Abstract
Immunization with recombinant serotype 5 adenoviral (rAd5) vectors or a combination of DNA plasmid priming and rAd5 boosting is known to elicit potent immune responses. However, little data exist regarding these immunization strategies and the development of anti-human immunodeficiency virus type 1 (HIV-1) neutralizing antibodies. We used DNA plasmids and rAd5 vectors encoding the HIV-1 89.6P or chimeric HxB2/BaL envelope glycoprotein to immunize macaque monkeys. A single rAd5 immunization elicited anti-Env antibody responses, but there was little boosting with subsequent rAd5 immunizations. In contrast, rAd5 boosting of DNA-primed monkeys resulted in a rapid rise in antibody titers, including the development of anti-HIV-1 neutralizing antibodies. The potency and breadth of neutralization were evaluated by testing plasma against a panel of 14 clade B primary isolates. Moderate levels of plasma neutralizing activity were detected against about one-third of the viruses tested, and immunoglobulin G fractionation demonstrated that virus neutralization was antibody mediated. After a challenge with a chimeric simian-human immunodeficiency virus (SHIV89.6P), an anamnestic neutralizing antibody response was observed, although the breadth of the response was limited to the subset of viruses that were neutralized after the primary immunization. These data are the first detailed description of the anti-HIV-1 neutralizing antibody response in nonhuman primates elicited by DNA and rAd5 immunization. In addition to the well-established ability of DNA priming and rAd5 boosting to elicit potent anti-HIV-1 cellular immune responses, this immunization strategy elicits anti-HIV-1 neutralizing antibodies and therefore can be used to study novel Env immunogens designed to elicit more potent neutralizing antibodies.
The design of vaccine immunogens that elicit anti-human immunodeficiency virus type 1 (HIV-1) neutralizing antibodies is a major goal of HIV-1-AIDS vaccine researchers. Since current envelope immunogens are not yet optimal for eliciting neutralizing antibodies, immunization platforms that elicit potent humoral immunity would facilitate the testing of novel vaccine immunogens. Gene-based immunization strategies are attractive because they can be readily manipulated to express novel proteins. Such gene-based approaches include DNA plasmids and recombinant viral vectors encoding immunogens under the control of potent eukaryotic promoters (9, 17, 19-21, 25, 26, 29, 30). DNA plasmid immunization can induce detectable immune responses in nonhuman primates (2, 3, 22), but more robust responses are generated after boosting with a viral vector (1, 2, 5, 6, 10, 22, 28). This bimodal strategy of DNA priming followed by viral vector boosting has become a common approach for eliciting humoral and cellular immune responses (16). Among replication-incompetent viral vectors, recombinant adenovirus type 5 (rAd5) has been shown to be particularly potent when administered alone or as a booster for DNA-primed animals (5, 6, 22). Ebola virus vaccination using DNA priming and rAd5 boosting or rAd5 vectors given alone can protect macaque monkeys against a lethal Ebola virus challenge (23, 24). Also, DNA priming and rAd5 boosting of macaques with vectors encoding the simian immunodeficiency virus (SIV) Gag protein produced robust CD4- and CD8-T-cell immunity and conferred partial protection against a chimeric simian-human immunogenicity virus (SHIV) challenge (22). However, few nonhuman primate data exist regarding the anti-HIV-1 antibody response elicited by DNA-rAd immunization (28).
Our ongoing studies with small animals have demonstrated that robust HIV-1-specific antibody responses can be elicited by DNA-rAd5 immunization (7, 31). In this report, we describe the anti-HIV-1 antibody response in macaque monkeys immunized with DNA plasmids and rAd5 vectors encoding either SHIV89P Env or chimeric HIV-1 HxB2/BaL Env. All monkeys also received a vector encoding an SIV Gag-Pol-Nef or SIV Gag-Pol fusion protein. In a pilot study of four animals, two monkeys received sequential inoculations of rAd5 and two were immunized with DNA followed by rAd5. Additionally, we evaluated the anti-HIV-1 antibody responses of a cohort of 24 DNA-rAd5-immunized macaques that were subsequently challenged with SHIV89.6P. The immunogenicity and protective efficacy of immunizations for these animals were recently described (12). Herein we describe the anti-HIV-1 binding and neutralizing antibody responses elicited by the 89.6P and HxB2/BaL Env immunogens in these 24 animals. Because this SHIV challenge study included animals that received the SIV Gag-Pol-Nef immunogen with or without an Env immunogen, we were able to evaluate the role of an Env immunogen in generating an anamnestic neutralizing antibody response. Our data show that the DNA-rAd5 immunization strategy elicits high levels of antibodies to the HIV-1 envelope glycoprotein and that these antibodies can neutralize some heterologous virus isolates. After a SHIV challenge, an anamnestic neutralizing antibody response was observed in Env-immunized animals. However, the breadth of the response was limited to those viruses that were neutralized after the primary immunization. Studies of the epitope specificities of the induced neutralizing antibodies may provide insight into the breadth and potency of the virus neutralization that was observed and may suggest strategies for eliciting broader neutralizing antibody responses. This DNA-rAd5 immunization platform elicits robust humoral immune responses and can be used to study novel gene-based Env immunogens designed to elicit neutralizing antibodies.
MATERIALS AND METHODS
Vaccine constructs.
