Abstract
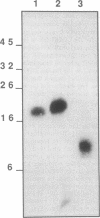
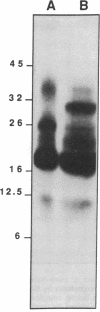
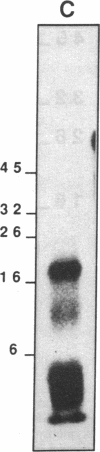
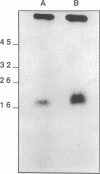
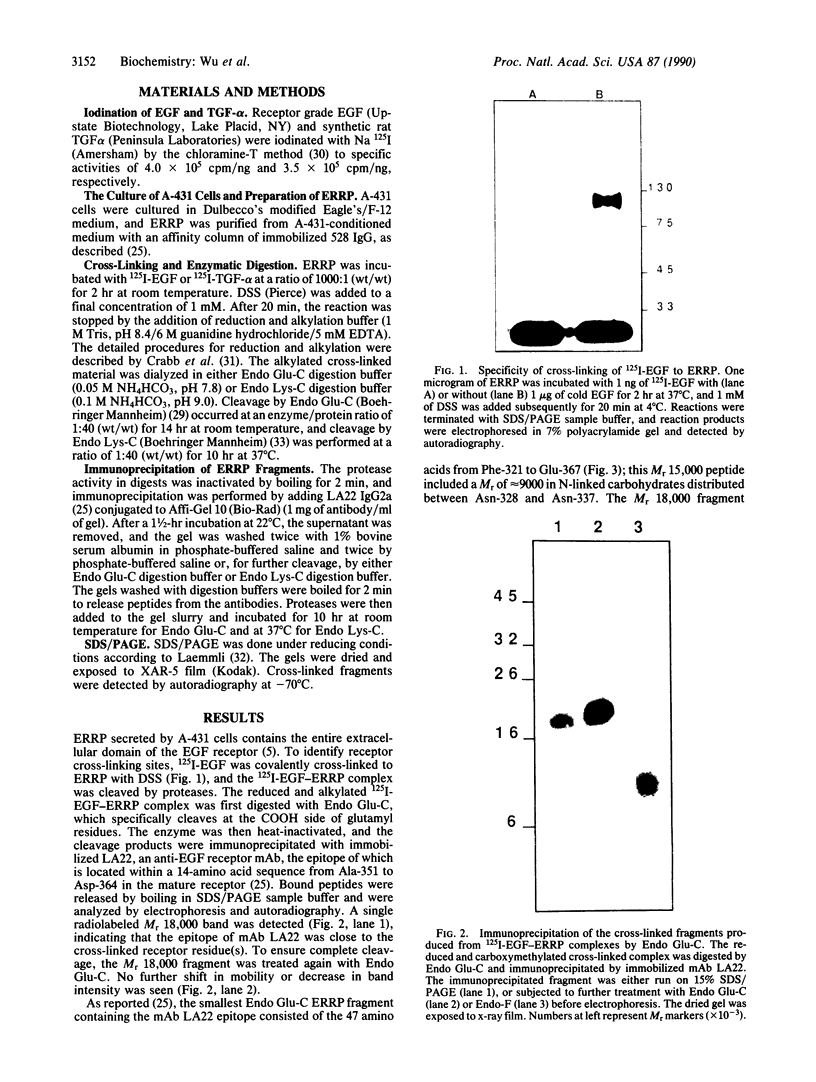
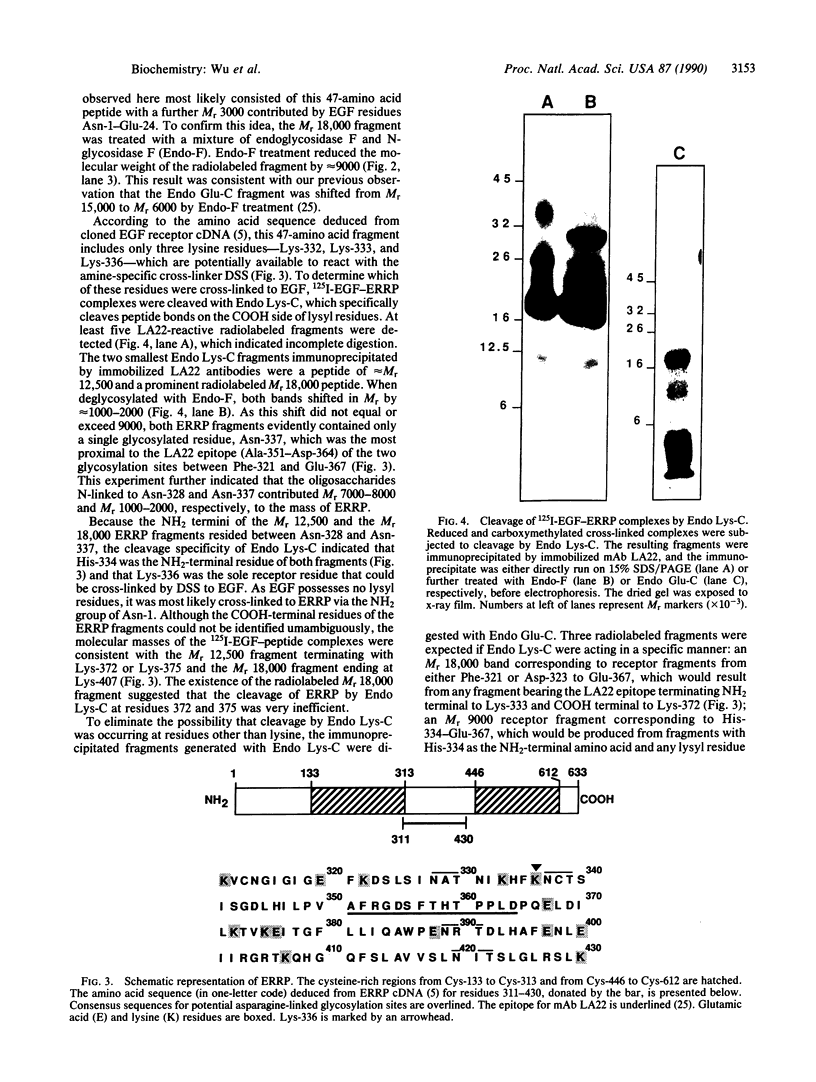
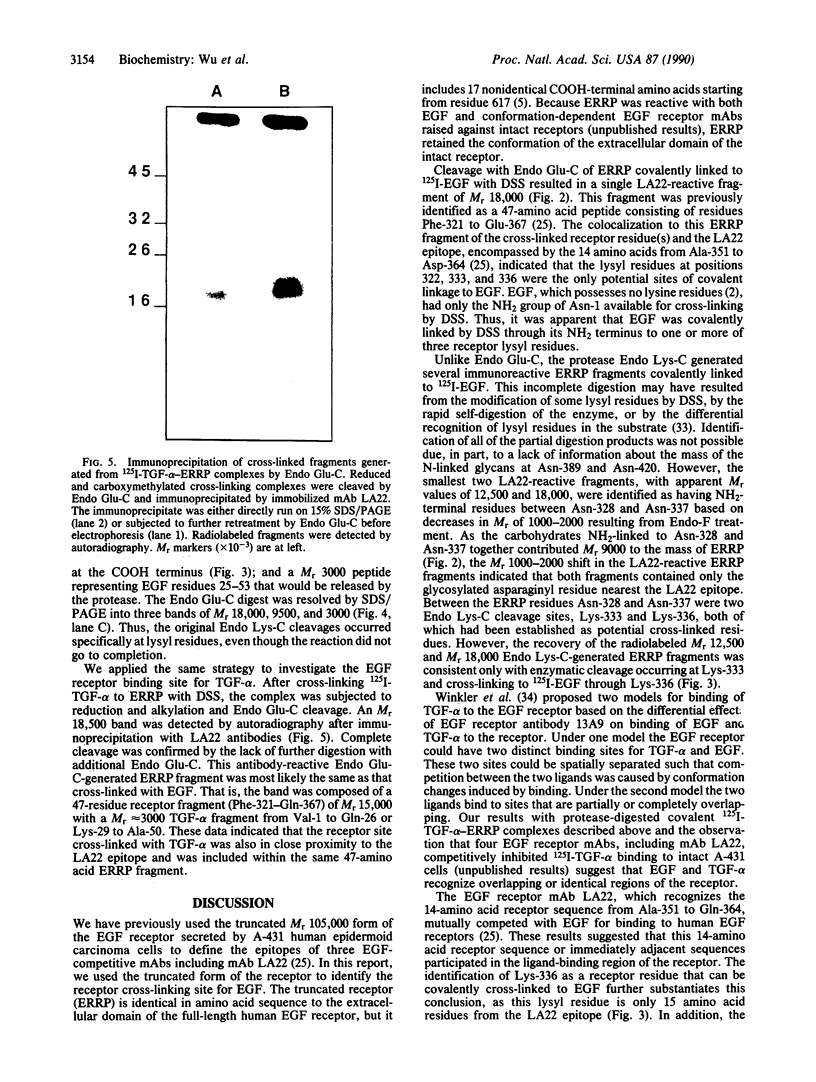
An epidermal growth factor (EGF) receptor monoclonal antibody (mAb), mAb LA22, was used to analyze the covalent coupling of human EGF receptors to mouse EGF by the amine-reactive cross-linking agent disuccinimidyl suberate. A soluble Mr 105,000 truncated form of the receptor secreted by A-431 epidermoid carcinoma cells and consisting of the ligand-binding extracellular domain was cross-linked to 125I-labeled EGF. Digestion of this complex with an endoproteinase that specifically cleaves at the COOH side of glutamyl residue released a single radiolabeled glycosylated fragment of Mr 18,000 that reacted with mAb LA22. As the epitope for mAb LA22 resided between Ala-351 and Asp-364 of the mature receptor, this result localized the cross-linked receptor residue(s) to the 47-amino acid interval from Phe-321 to Glu-367. The receptor residue(s) involved in the covalent coupling of rat 125I-labeled transforming growth factor alpha was similarly localized to this region of the receptor. This receptor interval, which included two glycosylated asparaginyl residues at positions 328 and 337, contained but three amino acid residues that were potentially reactive with disuccinimidyl suberate: Lys-332, Lys-333, and Lys-336. Characterization of mAb LA22-reactive 125I-EGF-labeled receptor fragments generated by an endoproteinase specific for the COOH side of lysyl residue placed the NH2 termini of the two smallest fragments between the glycosylated residues Asn-328 and Asn-337. These results indicated that disuccinimidyl suberate cross-linked the NH2 group of EGF residue Asn-1 to the human EGF receptor residue Lys-336. Our results further suggest that EGF and transforming growth factor alpha, two members of the EGF family of peptide growth factors, interact with closely apposed or identical features of the receptor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- Chen W. S., Lazar C. S., Poenie M., Tsien R. Y., Gill G. N., Rosenfeld M. G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. 1987 Aug 27-Sep 2Nature. 