of the 4 polyreactive clones to allogeneic PBMC by complement dependent cytotoxicity (CDC). However, all 4 clones were negative using this assay (data not shown).
Analysis of B cells clones’ immunoglobulin heavy chain variable regions
We had previously confirmed the monoclonality of 4G4 and 4G10 cells13. To verify that the two additional HLA reactive B cells (2B4 and 2D9) were also clonal, we analyzed the sequences of their rearranged immunoglobulin heavy chain genes by PCR. This analysis revealed a unique sequence with a distinct rearranged complementarity determining region 3 (CDR3) for each clone (Figure S6).
B cell clones reactivity to self-antigens and apoptotic cells
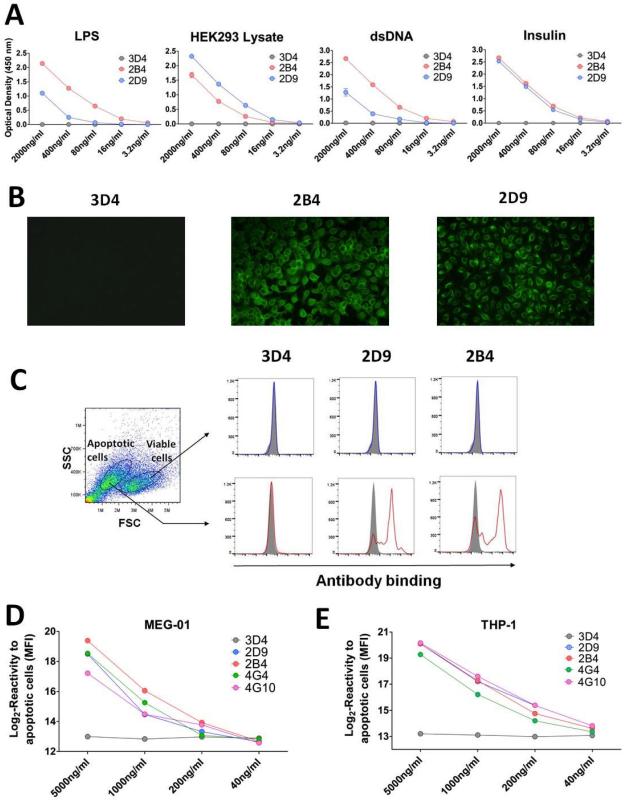
We assessed the capacity of HLA reactive clones to bind several generic autoantigens: lipopolysaccharide (LPS), double stranded DNA (dsDNA), insulin, as well as a whole protein lysate extracted from the human kidney line HEK293. As reported previously, both clones 4G4 and 4G10 reacted to multiple autoantigens, displaying a polyreactive profile 13. Likewise, clones 2B4 and 2D9 reacted to LPS, dsDNA, insulin and HEK293 lysate whereas control clone 3D4 did not (Figure 3A). In addition, as shown in Figure 3B, supernatants from clone 2B4 and 2D9 but not 3D4 also reacted to fixed/permeabilized Hep-2 cells. We have previously reported that polyreactive clones 4G4 and 4G10 bind to apoptotic Jurkat and HEK293 cells. Furthermore, we observed a close association between reactivity to apoptotic cells and polyreactivity17. As depicted in Figure 3C, clone 2B4 and 2D9 also reacted to apoptotic Jurkat cells. Next, we assessed the reactivity of culture supernatants from the 4 polyreactive clone 2B4, 2D9, 4G4 and 4G10 and control clone 3D4 to apoptotic cells induced from myelogenous leukaemia cell line MEG-01 and monocytic leukemia cell line THP-1. All polyreactive antibodies reacted to both apoptotic MEG-01 and THP-1 cells (Figure 3D and 3E). A summary of the reactivity profiles of all 5 clones is provided Table S2.
Figure 3.