DNA plasmids expressing HIV-1 and SIV proteins were made synthetically by previously described methods (7). cDNAs were cloned into the expression vector pVR1012 under control of the cytomegalovirus immediate-early enhancer, promoter, and first intron. The chimeric HxB2/BaL gp145 Env vector was previously described (7). To produce this R5-tropic version of the envelope glycoprotein, we replaced the env gene region encoding amino acids 275 to 361 of HxB2 (GenBank accession number K03455) with the corresponding BaL gene sequence (GenBank accession number M68893). A second Env plasmid expressed the 89.6P gp145 protein (GenBank accession number U89134). Both Env constructs were made by use of a strategy analogous to that used to create a previously described HIV-1 Env vector, including codon modifications to optimize mammalian cell expression and nucleotide deletions in the gp120 cleavage site, the gp41 fusogenic domain, and the spacing region between heptad repeats 1 and 2 (7). A synthetic codon-modified SIVmac239 Gag-Pol-Nef gene encoding a fusion protein was made by use of a strategy similar to that used to construct a previously described HIV-1 Gag-Pol vector (11). This genetic construct was also cloned into the pVF1012 expression vector.
Recombinant rAd5 viruses with deletions in E1 and E3 were generated by a modification of a previously published method (24). The HxB2/BaL and 89.6P Env rAd5 constructs expressed gp140 rather than the gp145 protein expressed by the corresponding DNA constructs. Our unpublished observations have shown that the combination of DNA priming with gp145 Env and boosting with rAd5 gp140 Env provides the most robust antibody response. Also, because of the instability of the SIV Gag-Pol-Nef gene insert in rAd5, the vector was constructed to express the SIVmac239 Gag-Pol fusion protein. rAd5 vectors were produced and amplified in 293 cells; viruses were purified on a cesium chloride gradient and stored in phosphate-buffered saline with 15% glycerol at −20°C.
Animal immunizations.
Rhesus macaques were housed and handled in accordance with guidelines set by the Association for the Assessment and Accreditation of Laboratory Animal Care. Each plasmid DNA was formulated in sterile saline and delivered into the quadriceps muscle as a 4-mg inoculum with a needleless bioinjector device (Bioject, Portland, Oreg.). DNA immunizations were given at 0, 4, and 8 weeks. rAd5 vectors were formulated in sterile saline and delivered into the quadriceps muscle by use of a needle and syringe. The dose of each rAd5 vector was 1012 particles.
Antibody binding and virus neutralization assays.
HIV-1 gp120- and gp140-specific binding antibodies were quantified by enzyme-linked immunosorbent assays (ELISAs) as described previously (8, 27). Immunoplates were coated with BAL-gp120 (Quality Biological, Inc., Columbia, Md.), KB9-gp120, or 89.6gp140 (both kindly provided by Patricia Earl, National Institute for Allergy and Infectious Diseases [NIAID], National Institutes of Health [NIH]). Antibody detection was accomplished with alkaline phosphatase- or horseradish peroxidase-conjugated goat anti-monkey immunoglobulin G (IgG) (Sigma Chemical Co., St. Louis, Mo.).
HIV-1 isolates (ADA, JRCSF, JRFL, BaL, SF162, US717, 89.6, and HxB2) were obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. The primary isolates 6101 (previously called P15), 1168, and 1196 were obtained from Larry Corey (University of Washington, Seattle) via David Montefiori of Duke University (4). CM237 (T237), a Thai clade B isolate, was obtained from the U.S. Military HIV Research Program (15). Viruses were expanded by two or three cycles of growth in phytohemagglutinin and interleukin-2-stimulated peripheral blood mononuclear cells (PBMC). Virus stocks were made cell-free by centrifugation at 1,000 × g and filtration through a 0.45-μm-pore-size filter. In some cases, viral stocks were concentrated as much as 10-fold by use of a 100-kDa-cutoff polyethersulfone filter (Centricon Plus Biomax filter; Millipore, Bedford, Mass.) according to the manufacturer's instructions. Virus aliquots were stored in the vapor phase of liquid nitrogen. The BL01 and BR07 viruses were provided by Dana Gabuzda of the Dana-Farber Cancer Institute. Both are chimeric infectious molecular clones of NL4-3 that contain nearly full-length env genes from primary HIV-1 isolates (18). After initial plasmid transfections of 293T cells, these viruses were expanded in PBMC as described above.
HIV-1 neutralization assays were performed by a flow cytometric assay based on a single round of infection according to previously described methods (14). This assay detects HIV-1-infected T cells by intracellular staining for the HIV-1 p24 Gag antigen (p24 Ag). A protease inhibitor was used to prevent secondary rounds of virus replication. Briefly, 40 μl of HIV-1 virus stock (multiplicity of infection, ∼0.1)/well was incubated with 10 μl of heat-inactivated monkey plasma for 30 min at 37°C. The plasma dilution was defined at this step. After incubation, 20 μl of phytohemagglutinin- and interleukin-2-stimulated CD4 T cells (1.5 × 105 cells) was added to each well. Approximately 48 h later, the T cells were harvested for intracellular p24 Ag staining. The cells were fixed and permeabilized by use of a Cytofix/Cytoperm kit (BD Pharmingen, San Diego, Calif.) and were stained with a phycoerythrin-conjugated mouse anti-p24 monoclonal antibody (KC57-RD1; Beckman Coulter, Inc.). HIV-1- or mock-infected T cells were analyzed with a FACSCalibur flow cytometer (Becton Dickinson), and data analyses were performed with FlowJo software (Tree Star, Inc.). Live cells initially gated by their forward and side scatter were analyzed for their intracellular expression of p24 Ag. The number of p24 Ag-positive cells was determined by use of a bivariate plot of fluorescence versus forward scatter; the gate was set for mock-infected cells. After forward and side scatter gating, 50,000 events were counted. The percentage of virus neutralization mediated by each immune plasma was derived by calculating the reduction in the number of p24 Ag-positive cells in the test wells with immune sera compared to the number of p24 Ag-positive cells in wells containing preimmune plasma from the corresponding animal. All plasma samples were also tested against an amphotropic murine leukemia virus (MuLV) to test for non-HIV-1-specific plasma effects. The MuLV reporter viruses encoded green fluorescent protein (GFP), and infected T cells were detected by their expression of GFP rather than p24 Ag (14). As a control for interassay variation, all assays included positive control wells containing well-characterized HIV immunoglobulin and monoclonal antibodies. For experiments with purified IgG, macaque plasma IgG was purified by thiophilic adsorption chromatography according to the manufacturer's instructions (T-Gel; Pierce Inc., Rockford, Ill.). After purification, the concentration of IgG was assayed by radial immunodiffusion according to a standardized methodology (The Binding Site, Inc., San Diego, Calif.). SHIV89.6P neutralizing antibodies were measured in an MT-2 cell killing assay as previously described (8). Neutralizing titers were calculated as reciprocal dilutions of plasma required to protect 50% of the cells from virus-induced killing, as measured by neutral red dye uptake.
