Abstract
Vitamin D insufficiency is widespread in HIV-infected patients. HIV and/or antiretroviral therapy (ART), particularly efavirenz (EFV), may interfere with vitamin D metabolism. However, few data from randomized, controlled trials exist. Here, we investigate changes in vitamin D metabolites and binding protein (VDBP) after 6 months of supplementation in a randomized, active-control, double-blind trial investigating 2 different monthly cholecalciferol (vitamin D3) doses [60,000 (medium) or 120,000 (high) IU/month] vs. a control arm of 18,000 IU/month in 8–25 year old HIV-infected youth on ART with HIV-1 RNA <1000 copies/mL and baseline 25-hydroxycholecalciferol (25(OH)D3) ≤30 ng/mL. A matched healthy uninfected group was enrolled in a similar parallel study for comparison. Changes after 6 months were analyzed as intent-to-treat within/between groups [control group (low dose) vs. combined supplementation doses (medium+high)]. At 6 months, 55% vs. 82% of subjects in control and supplementation groups, respectively, reached 25(OH)D3 ≥30 ng/mL (P=0.01) with no difference between medium and high doses (both 82% ≥30 ng/mL). There were few differences for those on EFV vs. no-EFV, except serum VDBP decreased in EFV-treated subjects (both within- and between-groups P≤0.01). There were no significant differences between the HIV-infected vs. healthy uninfected groups. The major finding of the present study is that cholecalciferol supplementation (60,000 or 120,000 IU/month) effectively raises serum 25(OH)D3 in the majority of HIV-infected subjects, regardless of EFV use. Notably, response to supplementation was similar to that of uninfected subjects.
Keywords: HIV, vitamin D metabolites, vitamin D binding protein, cholecalciferol supplementation, randomized-controlled trial, pediatrics and adolescents, vitamin D
1. Introduction
While viral suppression with combination antiretroviral therapy (cART) dramatically restores health, HIV-1-infected individuals have more age-related co-morbidities, such as osteoporosis and an increased risk of fractures, cardiovascular disease, neurocognitive impairment, renal disease and non-AIDS-defining malignancies and occur at an earlier age than individuals in the general population [1–3]. Similar to their adult counterparts, data show that HIV-1-infected youth are also at an increased risk of development of these HIV-related co-morbidities later in life, despite few clinical manifestations at their younger age [4–9]. Given that they will live for many decades with exposure to this state of chronic infection and immune dysfunction, the implications could be profound as the population ages. Developing complementary strategies to cART aimed at decreasing the risk of HIV-associated co-morbidities before clinical manifestations develop may greatly improve quality and length of length in this vulnerable population.
Vitamin D supplementation is arguably an under-utilized potential adjuvant to cART based on the currently available data in both the HIV and general populations. The naturally-synthesized vitamin D hormone, 1,25-dihydroxyvitamin D (1,25(OH)2D), calcitriol), plays a substantial role in many of the complications related to chronic HIV infection [10–14]. In addition, vitamin D endocrine system has long been known to play a key role in the immune system, making it even more important to be studied in the HIV-infected population. A number of observational and cross-sectional studies have shown that vitamin D deficiency, as measured by the concentration of circulating 25-hydroxyvitamin D (25(OH)D), an established marker of overall vitamin D status [15], hastens HIV disease progression and mortality. Higher 25(OH)D concentrations contribute to a more favorable immune restoration after initiating cART and are associated with higher CD4+ T-cell counts and lower HIV-1 RNA levels [16–24]. Likewise, we have previously shown a significant association between vitamin D status and common carotid artery intima-media thickness, a surrogate marker of subclinical atherosclerosis, in a group of HIV-infected subjects on stable cART [18]. In the few randomized-controlled trials (RCTs) conducted in the HIV-infected population, limited data suggest that vitamin D supplementation may improve immune function, decrease immune activation, and increase bone mineral density [25–30].
Importantly, the prevalence of vitamin D insufficiency is very high and widespread in both HIV-infected children and adults. Some studies have demonstrated >90% of subjects with HIV have 25(OH)D concentrations <30 ng/mL [18, 22, 31–34], a blood concentration suggested by the Endocrine Society to represent a suboptimal vitamin D status [35]. In addition, data suggest that HIV infection and cART, especially the non-nucleoside reverse transcriptase inhibitor (NNRTI), efavirenz (EFV), may interfere with vitamin D metabolism [32, 33, 36–43]; however, data from RCTs are limited and none of these studies showed a negative effect of EFV on raising 25(OH)D concentrations [44–46]. In addition, the precise mechanisms whereby HIV and ART may alter vitamin D pathways are poorly-defined. In fact, to date, no vitamin D supplementation study has investigated the mechanisms by which EFV may interfere with vitamin D metabolism. Likewise, there are relatively few RCTs in HIV that have evaluated dosing regimens needed to raise 25(OH)D to optimal blood concentrations [26, 45, 47–50].
Thus, the primary objectives of this study were to 1) comprehensively investigate the changes in serum and urine vitamin D metabolites and vitamin D binding protein (VDBP) with three different monthly dosing regimens in cART-stable, virally-suppressed HIV-infected subjects with vitamin D insufficiency within a RCT of cholecalciferol supplementation, 2) evaluate the efficacy of raising blood concentrations of 25(OH)D3 to >30 ng/mL with these different dosing regimens in this specific HIV population, and 3) determine if the changes in serum and urine vitamin D metabolites and VDBP proteins differ among HIV-infected subjects on EFV compared to those not on EFV. Secondary objectives included to: 1) determine if the results differed between HIV-infected subjects and healthy uninfected controls; and 2) examine factors associated with changes in vitamin D metabolites and VDBP within the HIV-infected subjects. Our focus on HIV-1-infected youth represents an innovative approach to potentially identify efficacious strategies to prevent the development of HIV-related co-morbidities before the onset of established disease.
2. Materials and Methods
2.1 Study Design/Population
This is a randomized, active-control, double-blinded trial designed to measure the effect of cholecalciferol supplementation in HIV-infected youth. HIV-infected subjects were recruited from the HIV clinics of University Hospitals Case Medical Center, Cleveland, OH and Grady Health System, Atlanta, GA via electronic medical record system queries and case manager/provider referrals. HIV-infected subjects were eligible if they were between 8–25 years of age with documented HIV-1 infection on a stable cART regimen for ≥12 weeks, with ≥6 months cumulative cART duration, HIV-1 RNA level <1,000 copies/mL, with no intent to change cART regimen.
Controls were 8–25 years of age and healthy and were selected so that the group matched the HIV-infected subjects in regards to sex, race, and age. Controls were recruited in multiple ways, including a) friends or family members of the HIV-infected subjects, b) physician referrals from local pediatric and adult clinics, c) extensive outreach to various local organizations, churches, and schools, and d) recruitment flyers in targeted locations throughout the two cities. Documentation of absence of HIV infection was obtained in controls ≥13 years of age prior to study inclusion with OraQuick Advance Rapid HIV Test (OraSure Technologies, Inc., Bethlehem, PA, USA) for subjects at the Emory University site, given the high prevalence of HIV in this age group in Atlanta, Georgia.
Additional inclusion criteria for both HIV-infected subjects and healthy controls included baseline serum 25(OH)D concentration ≤30 ng/mL with no intent to change diet, sun exposure or exercise routine during study period. Exclusion criteria included regular daily use of vitamin D supplementation of >400 IU/day, pregnancy or lactation, acute illness or inflammatory condition, malignancy, parathyroid or calcium disorder, diabetes, creatinine clearance <50 mL/min, liver enzymes ≥2.5 times the upper limit of normal, hemoglobin ≤9.0 g/dL, medication use (e.g., chemotherapy agents, systemic steroids) which could affect results, or unwillingness/inability to comply with study procedures. Enrollment into the study occurred ≤30 days after screening.
Intervention consisted of 2 different monthly cholecalciferol doses [60,000 (medium) or 120,000 (high) IU/month] vs. a control arm of 18,000 IU/month (Tischon Corp., Salisbury, MD). A monthly dosing strategy was chosen to minimize additional pill burden, given the risk of poor adherence to medication among HIV-infected adolescents and young adults. These particular monthly doses were designed to represent doses of 600 IU/day (control arm: Institute of Medicine’s current recommended daily allowance for those ages 1–70 years), 4,000 IU/day (high dose: Institute of Medicine’s recommended upper daily limit), and 2,000 IU/day (medium dose) [51].
The randomization scheme was computer-generated, stratified by EFV use at entry and administered by an investigational pharmacist. Regardless of randomized group, subjects took two capsules of cholecalciferol orally at baseline and then monthly after being prompted by a reminder phone call from study staff; capsules looked identical regardless of dose. Representative capsules were sent to an independent laboratory (Analytical Research Laboratories, Oklahoma City, OK) at regular intervals during the study period to ensure continued potency of each dose.
The study was reviewed and approved by the Institutional Review Boards of University Hospital Case Medical Center, Emory University and Grady Health System. All parents or legal guardians and participants ≥18 years of age gave written informed consent to participate in the study. Participants aged 17 years of age signed a written consent along with their parent or legal guardian. Participants between the ages of 8–10 years gave verbal assent and those 11–16 years gave written assent. The study was registered on clinicaltrials.gov (NCT01523496).
Serum vitamin D parameters were measured in subjects who were still in the study at the 6-month time point and had available stored serum. Urine vitamin D parameters were measured in a subset of subjects who were still in the study at the 6-month time point and had available stored urine. Here, we present the pre-planned analysis that assessed changes in serum and urine vitamin D metabolites and VDBP from baseline to month 6 in HIV-infected subjects and healthy controls.
