Abstract
Rsv1, a single dominant resistance gene in soybean PI 96983 (Rsv1), confers extreme resistance against all known American strains of Soybean mosaic virus (SMV), except G7 and G7d. SMV-G7 provokes a lethal systemic hypersensitive response (LSHR), whereas SMV-G7d, an experimentally evolved variant of SMV-G7, induces systemic mosaic. To identify the elicitor of Rsv1-mediated LSHR, chimeras were constructed by exchanging fragments between the molecularly cloned SMV-G7 (pSMV-G7) and SMV-G7d (pSMV-G7d), and their elicitor functions were assessed on PI 96983 (Rsv1). pSMV-G7-derived chimeras containing only P3 of SMV-G7d lost the elicitor function, while the reciprocal chimera of pSMV-G7d gained the function. The P3 regions of the two viruses differ by six nucleotides, of which two are translationally silent. The four amino acid differences are located at positions 823, 915, 953, and 1112 of the precursor polypeptide. Analyses of the site-directed point mutants of both the viruses revealed that nucleotide substitutions leading to translationally silent mutations as well as reciprocal amino acid substitution at position 915 did not influence the loss or gain of the elicitor function. pSMV-G7-derived mutants with amino acid substitutions at any of the other three positions lost the ability to provoke LSHR but induced SHR instead. Two concomitant amino acid substitutions at positions 823 (V to M) and 953 (K to E) abolished pSMV-G7 elicitor function, provoking Rsv1-mediated SHR. Conversely, pSMV-G7d gained the elicitor function of Rsv1-mediated LSHR by a single amino acid substitution at position 823 (M to V), and mutants with amino acid substitutions at position 953 or 1112 induced SHR instead of mosaic. Taken together, the data suggest that strain-specific P3 of SMV is the elicitor of Rsv1-mediated LSHR.
Plants have evolved surveillance mechanisms to recognize invading pathogenic intruders, including viruses, and initiate defense responses. One such detection mechanism involves resistance (R) genes (61). R-mediated recognition of a pathogen is conditioned through matched specificity between products of an R gene and a pathogen avirulence (Avr) gene, which is achieved by either direct or indirect interactions (8, 18, 25). Following the recognition, host defense responses are triggered, resulting in suppression of the invading pathogen and its confinement to the point of entry (32). On their part, plant pathogens have evolved different means to evade R-mediated recognition or suppress the resistance response. Bacteria and fungi introduce diverse mutations into their Avr genes, including point mutations, insertions, and even deletions (3, 34, 47, 60, 65). However, due to multifunctionality of viral genes, minor modification of Avr genes is the only affordable mechanism of evasion by plant viruses (7, 16, 26, 40, 41, 50, 53, 66). Thus, in a plant genotype harboring an R gene against a plant virus, the elicitation of R-mediated defense responses is dependent upon the elicitor function of the invading virus. R-dependent elicitor function for a number of plant viral genes has been established (12). However, the underlying mechanism of evasion of R-mediated recognition by viruses has not been elucidated, and it is not known whether it is an active or a passive process.
Phenotypically, R-mediated defense responses against a virus harboring a complementary Avr gene consist of either extreme resistance (ER) or a hypersensitive response (HR) (33), both of which are considered the consequence of the same defense response (5). The arrest of an avirulent virus in ER-expressing tissues is not associated with any visible symptoms or virus accumulation (5, 28). However, in HR-expressing tissues, the arrest is associated with rapid, localized death of infected and neighboring cells; induction of a variety of defense-related genes including pathogenesis-related (PR) proteins; and a wide range of physiological changes (32).
Perturbation of Avr or R genes (15, 20, 41), graft inoculation instead of mechanical inoculation (6, 28, 69, 72), change in genetic background of the R gene-carrying plant (45), heterozygosity rather than homozygosity of the R-genotype plant (10, 52, 56), prevention of salicylic acid accumulation in the R-genotype plant (19, 42), or temperature shift (30, 70) can result in virus escape to distant tissues, provoking systemic HR (SHR) in resistant genotypes. Occasionally, SHR will result in the death of the infected plant, a condition termed lethal SHR (LSHR) (28, 29). Elicitation of SHR, instead of localized HR, is not unique to the interactions involving avirulent viruses and resistant plants. Interactions of specific plant fungal and bacterial pathogens harboring Avr genes with their matching R genes also are known to trigger “trailing HR” (24, 38). Furthermore, transient expression of fungal and bacterial Avr genes via a heterologous system (i.e., virus-based expression vector) can elicit SHR instead of localized HR (9, 31, 39, 46, 64). In any case, elicitation of SHR, instead of localized HR, represents a failure of R-mediated defense responses of a host to confine the invading virus to its entry points. Currently, the underlying molecular and biochemical mechanisms leading to SHR are not understood. Culver et al. (15) have suggested that SHR is indicative of “weak elicitor” function of an Avr gene, and Dinesh-Kumar et al. (20) have postulated that SHR is a consequence of delayed occurrence of biochemical and physiological events that are associated with localized HR.