328(6133):820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- Childs R. A., Gregoriou M., Scudder P., Thorpe S. J., Rees A. R., Feizi T. Blood group-active carbohydrate chains on the receptor for epidermal growth factor of A431 cells. EMBO J. 1984 Oct;3(10):2227–2233. doi: 10.1002/j.1460-2075.1984.tb02120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb J. W., Johnson C. M., Carr S. A., Armes L. G., Saari J. C. The complete primary structure of the cellular retinaldehyde-binding protein from bovine retina. J Biol Chem. 1988 Dec 15;263(35):18678–18687. [PubMed] [Google Scholar]
- Cummings R. D., Soderquist A. M., Carpenter G. The oligosaccharide moieties of the epidermal growth factor receptor in A-431 cells. Presence of complex-type N-linked chains that contain terminal N-acetylgalactosamine residues. J Biol Chem. 1985 Oct 5;260(22):11944–11952. [PubMed] [Google Scholar]
- Derynck R., Roberts A. B., Winkler M. E., Chen E. Y., Goeddel D. V. Human transforming growth factor-alpha: precursor structure and expression in E. coli. Cell. 1984 Aug;38(1):287–297. doi: 10.1016/0092-8674(84)90550-6. [DOI] [PubMed] [Google Scholar]
- Derynck R. Transforming growth factor alpha. Cell. 1988 Aug 26;54(5):593–595. doi: 10.1016/s0092-8674(88)80001-1. [DOI] [PubMed] [Google Scholar]
- Derynck R. Transforming growth factor-alpha: structure and biological activities. J Cell Biochem. 1986;32(4):293–304. doi: 10.1002/jcb.240320406. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Honegger A. M., Dull T. J., Felder S., Van Obberghen E., Bellot F., Szapary D., Schmidt A., Ullrich A., Schlessinger J. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987 Oct 23;51(2):199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- Honegger A. M., Szapary D., Schmidt A., Lyall R., Van Obberghen E., Dull T. J., Ullrich A., Schlessinger J. A mutant epidermal growth factor receptor with defective protein tyrosine kinase is unable to stimulate proto-oncogene expression and DNA synthesis. Mol Cell Biol. 1987 Dec;7(12):4568–4571. doi: 10.1128/mcb.7.12.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekel P. A., Weijer W. J., Beintema J. J. Use of endoproteinase Lys-C from Lysobacter enzymogenes in protein sequence analysis. Anal Biochem. 