3.0 Study Assessments
3.1 Clinical evaluations
Relevant data including demographics, current and past medical history, and tobacco use were obtained by questionnaire. Further information was also collected from the participants’ medical records including past and current medical diagnoses, CD4 nadir, detailed past and current ARV and non-ARV medication use, HIV diagnosis date, and acquisition method (perinatal or horizontal). Targeted physical examination and weight and height measurements were obtained in all participants.
3.2 Laboratory evaluations
Blood and urine were collected from all participants after at least an 8-hour fast. Whole blood was collected in serum separator tubes for immediate serum isolation and cryopreservation without prior thawing until analysis. For all laboratory assessments, laboratory personnel were blinded to clinical information and HIV status.
Screening serum 25(OH)D concentrations were measured at the local site of the respective participant using either an automated chemiluminescent technique (IDS-iSYS automated machine, Immunodiagnostic Systems, Inc., Fountain Hills, AZ) or a competitive immunoassay (ADVIA Centaur XP System, Siemens Healthcare Diagnostics, Inc., Tarrytown, NY).
Study entry (i.e. baseline) and 6-month study visit samples were utilized for the measurement of all serum and urine vitamin D metabolites and VDBP analyzed in this current study. Serum metabolites and serum VDBP were measured in the same co-investigator’s laboratory (RS) at the Mayo Clinic. Urine metabolites and urine VDBP were measured in the same co-investigator’s laboratory (MTP) at Morehouse University.
Serum 25(OH)D3 was measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) per manufacturers’ product manuals (ThermoFisher Scientific, Franklin, MA and Applied Biosystems-MDS Sciex, Foster City, CA). Intra-assay coefficients of variance (CV) were 3.8%, 2.4% and 4.7% at 25, 54 and 140 ng/mL respectively. Inter-assay CV were 6.4%, 6.8% and 5.0% at 24, 52 and 140 ng/mL respectively.
Serum 1α,25-dihydroxycholecalciferol (1,25(OH)2D3) was measured by LC-MS/MS as described by Strathmann, et al [52]. Intra- and inter-assay CV were 5.6% and 8.0% at 53.4 ng/mL, respectively, and 8.7% and 13% at 21.4 ng/mL, respectively. Limits of detection and quantification were 0.67 ng/mL and 1.3 ng/mL, respectively.
Serum 24,25-dihydroxycholecalciferol (24,25(OH)2D3) was measured via a LC-MS/MS assay we recently developed and validated [53]. The limit of detection was 0.03 ng/mL; the corresponding limit of quantification was 0.1 ng/mL. On the basis of the limit of quantification and the highest calibrators used, the analytical measurement range for undiluted samples was set at 0.1–25 ng/mL. Across this range, intra-assay CV was 3.1%–6.2% and the inter-assay CV was 4.5%–8.3%. Recovery of exogenous 24,25(OH)2D3 spiked into samples was 94%–100%. 24,25-dihydroxyvitamin D3 showed very low cross-reactivity (0.6%) with the spiked total 25(OH)D, and we observed <5% signal suppression.
Serum VDBP was measured by a quantitative sandwich enzyme immunoassay ELISA kit (R&D Systems, Minneapolis, MN). Intra-assay CV were 3.6%, 4.3%, and 3.0% at 32.9, 110.8, and 158.7 ng/mL, respectively. Inter-assay CV were 4.4%, 8.4%, and 4.8% at 34.1, 119.2, and 171.9 ng/mL, respectively.
Urinary vitamin D metabolites (25(OH)D + 24,25(OH)2D) were measured by a quantitative enzyme immunoassay ELISA kit (Immuno Diagnostic Systems, Scottsdale, AZ). Intra-assay CV were 5.3%, 5.6% and 6.7% at 15.6, 26.9 and 66.1 ng/mL, respectively. Inter-assay CVs were 4.6%, 6.4% and 8.7% at 16.2, 28.9 and 52.9 ng/mL, respectively.
Urinary VDBP was measured by a quantitative sandwich enzyme immunoassay ELISA kit (ALPCO, Salem, NH). Intra-assay CVs were 5.0% and 3.2% at 24.2 and 42.9 mg/dL, respectively. Inter-assay CV was 12.7% at 19.3 mg/dL.
Parathyroid hormone was measured via ELISA (Immutopics, Inc., Athens, OH). Absolute CD4+ T-cell count, CD8+ T-cell count and plasma HIV-1 RNA level were concomitantly measured as markers of HIV disease activity.
3.3 Statistical Analyses
Variables are described first by each study group (all HIV-infected subjects combined vs. all control subjects combined). Then, within the HIV-infected group, subjects in the medium and high dose arms were considered together (supplementation dose) and compared to subjects in the control arm. Data from the medium and high dose arms were analyzed together as a single group after it was determined in preliminary data analyses that there were no statistically significant changes between these two dosing arms for any of the vitamin D metabolites except 25(OH)D3 (see results section below). This process was then repeated within the healthy uninfected control group, where subjects in the medium and high dose arms were considered together (supplementation dose) and compared to subjects in the control arm.
Analyses were performed using intent-to-treat principles based on randomized treatment assignment which used all available data. Continuous measures are described by medians and interquartile ranges, and nominal variables are described with frequencies and percents.
Nominal variables were compared using χ2 analysis or Fisher’s exact test. Continuous measures were tested for normality. For between-group comparisons (baseline and changes from baseline to 6 months), normally-distributed variables were compared using the t-test, and non-normally-distributed variables were compared using Wilcoxon rank sum test. For within-group changes from baseline to 6 months, normally-distributed variables were compared with the paired t-test, and non-normally-distributed variables were compared with Wilcoxon signed rank test.
Correlations between variables of interest were assessed using Spearman correlation coefficients. Appropriate two-sample tests were used to assess differences in sub-groups for dichotomous variables (e.g. number of subjects with 25(OH)D3 concentration <30 vs. ≥30 ng/mL at the 6-month time point).
All statistical tests were two-sided with a 0.05 significance level. Analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC).
4.0 Results
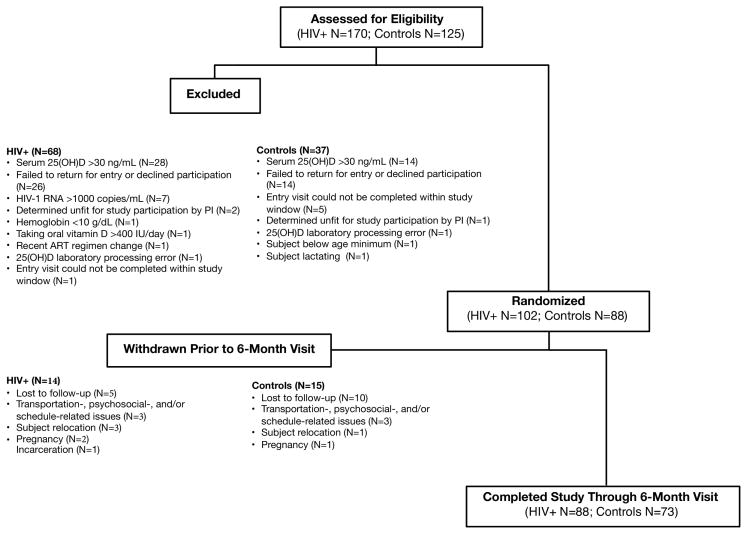
Participants were recruited from January 2012–July 2014. One hundred and two HIV-infected participants were enrolled into the study; 88 (86%) participants completed their 6-month visit (Figure 1). Eighty-eight healthy uninfected controls were enrolled; 73 (83%) completed their 6-month visit. By design, groups were well-matched for age, sex, and race (Table 1). There were also no statistically significant differences between groups for baseline serum and urine vitamin D metabolites or VDBP, except that 1,25(OH)2D3 concentrations were higher in the HIV-infected group.
Figure 1. Consort Diagram of Study Participants.
One hundred-seventy HIV-infected patients were screened for enrollment. Sixty-eight subjects screen-failed, resulting in 102 subjects enrolled. Fourteen subjects were withdrawn before their 6-month study visit, leaving 88 subjects. One hundred twenty-five controls were screened for enrollment. Thirty-seven screen-failed, resulting in 88 enrolled. Of these 88 subjects, 15 subjects were withdrawn before their 6-month study visit, leaving 73 subjects. 25(OH)D, 25-hydroxyvitamin D; ART, antiretroviral therapy
Table 1.