Rsv1, a genetically mapped single dominant gene in soybean line PI 96983 (Rsv1) (71), confers ER against all naturally occurring American strains of Soybean mosaic virus (SMV), including those in groups G1 to G6 and C14, but not G7, when leaf tissues are challenged by mechanical inoculation (11, 48). However, an isolate belonging to the G2 group of SMV strains (11), SMV-N (68), has the potential to induce Rsv1-mediated restricted SHR when the virus is introduced continuously into Rsv1-bearing scions from infected rootstocks (28). SMV-G7 overcomes Rsv1-mediated ER and spreads systemically in infected PI 96983 (Rsv1) tissues, where it provokes SHR, which eventually progresses to LSHR (28, 29). Induction of LSHR by pSMV-G7 is consistently associated with up regulation of a soybean PR-1 gene transcript (28, 29), which is considered a universal molecular marker of HR (17).
Based on our previous finding that an isolate of a G2 group of SMV strains, SMV-N, can induce both ER and restricted SHR in Rsv1-genotype soybean and assuming that Rsv1-mediated resistance against all avirulent strains of SMV operates via a common resistance mechanism, we proposed that Rsv1-mediated resistance operates based on two-tiered mechanisms of resistance (28). Primary resistance or ER, which is operational at the point of inoculation, is HR independent. Secondary resistance (HR mediated) activates when the primary tier of resistance is bypassed and the HR elicitor is allowed to accumulate. The two-tiered mechanism of resistance is not unique to Rsv1. Rx-mediated resistance in potato against specific Potato virus X (PVX) strains containing the matching Avr gene also involves a two-tiered defense mechanism, ER and HR (5). Furthermore, in the case of the Rx-PVX pathosystem, the coat protein (CP) is the elicitor of both phenotypes (4, 5) and Rx controls the separate expression of ER and HR (5). Rsv1-mediated resistance shares a number of superficial similarities to that of Rx (28). Thus, we have hypothesized that the SMV-N elicitor of ER and that of restricted SHR are the same SMV gene and that the corresponding region of SMV-G7, albeit modified, is also the elicitor of Rsv1-mediated LSHR.
To facilitate identification of the SMV-N elicitor of Rsv1-mediated ER and restricted SHR, we have focused on identification of the SMV-G7 elicitor of Rsv1-mediated LSHR as the initial step. This is mainly because, unlike SMV-N, which does not infect Rsv1-soybean genotype by mechanical inoculation (28), SMV-G7 readily infects this genotype (11, 28, 48). To achieve this goal, we have generated an experimentally evolved variant of SMV-G7 (SMV-G7d), which, unlike its progenitor, SMV-G7, induces mosaic in the context of Rsv1-genotype soybean plants (29). Hence, it bypasses both ER and HR tiers of Rsv1-mediated defense responses, as its replication in PI 96983 (Rsv1) is not associated with PR-1 gene transcript up regulation (29). Thus, pSMV-G7d has acquired the capability to evade Rsv1-mediated recognition.
The molecular characteristics of an infectious clone of SMV-G7d (pSMV-G7d) and its progenitor, an infectious clone of SMV-G7 (pSMV-G7), were reported earlier (29). The genome of pSMV-G7d differs from its progenitor (pSMV-G7) genome by 17 substitutions, of which 10 are translationally silent. The seven amino acid substitutions in deduced sequences of pSMV-G7d differ from that of pSMV-G7 by one each in P1 proteinase, helper component-proteinase (HC-Pro), and CP, respectively, and by four in P3 (29). In this paper, we demonstrate that loss and gains of the elicitor function of the two strains provoking Rsv1-mediated LSHR are encoded in the P3 gene sequences, and we identify the causal residues involved.
MATERIALS AND METHODS
Virus strains, soybean genotypes, inoculation, and SMV detection.
Plasmids containing infectious full-length cDNA clones of SMV-G7 (pSMV-G7) and SMV-G7d (pSMV-G7d) (29) served as the virus sources. Unless otherwise stated, both plasmids were propagated in ElectroMax DH5α-E cells (Invitrogen) and purified using the QiaPrep Spin MiniPrep kit (Qiagen). The soybean (Glycine max) cultivar Williams 82 (rsv1) and line PI 96983 (Rsv1) were used in this study (28, 29). All the seeds were obtained from virus-indexed greenhouse-grown plants. To establish infection, plasmid DNA was bombarded onto hypocotyles of the soybean PI 96983 (Rsv1) by biolistic delivery (29). Infectious sap from the infected PI 96983 (Rsv1) was used to mechanically inoculate the carborundum-dusted (600-mesh) soybeans (28). The inoculated plants were maintained in a growth chamber operating at 20°C with a photoperiod of 18 h. The presence of SMV in the inoculated soybean leaf tissues was confirmed by squash immunoblotting (28).
Construction of artificial chimeras.
Chimera viruses were constructed by exchanging DNA fragments between pSMV-G7 and pSMV-G7d, taking advantage of single restriction sites common to the two viruses (Fig. 1). The pSMV-G7 and pSMV-G7d plasmids propagated in ElectroMax DH5α-E cells were found to be insensitive to digestion with StuI located at position 5793 of the genome (Fig. 1). To obtain StuI-sensitive templates, Escherichia coli strain GM2163 (New England Biolabs) was transformed with pSMV-G7 or pSMV-G7d, and the plasmids were purified as described above. To exchange DNA fragments representing nucleotides 1 to 2337 of each genome, the KpnI site (Fig. 1) and a NotI site located 699 nucleotides upstream of the viral genome were used. ElectroMax DH5α-E cells were transformed by electroporation with a MicroPulser (Bio-Rad Laboratories). Positive transformants were identified by a PCR-based screening method with SMV-specific primers for the exchanged genomic fragments. The recombinant plasmids were purified as described above, and their identities were further confirmed by sequencing of two regions: one representing the recipient virus and the other representing the exchanged genomic fragment from the donor virus. The chimera viruses were tested for infectivity on PI 96983 (Rsv1) by biolistic delivery (29).