1983 Oct 15;134(2):347–354. doi: 10.1016/0003-2697(83)90308-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lax I., Bellot F., Howk R., Ullrich A., Givol D., Schlessinger J. Functional analysis of the ligand binding site of EGF-receptor utilizing chimeric chicken/human receptor molecules. EMBO J. 1989 Feb;8(2):421–427. doi: 10.1002/j.1460-2075.1989.tb03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax I., Burgess W. H., Bellot F., Ullrich A., Schlessinger J., Givol D. Localization of a major receptor-binding domain for epidermal growth factor by affinity labeling. Mol Cell Biol. 1988 Apr;8(4):1831–1834. doi: 10.1128/mcb.8.4.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax I., Johnson A., Howk R., Sap J., Bellot F., Winkler M., Ullrich A., Vennstrom B., Schlessinger J., Givol D. Chicken epidermal growth factor (EGF) receptor: cDNA cloning, expression in mouse cells, and differential binding of EGF and transforming growth factor alpha. Mol Cell Biol. 1988 May;8(5):1970–1978. doi: 10.1128/mcb.8.5.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh E., Reiss N., Berent E., Ullrich A., Schlessinger J. An insertional mutant of epidermal growth factor receptor allows dissection of diverse receptor functions. EMBO J. 1987 Sep;6(9):2669–2676. doi: 10.1002/j.1460-2075.1987.tb02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Todaro G. J. Rat transforming growth factor type 1: structure and relation to epidermal growth factor. Science. 1984 Mar 9;223(4640):1079–1082. doi: 10.1126/science.6320373. [DOI] [PubMed] [Google Scholar]
- Massague J., Czech M. P., Iwata K., DeLarco J. E., Todaro G. J. Affinity labeling of a transforming growth factor receptor that does not interact with epidermal growth factor. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6822–6826. doi: 10.1073/pnas.79.22.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. Epidermal growth factor-like transforming growth factor. I. Isolation, chemical characterization, and potentiation by other transforming factors from feline sarcoma virus-transformed rat cells. J Biol Chem. 1983 Nov 25;258(22):13606–13613. [PubMed] [Google Scholar]