Statistical analysis.
Comparisons of percent neutralization, or 50% infective concentrations (IC50 values), among groups of monkeys were performed by Mann-Whitney or Wilcoxon rank sum tests in the statistical package within GraphPad Prism software, version 4.0. Data in figures with bar graphs showing error bars represent means and standard errors of the means (SEM).
RESULTS
Pilot study of rAd5 immunization compared to DNA priming and rAd5 boosting.
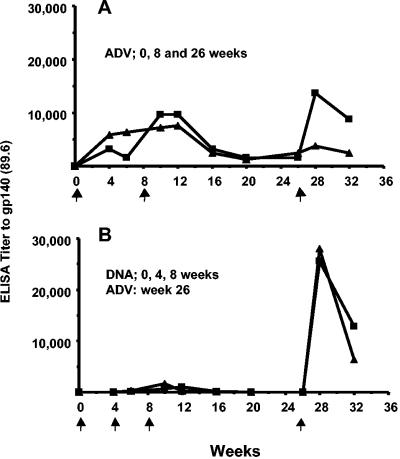
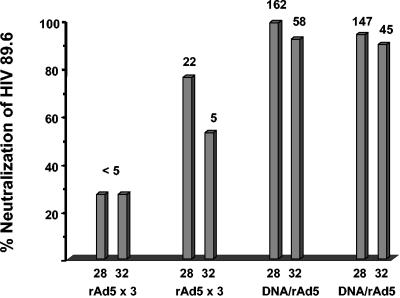
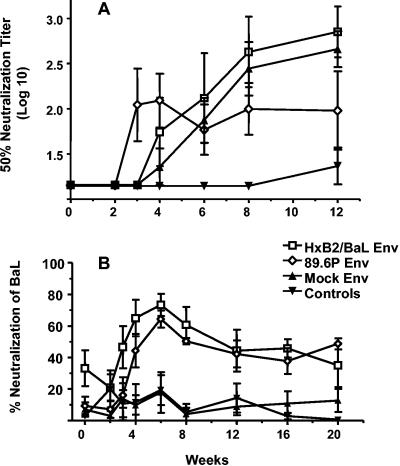
Two macaques received rAd5 at 0, 8, and 26 weeks, and two received DNA at 0, 4, and 8 weeks followed by rAd5 at week 26. A single immunization with rAd5 elicited ELISA titers to HIV-1 gp140 that were substantially higher than those elicited by three immunizations with DNA. However, only modest boosting was observed with subsequent rAd5 immunizations (Fig. 1A). The highest antibody titer occurred in the two animals that were primed with DNA and boosted with rAd5 (Fig. 1B). Plasma samples from 2 and 6 weeks after the last rAd5 immunization were tested for the ability to neutralize HIV-1 89.6. As shown in Fig. 2, neutralizing antibodies to this homologous virus were detected in all four animals. Only DNA-rAd5 immunization elicited virus neutralization levels of >90%, and there was only a small decrement in neutralization between weeks 28 and 32. This contrasted with a two- to fourfold decrease in binding antibody titers during the same time (Fig. 1B). These pilot data showed that DNA-rAd5 immunization produced higher levels of anti-Env binding and neutralizing antibodies than repeated rAd5 immunization. Thus, the additional studies described herein were performed with macaques that were primed with DNA and boosted with rAd5.
FIG. 1.
End-point anti-Env antibody titers in plasma against 89.6 gp140 were measured during the course of immunizations. (A) Data for two animals immunized with rAd5 encoding SIVmac239 Gag-Pol and HIV89.6P Env at 0, 8, and 26 weeks. rAd5 SIVmac239 and HIV89.6P Env were each administered at a dose of 1012 particle units. (B) Data for two animals immunized with DNA plasmids at 0, 4, and 8 weeks and boosted with rAd5 at week 26. DNA plasmids encoded SIVmac239 Gag-Pol-Nef and HIV89.6P Env. Each plasmid was administered at a dose of 4.5 mg; the rAd5 constructs were administered as described above.
FIG. 2.