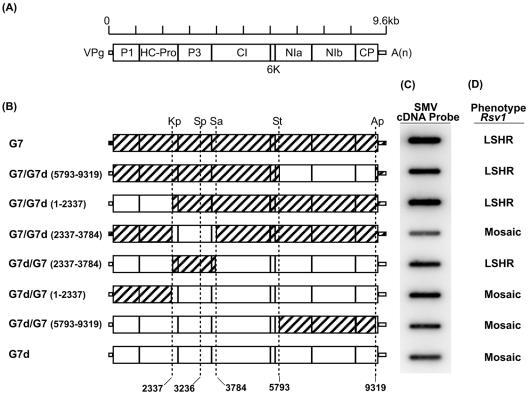
FIG. 1.
Chimeric viruses and their phenotypic responses on soybean line PI 96983 (Rsv1). (A) Proposed genomic map of SMV (35). (B) Schematic representation of chimeric viruses constructed by exchanging fragments between pSMV-G7 (G7) and pSMV-G7d (G7d) utilizing the single restriction sites KpnI (Kp), SpeI (Sp), SalI (Sa), StuI (St), and ApaI (Ap) common between the two viruses. For construction of G7/G7d(1-2337) and G7d/G7(1-2337), the unique restriction site NotI located 669 nucleotides upstream of SMV sequences within vector sequences (data not shown) and the KpnI site were utilized. (C) Detection of progenies of the chimeric viruses in infected PI 96983 (Rsv1) soybean plants by slot blot hybridization. Following inoculation, plants were maintained in a growth chamber (20°C) until a leaflet from trifoliolates 3 and 4 of infected plants was collected 4 weeks postinoculation. Samples from corresponding trifoliolate leaflets of four independent replicate plants were combined; total RNA was isolated and denatured, and 10 μg was slot blotted onto a membrane and hybridized with 32P-labeled cDNA probes. (D) Phenotypic responses of soybean line PI 96983 (Rsv1) to mechanical inoculation with infectious sap containing progenies of parental or chimeric viruses. Following inoculation, the plants were maintained in a growth chamber (20°C) until virus-induced symptoms, mosaic or LSHR, were recorded about 6 weeks postinoculation.
Site-directed mutagenesis of P3.
To introduce reciprocal point mutations into the P3 regions of pSMV-G7 and pSMV-G7d by site-directed mutagenesis, the megaprimer PCR-based mutagenesis method (59) was used. To generate pSMV-G7-derived site-directed mutants, the targeted P3 regions were PCR amplified in the presence of pSMV-G7 with antisense mutagenic primers G7d-2607a, G7d-2889a, G7d-3008a, G7-2608a1, and G7-2608a2 and the forward primer 2276s or 2289s (Table 1). Each of the mutagenized amplified PCR products was gel purified and then used as a forward megaprimer in the presence of pSMV-G7 together with primer 3263a (Table 1), in order to PCR amplify the mutagenized P3 fragment. The mutagenized fragment was subsequently gel purified, digested with KpnI and SpeI, and ligated into similarly digested sites of pSMV-G7. The pSMV-G7d-derived site-directed mutants were generated as described above, except that antisense primers G7-2602a, G7-2878a, G7-2997a, G7-2507a, and G7-2621a were used as the mutagenic primers (Table 1), and pSMV-G7d served as a template. The final PCR products were digested as described above and ligated into similarly digested sites of pSMV-G7d. To generate mutant viruses harboring two point mutations, an already constructed site-directed mutant virus containing a single point mutation served as a template in a PCR along with a desired mutagenic primer as a reverse primer (Table 1) in order to introduce the second point mutation. ElectroMax DH5α-E cells were transformed with the ligation mixtures, and positive transformants were identified by PCR amplification of the exchanged fragment with SMV-specific primers flanking regions of the mutation sites. The plasmids were purified as described above, and to ensure the absence of any unwanted PCR-generated mutations in the amplified fragment, the entire PCR-amplified sequences corresponding to nucleotides 2337 to 3236 of each of the mutant viruses were determined. The site-directed mutant viruses were subsequently tested for infectivity on PI 96983 (Rsv1) by biolistic delivery (29).
TABLE 1.