Baseline Characteristics
| Median (Q1, Q3) or no. (%) | HIV-infected Subjects | Healthy Uninfected Subjects | P‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Combined (N=102) | Supplementation Dose (N=66) | Control Dose (N=36) | P† | Combined (N=88) | Supplementation Dose (N=58) | Control Dose (N=30) | P† | ||
|
| |||||||||
|
Demographics & Clinical Variables
| |||||||||
| Age, years | 20.3 (16.6, 22.8) | 20.9 (17.0, 23.6) | 19.9 (15.9, 21.6) | 0.27 | 19.3 (14.4, 23.2) | 19.6 (14.5, 23.7) | 18.4 (14.4, 22.2) | 0.54 | 0.34 |
| Male sex | 65 (64%) | 44 (67%) | 21 (58%) | 0.07 | 53 (60%) | 38 (66%) | 15 (50%) | 0.18 | 0.66 |
| Black race | 91 (89%) | 57 (86%) | 34 (94%) | 0.52 | 76 (86%) | 47 (81%) | 29 (97%) | 0.05 | 0.66 |
| Body mass index, kg/m2 | 22.5 (19.5, 25.3) | 23.2 (19.6, 25.8) | 21.1 (18.9, 21.6) | 0.32 | 23.6 (19.8, 28.1) | 23.6 (19.7, 28.1) | 23.3 (20.2, 28.3) | 0.62 | 0.17 |
| Current smoking | 27 (27%) | 35 (47%) | 19 (53%) | 0.04 | 13 (15%) | 11 (19%) | 2 (7%) | 0.20 | 0.05 |
| Corrected calcium, mg/dL | 9.0 (8.7, 9.2) | 8.9 (8.7, 9.1) | 9.0 (8.8, 9.3) | 0.25 | 9.0 (8.8, 9.2) | 9.0 (8.7, 9.2) | 9.1 (8.9, 9.3) | 0.10 | 0.47 |
| Magnesium, mg/dL | 2.0 (1.8, 2.1) | 2.0 (1.8, 2.0) | 2.0 (1.8, 2.2) | 0.59 | 2.0 (1.9, 2.1) | 2.0 (1.9, 2.1) | 1.9 (1.9, 2.0) | 0.18 | 0.94 |
| Phosphorus, mg/dL | 3.7 (3.3, 4.2) | 3.6 (3.3, 4.2) | 3.8 (3.4, 4.2) | 0.44 | 4.0 (3.5, 4.6) | 3.9 (3.5, 4.5) | 4.3 (3.5, 4.6) | 0.42 | 0.04 |
| ALT, U/L | 23 (19, 30) | 24 (19, 32) | 21 (16, 24) | 0.02 | 22 (18, 29) | 23 (19, 29) | 19 (16, 26) | 0.08 | 0.32 |
| AST, U/L | 21 (17, 24) | 21 (18, 24) | 21 (17, 25) | 0.87 | 20 (15, 24) | 20 (16, 25) | 20 (13, 22) | 0.47 | 0.09 |
| PTH, pg/mL | 55.1 (40.7, 74.9) | 53.4 (39.8, 74.9) | 58.1 (44.9, 72.2) | 0.50 | 50.6 (35.3, 71.5) | 53.3 (38.1, 72.2) | 42.5 (30.2, 66.8) | 0.17 | 0.25 |
| Albumin, g/dL | 4.3 (4.1, 4.3) | 4.3 (4.1, 4.5) | 4.4 (4.0, 4.4) | 0.97 | 4.2 (4.0, 4.4) | 4.2 (4.0, 4.4) | 4.2 (4.0, 4.3) | 0.87 | 0.02 |
| Serum TP, g/dL | 7.8 (7.5, 8.2) | 7.8 (7.5, 8.1) | 7.9 (7.5, 8.3) | 0.63 | 7.5 (7.2, 7.7) | 7.5 (7.1, 7.7) | 7.5 (7.2, 7.8) | 0.20 | <0.0001 |
| Urine TP, mg/L | 173 (125, 268) | 174 (125, 283) | 167 (119, 256) | 0.72 | 157 (92, 226) | 139 (88, 196) | 172 (130, 276) | 0.07 | 0.05 |
| Urine TP/cr ratio, mg/g | 109 (84, 139) | 105 (79, 134) | 124 (92, 168) | 0.03 | 82 (64, 129) | 91, 64, 129) | 78 (60, 125) | 0.79 | 0.02 |
| Urine calcium, mg/dL | 6.8 (5.0, 11.8) | 7.4 (5.0, 11.7) | 5.6 (5.0, 12.4) | 0.33 | 7.3 (5.0, 12.2) | 7.9 (5.0, 12.3) | 7.1 (5.0, 12.1) | 0.89 | 0.93 |
| Urine calcium/cr ratio, mg/g | 44 (30, 71) | 42 (27, 76) | 53 (35, 71) | 0.29 | 50 (29, 80) | 56 (35, 83) | 37 (24, 69) | 0.06 | 0.86 |
|
| |||||||||
|
Serum Vitamin D Status*
| |||||||||
| 25(OH)D3, ng/mL | 18 (11, 23) (N=87) | 18 (11, 22) (N=55) | 15 (11, 26) (N=32) | 0.66 | 16 (12, 20) (N=68) | 15 (12, 21) (N=44) | 16 (14, 20) (N=24) | 0.67 | 0.28 |
| 1,25(OH)2D3, pg/mL | 79 (62, 101) (N=86) | 75 (59, 106) (N=54) | 82 (66, 95) (N=32) | 0.39 | 65.6 (52.5, 87.7) (N=67) | 65.7 (52.7, 96.0) (N=44) | 65.6 (49.5, 85.6) (N=23) | 0.60 | 0.01 |
| 24,25(OH)2D3, ng/mL | 0.8 (0.6, 1.2) (N=39) | 0.8 (0.6, 1.2) (N=24) | 0.8 (0.5, 1.0) (N=15) | 0.61 | 0.9 (0.5, 1.4) (N=42) | 1.0 (0.5, 1.6) (N=29) | 0.9 (0.7, 1.1) (N=13) | 0.37 | 0.53 |
| 24,25(OH)2D3/25(OH)D3 ratio | 0.05 (0.04, 0.08) (N=39) | 0.05 (0.04, 0.09) (N=24) | 0.06 (0.03, 0.08) (N=15) | 0.79 | 0.06 (0.04, 0.07) (N=42) | 0.06 (0.04, 0.08) (N=29) | 0.06 (0.04, 0.07) (N=13) | 0.78 | 0.66 |
| VDBP, μg/mL | 132 (89, 283) (N=86) | 130 (88, 2996) (N=54) | 154 (93, 222) (N=32) | 0.99 | 176 (74, 265) (N=68) | 177 (81, 266) (N=44) | 137 (71, 247) (N=24) | 0.23 | 0.38 |
|
| |||||||||
|
Urine Vitamin D Status*
| |||||||||
| Metabolites, ng/mL | 9.6 (8.4, 11.4) (N=49) | 9.4 (8.4, 12.0) (N=33) | 9.8 (7.7, 11.2) (N=16) | 0.81 | 9.7 (8.6, 12.2) (N=48) | 9.6 (8.5, 12.0) (N=31) | 10.1 (9.1, 12.4) (N=17) | 0.56 | 0.51 |
| VDBP, ng/mL | 104.2 (58.5, 187.1) (N=47) | 102.3 (57.0, 187.2) (N=32) | 115.3 (73.9, 173.2) (N=15) | 0.40 | 81.1 (50.7, 124.4) (N=48) | 63.9 (38.7, 113.4) (N=31) | 103.5 (61.6, 251.9) (N=17) | 0.03 | 0.09 |
|
| |||||||||
|
HIV Variables
| |||||||||
| Current CD4, cells/mm3 | 652 (449, 872) | 652 (451, 879) | 608 ( 417, 792) | 0.58 | -- | -- | -- | -- | -- |
| Nadir CD4, cells/mm3 | 293 (174, 424) | 317 (190, 472) | 246 (109, 363) | 0.06 | -- | -- | -- | -- | -- |
| Undetectable HIV RNA | 91 (89%) | 59 (89%) | 32 (89%) | 1.00 | -- | -- | -- | -- | -- |
| HIV RNA, copies/mL (N=11) | 190 (127, 630) | 143 (90, 590) | 654 (382, 805) | 0.10 | -- | -- | -- | -- | -- |
| Perinatal transmission | 50 (49%) | 35 (47%) | 19 (53%) | 0.68 | -- | -- | -- | -- | -- |
| HIV duration, years | 8.1 (3.4, 15.6) | 8.2 (2.1, 15.8) | 8.0 (2.7, 15.6) | 0.86 | -- | -- | -- | -- | -- |
| ARV duration, years | 3.2 (1.3, 10.0) | 3.2 (1.3, 10.0) | 2.7 (1.3, 10.1) | 1.00 | -- | -- | -- | -- | -- |
| NRTI duration, years | 3.1 (1.3, 9.5) | 3.2 (1.3, 9.7) | 2.6 (1.3, 7.7) | 0.84 | -- | -- | -- | -- | -- |
| PI duration, years | 2.3 (0.6, 7.3) | 2.8 (0.5, 8.3) | 1.8 (0.8, 6.5) | 0.87 | -- | -- | -- | -- | -- |
| EFV duration, months | 2 (0, 16) | 6 (0, 16) | 0 (0, 15) | 0.34 | -- | -- | -- | -- | -- |
| TDF duration, months | 18 (7, 32) | 18 (7, 33) | 18 (8, 30) | 0.95 | -- | -- | -- | -- | -- |
| Current EFV use | 27 (25%) | 18 (27%) | 9 (25%) | 1.00 | -- | -- | -- | -- | -- |
| Current TDF use | 82 (80%) | 55 (83%) | 27 (75%) | 0.31 | -- | -- | -- | -- | -- |
P value between supplementation and control dosing arms;
P value between combined HIV+ and combined healthy uninfected subjects
Serum vitamin D parameters were measured in subjects who were still in the study at the 6-month time point and had available stored serum. Urine vitamin D parameters were measured in a random sub-set of subjects who were still in the study at the 6-month time point and had available stored urine. There was no significant differences between subjects with these measurements and the rest of the HIV subjects in terms of age, sex, race, CD4 count, % with an undetectable HIV RNA and 25(OH)D concentration.
Q, quartile; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PTH, parathyroid hormone; TP, total protein; cr, creatinine; 25(OH)D3, 25-hydroxycholecalciferol; 1,25(OH)2D3, 1,25-dihydroxycholecalciferol; 24,25(OH)2D3, 24,25-dihydroxycholecalciferol; VDBP, vitamin D binding protein; ARV, antiretroviral; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; EFV, efavirenz; TDF, tenofovir
Medium and high dose arms were considered together as one group (supplementation arm) after it was determined that there were no statistically significant changes between these two dosing arms after 6 months of supplementation for any of the vitamin D metabolites except median 25(OH)D3 concentration among the HIV-infected subjects (Table 2). Although the median 25(OH)D3 concentration increased more in the high dose group compared to the medium dose group, there was no difference in the % of subjects with 25(OH)D3 ≥30 ng/mL (both 82%; P=1.00).