Neutralization of HIV89.6 mediated by a 1:5 dilution of plasma. The y axis shows the percent neutralization value for each plasma sample at a 1:5 dilution (based on a comparison to the corresponding preimmune plasma). The value above each bar is the reciprocal plasma titer that produced 50% neutralization (i.e., the IC50 value). For each of four animals, the bars show neutralization at weeks 28 and 32, which were 2 and 6 weeks, respectively, after the last immunization.
Neutralizing antibody response after DNA-rAd5 immunization.
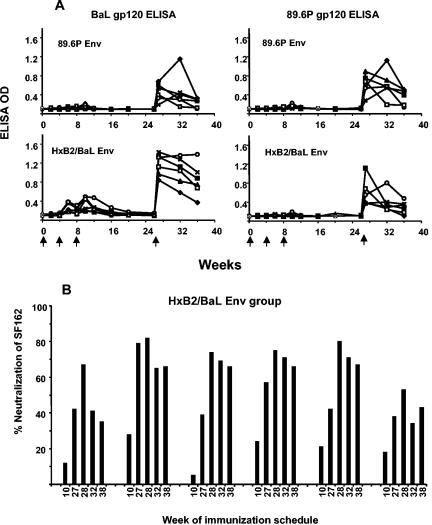
We recently reported the results of a SHIV89.6P challenge study that included four groups of six macaques each (12). All monkeys were immunized with DNA plasmids at 0, 4, and 8 weeks and boosted with rAd5 at week 26. In brief, the four immunization groups were immunized with (i) a control, (ii) SIV Gag-Pol-Nef plus mock Env, (iii) SIV Gag-Pol-Nef plus HxB2/BaL Env, and (iv) SIV Gag-Pol-Nef plus 89.6P Env. Figure 3 shows the ELISA binding antibody responses to BaL gp120 and 89.6P gp120 for the two groups that received Env immunogens. Anti-Env antibodies were detected at low levels after DNA immunizations, and a rapid boost was observed after rAd5 immunization. To evaluate the kinetics of the neutralizing antibody responses during the course of immunizations, we used the neutralization-sensitive SF162 viral strain. Plasma samples from the six monkeys that were immunized with the HxB2/BaL Env vector were tested (Fig. 3B). With preimmune plasma as the baseline control, the low levels of neutralization observed at week 10 (2 weeks after the third DNA inoculation) were rapidly increased by rAd5 immunization. The peak response occurred 1 to 2 weeks after the week 26 rAd5 inoculation, and neutralization of SF162 was still observed 12 weeks later, at week 38. Since the animals were challenged at this time (week 38), no further prechallenge time points could be measured.
FIG. 3.
(A) Anti-Env plasma antibody ELISA performed with BaL gp120 and 89.6P gp120. The immunization groups, immunized with either 89.6P Env or HxB2/BaL Env, are indicated within each graph. Data for all six animals in each group are shown. Low levels of anti-Env antibodies were detected after DNA plasmid immunization; a substantial boost was observed after rAd5 immunization. (B) Kinetics of neutralizing antibody responses in six monkeys that received the HxB2/BaL Env immunogen. Plasma samples from weeks 10, 27, 28, 32, and 38 were tested at a 1:5 dilution against SF162. The week 10 time point was 2 weeks after the third DNA immunization; subsequent time points were after the rAd5 booster immunization that occurred at week 26.
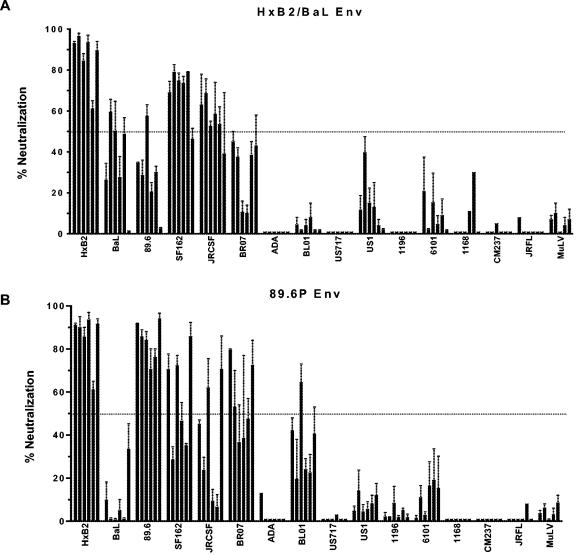
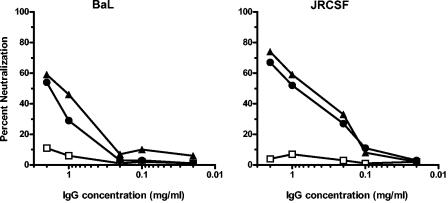
To evaluate the breadth of the neutralizing antibody responses, we assayed week 28 plasma samples from all 12 monkeys that received an Env immunogen against a panel of 15 viruses. This virus panel included HxB2 and 14 primary clade B isolates. We used a flow cytometric neutralization assay based on a single round of infection, which gives more precise and reproducible data than our prior multiple-round PBMC neutralization assay (14). As shown in Fig. 4, reproducible neutralization could be demonstrated against several primary isolates. For the HxB2/BaL Env-immunized group, the laboratory strain HxB2 and a homologous BaL isolate were neutralized, as well as several heterologous viruses. Compared to the HxB2/BaL Env-immunized monkeys, the 89.6P Env-immunized animals demonstrated better HIV89.6 neutralization but weaker BaL neutralization. However, the overall patterns of heterologous virus neutralization for the two groups were similar. Some heterologous viruses, such as SF162, JRCSF, and BR07, were neutralized by immune plasma, while other viruses were either weakly neutralized or were resistant to neutralization. Serial plasma dilutions were tested against HxB2 and four of the more neutralization-sensitive viruses, and the plasma dilution that produced 50% virus neutralization was determined (Table 1). While plasma-mediated neutralization of the primary viruses was clearly detectable, the titers were generally low. None was higher than 1:100. Regarding controls, all immune sera were compared to corresponding preimmune samples, and none of the immune plasma samples had a significant effect on the irrelevant MuLV-pseudotyped GFP reporter virus. In addition, the purification of IgG from two plasma samples from week 28 demonstrated that the neutralization of BaL and JRCSF was mediated by IgG (Fig. 5). Additionally, we assayed all six plasma samples from the mock Env-immunized group against HxB2, BaL, 89.6, SF162, JRCSF, and BR07. In all cases, the mean percent neutralization for two independent experiments was <20% (data not shown). Thus, our data clearly demonstrate that this vaccine platform and these immunogens elicit IgG-mediated virus neutralization but that the potency and breadth of neutralization are limited.