Sequences of sense and antisense oligonucleotide primers used for synthesis of the site-directed mutants
| Name | Sequencea (5′ to 3′) | Positionb |
|---|---|---|
| G7d-2607a | TCCACAACTCCATCCCTTTCTCAAA | 2610-2586 |
| G7d-2889a | TTTTTTCATATGCCACGTACAATTTGTC | 2889-2862 |
| G7d-3008a | GCTTTCTTTGTCAAACATTCTTCCGTATGTGGAGT | 3007-2973 |
| G7-2608a1 | TCCACAACTCTACCCCTTTCTCAAA | 2610-2586 |
| G7-2608a2 | TCCACAACTCGACCCCTTTCTCAAA | 2610-2586 |
| G7-2602a | AACTCCACCCCTTTC | 2605-2591 |
| G7-2878a | TATGCCATGTACAATTTGT | 2881-2863 |
| G7-2997a | TTGTCAAACATTTTTCCGTATG | 3000-2979 |
| G7-2507a | AAGGATCTGAACCATTATC | 2510-2492 |
| G7-2621a | ATGTTCCTTACTTATCCACAAC | 2624-2603 |
| 2276s | GGTTGACCATGCGTATCA | 2279-2296 |
| 2289s | ATTTTGGTTGACCATGCGT | 2274-2292 |
| 3263a | TGTATTGATAATACCTTGC | 3266-3248 |
RNA isolation, RT-PCR, and sequencing.
Total soybean RNA was isolated (55) from systemically infected liquid nitrogen-frozen soybean PI 96983 (Rsv1) leaf tissues kept at −85°C. The isolated RNAs, however, were not efficiently transcribed by using SuperScript reverse transcriptase (RT) (Invitrogen); thus, they were further purified using an RNeasy Plant minikit (Qiagen). The genomic regions of progeny viruses derived from each of the chimeras or site-directed mutants were reverse transcribed in the presence of antisense SMV-specific primers. To confirm the identity of the progeny viruses as chimeras, two different regions, one representing the recipient strain and the other representing the genomic fragment from the donor strain, were RT-PCR amplified using SMV-specific primers. RT-PCR amplification of progeny viruses derived from site-directed mutants targeted the mutated site flanked by surrounding sequences. Occasionally, another pair of nested PCR primers was used to reamplify the PCR products. The PCR products were eventually purified by using a QIAquick PCR purification kit (Qiagen) and directly sequenced using SMV-specific primers. Sequencing was done at the Iowa State University DNA Sequencing and Synthesis Facility. The sequences were edited by Factura (Applied Biosystems) and analyzed by using Autoassembler software (Applied Biosystems).
Probes and slot blot hybridization.
A cloned fragment of a soybean PR-1 protein gene (GenBank accession no. AI 930866) was PCR amplified with vector-specific T7 and T3 primers, gel purified, and reamplified in the presence of [32P]dCTP to generate a cDNA-labeled probe (28). A probe against soybean 18S rRNA (21) was synthesized with a random-primed DNA labeling kit (Life Technologies) according to the manufacturer's instructions. The SMV probe was PCR synthesized in the presence of [32P]dCTP with SMV-specific primers and supercoiled plasmid pSMV-G7d as described previously (29). The PCR-amplified SMV sequences used as probes were identical between pSMV-G7 and pSMV-G7d (29). Unincorporated nucleotides were removed using Probe Quant G-50 Microcolumns (Pharmacia, Piscataway, N.J.). RNA denaturation, slot blot hybridization, and detection of hybridization signals were performed as described previously (29). Images were reformatted for publication with Adobe PhotoShop (Adobe Systems, San Jose, Calif.).
RESULTS
Ability of the chimeric viruses to provoke Rsv1-mediated LSHR.
SMV is a single-stranded positive-sense RNA virus belonging to the virus family Potyviridae. Its genome is 9,588 nucleotides long and encodes a single polypeptide, which is proteolytically cleaved by three virus-encoded proteases to produce eight to nine mature proteins (35). The construction of pSMV-G7- and pSMV-G7d-derived chimeras resulted in the exchange of 9,318 of 9,588 nucleotides in each genome (Fig. 1B). The remaining genomic region (nucleotides 9319 to 9588) is identical between the two viruses (29). Symptoms induced in Willams 82 (rsv1) by all chimeric viruses were indistinguishable from those induced by each other as well as from those induced by the parental viruses (29) and were characterized by very mild mosaic, mild leaf distortion, and no visible necrosis (data not shown). All the chimeric viruses, similarly to their parents, infected PI 96983 (Rsv1) soybean plants (Fig. 1C and D), and the identities of all the chimeras in infected PI 96983 (Rsv1) leaf tissues were confirmed by sequencing of two regions of RT-PCR-derived product, one representing the recipient strain and the other representing the genomic fragment from the donor strain, from the progeny viruses.
The chimeras displayed different phenotypes on PI 96983 (Rsv1) (Fig. 2). The symptoms induced by pSMV-G7/pSMV-G7d(5793-9319) appeared with some delay compared to those induced by pSMV-G7; however, 6 to 8 weeks postinoculation the chimera eventually provoked LSHR (Fig. 2). This was associated with a C-to-U point mutation in P3 at nucleotide 2764 and changed the encoded amino acid from Ser to Leu. The severity of LSHR induced by pSMV-G7/pSMV-G7d(1-2337) was similar to that of pSMV-G7 (Fig. 2), and its symptoms developed faster than those of LSHR induced by pSMV-G7. Among pSMV-G7-derived chimeric viruses, only pSMV-G7/pSMV-G7d(2337-3784) failed to elicit LSHR. It induced a phenotype similar to that of pSMV-G7d, consisting of mosaic with no visible necrosis (Fig. 2). Conversely, when nucleotides 2337 to 3784 of pSMV-G7d were replaced with the corresponding region of pSMV-G7, pSMV-G7d/pSMV-G7(2337-3784) induced Rsv1-mediated LSHR instead of mosaic (Fig. 2). The replacement of nucleotides 5793 to 9319 and 1 to 2337 of pSMV-G7d with those of pSMV-G7 did not enhance the ability of pSMV-G7d to elicit LSHR (Fig. 2).