(A) Reactivity of cell culture supernatants to multiple antigens. The reactivity of polyreactive antibodies 2B4 and 2D9 and non-polyreactive antibody 3D4 to LPS, dsDNA, insulin and HEK293 lysate was assessed by ELISA using an anti-IgM secondary antibody. (B) Reactivity of cell culture supernatants to Hep-2 cells. Staining of Hep-2 cells was carried out with polyreactive clones 2B4 and 2D9 and control non-polyreactive clone 3D4. Staining was revealed using an anti-IgM secondary antibody conjugated to fluorescein isothiocyanate. All pictures were acquired using the same settings for consistency. (C) Reactivity of cell culture supernatants to Jurkat cells. Cell culture supernatants from polyreactive clones 2B4 and 2D9 as well as control non-polyreactive clone 3D4, were used to stain a mixture of viable and apoptotic Jurkat cells. Staining was revealed using an anti-IgM secondary antibody. Results are reported after gating on viable cells (upper panels) or apoptotic cells (lower panels). Filled gray histograms correspond to the signal generated by staining with the secondary antibody alone. (D) Reactivity of cell culture supernatants to apoptotic MEG-01 cell line. Cell culture supernatants at different concentration from polyreactive clones 2B4, 2D9, 4G4 and 4G10 as well as control non-polyreactive clone 3D4, were used to stain apoptotic MEG-01. Staining was revealed using an anti-IgM secondary antibody. Reactivity is reported as Log2 values of MFI. (E) Reactivity of cell culture supernatants to apoptotic THP-1 cell line. Cell culture supernatants at different concentration from polyreactive clones 2B4, 2D9, 4G4 and 4G10 as well as control non-polyreactive clone 3D4, were used to stain apoptotic THP-1 cell line. Staining was revealed using an anti-IgM secondary antibody. Reactivity is reported as Log2 values of MFI.
Correlation between serum IgG reactivity to apoptotic cells and reactivity to HLA
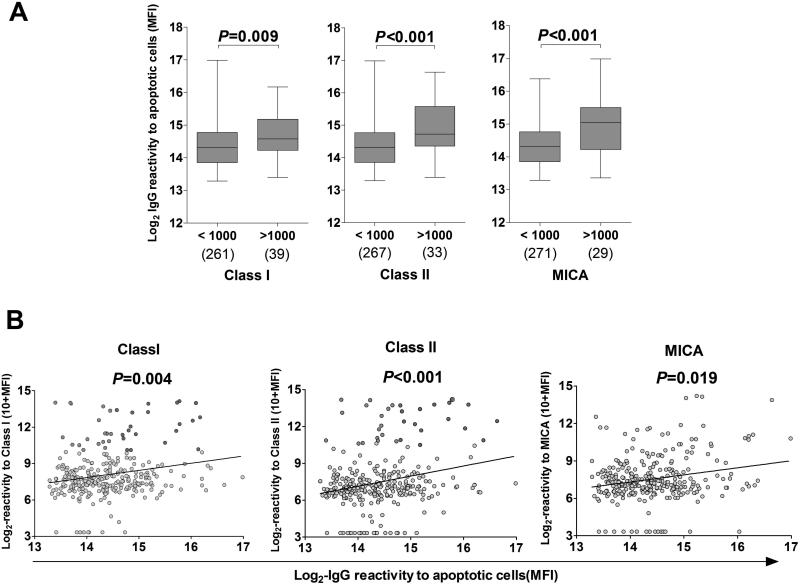
Based on the previous findings we hypothesized that polyreactive antibodies could also contribute to the serum reactivity to HLA in kidney transplant candidates. To test this hypothesis, we compared IgG reactivity to HLA class I and class II as detected by Luminex and reactivity to apoptotic cells measured by flow cytometry in 300 pre-transplant serum samples. As shown in Figure 4A, serum specimens with reactivity to HLA class I, HLA class II or MICA exceeding 1000 MFI, also showed higher IgG reactivity to apoptotic cells (P = 0.009, P < 0.001, P < 0.001, respectively). Overall, we observed a strong positive correlation between IgG reactivity to apoptotic cells and reactivity to HLA class I, class II and MICA (Figure 4B; P = 0.004, P < 0.001, P = 0.019, respectively).
Figure 4. IgG reactivity to apoptotic cells and HLA.
(A) Comparison of IgG reactivity to apoptotic cells in pre-transplant patients. Patients whose serum reacted to HLA Class I, HLA Class II, or MICA (MFI ≥ 1000) were separated from patients whose serum did not react strongly (MFI < 1000). The IgG reactivity to apoptotic cells (Log2 MFI) was compared between the 2 groups. The numbers of samples analyzed in each group are shown below the box plot. P values from two-tailed Mann Whitney tests are shown for corresponding box pairs. The horizontal bar represents the median value; the bottom and top of each box represent the 25th and 75th percentiles; the lower and upper bars of each box represent the minimum and maximum values. (B) Correlation between IgG reactivity to apoptotic cells and IgG reactivity to HLA or MICA in pre-transplant patients (N=300). IgG reactivity to apoptotic Jurkat cells (X axis) and reactivity to HLA class I, HLA class II or MICA (Y axis) are depicted as log2 MFI and log2 (10+MFI), respectively. Each circle represents the serum of one patient. Dots corresponding to sera reactive to HLA class I, class II or MICA (>1000) are colored in green or red if their reactivity to apoptotic cells was below or above the median respectively. P values from Spearman correlation tests are shown for corresponding groups.