FIG. 4.
Plasma neutralization at week 28 of a panel of 15 clade B HIV-1 isolates. Plasma samples were tested at a 1:5 dilution, and the percent neutralization was calculated by a comparison to the corresponding preimmune plasma sample for each animal. HxB2 is a T-cell-adapted isolate, and all other HIV-1 strains are primary isolates. Each bar shows the neutralization value for one of six animals. (A) Animals that received the HxB2/BaL Env immunogen; (B) animals that received the 89.6P Env immunogen. Plasma samples demonstrating virus neutralization were retested in two to four independent neutralization experiments. For these cases, the means and SEM are shown. In some cases, little or no neutralization was observed in the first set of assays, and the assay was not repeated. An arbitrary horizontal line was drawn to show 50% neutralization.
TABLE 1.
Neutralization titers in plasma
| Vaccine group | Animal no. | Neutralization titer against HIV-1 isolatea
|
|||||
|---|---|---|---|---|---|---|---|
| HxB2 | BaL | 89.6 | SF162 | JRCSF | MuLV-GFP | ||
| HxB2/BaL Env | 1 | 105 | <5 | <5 | 25 | 15 | <5 |
| 2 | 155 | 10 | <5 | 65 | 20 | <5 | |
| 3 | 80 | 5 | 10 | 40 | 5 | <5 | |
| 4 | 120 | <5 | <5 | 35 | 10 | <5 | |
| 5 | 30 | 5 | <5 | 50 | 5 | <5 | |
| 6 | 90 | <5 | <5 | 5 | <5 | <5 | |
| 89.6P Env | 1 | 75 | <5 | 90 | 25 | <5 | <5 |
| 2 | 90 | <5 | 45 | <5 | <5 | <5 | |
| 3 | 65 | <5 | 60 | 20 | 15 | <5 | |
| 4 | 115 | <5 | 40 | 5 | <5 | <5 | |
| 5 | 25 | <5 | 45 | <5 | <5 | <5 | |
| 6 | 80 | <5 | 80 | 20 | 20 | <5 | |
Values are reciprocal plasma dilutions that produced 50% virus neutralization, based on serial plasma dilutions starting at 1:5. A dose-response curve was fit with a nonlinear function, and the dilutions producing 50% effects were calculated from the curve fit. MuLV is an Env-pseudotyped GFP reporter virus, as described in Materials and Methods.
FIG. 5.
Antibody-mediated neutralization of HIV-1 BaL and JRCSF. IgG was purified from the week 28 plasma samples of two macaques that received the HxB2/BaL Env immunogen (closed symbols) and of one animal that received the mock Env immunogen (open symbols). The Hx/BaL Env-immunized macaques were the same two animals represented by the second and third bars in Fig. 4A. Note that the starting IgG concentration of 2 mg/ml is roughly equivalent to a 1:5 plasma dilution.
Secondary antibody response after SHIV89.6P challenge.
Twelve weeks after rAd5 immunization, the four groups of monkeys were challenged by intravenous inoculation of SHIV89.6P. All animals became infected by SHIV, but as described previously (12), the immunized animals displayed lower levels of viremia and higher CD4-T-cell levels than control animals. Notably, the SIV Gag-Pol-Nef- and mock Env-immunized animals were protected from the acute-phase CD4-T-cell decline that is characteristic of an uncontrolled SHIV89.6P infection. Thus, the preserved CD4 T cells and the relative protection for the mock Env-immunized group allowed us to compare this group to animals that received Env immunogens.
Our previous publication included data on neutralizing antibody responses to the challenge virus, SHIV89.6P (12). At 3 weeks postchallenge, three animals in the 89.6P Env-immunized group, but none in the mock- or HxB2/BaL Env-immunized groups, showed an anamnestic response to the challenge virus. Despite this trend suggesting a more rapid neutralizing antibody response to the homologous challenge virus for the 89.6P Env group (Fig. 6A), the postchallenge SHIV89.6P neutralization curves for the 89.6P, BaL/HxB2, and mock Env groups were not statistically different (P > 0.05 for comparisons of IC50 values by Mann-Whitney and Wilcoxon rank order tests for all weeks tested). To further evaluate the anamnestic neutralizing antibody response, we evaluated plasma samples at 20 weeks postchallenge for neutralization of HIV-1 BaL (Fig. 6B). Despite a relatively preserved CD4-T-cell level in the Gag-Pol-Nef- and mock Env-immunized macaques, these animals did not develop neutralizing antibodies to the heterologous BaL strain. In contrast, both the HxB2/BaL and 89.6P Env groups developed anamnestic neutralizing antibody responses to HIV BaL (P < 0.01 by Mann-Whitney tests for comparisons of either of the Env-immunized groups to the mock Env group). Notably, compared to monkeys that were immunized with HxB2/BaL Env, those immunized with 89.6P Env displayed a weak response against BaL after DNA-rAd immunization. Despite this, at week 6 postchallenge, both groups had similar neutralizing antibody responses to HIV BaL.