FIG. 2.
Phenotypic differences in responses of soybean line PI 96983 (Rsv1) to inoculation with infectious sap containing progenies of pSMV-G7 (G7), pSMV-G7d (G7d), or their derivative chimeras. Following inoculation, the plants were maintained in a growth chamber (20°C) until photographed about 6 weeks postinoculation.
Strain-specific P3 of SMV-G7 is the elicitor of Rsv1-mediated LSHR.
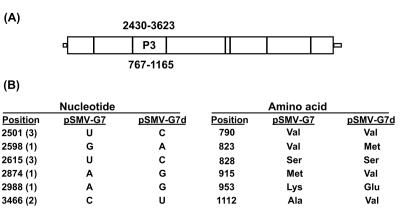
Analyses of the chimeric viruses on PI 96983 (Rsv1) showed that the loss and gain of elicitor function of SMV provoking Rsv1-mediated LSHR map to the KpnI/SalI fragments representing nucleotides 2337 to 3784 of the two genomes (Fig. 1). This region of the SMV genome consists of the 3′ end of the HC-Pro cistron (nucleotides 2337 to 2430), the full-length P3 cistron (2430 to 3623) that encodes amino acids 767 to 1165 of the polypeptide precursor (Fig. 3), and the 5′ end of the cytoplasmic inclusion cistron (nucleotides 3623 to 3784) (35). The nucleotide sequences of both the 3′ terminus of HC-Pro and the 5′ terminus of the cytoplasmic inclusion cistron, corresponding to nucleotides 2337 to 2430 and 3623 to 3784, respectively, are identical between pSMV-G7 and pSMV-G7d (29). Thus, the elicitor functions of pSMV-G7 provoking Rsv1-mediated LSHR map to P3. The P3 regions of pSMV-G7 and pSMV-G7d differ by six nucleotides, of which two are translationally silent (29). The four amino acid differences between P3 of pSMV-G7 and that of pSMV-G7d and their locations on the SMV polyprotein precursor are shown in Fig. 3.
FIG. 3.
Genetic differences between P3 of pSMV-G7 and that of pSMV-G7d. (A) Proposed genomic map of SMV (35) indicating the size and the position of P3. (B) Nucleotide and amino acid differences between P3 of pSMV-G7 and that of pSMV-G7d. The positions of nucleotides and amino acids on the SMV genomes are based on sequences of SMV strains G7 and G7d (GenBank accession no. AY216010 and AY216987, respectively). Numbers in parentheses show positions of nucleotides within the codon.
Translationally silent mutations do not alter Rsv1-mediated elicitor function of P3.
The nucleotides at positions 2501 and 2615, which are both translationally silent, differ between pSMV-G7 and pSMV-G7d (Fig. 3). To examine if either of these two silent mutations is responsible for the loss of elicitor function of P3 of pSMV-G7d, the nucleotides corresponding to those of P3 of pSMV-G7 at these positions were incorporated into the P3 of pSMV-G7d by using primers G7-2507a and G7-2621a (Table 1). When the two site-directed mutants were inoculated onto PI 96983 (Rsv1), both pSMV-G7dC2501U and pSMV-G7dC2615U, similarly to pSMV-G7d, failed to elicit Rsv1-mediated LSHR (data not shown). To examine the impact of silent point mutations on the elicitor function of P3 of pSMV-G7, we also introduced two silent mutations into its corresponding P3. The G at position 2600 of the genome of pSMV-G7 was targeted by using primers G7-2608a1 and G7-2608a2 (Table 1). This is the third position in the codon (2998GUG2600), which codes for a valine, which is located at position 823 of the precursor polyprotein of pSMV-G7 (29). The corresponding codon in the context of pSMV-G7d codes for a Met (Fig. 3). However, both pSMV-G7G2600A and pSMV-G7G2600C retained their elicitor function when they were inoculated onto PI 96983 (Rsv1) and, like pSMV-G7, provoked Rsv1-mediated LSHR (data not shown). These observations suggest that translationally silent mutations in P3 of both pSMV-G7 and pSMV-G7d do not influence the loss or gain of Rsv1-mediated elicitor function of their respective P3 regions.
Amino acid substitutions affect the elicitor function of P3 provoking Rsv1-mediated LSHR.