Reactivity to apoptotic cells associates with broader reactivity to HLA class I
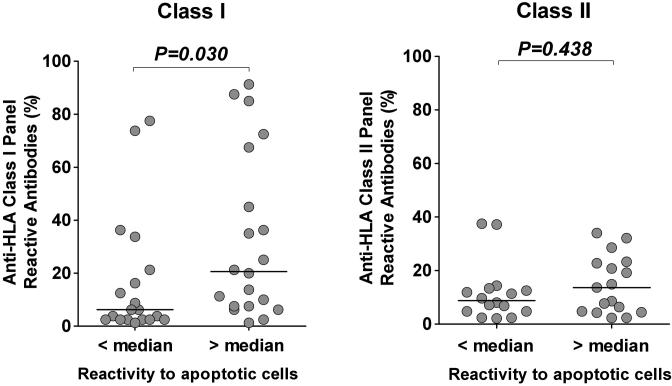
Out of the 300 pre-transplant serum samples, 39 samples reacted to HLA class I and 33 samples reacted to HLA class II (MFI > 1000). We further examined the reactivity of these samples to HLA class I and class II alleles by Luminex using regular SAB. As a surrogate marker of PRA, we calculated the percentage of HLA class I and class II alleles recognized by these samples and compared the values between samples with high reactivity or low reactivity to apoptotic cells (red and green dots in Figure 4B). As depicted in Figure 5, samples with reactivity to apoptotic cells above the median value recognized more HLA class I molecules, therefore displaying a higher PRA compared to samples with reactivity to apoptotic cells below the median value (P = 0.030, left panel). In contrast, higher reactivity to apoptotic cells was not associated with increased HLA class II PRA (P =0.438, right panel).
Figure 5. Comparison between reactivity to apoptotic cells and HLA class I and class II panel reactive antibodies.
Serum reactivity to HLA class I and class II molecules was assessed by Luminex using single antigen beads. HLA class I and class II PRA were calculated by dividing the number of beads recognized by the total number of corresponding class I or class II beads. All redundant beads were counted as one in the PRA calculation. Patients were separated into two groups depending on IgG reactivity to apoptotic cells below or above the median value. P values from two-tailed Mann Whitney tests are shown for corresponding group pairs. The horizontal bar represents the median value.
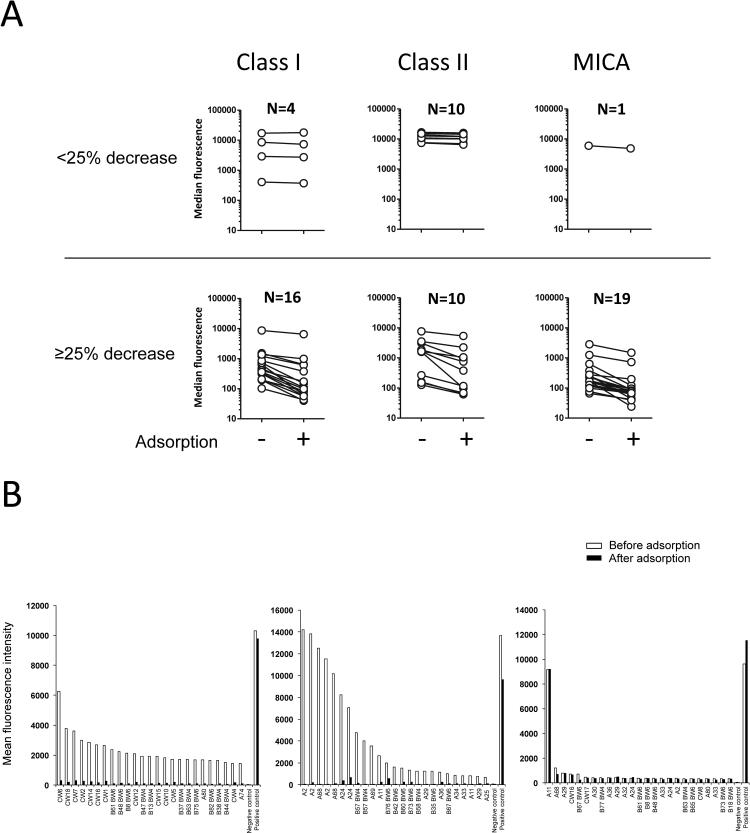
We then carried out adsorption experiments to verify that the same antibodies were responsible for the reactivity to apoptotic cells and the reactivity to HLA in some sera. We assessed the reactivity of serum specimens collected post-transplants from 20 kidney graft recipients to HLA class I, class II and MICA by Luminex before or after adsorption on apoptotic cells. As depicted in Figure 6A, the reactivity to HLA class I and MICA was reduced in most sera (16/20 and 19/20, respectively), while the reactivity to HLA class II was only reduced in half of the sera after adsorption on apoptotic Jurkat cells. Moreover, adsorption on apoptotic cells markedly decreased the reactivity to multiple HLA antigens in some broadly reactive serum specimens as revealed by Luminex SAB (Figure 6B left and central panels). In contrast, the reactivity of a serum showing high specificity to a single HLA class I antigen was unaffected (Figure 6B, right panel).
Figure 6. Adsorption of serum reactivity to HLA.