Table 2.
Changes after 6 Months of Cholecalciferol Supplementation for Medium- and High-Dose Groups Among HIV-infected Subjects
| Median (Q1, Q3) | High Dose | Medium Dose | P |
|---|---|---|---|
|
| |||
|
Serum Vitamin D Variables
| |||
| 25(OH)D3, ng/mL | +30 (+17, +37) (N=27) | +16 (+12, +23) (N=27) | <0.0001 |
| 1,25(OH)2D3, pg/mL | +6.0 (−4.8, +20.7) (N=25) | −7.0 (−27.4, +18.8) (N=27) | 0.14 |
| 24,25(OH)2D3, ng/mL | +0.02 (−2.0, +4.5) (N=7) | +1.2 (+0.6, +2.2) (N=10) | 0.33 |
| VDBP, μg/mL | −7.2 (−43.7, +13.9) (N=16) | −8.9 (−41.2, +12.0) (N=18) | 0.97 |
|
| |||
|
Urine Vitamin D Variables
| |||
| Metabolites, ng/mL | +12.3 (+5.0, +32.4) (N=16) | +7.1 (+2.9, +12.8) (N=17) | 0.20 |
| VDBP, ng/mL | −9.7 (−66.8, +26.2) (N=16) | −19.0 (−71.5, +43.5) (N=16) | 0.98 |
25(OH)D3, 25-hydroxycholecalciferol; 1,25(OH)2D3, 1,25-dihydroxycholecalciferol; 24,25(OH)2D3, 24,25-dihydroxycholecalciferol; VDBP, vitamin D binding protein
Among the HIV-infected subjects, the randomized groups were well-balanced. There were no statistically significant differences between the supplementation and control dose arms for any of the baseline concentrations of serum and urine vitamin D metabolites or VDBP. At baseline, 56 subjects were on a ritonavir-boosted protease inhibitor (PI) (darunavir = 29; atazanavir = 20; lopinavir = 7), 39 subjects were on a NNRTI (27 = EFV; rilpivirine = 9; nevirapine = 2; etravirine = 1), and 9 subjects were on an integrase inhibitor (elvitegravir = 7; dolutegravir = 1; raltegravir = 1). Nucleoside reverse transcriptase inhibitor (NRTI) backbones included emtricitabine/tenofovir (N=91), lamivudine/abacavir (N=7), lamivudine/zidovudine (N=2), stavudine/abacavir (N=1), and lamivudine/zidovudine/abacavir (N=1). HIV variables were well-balanced between dosing groups at baseline, with an equal number of subjects on EFV and with an undetectable HIV-1 viral load.
Likewise, the randomized groups within the healthy controls were well-balanced. The only significant difference at baseline was a statistically lower urine VDBP in the supplementation arm.
Over the 6-month study period, 9 HIV-infected subjects changed cART regimens, reflecting in part updates in the Guidelines for the Use of Antiretroviral Agents in HIV-1-infected Adults and Adolescents [54]. Five subjects stopped a boosted PI and switched to elvitegravir (N=4) or EFV (N=1; from the control dose group). Three subjects stopped EFV (2 subjects from the control dose arm and 1 subject from the supplementation arm) and switched to elvitegravir (N=1), dolutegravir (N=1), or rilpivirine (N=1). One subject on a boosted PI and emtricitabine/tenofovir stopped all ARVs about 1 month after entry. There were no other changes in NRTI backbones. CD4 counts at the 6-month time point had not changed significantly from baseline for either group (supplementation dose arm: 655 (471, 889) cells/mm3, P=0.57; control dose arm: 657 (511, 809) cells/mm3, P=0.15). Likewise, the % of subjects with an undetectable HIV-1 RNA level at 6 months (84%) was similar to baseline.
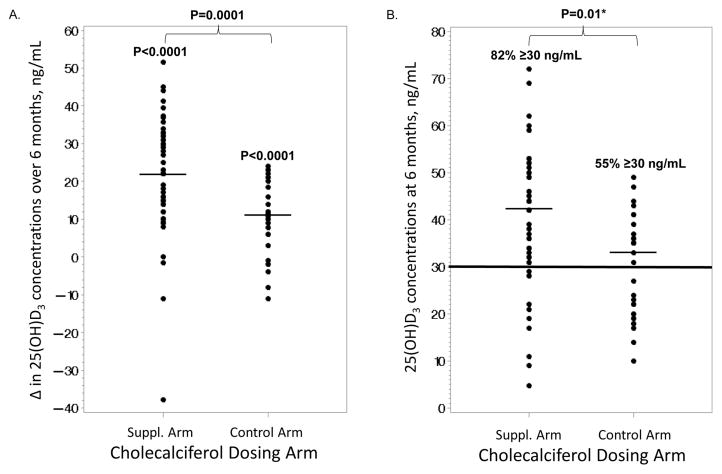
Changes in variables after 6 months of vitamin D supplementation are shown in Table 3. HIV-infected subjects combined and within each of the two dosing groups had a statistically significant within-group rise in their serum 25(OH)D3 concentrations (Table 3A). There were also significant differences in the 25(OH)D3 increases among the two dosing groups, where the supplementation group had a greater increase in 25(OH)D3 (+22 ng/mL) compared to the control dose (+11 ng/mL) (Figure 2A). At the 6-month time point, 82% of the supplementation arm had attained a 25(OH)D3 concentration ≥30 ng/mL compared to 55% in the control arm (P=0.01; Figure 2B).
Table 3.
Changes in Variables after 6 Months of Cholecalciferol Supplementation
| A. HIV-infected Subjects
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Median (Q1, Q3) | All Subjects (N=88) | P† | Suppl. Dose (N=56) | P† | Control Dose (N=32) | P† | P* | P** |
|
Clinical Variables
| ||||||||
| Body mass index, kg/m2 | +0.2 (−0.6, 1.0) | 0.04 | +0.4 (−0.8, +1.1) | 0.17 | +0.2 (−0.2, 0.9) | 0.09 | 0.89 | 0.41 |
| Corrected calcium, mg/dL | +0.19 (−0.12, +0.35) | 0.0018 | +0.24 (−0.01, +0.35) | 0.0029 | +0.07 (−0.19, +0.35) | <0.0001 | 0.23 | 0.71 |
| Magnesium, mg/dL | −0.01 (−0.2, +0.05) | <0.0001 | −0.06 (−0.19, +0.08) | 0.02 | −0.14 (−0.26, −0.01) | 0.0003 | 0.13 | 0.07 |
| ALT, U/L | −3 (−10, −3) | 0.0001 | −4 (−10, +1) | 0.0004 | −2 (−9, +1) | 0.09 | 0.29 | 0.19 |
| PTH, pg/mL | −7.7 (−26.9, +15.9) | 0.045 | −9.4 (−30.1, +15.9) | 0.04 | −1.5 (−21.9, +18.4) | 0.45 | 0.55 | 0.50 |
| Serum TP, g/dL | −0.2 (−0.5,+ 0.1) | 0.0002 | −0.1 (−0.6, +0.2) | 0.04 | −0.2 (−0.4, −0.1) | <0.0001 | 0.27 | 0.10 |
| Urine calcium, mg/dL | 0.0 (−1.3, +3.8) | 0.04 | 0.0 (−2.1, +3.8) | 0.10 | 0.25 (−0.75, +3.70) | 0.22 | 0.94 | 0.71 |
|
| ||||||||
|
Serum Vitamin D Variables
| ||||||||
| 25(OH)D3, ng/mL | +17 (+10, +28) (N=85) | <0.0001 | +22 (+14, +32) (N=54) | <0.0001 | +11 (+6, +20) (N=31) | <0.0001 | 0.0001 | 0.72 |
| 1,25(OH)2D3, pg/mL | +2.2 (−14.6, +21.0) (N=83) | 0.31 | +2.2 (−15.5, +20.2) (N=52) | 0.63 | +2.2 (−10.0, +25.3) (N=31) | 0.25 | 0.59 | 0.07 |
| 24,25(OH)2D3, ng/mL | +0.95 (+0.58, +2.21) (N=29) | 0.0001 | +0.98 (+0.30, +2.21) (N=17) | 0.11 | +0.89 (+0.67, +2.05) (N=12) | 0.0014 | 0.88 | 0.10 |
| 24,25(OH)2D3/25(OH)D3 ratio | +0.01 (−0.004, +0.03) (N=29) | 0.20 | +0.007 (−0.02, +0.02) (N=66) | 0.67 | +0.02 (+0.005, +0.04) (N=12) | 0.10 | 0.23 | 0.11 |
| VDBP, μg/mL | −3.0 (−24.8, 12.0) (N=53) | 0.07 | −8.9 (−41.3, +13.3) (N=17) | 0.07 | +3.9 (−17.9, +12.0) (N=19) | 0.68 | 0.54 | 0.09 |
|
| ||||||||
|
Urine Vitamin D Variables
| ||||||||
| Metabolites, ng/mL | +5.3 (+1.8, +12.8) (N=49) | <0.0001 | +9.7 +(3.5, +18.6) (N=33) | <0.0001 | +1.8 (0.2, 4.4) (N=16) | 0.04 | 0.0016 | 0.66 |
| VDBP, ng/mL | −5.5 (−87.1, +39.7) (N=47) | 0.30 | −15.5 (−71.6, +36.7) (N=32) | 0.19 | +12.1 (−128.7, +104.7) (N=15) | 0.85 | 0.38 | 0.98 |
| B. Healthy Uninfected Subjects
| |||||||
|---|---|---|---|---|---|---|---|
| Median (Q1, Q3) | All Subjects (N=73) | P† | Suppl. Dose (N=46) | P† | Control Dose (N=27) | P† | P* |
|
Clinical Variables
| |||||||
| BMI, kg/m2 | +0.1 (−0.6, +0.1) | 0.33 | +0.08 (−0.6, +0.85) | 0.68 | +0.09 (−0.64, +0.86) | 0.47 | 0.74 |
| Corrected calcium, mg/dL | +0.16 (−0.1-, +0.34) | 0.0072 | +0.23 (−0.04, +0.40) | 0.0017 | +0.02 (−0.14, +0.20) | 0.67 | 0.03 |
| Magnesium, mg/dL | −0.03 (−0.16, +0.06) | 0.09 | −0.04 (−0.16, +0.05) | 0.03 | 0.0 (−0.15, +0.08) | 0.85 | 0.13 |