To assess which of the four amino acid differences between P3 of pSMV-G7 and P3 of pSMV-G7d (Fig. 3) plays a role in elicitation of Rsv1-mediated LSHR by pSMV-G7, each of the amino acids at positions 823, 915, and 953 was reciprocally replaced between the two viruses by site-directed mutagenesis. The replacement of the amino acid at position 1112 of precursor polyprotein was done by reciprocal exchanges of SpeI/SalI fragments (nucleotides 3236 to 3784) (Fig. 1) between the two viruses. This region of P3 differs between the two viruses by only one nucleotide at position 3466, which is responsible for the amino acid difference (Fig. 3). To assess the additive effect of the amino acid changes at positions 823, 915, and 953 on the elicitor function of P3, two additional chimeras were constructed by reciprocal exchanges of the KpnI/SpeI fragment, which represents nucleotides 2337 to 3236 (Fig. 1). The elicitor functions of all site-directed mutants were evaluated on PI 96983 (Rsv1) for both induction of Rsv1-mediated LSHR and up regulation of PR-1 protein gene transcript (Fig. 4 and 5). Results showed that the reciprocal amino acid substitutions at position 915 did not influence the loss or gain of the elicitor function of P3, as the phenotype induced on PI 96983 (Rsv1) by both pSMV-G7M915V and pSMV-G7dV915 M was similar to that for the parental viruses (Fig. 4 and 5). Nevertheless, pSMV-G7dV915 M accumulated poorly in PI 96983 (Rsv1) leaf tissues compared to parental or other point mutant viruses (Fig. 5). The pSMV-G7-derived point mutants with V823M, K953E, or A1112V lost the ability to elicit LSHR, but instead induced SHR, which was more pronounced on the second trifoliates (Fig. 4). pSMV-G7K953E accumulated poorly compared with parental or other pSMV-G7-derived point mutants (Fig. 5). However, two concomitant amino acid substitutions at positions 823 and 953 abolished the elicitor function of P3 of pSMV-G7 provoking Rsv1-mediated SHR as well as its ability to up regulate PR-1 protein gene transcript (Fig. 4 and 5). The chimera pSMV-G7/pSMV-G7d(2337-3263), which contains all three amino acid substitutions at positions 823, 915, and 953, also exhibited a similar phenotype on PI 96983 (Rsv1) (data not shown), which further confirms the additive effect of amino acids at positions 823 and 953 of knocking out the elicitor function of P3. Conversely, P3 of pSMV-G7d gained the elicitor function of Rsv1-mediated LSHR by a single amino acid substitution at position 823 (M to V) (Fig. 4 and 5). However, the other two pSMV-G7d-derived mutants, E953K and V1112A, induced SHR instead of mosaic (Fig. 4). Surprisingly, the incorporation of two concomitant amino acid substitutions at positions 823 and 953 of P3 of pSMV-G7d delayed the full expression of LSHR about 2 weeks when inoculated on PI 96983 (Rsv1) (Fig. 4). The chimera pSMV-G7d/pSMV-G7(2337-3236) also displayed a phenotype similar to that of pSMV-G7 when it was inoculated on PI 96983 (Rsv1) (data not shown).
FIG. 4.
Phenotypic differences in responses of soybean line PI 96983 (Rsv1) to inoculation with sap containing progenies of pSMV-G7 (G7), pSMV-G7d (G7d), or their derivative point mutants generated by site-directed mutagenesis of their respective P3. Following inoculation, the plants were maintained in a growth chamber (20°C) until photographed about 6 weeks postinoculation.
FIG. 5.
Slot blot hybridization analysis of accumulation of SMV RNA, soybean PR-1 protein gene transcript up regulation (PR1), and soybean 18S rRNA in soybean trifoliate leaves. Primary leaves of PI 96983 (Rsv1) were mechanically inoculated with buffer (mock) or infectious sap containing progenies of pSMV-G7 (G7), pSMV-G7d (G7d), or their derivative point mutants generated by site-directed mutagenesis. Following inoculation, plants were maintained in a growth chamber (20°C) until a leaflet from trifoliolates 3 and 4 of infected plants was collected about 6 weeks postinoculation. Samples from corresponding trifoliolate leaflets of four independent replicate plants were combined, total RNA was isolated and denatured, and 10 μg was slot blotted onto a membrane and hybridized with 32P-labeled cDNA probes.
DISCUSSION
In this paper we exploited the differential interactions of pSMV-G7 and pSMV-G7d with Rsv1-genotype soybean and demonstrated that the loss or gain of Rsv1-mediated elicitor function provoking LSHR resides on P3. P3 is one of the least conserved regions among SMV strains (35) as well as among other potyviruses (67). However, it is an essential protein for potyvirus replication (43), and in a number of pathosystems involving potyviruses, it has been demonstrated that it is involved in pathogenicity as well (67). It remains to be seen whether P3 of SMV is also involved in pathogenicity of the virus in soybean genotypes lacking Rsv1. In the case of the Turnip mosaic virus-Brassica pathosystem, strain-specific P3 of the virus has been identified as the virus elicitor for both TuRB03- and TuRB04-mediated resistance responses, and it has been proposed that it also plays a role in pathogenicity (36, 37).
The impact of six nucleotide differences in the P3 regions of pSMV-G7 and pSMV-G7d on the elicitor function of each virus was examined by site-directed mutagenesis. Results showed that changes in the nucleotide sequences leading to silent mutations do not influence the loss or gain of the elicitor function of Rsv1-mediated LSHR. Silent mutations in the CP of strain-specific PVX also did not affect the sensitivity of the PVX to Rx-mediated resistance (26). Thus, our results are consistent with the generally accepted view that a gene product, and not the gene nucleotide structure, is the elicitor of R-mediated defense responses (13, 25, 44). This was confirmed by analyses of the site-directed mutants of both viruses containing mutations leading to amino acid changes (Fig. 4).