(A) Reactivity of sera from 20 kidney transplant recipients to Class I, Class II, and MICA was measured by Luminex (Labscreen mixed beads) before and after adsorption on apoptotic cells. For clarity, sera for which the reactivity was not reduced by adsorption (<25% decrease; upper panels) are presented separately from sera for which the reactivity was significantly decreased after adsorption (≥ 25%, lower panel). (B) The reactivity of three representative sera to HLA Class I was measured by Luminex (SAB) before and after adsorption on apoptotic cells. Only the 25 antigens towards which the supernatants reacted the most are depicted. Antigens are sorted based on the supernatant reactivity to regular single antigen beads.
Discussion
It is often assumed that monoclonality is equivalent to monoreactivity. Along this line, a monoclonal antibody is expected to recognize a unique antigen or epitope even if this one can be shared among different molecules. However, the existence of antibodies with a polyreactive profile refutes this assumption18-21. Here we present an in-depth characterization of four such polyreactive clones cross-reacting to HLA antigens coated on SAB routinely used in Luminex based assays to monitor transplant recipients for sensitization. Our findings raise several questions that we discuss below.
The four clones described in this study were isolated from two kidney transplant recipients through B cell immortalization and cloning on feeder cells composed of irradiated Jurkat cells. However, none of these clones appeared to react to the HLA class I antigens expressed by Jurkat cells (HLA-A9, -A25, -B702, -B41), ruling out the hypothesis that the feeder cells preferentially promoted the growth of these specific clones among others. For the same reason, it is also unlikely that the polyreactive monoclonal antibodies produced by these B cell clones react to HLA class I on apoptotic Jurkat cells. In previous studies we generated HLA class I-negative Jurkat cells by transduction with a retroviral vector containing an shRNA specific for the human β2-microglobulin. Serum polyreactive IgG reacted equally to apoptotic wild type and HLA negative-Jurkat cells, indicating the recognition of antigens other than HLA molecules at the surface of apoptotic cells. On the other hand, all 4 clones presented in the present manuscript cross-reacted to at least one donor HLA. As we have previously described, the first patient had low levels of DSA at the time the specimen that was used to generate B cells clones was collected13. In contrast, we could not detect anti-HLA antibodies in the serum of the second patient at the time of blood collection. Since these clones cross-react to multiple antigens, it is virtually impossible to know whether they were primed by donor antigens or self-antigens in vivo.