- Mayes E. L., Waterfield M. D. Biosynthesis of the epidermal growth factor receptor in A431 cells. EMBO J. 1984 Mar;3(3):531–537. doi: 10.1002/j.1460-2075.1984.tb01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelione G. T., Winkler M. E., Burton L. E., Rinderknecht E., Sporn M. B., Wagner G. Sequence-specific 1H-NMR assignments and identification of two small antiparallel beta-sheets in the solution structure of recombinant human transforming growth factor alpha. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1519–1523. doi: 10.1073/pnas.86.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Bierman A. J., Tilly B. C., Verlaan I., Defize L. H., Honegger A. M., Ullrich A., Schlessinger J. A point mutation at the ATP-binding site of the EGF-receptor abolishes signal transduction. EMBO J. 1988 Mar;7(3):707–710. doi: 10.1002/j.1460-2075.1988.tb02866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. Interaction of cross-linking agents with the insulin effector system of isolated fat cells. Covalent linkage of 125I-insulin to a plasma membrane receptor protein of 140,000 daltons. J Biol Chem. 1979 May 10;254(9):3375–3381. [PubMed] [Google Scholar]
- Schlessinger J. The epidermal growth factor receptor as a multifunctional allosteric protein. Biochemistry. 1988 May 3;27(9):3119–3123. doi: 10.1021/bi00409a002. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Weber W., Bertics P. J., Gill G. N. Immunoaffinity purification of the epidermal growth factor receptor. Stoichiometry of binding and kinetics of self-phosphorylation. J Biol Chem. 1984 Dec 10;259(23):14631–14636. [PubMed] [Google Scholar]
- Weber W., Gill G. N., Spiess J. Production of an epidermal growth factor receptor-related protein. Science. 1984 Apr 20;224(4646):294–297. doi: 10.1126/science.6324343. [DOI] [PubMed] [Google Scholar]
- Winkler M. E., O'Connor L., Winget M., Fendly B. Epidermal growth factor and transforming growth factor alpha bind differently to the epidermal growth factor receptor. Biochemistry. 1989 Jul 25;28(15):6373–6378. doi: 10.1021/bi00441a033. [DOI] [PubMed] [Google Scholar]
- Wu D. G., Wang L. H., Sato G. H., West K. A., Harris W. R., Crabb J. W., Sato J. D. Human epidermal growth factor (EGF) receptor sequence recognized by EGF competitive monoclonal antibodies. Evidence for the localization of the EGF-binding site. J Biol Chem. 1989 Oct 15;264(29):17469–17475. [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Molecular analysis of signal transduction by growth factors. Biochemistry. 1988 May 3;27(9):3113–3119. doi: 10.1021/bi00409a001. [DOI] [PubMed] [Google Scholar]