Amino acid substitutions at position 915 of the protein precursor of both pSMV-G7 and pSMV-G7d, however, did not influence the elicitor function of Rsv1-mediated LSHR (Fig. 4 and 5). This was expected, because the amino acid at this position was identical between pSMV-G7 and the viral population from which pSMV-G7d was cloned, vSMV-G7d, and no phenotypic differences were observed between pSMV-G7d and vSMV-G7d when they were inoculated on PI 96983 (Rsv1) (29). Hence, it is likely that this amino acid is not a part of the elicitor site.
The Ala/Val difference at position 1112 represents a conservative substitution, since both amino acids have small hydrophobic side chains, and hence, the impact on tertiary structure of P3 is predicted to be minimal. Indeed, in the context of pSMV-G7d, the amino acid substitution resulted in induction of a mild SHR (Fig. 4 and 5). However, in the context of pSMV-G7, the substitution induced only Rsv1-mediated SHR rather than LSHR (Fig. 4). On the other hand, the amino acids at position 953 (Lys/Glu) are both hydrophilic but oppositely charged, and if surface residues they may participate in protein-protein interactions. Thus, reciprocal substitutions at this position might be expected to disrupt the tertiary structure or affect the net charge of P3 more than would any other amino acid substitutions between the two viruses. The substitutions at this location did indeed affect the elicitor functions of both viruses (Fig. 4) but not to the extent of the exchange at position 823 (Val/Met and vice versa) (Fig. 4). It is interesting that Val/Met exchanges at position 823 involve the same pair of amino acids as those at position 915 (Met to Val and vice versa), which resulted in no noticeable effect on the elicitor function (Fig. 4 and 5). Thus, it is likely that, within the tertiary structure of P3, the amino acid at position 823 participates in a site different from that of 915.
Concomitant substitutions at positions 823 and 953 showed that they have an additive effect on the loss of elicitor function of pSMV-G7 (Fig. 4), which suggests that the two amino acids contribute independently to evasion of Rsv1-mediated recognition. A similar observation has been reported for N′-mediated recognition of strain-specific CP of TMV (62). However, it is equally likely that they may contribute as a pair, where the sum of the two is more efficient than the individual residues.
The participation of multiple amino acids in Rsv1-mediated elicitor activity of P3 is not unique to Rsv1-SMV interactions. Tobacco mosaic virus (TMV) strain U1 gained elicitor function provoking N′-mediated HR following four different amino acid substitutions in its CP, and each mutant produced phenotypically distinct HR (14). Furthermore, it has been shown elsewhere that at least four domains of the TMV replicase protein can induce full HR in N-carrying tobacco (2).
All the substitutions in P3 of SMV-G7 leading to knockout of Rsv1-mediated LSHR are noncontiguous. Currently, there is no structural information available on P3 of SMV. Hence, their positions within the three-dimensional structure of P3 are unknown. It is possible that, similarly to CP of TMV (63), all these amino acids are positioned on the same structural site, which is involved in elicitation of Rsv1-mediated LSHR.
The analyses of chimeras and the reciprocal point mutants reported here clearly show that the SMV elicitor functions of Rsv1-mediated LSHR reside on P3. However, it is not clear if P3 alone is the elicitor. Our data do not exclude the possibility that additional viral factors may be involved, as the mature P3 is produced following proteolytic processing of a polypeptide precursor (57). Furthermore, it is not known at this stage whether the elicitor function of P3 is dependent on virus replication. However, for a number of other viruses, direct evidence has been presented that the transient expression of a single viral Avr gene in plant tissues harboring their matching R genes is sufficient for HR elicitation (1, 12, 22, 54). In the case of potyviruses, transient expression of NIa of Potato virus Y and NIb replicase of potato virus Y MSNR in RY and Rk genotype plants, respectively, leads to elicitation of HR (23, 51). Further research is needed to demonstrate analogy to strain-specific P3 of SMV.
The underlying mechanism by which pSMV-G7d, as well as the pSMV-G7 mutant containing two concomitant amino acid changes, evades Rsv1-mediated recognition remains to be elucidated. Such a mechanism may be passive or active. It has been proposed that TMV has evolved a unique aggregation-assembly pattern that masks its elicitor active site from N′-mediated recognition machinery (63). Thus, it utilizes a passive mechanism for evasion of N′-mediated recognition. P3 is a nonstructural protein of potyviruses; however, it has been shown that it interacts with other virus-encoded proteins in a yeast two-hybrid system (27, 49) or in infected cells (58) and possibly interacts with certain host factors during virus replication or movement as well. It is possible that pSMV-G7d evades Rsv1-mediated recognition by masking its elicitor site. However, other mechanisms such as changes in P3 localization site, enhancement or reduction in physical stability, and suppression of Rsv1-mediated defense responses cannot be excluded.
Acknowledgments
We are grateful to W. A. Miller and S. A. Whitham (Iowa State University, Ames) and E. J. Fernandez (The University of Tennessee, Knoxville) for useful comments on the manuscript.
This project was supported, in part, by the Iowa Soybean Promotion Board. This journal paper of the Iowa Agriculture and Home Economics Experiment Station, Ames, project no. 2428, was supported by the Hatch Act and State of Iowa funds.