Most studies examining the reactivity of anti-HLA antibodies thus far have identified different types of epitopes12, 16. Native epitopes are presented on intact HLA molecules and can be shared by different HLA-A, B and C antigens. These epitopes are present on regular SAB as well as iBEADS. Denatured epitopes have also often been reported that presumably correspond to misfolded recombinant proteins present on regular SAB but removed from iBEADS. Lastly, antibodies can also recognize additional denatured epitopes not usually present on SAB but made accessible through acid treatment. These unusual denatured epitopes have been designated as cryptic epitopes12. Remarkably, the four monoclonal polyreactive antibodies we report here display unconventional reactivity profiles that challenge the rules whereby antibodies are thought to bind to HLA. All four clones reacted primarily to denatured, i.e. non-native HLA molecules as detected using acid-treated beads. These findings suggested that polyreactive antibodies are not truly HLA-specific. However, the reactivity to iBEADS for 3 of the clones was particularly informative as it indicated some level of recognition of native epitopes, even if minimal. While we cannot categorically rule out the possibility that some denatured antigens are still present on iBEADS, this is very unlikely. Clone 4G10, in particular, did not react to any antigen on iBEADS but strongly reacted to a wide range of antigens on regular and acid treated SAB. Moreover, none of these 4 clones seemed to react to known shared epitopes12, 16, suggesting a distinct pattern of reactivity. Although we cannot rule out the existence of additional shared epitopes that have not been identified yet, it is unlikely that all 4 clones would bind to them. The negative reactivity observed by CDC is also intriguing. Either these polyreactive antibodies do not react to HLA in their native conformation at the surface of viable cells or their affinity is too low to be detected using this type of assay.
The association between IgG reactivity to apoptotic cells and reactivity to HLA in the serum of transplant candidates is striking. Two main hypotheses can be proposed to explain this association: 1) polyreactive antibodies and anti-HLA antibodies developed concomitantly, hence their detection in the same serum samples or 2) the serum specimens contain antibodies that cross react between apoptotic cells and multiple HLA. The identification of polyreactive clones cross-reactive to HLA and apoptotic cells in the peripheral blood of kidney transplant recipients strongly supports the latter hypothesis. Moreover, we isolated 4 such clones out of ~200 clones tested, indicating that B cells displaying this type cross-reactivity profile are not exceptional. In a previous study we even demonstrated that one of these clones, 4G10, was highly expanded in the patient blood13. Lastly, our adsorption experiments further supported the existence of antibodies cross-reacting to apoptotic cells and HLA in some serum samples. A longitudinal assessment of anti-HLA and polyreactive antibodies in a large group of kidney transplant recipients is now warranted to clarify their kinetics of development and further confirm their relatedness.
Our findings have direct implications for the interpretation of anti-HLA detection assays. Since polyreactive antibodies cross-reactive to HLA are detected by Luminex, it is anticipated that their presence in the serum will contribute to the overall reactivity to HLA. This could explain why the serum of some healthy subjects or transplant candidates react to HLA without evidence of prior immunizing events2. Likewise polyreactive antibodies could also be responsible for the mysterious development of non-donor specific antibodies (NDSA) following transplantation5-9. Furthermore, as thoroughly reviewed and discussed by Gebel and Bray, most transplant teams have experienced conflicting results using different techniques to detect anti-HLA antibodies 22. The existence of polyreactive antibodies cross-reactive to HLA could provide a simple explanation for some of these observations. The discrepancy between the positive Luminex assay results and the negative reactivity by CDC using the 4 polyreactive monoclonal antibodies is particularly revealing. Gombos et al. recently described significant differences between results obtained by Luminex and CDC methods1. The additional reactivity picked up by Luminex but not by CDC in individuals with no history of sensitization is often considered “false positive” and is possibly due to the presence of polyreactive antibodies in the serum. Distinguishing between the two components i.e., polyreactive and monospecific, is important as the HLA reactivity often guides medical decision. Patients on the wait list are allocated organs based on the identification of acceptable and unacceptable antigens, which are routinely determined by Luminex using SAB. It is also important to note that polyreactive antibodies can generate strong signals with high MFI on SAB. Discerning which components of the serum reactivity correspond to polyreactive or monospecific antibodies based solely on the MFI can therefore be misleading.
The pathogenic potential of polyreactive antibodies, whether they cross-react to HLA or not, remains an important yet unanswered question. It is tempting to dismiss them as irrelevant “false positive” antibodies without any biological significance, whereas monospecific antibodies targeting donor HLA molecules would be truly pathogenic. However, a recent study from our lab suggests otherwise. Using serum samples collected pre-transplant from 300 kidney transplant recipients, we found a significant correlation between the presence of polyreactive antibodies cross-reactive to apoptotic cells and late kidney graft loss14. Furthermore, the serum reactivity to apoptotic cells was almost exclusively attributable to IgG1 and IgG3, which displayed complement activation properties. Based on these results it is plausible that polyreactive antibodies have important even if unrecognized effector capacities that may be distinct from that of monospecific anti-HLA antibodies. Until their function is clarified, it may therefore be imprudent to disregard them as clinically insignificant.