| ALT, U/L | −2 (−6, +1) | 0.0027 | −1 (−6, +1) | 0.04 | −3 (−8, +2) | 0.046 | 0.45 |
| PTH, pg/mL | −10.2 (−23.8, +4.2) | 0.0022 | −12.4 (−24.5, +8.6) | 0.02 | −4.0 (−21.6, +2.1) | 0.04 | 0.65 |
| Serum TP, g/dL | −0.1 (−0.4, +0.4) | 0.31 | 0.0 (−0.4, +0.5) | 0.78 | −11.7 (−183.6, −2.5) | 0.03 | 0.09 |
| Urine calcium, mg/dL | 0.0 (−3.5, +4.2) | 0.29 | +0.7 (−0.7, +4.3) | 0.12 | 0.0 (−3.7, +2.8) | 0.73 | 0.21 |
|
| |||||||
|
Serum Vitamin D Variables
| |||||||
| 25(OH)D3, ng/mL | +16 (+7, +27) (N=64) | <0.0001 | +25 (+15, +35) (N=40) | <0.0001 | +7.5 (+4.0, +13.0) (N=24) | <0.0001 | <0.0001 |
| 1,25(OH)2D3, pg/mL | +12.2 (−4.5, +27.1) (N=62) | 0.0008 | +9.0 (−9.2, +24.2) (N=39) | 0.09 | +18.8 (+7.8, +32.6) (N=23) | 0.0004 | 0.09 |
| 24,25(OH)2D3, ng/mL | +1.57 (−0.23, +3.19) (N=21) | <0.0001 | +2.25 (+0.97, +5.37) (N=14) | 0.0006 | +1.01 (+0.81, +1.58) (N=7) | 0.0003 | 0.23 |
| 24,25(OH)2D3/25(OH)D3 ratio | +0.02 (+0.01, +0.05) (N=21) | 0.008 | +0.02 (−0.01, +0.05) (N=14) | 0.07 | +0.02 (+0.01, +0.03) (N=7) | 0.001 | 1.00 |
| VDBP, μg/mL | −11.5 (−187.3, +0.9) (N=22) | 0.0010 | −11.3 (−202, +5.4) (N=11) | 0.08 | −11.7 (−183.6, −2.5) (N=11) | 0.02 | 1.00 |
|
| |||||||
|
Urine Vitamin D Variables
| |||||||
| Metabolites, ng/mL | +4.9 (+0.6, +12.8) (N=48) | <0.0001 | +7.0 (+2.3, +17.9) (N=31) | <0.0001 | +2.2 (−1.1, +7.6) (N=17) | 0.13 | 0.04 |
| VDBP, ng/mL | −22.6 (−56.4, +36.5) (N=47) | 0.21 | −5.6 (−38.3, +43.4) (N=30) | 0.88 | −33.5 (−178.6, −3.2) (N=17) | 0.06 | 0.12 |
P value within group;
P value between the two dosing groups;
P value between HIV-infected subjects and healthy uninfected controls
N.B. There were no significant changes in any group for phosphorus, AST, urine TP, urine TP/cr ratio, urine calcium/cr ratio, or CD4 count. P-values with bolded font designate those <0.05.
N.B. There were no significant changes in any group for phosphorus, AST, urine TP, urine TP/cr ratio, urine calcium/cr ratio. P-values with bolded font designate those <0.05. Q, quartile; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PTH, parathyroid hormone; TP, total protein; cr, creatinine; 25(OH)D3, 25-hydroxycholecalciferol; 1,25(OH)2D3, 1,25-dihydroxycholecalciferol; 24,25(OH)2D3, 24,25-dihydroxycholecalciferol; VDBP, vitamin D binding protein
Figure 2. Serum 25(OH)D3 Changes over 6 Months and at 6 Months in the Supplementation and Control Dose Arms in the HIV-infected Subjects.
These scatter plots show the distribution of A. the changes in serum 25(OH)D3 concentrations after 6 months of oral monthly vitamin D3 in the supplementation dose arm (60,000 or 120,000 IU/month) vs. the control arm of 18,000 IU/month and B. the serum 25(OH)D3 concentrations at the 6-month time point. Thin horizontal lines denote medians. Thick horizontal line represents the 25(OH)D3 concentration chosen to define an optimal vitamin D status. 25(OH)D3, 25-hydroxycholecalciferol
There was also a significant increase in 24,25(OH)2D3 for the HIV-infected subjects combined and within the control dose, but there were no significant between-group differences between the control and supplementation groups. There were no significant within-group changes for serum concentrations of 1,25(OH)2D3 or 24,25(OH)2D3/25(OH)D3 ratio. Decreases in serum VDBP approached significance within the HIV-infected group combined and within the supplementation arm (both P=0.07), but not within the control arm (P=0.68). Urine vitamin D metabolites increased in both the supplementation and control dose groups with a statistically greater increase in the supplementation arm. There were no changes in urine VDBP. Parathyroid hormone (PTH) decreased significantly within the supplementation arm and not within the control arm, but between-group differences did not reach statistical significance.
Changes in the serum and urine vitamin D parameters over the 6 months were evaluated for the HIV-infected subjects who were on an EFV-based regimen at baseline compared to those who were not on EFV (Table 4). The only statistically significant difference between the two groups was that serum VDBP decreased 69.3 μg/mL (P=0.01) in those subjects on EFV compared to a non-significant increase of 4.9 μg/mL (P=0.80) in subjects not on EFV. As the ELISA used to measure VDBP has been shown to have some skewing due to the racial distribution of VDBP genotype, the racial composition of each group was compared. Black subjects comprised 85% and 91% of the groups on EFV vs. not on EFV (P=0.87). Urine metabolites also increased significantly in those subjects not on EFV, but this increase was not statistically different than the subjects on EFV.
Table 4.
Changes over 6 Months in HIV-infected Subjects on vs. not on Efavirenz at Baseline
| Variable | Current Efavirenz Use (N=23) | P† | No Current Efavirenz Use (N=65) | P† | P* |
|---|---|---|---|---|---|
|
Serum Vitamin D Variables
| |||||
| 25(OH)D3, ng/mL | +15 (+7.8, +24) (N=22) | <0.0001 | +18.5 (+10.8, +29.0) (N=63) | <0.0001 | 0.30 |
| 1,25(OH)2D3, pg/mL | −2.6 (−15.5, +8.6) (N=22) | 0.36 | +3.8 (−11.4, +26.1) (N=61) | 0.20 | 0.19 |
| 24,25(OH)2D3, ng/mL | +0.83 (+0.75, +2.4) (N=9) | 0.049 | +0.96 (+0.23, +2.2) (N=20) | 0.003 | 0.73 |
| VDBP, μg/mL | −69.3 (−94.0, −13.7) (N=11) | 0.01 | +4.9 (−16.8, +13.3) (N=42) | 0.80 | 0.01 |
|
| |||||
|
Urine Vitamin D Variables
| |||||
| Metabolites, ng/mL | +2.9 (−0.8, +9.8) (N=19) | 0.11 | +5.9 (+2.4, +18.6) (N=30) | <0.0001 | 0.10 |
| VDBP, ng/mL | −17.2 (−119.5, +56.2) (N=17) | 0.32 | −1.2 (−77.0, +36.8) (N=30) | 0.46 | 0.87 |
P value within group;
P value between the two dosing groups;
N.B. P-values with bolded font designate those <0.05. 25(OH)D3, 25-hydroxcholecalciferol; 1,25(OH)2D3, 1,25-dihydroxycholecalciferol; 24,25(OH)2D3, 24,25-dihydroxycholecalciferol; VDBP, vitamin D binding protein
Among the healthy controls (Table 3B), all serum vitamin D parameters changed significantly when all control subjects were considered together and within the control dose arm. Within the supplementation dose arm, changes in 25(OH)D3 and 24,25(OH)2D3 reached statistical significance, and changes in 1,25(OH)2D3, 24,25(OH)2D3/25(OH)D3 ratio, and serum VDBP approached significance. Urine metabolites increased within the supplementation arm but not within the control arm. There were no significant changes in urine VDBP for either the supplementation or control arms. Parathyroid hormone decreased significantly within both the supplementation and control arms, but the decreases between groups were not statistically significant.