FIG. 6.
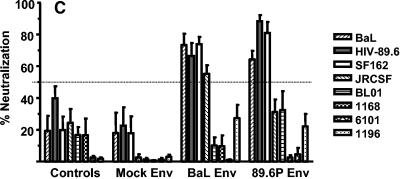
Neutralizing antibodies measured after SHIV89.6P challenge. (A) IC50 values (50% neutralization titers) in plasma against the SHIV89.6P challenge virus. Values are means ± SEM for six animals in each group. (B) Percent neutralization of HIV BaL mediated by a 1:5 dilution of plasma. Values are means ± SEM for six animals in each group. (C) A 1:5 dilution of plasma from week 6 postchallenge was tested against a panel of eight HIV-1 strains. The four immunization groups are shown on the x axis; each bar represents one of the eight viruses. The bars show the mean percentages of neutralization ± SEM for six animals per group against each virus. An arbitrary horizontal line was drawn to show 50% neutralization.
In order to evaluate the breadth of the anamnestic neutralizing antibody response, we tested plasma samples from all monkeys at weeks 6 and 12 postchallenge for neutralization of a panel of eight viruses. While the Gag-Pol-Nef- and mock Env-immunized group of monkeys eventually developed neutralizing antibodies specific for the challenge virus (Fig. 6A), these plasma samples displayed little or no neutralization of primary isolates at 6 weeks postchallenge (Fig. 6C). The data were similar at 12 weeks postchallenge (data not shown). In contrast, both of the Env-immunized groups displayed some neutralization of primary isolates at week 6 postchallenge. We have emphasized these early time points because it seems reasonable to assume that the early anamnestic response may affect acute-phase viremia and alter the disease course after virus challenge. We observed that postchallenge heterologous virus neutralization appeared to be restricted to those viruses that were initially neutralized by the prechallenge immune plasma. For example, the BaL, 89.6, SF162, and JRCSF viruses were neutralized by immune plasma samples from week 28 (Fig. 4) and by postchallenge plasma samples (Fig. 6C), but the BL01, 1168, 6101, and 1196 viruses were not neutralized by either set of plasma samples. The data for samples from 12 weeks postchallenge are not shown, but they were similar to the data for samples from week 6. Thus, while Env immunization primed the animals for an anamnestic neutralizing antibody response, the breadth of the antibody repertoire appears to be limited to the types of neutralizing antibodies that were elicited by the primary immunization regimen.
DISCUSSION
This study demonstrates that the DNA priming-rAd5 boosting immunization platform can elicit robust HIV-1-specific anti-Env antibody responses in nonhuman primates. Prior reports have described HIV-1-specific immune responses after DNA-rAd5 immunization of monkeys, but these have focused on cellular immunity. Our pilot study of four animals suggested that the antibody response generated by DNA-rAd5 immunization was larger than the response observed with sequential rAd5 immunizations. Since other studies have reported similar conclusions (5, 6, 22), we did not pursue the immunization strategy with rAd5 alone for this study. Our study focused on the kinetics, potency, and breadth of the neutralizing antibody response elicited by DNA-rAd5 immunization. Using the neutralization-sensitive SF162 virus, we observed low but detectable levels of neutralizing antibodies after three DNA inoculations and a rapid increase in neutralizing antibodies within 1 week of the rAd5 boost. The vaccine-induced neutralizing antibody response persisted for 12 weeks after the rAd5 boost, which was the time of SHIV89.6P challenge.
The neutralization of heterologous primary HIV-1 isolates was tested with plasma samples from 2 weeks after the rAd5 boost. Data for all 12 animals that received Env immunogens are shown in Fig. 4. Immune plasma samples were tested at a dilution of 1:5 and were compared directly to the corresponding preimmune plasma samples in each assay. The bars in Fig. 4 show means ± SEM for two to four independent neutralization assays for each plasma sample measured against each virus. Thus, the neutralization shown was reproducible.
Regarding the precision of neutralization, our single-round flow cytometric assay directly enumerates the number of HIV-1-infected CD4 T cells (14). Since we counted 50,000 events by flow cytometry and since the infection rate in preimmune plasma is typically about 1 to 2% of the T cells in culture, this assay can readily distinguish a 50% decrease in the number of infected target cells. The fact that immune plasma demonstrated the neutralization of some viruses, but not others, strongly supports our conclusion of antibody-mediated virus neutralization. For example, HxB2/BaL immune plasma neutralized HIV-1 BaL more strongly than 89.6P immune plasma, while the reverse was true for the neutralization of HIV89.6. In addition, some viruses were not neutralized at all by immune plasma, and we included a MuLV control to test for nonspecific plasma effects on virus entry. Lastly, we purified IgG from two immune plasma samples and confirmed that the modest levels of neutralization observed (50 to 75% against HIV-1 BaL and JRCSF) were mediated by IgG. Thus, we are confident that the Env immunogens encoded by the DNA and rAd5 vectors elicited HIV-1-specific neutralizing antibodies.