The source of polyreactive antibodies cross-reactive to HLA is uncertain. Since these antibodies bind to multiple antigenic targets including that exposed on apoptotic cells, the identity of the antigen responsible for priming the corresponding B cells is difficult to ascertain. Whether these B cells were even primed by a specific antigen is unclear. Another scenario is that “innate” B cells known to constitutively produce polyreactive “natural IgM”, would undergo class switch recombination (CSR) following antigen non-specific activation. This alternate B cell differentiation pathway involving stimulation through Toll Like Receptors and leading to CSR is now well characterized23. After undergoing CSR, innate B cells would secrete polyreactive IgG, a proportion of which cross-react to HLA on SAB. Chronic inflammation associated with prolonged hemo-dialysis could provide a suitable context for the differentiation of innate B cells into polyreactive IgG secreting cells.
In conclusion, our findings provide further evidence at the clonal level that human polyreactive antibodies can cross-react to HLA, self-antigens and apoptotic cells. The characterization of such clones together with the association between reactivity to HLA and apoptotic cells in our patient series suggests that polyreactive antibodies contribute to the overall serum reactivity to HLA. Polyreactive antibodies should be taken into consideration when interpreting the results of Luminex-based assays for the detection of anti-HLA antibodies.
Supplementary Material
Acknowledgements
The authors are indebted to Dr. Nadim El-Awar for his contribution to the characterization of the clones’ reactivity to HLA epitopes as well as One Lambda/ThermoFisher for providing HLA testing kits used in this study.
Disclosure
The authors received support in form of HLA testing kits from One Lambda/ThermoFisher to conduct this study.
This work was supported by the Roche Organ Transplantation Research Foundation (ROTRF) and the National Institute of Health, National Institute of Diabetes, Digestive and Kidney Diseases Grant DK083352.
Abbreviations
- DSA
donor-specific antibodies
- NDSA
non donor-specific antibodies
- PRA
panel reactive antibodies
- MFI
mean fluorescence intensity
- CDR3
complementarity-determining region 3
- SAB
single antigen beads
Footnotes
EZ was the principal investigator who coordinated the research. BG, CR, FP and CM performed the laboratory work for this study. TCG, SLS, WW and YF recruited the patients, provided clinical data and participated in the design of the study. BG and EZ wrote the manuscript.
Supporting information
Figure S1-S5. Reactivity of B cell clones to HLA class I molecules.
Figure S6. Sequence analysis of B cell clones 2B4 and 2D9.
Table S1. Summary of patient characteristics.
Table S2. Summary of B cell clones reactivity.
References
- 1.Gombos P, Opelz G, Scherer S, et al. Influence of test technique on sensitization status of patients on the kidney transplant waiting list. Am J Transplant. 2013;13(8):2075–2082. doi: 10.1111/ajt.12332. [DOI] [PubMed] [Google Scholar]
- 2.Morales-Buenrostro LE, Terasaki PI, Marino-Vazquez LA, et al. "Natural" human leukocyte antigen antibodies found in nonalloimmunized healthy males. Transplantation. 2008;86(8):1111–1115. doi: 10.1097/TP.0b013e318186d87b. [DOI] [PubMed] [Google Scholar]
- 3.Sicard A, Amrouche L, Suberbielle C, et al. Outcome of kidney transplantations performed with preformed donor-specific antibodies of unknown etiology. Am J Transplant. 2014;14(1):193–201. doi: 10.1111/ajt.12512. [DOI] [PubMed] [Google Scholar]
- 4.Sigle JP, Thierbach J, Infanti L, et al. Anti-leucocyte antibodies in platelet apheresis donors with and without prior immunizing events: implications for TRALI prevention. Vox Sang. 2013;105(3):244–252. doi: 10.1111/vox.12045. [DOI] [PubMed] [Google Scholar]
- 5.Briggs D, Zehnder D, Higgins RM. Development of non-donor-specific HLA antibodies after kidney transplantation: frequency and clinical implications. Contrib Nephrol. 2009;162:107–116. doi: 10.1159/000170843. [DOI] [PubMed] [Google Scholar]