Both the HIV-infected group and healthy controls had some statistically significant changes in non-vitamin D blood and urine chemistry parameters (e.g. corrected calcium) (Table 3); however, no subject was deemed to have a clinically-significant abnormality. Likewise, among the subjects who were still in the study at the 6-month time point, there were no study-related adverse events, interruptions in study drug administration, or known non-adherence for any subject in any group. No subject achieved a 25(OH)D3 concentration ≥100 ng/mL. There was one subject who reported mild nausea after taking the cholecalciferol capsules; this subject withdrew from the study prior to the 6-month time point for non-study-related reasons.
There were no statistically significant differences in changes for any variable between the HIV-infected group and healthy controls.
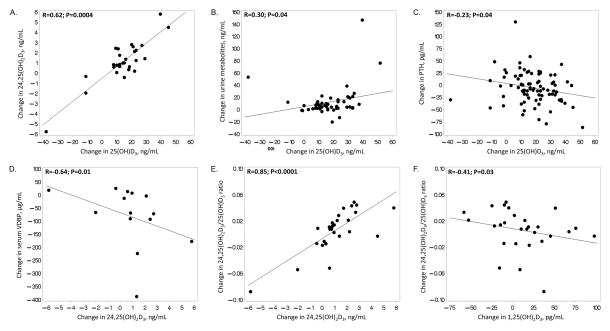
Next, correlations among changes in serum and urine metabolites and VDBP were evaluated within the HIV-infected subjects (Figure 3). Increases in serum 25(OH)D3 concentrations were significantly correlated with increases in serum 24,25(OH)D3 and urine metabolites (Figure 3A, 3B). Increases in serum 25(OH)D3 were significantly correlated with decreases in PTH (Figure 3C). Changes in serum VDBP were negatively correlated with changes in 24,25(OH)2D3 (Figure 3D). Changes in the serum 24,25(OH)2D3/25(OH)D3 ratio were also negatively correlated with changes in serum 1,25(OH)2D3 and positively correlated with changes in 24,25(OH)2D3 (Figure 3E, 3F). There were no other significant correlations between changes in other serum or urine metabolites and VDBP.
Figure 3. Significant Correlations with Changes in Serum 25(OH)D3 in HIV-infected Subjects.
Scatter plots depict the significant bivariate relationships between changes in serum 25(OH)D3 and changes in A. serum 24,25(OH)D3, B. urine vitamin D metabolites, and C. PTH after 6 months of oral monthly vitamin D3. There were no other significant correlations between changes in 25(OH)D3 and the other measured serum and urine vitamin D metabolites and binding proteins. 25(OH)D3, 25-hydroxycholecalciferol; 1,25(OH)2D3, 1,25-dihydroxycholecalciferol; 24,25(OH)2D3, 24,25-dihydroxycholecalciferol; VDBP, vitamin D binding protein; PTH, parathyroid hormone
5. Discussion
To our knowledge, this is the first RCT of vitamin D supplementation to comprehensively investigate changes in serum and urine vitamin D metabolites and VDBP among HIV-infected subjects. We demonstrated that doses of either 60,000 IU or 120,000 IU cholecalciferol given monthly resulted in a greater increase in serum concentrations of 25(OH)D3 compared to the control dose of 18,000 IU after 6 months of supplementation. Likewise, these higher doses allowed a greater percentage of subjects to achieve an optimal 25(OH)D3 concentration of ≥30 ng/mL compared to the control dose. Both high doses (60,000 IU/month or 120,000 IU/month) resulted in a similar percentage of subjects with an optimal 25(OH)D3 concentration after six months of supplementation.
The only other parameter besides 25(OH)D3 that had a statistically significant difference between the supplementation and control arms after 6 months of vitamin D3 was urine vitamin D metabolites, whereby urinary excretion of metabolites increased in both arms but increased to a greater degree with the higher doses. We also observed a statistically significant decrease in PTH in the supplementation arm that was not observed in the control arm. Importantly, there were no significant differences in terms of increases in serum 25(OH)D3 concentrations or changes in other serum/urinary metabolites or VDBP between the HIV-infected subjects and healthy controls. All three doses were safe and well-tolerated.
Multiple studies have demonstrated that HIV infection and ARVs interfere with vitamin D metabolism, citing modulation of the cytochrome P450 systems that control hydroxylation of vitamin D and its metabolites. For example, human hepatocyte and monocyte cell lines exposed to PIs, particularly ritonavir, showed markedly reduced activity of 25- and 1α-hydroxylase and a mild inhibitory effect on 24-hydroxylase in vitro [55]. Perhaps the best-described ARV that appears to interfere with vitamin D metabolism is the NNRTI, EFV. Many observational, cross-sectional and in vitro studies suggest that EFV impairs normal vitamin D metabolism [32, 33, 36–43], but with conflicting data especially among supplementation studies [44–46, 56–59].
These prior findings suggest that HIV-infected patients may require different dosing strategies to optimize their circulating 25(OH)D concentrations. However, only one supplementation trial has investigated the efficacy of dosing strategies in HIV-infected subjects compared to uninfected controls and showed that higher-dose repletion regimens may not be necessary. In Lake, et al, the authors evaluated a standardized regimen of 50,000 IU of cholecalciferol twice weekly for 5 weeks followed by 2,000 IU daily for a total of 12 weeks in an open-label, non-randomized study of HIV-infected adults. This regimen produced rates of 25(OH)D repletion comparable to HIV-uninfected historical controls [46]. Our results support and expand upon these data. To our knowledge, our current study is the only supplementation study that has compared repletion doses directly in HIV-infected subjects vs. healthy uninfected controls.
Moreover, in our study of HIV-infected subjects on cART with suppressed virus, we saw that the majority of subjects in the supplementation dose achieved a 25(OH)D3 concentration of ≥30 ng/mL. This is in sharp contrast to another RCT that evaluated 7,000 IU/daily for 12 months in a similar population. In Schall, et al [60], the authors reported only 52% and 33% of subjects who reached a 25(OH)D concentration of ≥20 ng/mL and ≥32 ng/mL, respectively. However, only 77% of subjects were on cART at baseline, and the authors did not report HIV-1 RNA levels. One could argue that our monthly dosing strategy with less of a pill burden resulted in a higher adherence to the study drugs, but they reported 92% adherence by pill count. Thus, these aforementioned studies and our current findings suggest that subjects with uncontrolled virus may necessitate different repletion dosing strategies compared to those who are already virally-suppressed on a stable cART regimen.
Similarly, as stated above, studies suggest that EFV in particular interferes with normal vitamin D metabolism. For example, in Brown, et al, initiation of ART with an EFV-containing regimen was associated with a significant decrease in 25(OH)D concentrations and a greater risk of vitamin D deficiency compared to a non-EFV-containing regimen [32]. Other ART-initiation and switch studies have shown similar results [37, 61–63], suggesting that perhaps higher repletion doses in HIV-subjects on EFV compared to those not on EFV are necessary. However, the supplementation studies that have evaluated changes in 25(OH)D or percentage of subjects reaching optimal 25(OH)D concentrations in ART-stable subjects have not shown any differences between those on or not on EFV [44, 46, 57]. Our current findings support this idea that once an individual is stable on an EFV-based regimen, no difference in repletion doses are necessary.
Notably, the one difference between the HIV-infected subjects on EFV compared to those not on EFV was that those on EFV had a greater decline in serum VDBP over the 6-month study period. Here, those subjects on EFV had a significant decline in VDBP of −69.3 μg/mL (P=0.01) compared to a non-significant increase of +4.9 μg/mL in those not on EFV (between-group changes P=0.01). Eighty-five to 90% of the body’s vitamin D metabolites circulate tightly bound to VDBP with an additional 10–15% less tightly bound to albumin. The bioavailable 25(OH)D is the sum of free 25(OH)D (unbound to proteins) and 25(OH)D loosely bound to albumin. It is the free form of 25OHD that acts as a substrate for local, paracrine production and action of 1,25(OH)2D on target cells and constitutes <0.1% of the total concentration [64].
We hypothesize that our observed decrease in serum VDBP in those subjects on EFV is a compensatory mechanism to increase the bioavailable/free portions of 25(OH)D and overcome the EFV-associated induction of hepatic enzymes that catabolize both 25(OH)D and 1,25(OH)2D to inactive vitamin D metabolites. The finding that those HIV-subjects not on EFV had a significant increase in urine vitamin D metabolites not seen in those on EFV may support this hypothesis. To date, this is the first vitamin D supplementation trial to attempt to elucidate the mechanism by which EFV interacts with vitamin D metabolism. This finding is novel and deserves further investigation.
Limitations to our current study include the lack of adherence measures to study drug such as pill counts and a small sample size. Overall, however, this study sheds light on the metabolism of vitamin D in HIV individuals. Our study suggests that HIV-infected individuals on stable cART with viral suppression require similar amounts of vitamin D supplementation compared to healthy uninfected individuals, and monthly doses of either 60,000 IU or 120,000 IU cholecalciferol appear effective and safe to raise serum 25(OH)D to optimal concentrations in most HIV-infected individuals. Importantly, EFV use does not seem to affect changes in 25(OH)D concentrations, perhaps by alterations in serum VDBP. Larger studies should be done to validate and expand upon these findings.
HIGHLIGHTS.