Overall, the potency and breadth of neutralization elicited by our Env immunogens were limited. Even at a 1:5 dilution, immune plasma displayed ≥80% neutralization against only a few virus isolates. This is generally considered to be a low or modest level of antibody, but it may be possible that moderate levels of virus-neutralizing antibodies act in concert with anti-HIV-1 cellular responses to affect the acquisition of HIV-1 in humans or the disease course after infection (13). Perhaps more important is the restriction in the breadth of virus neutralization. More than one-half of the viruses tested were not neutralized by any of the immune plasma samples. Our immunization strategy included a single clade B Env immunogen (either 89.6P or HxB2/BaL), and we do not know if a single Env immunogen presenting more conserved epitopes or a combination of Env immunogens would elicit a broader response. This is an active area of investigation in several laboratories.
An effective secondary antibody response might act during the early phase of viremia to limit viral spread. To assess if Env immunization primed animals for an anamnestic virus-neutralizing antibody response, we evaluated plasma neutralizing activity after a SHIV89.6P challenge. Our prior data suggested that the postchallenge neutralizing antibody response against the SHIV89.6P challenge virus was more rapid in 89.6P Env-immunized animals than in mock Env-immunized animals, but this difference was not statistically significant (12). In this study, we extended these observations by evaluating the kinetics of the neutralizing antibody response to BaL and by assessing the breadth of the neutralizing response against a panel of eight viruses. While the mock Env-immunized animals (those that received only SIV Gag-Pol-Nef) developed neutralizing antibodies to SHIV89.6P within about 6 weeks postchallenge, these mock Env-immunized animals did not develop neutralizing antibodies to BaL (Fig. 6B) or the other heterologous viruses tested (Fig. 6C). Thus, without Env priming, the neutralizing antibody response was highly strain specific. In contrast, the HxB2/BaL and 89.6P Env-immunized animals developed anamnestic neutralizing antibody responses to several heterologous viruses (Fig. 6C). Since this secondary antibody response may potentially impact the early stages of viral spread, it is important that the breadth of the response seen at week 6 postchallenge was limited to those viruses that were initially neutralized by prechallenge plasma. These data suggest that the breadth of immunity elicited by the original vaccine immunogen will be the limiting factor, even during the secondary antibody response. There is an additional question of the impact of Env immunization on the long-term neutralizing antibody response, including the neutralization of autologous plasma viruses. We are currently investigating this question.
In summary, this report provides the first detailed description of the anti-HIV-1 neutralizing antibody response in nonhuman primates elicited by DNA-rAd5 immunization. In addition to eliciting potent HIV-1-specific cellular immunity, this immunization platform generates robust HIV-1-specific antibody responses that allow for quantitative measurements of HIV-1 neutralization. The HxB2/BaL and 89.6P Env immunogens tested elicited modest levels of plasma antibodies that neutralized several heterologous HIV-1 strains. Ongoing research is focusing on strategies to improve both the potency and breadth of Env immunization. Future approaches with novel Env immunogens can be tested with this immunization platform.
Acknowledgments
We thank Srini Rao, Vi Dang, and Alida Ault for outstanding conduct of animal studies and Ranjani Prabhakara for technical assistance.
REFERENCES
- 1.Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 4.Bures, R., A. Gaitan, T. Zhu, C. Graziosi, K. M. McGrath, J. Tartaglia, P. Caudrelier, R. El Habib, M. Klein, A. Lazzarin, D. M. Stablein, M. Deers, L. Corey, M. L. Greenberg, D. H. Schwartz, and D. C. Montefiori. 2000. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 16:2019-2035. [DOI] [PubMed] [Google Scholar]
- 5.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casimiro, D. R., A. Tang, L. Chen, T. M. Fu, R. K. Evans, M. E. Davies, D. C. Freed, W. Hurni, J. M. Aste-Amezaga, L. Guan, R. Long, L. Huang, V. Harris, D. K. Nawrocki, H. Mach, R. D. Troutman, L. A. Isopi, K. K. Murthy, K. Rice, K. A. Wilson, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Vaccine-induced immunity in baboons by using DNA and replication-incompetent adenovirus type 5 vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:7663-7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakrabarti, B. K., W. P. Kong, B. Y. Wu, Z. Y. Yang, J. Friborg, X. Ling, S. R. King, D. C. Montefiori, and G. J. Nabel. 2002. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J. Virol. 76:5357-5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford, J. M., P. L. Earl, B. Moss, K. A. Reimann, M. S. Wyand, K. H. Manson, M. Bilska, J. T. Zhou, C. D. Pauza, P. W. Parren, D. R. Burton, J. G. Sodroski, N. L. Letvin, and D. C. Montefiori. 1999. Characterization of primary isolate-like variants of simian-human immunodeficiency virus. J. Virol. 