- 6.Cai J, Terasaki PI, Mao Q, et al. Development of nondonor-specific HLA-DR antibodies in allograft recipients is associated with shared epitopes with mismatched donor DR antigens. Am J Transplant. 2006;6(12):2947–2954. doi: 10.1111/j.1600-6143.2006.01560.x. [DOI] [PubMed] [Google Scholar]
- 7.Fotheringham J, Angel C, Goodwin J, et al. Natural history of proteinuria in renal transplant recipients developing de novo human leukocyte antigen antibodies. Transplantation. 2011;91(9):991–996. doi: 10.1097/TP.0b013e3182126ed0. [DOI] [PubMed] [Google Scholar]
- 8.Lee PC, Chen YL, Wang WM, et al. Clinical relevance of pre- and post-transplant HLA antibodies, donor-specific, and nondonor-specific HLA antibodies detected by ELISA in renal transplantation. Clin Transpl. 2013:385–391. [PubMed] [Google Scholar]
- 9.Mao Q, Terasaki PI, Cai J, et al. Extremely high association between appearance of HLA antibodies and failure of kidney grafts in a five-year longitudinal study. Am J Transplant. 2007;7(4):864–871. doi: 10.1111/j.1600-6143.2006.01711.x. [DOI] [PubMed] [Google Scholar]
- 10.Mao Q, Terasaki PI, Cai J, et al. Analysis of HLA class I specific antibodies in patients with failed allografts. Transplantation. 2007;83(1):54–61. doi: 10.1097/01.tp.0000250492.55775.83. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki N, Idica A, Terasaki PI. Mimetic human leukocyte antigen epitopes: shown by monoclonal antibodies and extra antibodies produced on transplantation. Transplantation. 2008;86(7):912–918. doi: 10.1097/TP.0b013e318183783b. [DOI] [PubMed] [Google Scholar]
- 12.El-Awar N, Terasaki PI, Nguyen A, et al. Epitopes of human leukocyte antigen class I antibodies found in sera of normal healthy males and cord blood. Hum Immunol. 2009;70(10):844–853. doi: 10.1016/j.humimm.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Porcheray F, Devito J, Helou Y, et al. Expansion of Polyreactive B Cells Cross-Reactive to HLA and Self in the Blood of a Patient With Kidney Graft Rejection. Am J Transplant. 2012;12(8):2088–2097. doi: 10.1111/j.1600-6143.2012.04053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao B, Moore C, Porcheray F, et al. Pretransplant IgG reactivity to apoptotic cells correlates with late kidney allograft loss. Am J Transplant. 2014;14(7):1581–1591. doi: 10.1111/ajt.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porcheray F, DeVito J, Yeap BY, et al. Chronic humoral rejection of human kidney allografts associates with broad autoantibody responses. Transplantation. 2010;89(10):1239–1246. doi: 10.1097/TP.0b013e3181d72091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Awar N, Terasaki PI, Cai J, et al. Epitopes of HLA-A, B, C, DR, DQ, DP and MICA antigens. Clin Transpl. 2009:295–321. [PubMed] [Google Scholar]
- 17.Porcheray F, Fraser JW, Gao B, et al. Polyreactive antibodies developing amidst humoral rejection of human kidney grafts bind apoptotic cells and activate complement. Am J Transplant. 2013;13(10):2590–2600. doi: 10.1111/ajt.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casali P, Notkins AL. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu Rev Immunol. 1989;7:513–535. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- 19.Notkins AL. Polyreactivity of antibody molecules. Trends Immunol. 2004;25(4):174–179. doi: 10.1016/j.it.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Zhou ZH, Notkins AL. Polyreactive antigen-binding B (PAB−) cells are widely distributed and the PAB population consists of both B-1+ and B-1− phenotypes. Clin Exp Immunol. 2004;137(1):88–100. doi: 10.1111/j.1365-2249.2004.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou ZH, Tzioufas AG, Notkins AL. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun. 2007;29(4):219–228. doi: 10.1016/j.jaut.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gebel HM, Bray RA. HLA Antibody Detection With Solid Phase Assays: Great Expectations or Expectations Too Great? Am J Transplant. 2014;14(9):1964–1975. doi: 10.1111/ajt.12807. [DOI] [PubMed] [Google Scholar]
- 23.Pone EJ, Xu Z, White CA, et al. B cell TLRs and induction of immunoglobulin class-switch DNA recombination. Front Biosci (Landmark Ed) 2012;17:2594–2615. doi: 10.2741/4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.