Cholecalciferol supplementation (60,000 or 120,000 IU/month) effectively raises serum 25-hydroxyvitamin D in the majority of HIV-infected youth
There is no significant difference in vitamin D metabolites changes between HIV-infected individuals and healthy uninfected controls with cholecalciferol supplementation
Efavirenz use does not appear to interfere with high-dose cholecalciferol supplementation in raising 25-hydroxyvitamin D in HIV-infected youth
Acknowledgments
Sources of Support: This work was made possible by the National Institute of Child Health and Development at the National Institutes of Health [K23 HD069199 to ARE; R01 HD070490 to GAM], National Institutes of Health/Clinical Research Center Grant [8G12MD007602-27 to Morehouse School of Medicine]. Case Western Reserve University’s Center for AIDS Research (P30 AI36219), Emory University’s Center for AIDS Research (P30 AI050409), Emory+Children’s Pediatric Research Center (Immunology Cores), Clinical and Translational Science Award and the Clinical and Translational Science Collaborative of Cleveland (UL1TR000439) from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- 1,25(OH)2D3
1,25-dihydroxycholecalciferol
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- 24,25(OH)2D3
24,25-dihydroxycholecalciferol
- 24,25(OH)2D
24,25-dihydroxyvitamin D
- 25(OH)D3
25-hydroxycholecalciferol
- 25(OH)D
25-hydroxyvitamin D
- vitamin D3
cholecalciferol
- VDBP
vitamin D binding protein
- PTH
parathyroid hormone
- cART
combination antiretroviral therapy (cART)
- EFV
efavirenz
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- PI
protease inhibitor
- NRTI
nucleoside reverse transcriptase inhibitor
- RCT
randomized-controlled trial
- CV
coefficients of variance
Footnotes
Prior presentation: These data were presented at the 19th Vitamin D Workshop, March 2016, Boston, MA (abstract #52).
ClinicalTrials.gov Identifier: NCT01523496
Conflicts of Interest: ARE has received research funding from Bristol-Myers Squibb, Cubist Pharmaceuticals, and GlaxoSmithKline and has served as an advisor for Gilead. ARE has received research funding from Bristol-Myers Squibb, Cubist Pharmaceuticals, and GlaxoSmithKline and has served as an advisor and speaker for Gilead. GAM serves as a consultant for Bristol-Myers Squibb, ViiV/GlaxoSmithKline, Gilead, Pfizer, and ICON, and has received grant funding from Bristol-Myers Squibb, ViiV/GlaxoSmithKline, Merck, AstraZeneca, and Gilead. All other declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 2.Denburg MR, Hoofnagle AN, Sayed S, Gupta J, de Boer IH, Appel LJ, et al. Comparison of Two ELISA Methods and Mass Spectrometry for Measurement of Vitamin D-Binding Protein: Implications for the Assessment of Bioavailable Vitamin D Concentrations Across Genotypes. J Bone Miner Res. 2016;31:1128–1136. doi: 10.1002/jbmr.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielson CM, Jones KS, Chun RF, Jacobs JM, Wang Y, Hewison M, et al. Free 25-Hydroxyvitamin D: Impact of Vitamin D Binding Protein Assays on Racial-Genotypic Associations. J Clin Endocrinol Metab. 2016;101:2226–2234. doi: 10.1210/jc.2016-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross AC, Storer N, O’Riordan MA, Dogra V, McComsey GA. Longitudinal changes in carotid intima-media thickness and cardiovascular risk factors in human immunodeficiency virus-infected children and young adults compared with healthy controls. Pediatr Infect Dis J. 2010;29:634–638. doi: 10.1097/inf.0b013e3181d770c4. [DOI] [PubMed] [Google Scholar]
- 5.Mora S, Zamproni I, Beccio S, Bianchi R, Giacomet V, Vigano A. Longitudinal changes of bone mineral density and metabolism in antiretroviral-treated human immunodeficiency virus-infected children. J Clin Endocrinol Metab. 2004;89:24–28. doi: 10.1210/jc.2003-030767. [DOI] [PubMed] [Google Scholar]
- 6.Uban KA, Herting MM, Williams PL, Ajmera T, Gautam P, Huo Y, et al. White matter microstructure among youth with perinatally acquired HIV is associated with disease severity. AIDS. 2015;29:1035–1044. doi: 10.1097/QAD.0000000000000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsuran D, Bhimma R, Ramdial PK, Naicker E, Adhikari M, Deonarain J, et al. The spectrum of HIV-related nephropathy in children. Pediatr Nephrol. 2012;27:821–827. doi: 10.1007/s00467-011-2074-8. [DOI] [PubMed] [Google Scholar]
- 8.DiMeglio LA, Wang J, Siberry GK, Miller TL, Geffner ME, Hazra R, et al. Bone mineral density in children and adolescents with perinatal HIV infection. AIDS. 2013;27:211–220. doi: 10.1097/QAD.0b013e32835a9b80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McComsey GA, O’Riordan M, Hazen SL, El-Bejjani D, Bhatt S, Brennan ML, et al. Increased carotid intima media thickness and cardiac biomarkers in HIV infected children. AIDS. 2007;21:921–927. doi: 10.1097/QAD.0b013e328133f29c. [DOI] [PubMed] [Google Scholar]
- 10.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review [see comment] AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 11.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. Journal of Clinical Endocrinology & Metabolism. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez E, Milinkovic A, Buira E, de Lazzari E, Leon A, Larrousse M, et al. Incidence and causes of death in HIV-infected persons receiving highly active antiretroviral therapy compared with estimates for the general population of similar age and from the same geographical area. HIV Medicine. 2007;8:251–258. doi: 10.1111/j.1468-1293.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovannucci E. Vitamin D status and cancer incidence and mortality. Advances in Experimental Medicine & Biology. 2008;624:31–42. doi: 10.1007/978-0-387-77574-6_3. [DOI] [PubMed] [Google Scholar]
- 15.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 16.Viard JP, Souberbielle JC, Kirk O, Reekie J, Knysz B, Losso M, et al. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS. 2011;25:1305–1315. doi: 10.1097/QAD.0b013e328347f6f7. [DOI] [PubMed] [Google Scholar]
- 17.Mehta S, Giovannucci E, Mugusi FM, Spiegelman D, Aboud S, Hertzmark E, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS One. 2010;5:e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross AC, Judd S, Kumari M, Hileman C, Storer N, Labbato D, et al. Vitamin D is linked to carotid intima-media thickness and immune reconstitution in HIV-positive individuals. Antivir Ther. 2011;16:555–563. doi: 10.3851/IMP1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta S, Giovannucci E, Mugusi FM, Spiegelman D, Aboud S, Hertzmark E, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS One. 5:e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vescini F, Cozzi-Lepri A, Borderi M, Re MC, Maggiolo F, De Luca A, et al. Prevalence of hypovitaminosis D and factors associated with vitamin D deficiency and morbidity among HIV-infected patients enrolled in a large Italian cohort. J Acquir Immune Defic Syndr. 2011;58:163–172. doi: 10.1097/QAI.0b013e31822e57e9. [DOI] [PubMed] [Google Scholar]
- 21.Sudfeld CR, Wang M, Aboud S, Giovannucci EL, Mugusi FM, Fawzi WW. Vitamin D and HIV progression among Tanzanian adults initiating antiretroviral therapy. PLoS One. 2012;7:e40036. doi: 10.1371/journal.pone.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutstein R, Downes A, Zemel B, Schall J, Stallings V. Vitamin D status in children and young adults with perinatally acquired HIV infection. Clin Nutr. 2011;30:624–628. doi: 10.1016/j.clnu.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Gandhi V, Psevdos G, Jr, Espinoza F, Park J, Sharp V. Evaluation of vitamin D levels among HIV-infected patients in New York City. AIDS Res Hum Retroviruses. 2012;28:235–241. doi: 10.1089/AID.2011.0040. [DOI] [PubMed] [Google Scholar]
- 24.Aziz M, Livak B, Burke-Miller J, French AL, Glesby MJ, Sharma A, et al. Vitamin D insufficiency may impair CD4 recovery among Women’s Interagency HIV Study participants with advanced disease on HAART. AIDS. 2013;27:573–578. doi: 10.1097/QAD.0b013e32835b9ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giacomet V, Vigano A, Manfredini V, Cerini C, Bedogni G, Mora S, et al. Cholecalciferol supplementation in HIV-infected youth with vitamin D insufficiency: effects on vitamin D status and T-cell phenotype: a randomized controlled trial. HIV Clin Trials. 2013;14:51–60. doi: 10.1310/hct1402-51. [DOI] [PubMed] [Google Scholar]
- 26.Stallings VA, Schall JI, Hediger ML, Zemel BS, Tuluc F, Dougherty KA, et al. High-dose vitamin D3 supplementation in children and young adults with HIV: a randomized, placebo-controlled trial. Pediatr Infect Dis J. 2015;34:e32–40. doi: 10.1097/INF.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabre-Mersseman V, Tubiana R, Papagno L, Bayard C, Briceno O, Fastenackels S, et al. Vitamin D supplementation is associated with reduced immune activation levels in HIV-1-infected patients on suppressive antiretroviral therapy. AIDS. 2014;28:2677–2682. doi: 10.1097/QAD.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 28.Bang U, Kolte L, Hitz M, Dam Nielsen S, Schierbeck LL, Andersen O, et al. Correlation of increases in 1,25-dihydroxyvitamin D during vitamin D therapy with activation of CD4+ T lymphocytes in HIV-1-infected males. HIV Clin Trials. 2012;13:162–170. doi: 10.1310/hct1303-162. [DOI] [PubMed] [Google Scholar]