73:10199-10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, A. R., B. Kostek, B. B. Mason, C. L. Hsiao, J. Morin, S. K. Dheer, and P. P. Hung. 1985. Expression of hepatitis B surface antigen with a recombinant adenovirus. Proc. Natl. Acad. Sci. USA 82:7560-7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doria-Rose, N. A., C. Ohlen, P. Polacino, C. C. Pierce, M. T. Hensel, L. Kuller, T. Mulvania, D. Anderson, P. D. Greenberg, S. L. Hu, and N. L. Haigwood. 2003. Multigene DNA priming-boosting vaccines protect macaques from acute CD4+-T-cell depletion after simian-human immunodeficiency virus SHIV89.6P mucosal challenge. J. Virol. 77:11563-11577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang, Y., W. P. Kong, and G. J. Nabel. 2001. Human immunodeficiency virus type 1-specific immunity after genetic immunization is enhanced by modification of Gag and Pol expression. J. Virol. 75:4947-4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Letvin, N. L., Y. Huang, B. K. Chakrabarti, X. Ling, M. S. Seaman, K. Beaudry, B. Korioth-Schmitz, F. Yu, D. Rohne, K. J. Martin, A. Miura, W. P. Kong, Z. Y. Yang, R. S. Gelman, O. G. Golubeva, D. C. Montefiori, J. R. Mascola, and G. J. Nabel. 2004. Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys. J. Virol. 78:7490-7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mascola, J. R. 2003. Defining the protective antibody response for HIV-1. Curr. Mol. Med. 3:209-216. [DOI] [PubMed] [Google Scholar]
- 14.Mascola, J. R., M. K. Louder, C. Winter, R. Prabhakara, S. C. De Rosa, D. C. Douek, B. J. Hill, D. Gabuzda, and M. Roederer. 2002. Human immunodeficiency virus type 1 neutralization measured by flow cytometric quantitation of single-round infection of primary human T cells. J. Virol. 76:4810-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mascola, J. R., J. Louwagie, F. E. McCutchan, C. L. Fischer, P. A. Hegerich, K. F. Wagner, A. K. Fowler, J. G. McNeil, and D. S. Burke. 1994. Two antigenically distinct subtypes of human immunodeficiency virus type 1: viral genotype predicts neutralization serotype. J. Infect. Dis. 169:48-54. [DOI] [PubMed] [Google Scholar]
- 16.Nabel, G. J. 2001. Challenges and opportunities for development of an AIDS vaccine. Nature 410:1002-1007. [DOI] [PubMed] [Google Scholar]
- 17.Natuk, R. J., P. K. Chanda, M. D. Lubeck, A. R. Davis, J. Wilhelm, R. Hjorth, M. S. Wade, B. M. Bhat, S. Mizutani, S. Lee, et al. 1992. Adenovirus-human immunodeficiency virus (HIV) envelope recombinant vaccines elicit high-titered HIV-neutralizing antibodies in the dog model. Proc. Natl. Acad. Sci. USA 89:7777-7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohagen, A., A. Devitt, K. J. Kunstman, P. R. Gorry, P. P. Rose, B. Korber, J. Taylor, R. Levy, R. L. Murphy, S. M. Wolinsky, and D. Gabuzda. 2003. Genetic and functional analysis of full-length human immunodeficiency virus type 1 env genes derived from brain and blood of patients with AIDS. J. Virol. 77:12336-12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 20.Sedegah, M., R. Hedstrom, P. Hobart, and S. L. Hoffman. 1994. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc. Natl. Acad. Sci. USA 91:9866-9870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sedegah, M., T. R. Jones, M. Kaur, R. Hedstrom, P. Hobart, J. A. Tine, and S. L. Hoffman. 1998. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 95:7648-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan, N. J., T. W. Geisbert, J. B. Geisbert, L. Xu, Z. Y. Yang, M. Roederer, R. A. Koup, P. B. Jahrling, and G. J. Nabel. 2003. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 424:681-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z. Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605-609. [DOI] [PubMed] [Google Scholar]
- 25.Tang, D. C., M. DeVit, and S. A. Johnston. 1992. Genetic immunization is a simple method for eliciting an immune response. Nature 356:152-154. [DOI] [PubMed] [Google Scholar]
- 26.Ulmer, J. B., J. J. Donnelly, S. E. Parker, G. H. Rhodes, P. L. Felgner, V. J. Dwarki, S. H. Gromkowski, R. R. Deck, C. M. DeWitt, A. Friedman, et al. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745-1749. [DOI] [PubMed] [Google Scholar]
- 27.VanCott, T. C., J. R. Mascola, L. D. Loomis-Price, F. Sinangil, N. Zitomersky, J. McNeil, M. L. Robb, D. L. Birx, and S. Barnett. 1999. Cross-subtype neutralizing antibodies induced in baboons by a subtype E gp120 immunogen based on an R5 primary human immunodeficiency virus type 1 envelope. J. Virol. 73:4640-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinner, L., E. G. Wee, S. Patel, S. Corbet, G. P. Gao, C. Nielsen, J. M. Wilson, H. C. Ertl, T. Hanke, and A. Fomsgaard. 2003. Immunogenicity in Mamu-A*01 rhesus macaques of a CCR5-tropic human immunodeficiency virus type 1 envelope from the primary isolate (Bx08) after synthetic DNA prime and recombinant adenovirus 5 boost. J. Gen. Virol. 84:203-213. [DOI] [PubMed] [Google Scholar]
- 29.Wang, B., K. E. Ugen, V. Srikantan, M. G. Agadjanyan, K. Dang, Y. Refaeli, A. I. Sato, J. Boyer, W. V. Williams, and D. B. Weiner. 1993. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 90:4156-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiang, Z. Q., Y. Yang, J. M. Wilson, and H. C. Ertl. 1996. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology 219:220-227. [DOI] [PubMed] [Google Scholar]
- 31.Yang, Z. Y., B. K. Chakrabarti, L. Xu, B. Welcher, W. P. Kong, K. Leung, A. Panet, J. R. Mascola, and G. J. Nabel. 2004. Selective modifications of variable loops alters tropism and enhances immunogenicity of HIV-1 envelope. J. Virol. 78:4029-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]