- 29.Eckard AR, Chahroudi A, Rosebush JC, O’Riordan MA, Daniels JE, Uribe-Leitz M, et al. Effects of vitamin D supplementation on BMD and bone markers in HIV+ youth. In. 23rd Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2016. [Google Scholar]
- 30.Eckard AR, CA, Rosebush JC, O’Riordan MA, Daniels JE, Uribe-Leitz M, Kinley B, Labbato D, Habib J, Tangpricha V, McComsey GA. Vitamin D Supplementation Decreases Immune Activation and Exhaustion in HIV+ Youth. In. 23rd Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2016. [Google Scholar]
- 31.Eckard AR, Judd SE, Ziegler TR, Camacho-Gonzalez AF, Fitzpatrick AM, Hadley GR, et al. Risk factors for vitamin D deficiency and relationship with cardiac biomarkers, inflammation and immune restoration in HIV-infected youth. Antivir Ther. 2012;17:1069–1078. doi: 10.3851/IMP2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown TT, McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D. Antivir Ther. 2010;15:425–429. doi: 10.3851/IMP1502. [DOI] [PubMed] [Google Scholar]
- 33.Welz T, Childs K, Ibrahim F, Poulton M, Taylor CB, Moniz CF, et al. Efavirenz is associated with severe vitamin D deficiency and increased alkaline phosphatase. AIDS. 2010;24:1923–1928. doi: 10.1097/QAD.0b013e32833c3281. [DOI] [PubMed] [Google Scholar]
- 34.Stein EM, Yin MT, McMahon DJ, Shu A, Zhang CA, Ferris DC, et al. Vitamin D deficiency in HIV-infected postmenopausal Hispanic and African-American women. Osteoporos Int. 2011;22:477–487. doi: 10.1007/s00198-010-1299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 36.Dao CN, Patel P, Overton ET, Rhame F, Pals SL, Johnson C, et al. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D Levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 37.Wohl DA, Orkin C, Doroana M, Pilotto JH, Sungkanuparph S, Yeni P, et al. Change in vitamin D levels and risk of severe vitamin D deficiency over 48 weeks among HIV-1-infected, treatment-naive adults receiving rilpivirine or efavirenz in a Phase III trial (ECHO) Antivir Ther. 2014;19:191–200. doi: 10.3851/IMP2721. [DOI] [PubMed] [Google Scholar]
- 38.Hariparsad N, Nallani SC, Sane RS, Buckley DJ, Buckley AR, Desai PB. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J Clin Pharmacol. 2004;44:1273–1281. doi: 10.1177/0091270004269142. [DOI] [PubMed] [Google Scholar]
- 39.Dave JA, Cohen K, Micklesfield LK, Maartens G, Levitt NS. Antiretroviral Therapy, Especially Efavirenz, Is Associated with Low Bone Mineral Density in HIV-Infected South Africans. PLoS One. 2015;10:e0144286. doi: 10.1371/journal.pone.0144286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avihingsanon A, Kerr SJ, Ramautarsing RA, Praditpornsilpa K, Sophonphan J, Ubolyam S, et al. The Association of Gender, Age, Efavirenz Use, and Hypovitaminosis D Among HIV-Infected Adults Living in the Tropics. AIDS Res Hum Retroviruses. 2016;32:317–324. doi: 10.1089/AID.2015.0069. [DOI] [PubMed] [Google Scholar]
- 41.Theodorou M, Serste T, Van Gossum M, Dewit S. Factors associated with vitamin D deficiency in a population of 2044 HIV-infected patients. Clin Nutr. 2014;33:274–279. doi: 10.1016/j.clnu.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 42.Kwan CK, Eckhardt B, Baghdadi J, Aberg JA. Hyperparathyroidism and complications associated with vitamin D deficiency in HIV-infected adults in New York City, New York. AIDS Res Hum Retroviruses. 2012;28:1025–1032. doi: 10.1089/aid.2011.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cervero M, Agud JL, Garcia-Lacalle C, Alcazar V, Torres R, Jusdado JJ, et al. Prevalence of vitamin D deficiency and its related risk factor in a Spanish cohort of adult HIV-infected patients: effects of antiretroviral therapy. AIDS Res Hum Retroviruses. 2012;28:963–971. doi: 10.1089/AID.2011.0244. [DOI] [PubMed] [Google Scholar]
- 44.Longenecker CT, Hileman CO, Carman TL, Ross AC, Seydafkan S, Brown TT, et al. Vitamin D supplementation and endothelial function in vitamin D deficient HIV-infected patients: a randomized placebo-controlled trial. Antivir Ther. 2012;17:613–621. doi: 10.3851/IMP1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steenhoff AP, Schall JI, Samuel J, Seme B, Marape M, Ratshaa B, et al. Vitamin D(3)supplementation in Batswana children and adults with HIV: a pilot double blind randomized controlled trial. PLoS One. 2015;10:e0117123. doi: 10.1371/journal.pone.0117123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lake JE, Hoffman RM, Tseng CH, Wilhalme HM, Adams JS, Currier JS. Success of Standard Dose Vitamin D Supplementation in Treated Human Immunodeficiency Virus Infection. Open Forum Infect Dis. 2015;2:ofv068. doi: 10.1093/ofid/ofv068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arpadi SM, McMahon D, Abrams EJ, Bamji M, Purswani M, Engelson ES, et al. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics. 2009;123:e121–126. doi: 10.1542/peds.2008-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dougherty KA, Schall JI, Zemel BS, Tuluc F, Hou X, Rutstein RM, et al. Safety and Efficacy of High-Dose Daily Vitamin D3 Supplementation in Children and Young Adults Infected With Human Immunodeficiency Virus. J Pediatric Infect Dis Soc. 2014;3:294–303. doi: 10.1093/jpids/piu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Havens PL, Mulligan K, Hazra R, Flynn P, Rutledge B, Van Loan MD, et al. Serum 25-hydroxyvitamin D response to vitamin D3 supplementation 50,000 IU monthly in youth with HIV-1 infection. J Clin Endocrinol Metab. 2012;97:4004–4013. doi: 10.1210/jc.2012-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kakalia S, Sochett EB, Stephens D, Assor E, Read SE, Bitnun A. Vitamin D supplementation and CD4 count in children infected with human immunodeficiency virus. J Pediatr. 2011;159:951–957. doi: 10.1016/j.jpeds.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Institute of Medicine (U.S.) Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 52.Strathmann FG, Laha TJ, Hoofnagle AN. Quantification of 1alpha,25-dihydroxy vitamin D by immunoextraction and liquid chromatography-tandem mass spectrometry. Clin Chem. 2011;57:1279–1285. doi: 10.1373/clinchem.2010.161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ketha H, Kumar R, Singh RJ. LC-MS/MS for Identifying Patients with CYP24A1 Mutations. Clin Chem. 2016;62:236–242. doi: 10.1373/clinchem.2015.244459. [DOI] [PubMed] [Google Scholar]
- 54.at PoAGfAaAGftuoaaiH--iaaaDoHaHSA.
- 55.Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. AIDS. 2003;17:513–520. doi: 10.1097/00002030-200303070-00006. [DOI] [PubMed] [Google Scholar]
- 56.Gupta SK, Mi D, Moe SM, Dube MP, Liu Z. Effects of switching from efavirenz to raltegravir on endothelial function, bone mineral metabolism, inflammation, and renal function: a randomized, controlled trial. J Acquir Immune Defic Syndr. 2013;64:279–283. doi: 10.1097/qai.0b013e3182a97c39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Banon S, Rosillo M, Gomez A, Perez-Elias MJ, Moreno S, Casado JL. Effect of a monthly dose of calcidiol in improving vitamin D deficiency and secondary hyperparathyroidism in HIV-infected patients. Endocrine. 2015;49:528–537. doi: 10.1007/s12020-014-0489-2. [DOI] [PubMed] [Google Scholar]
- 58.Coelho L, Cardoso SW, Luz PM, Hoffman RM, Mendonca L, Veloso VG, et al. Vitamin D3 supplementation in HIV infection: effectiveness and associations with antiretroviral therapy. Nutr J. 2015;14:81. doi: 10.1186/s12937-015-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poowuttikul P, Thomas R, Hart B, Secord E. Vitamin D Insufficiency/Deficiency in HIV-Infected Inner City Youth. J Int Assoc Provid AIDS Care. 2014;13:438–442. doi: 10.1177/2325957413495566. [DOI] [PubMed] [Google Scholar]
- 60.Schall JI, Hediger ML, Zemel BS, Rutstein RM, Stallings VA. Comprehensive Safety Monitoring of 12-Month Daily 7000-IU Vitamin D3 Supplementation in Human Immunodeficiency Virus-Infected Children and Young Adults. JPEN J Parenter Enteral Nutr. 2016;40:1057–1063. doi: 10.1177/0148607115593790. [DOI] [PubMed] [Google Scholar]
- 61.Conesa-Botella A, Florence E, Lynen L, Colebunders R, Menten J, Moreno-Reyes R. Decrease of vitamin D concentration in patients with HIV infection on a non nucleoside reverse transcriptase inhibitor-containing regimen. AIDS Res Ther. 2010;7:40. doi: 10.1186/1742-6405-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamzah L, Tiraboschi JM, Iveson H, Toby M, Mant C, Cason J, et al. Effects on vitamin D, bone and the kidney of switching from fixed-dose tenofovir disoproxil fumarate/emtricitabine/efavirenz to darunavir/ritonavir monotherapy: a randomized, controlled trial (MIDAS) Antivir Ther. 2016;21:287–296. doi: 10.3851/IMP3000. [DOI] [PubMed] [Google Scholar]
- 63.Fox J, Peters B, Prakash M, Arribas J, Hill A, Moecklinghoff C. Improvement in vitamin D deficiency following antiretroviral regime change: Results from the MONET trial. AIDS Res Hum Retroviruses. 2011;27:29–34. doi: 10.1089/aid.2010.0081. [DOI] [PubMed] [Google Scholar]
- 64.Bouillon R. Free or Total 25OHD as Marker for Vitamin D Status? J Bone Miner Res. 2016;31:1124–1127. doi: 10.1002/jbmr.2871. [DOI] [PubMed] [Google Scholar]