REFERENCES
- 1.Abbink, T. E. M., P. A. Tjernberg, J. F. Bol, and H. J. M. Linthorst. 1998. Tobacco mosaic virus helicase domain induces necrosis in N gene-carrying tobacco in the absence of virus replication. Mol. Plant-Microbe Interact. 11:1242-1246. [Google Scholar]
- 2.Abbink, T. E. M., J. de Vogel, J. F. Bol, and H. J. M. Linthorst. 2001. Induction of a hypersensitive response by chimeric helicase sequences of tobamoviruses U1 and Ob in N-carrying tobacco. Mol. Plant-Microbe Interact. 14:1086-1095. [DOI] [PubMed] [Google Scholar]
- 3.Abramovitch, R. B., Y.-J. Kim, S. Chen, M. B. Dickman, and G. B. Martin. 2003. Pseudomonas type III effector AvrPtoB induces plant disease susceptibility by inhibition of host programmed cell death. EMBO J. 22:60-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendahmane, A., B. A. Kohm, C. Dedi, and D. C. Baulcombe. 1995. The coat protein of potato virus X is a strain-specific elicitor of Rx1-mediated virus resistance in potato. Plant J. 8:933-941. [DOI] [PubMed] [Google Scholar]
- 5.Bendahmane, A., K. Kanyuka, and D. C. Baulcombe. 1999. The Rx gene from potato controls separate virus resistance and cell death responses. Plant Cell 11:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson, A. P., and W. J. Hooker. 1960. Isolation of virus X from “immune” varieties of potato Solanum tuberosum. Phytopathology 50:231-234. [Google Scholar]
- 7.Berzal-Herranz, A., A. De La Cruz, F. Tenllado, J. R. Diaz-Ruiz, L. Lopez, A. I. Sanz, C. Vaquero, M. T. Serra, and I. Garcia-Luque. 1995. The Capsicum L3 gene-mediated resistance against the tobamoviruses is elicited by the coat protein. Virology 209:498-505. [DOI] [PubMed] [Google Scholar]
- 8.Bonas, U., and T. Lahaye. 2002. Plant disease resistance triggered by pathogen-derived molecules: refined models of specific recognition. Curr. Opin. Microbiol. 5:44-50. [DOI] [PubMed] [Google Scholar]
- 9.Cai, X., F. L. W. Takken, M. H. A. J. Joosten, and P. J. G. M. De Wit. 2001. Specific recognition of AVR4 and AVR9 results in distinct patterns of hypersensitive cell death in tomato, but similar patterns of defence-related gene expression. Mol. Plant Pathol. 2:77-86. [DOI] [PubMed] [Google Scholar]
- 10.Chen, P., G. R. Buss, C. W. Roane, and S. A. Tolin. 1994. Inheritance in soybean of resistant and necrotic reactions to soybean mosaic virus strains. Crop Sci. 34:414-422. [Google Scholar]
- 11.Cho, E.-K., and R. M. Goodman. 1979. Strains of soybean mosaic virus: classification based on virulence in resistant soybean cultivars. Phytopathology 69:467-470. [Google Scholar]
- 12.Culver, J. N. 1997. Viral avirulence genes, p. 196-219. In G. Stacey and N. T. Keen (ed.), Plant-microbe interactions, vol. 2. Chapman & Hall, New York, N.Y.
- 13.Culver, J. N., and W. O. Dawson. 1989. Tobacco mosaic virus coat protein: an elicitor of the hypersensitive reaction but not required for the development of mosaic symptoms in Nicotiana sylvestris. Virology 173:755-758. [DOI] [PubMed] [Google Scholar]
- 14.Culver, J. N., and W. O. Dawson. 1989. Point mutations in the coat protein gene of tobacco mosaic virus induce hypersensitivity in Nicotiana sylvestris. Mol. Plant-Microbe Interact. 2:209-213. [Google Scholar]
- 15.Culver, J. N., A. G. C. Lindbeck, and W. O. Dawson. 1991. Virus-host interactions: induction of chlorotic and necrotic responses in plants by tobamoviruses. Annu. Rev. Phytopathol. 29:193-217. [Google Scholar]
- 16.Culver, J. N., G. Stubbs, and W. O. Dawson. 1994. Structure-function relationship between tobacco mosaic virus coat protein and hypersensitivity in Nicotiana sylvestris. J. Mol. Biol. 242:130-138. [DOI] [PubMed] [Google Scholar]
- 17.Dangl, J. 1998. Plants just say NO to pathogens. Nature 394:525-527. [DOI] [PubMed] [Google Scholar]
- 18.Dangl, J. L., and J. D. G. Jones. 2001. Plant pathogens and integrated defense responses to infection. Nature 411:826-833. [DOI] [PubMed] [Google Scholar]
- 19.Delaney, T. P., S. Uknes, B. Vernooij, L. Friedrich, K. Weymann, D. Negrotto, T. Gaffney, M. Gut-Rella, H. Kessmann, E. Ward, and J. Ryals. 1994. A central role of salicylic acid in plant disease resistance. Science 266:1247-1250. [DOI] [PubMed] [Google Scholar]
- 20.Dinesh-Kumar, S. P., W.-H. Tham, and B. J. Baker. 2000. Structure-function analysis of the tobacco mosaic virus resistance gene N. Proc. Natl. Acad. Sci. USA 97:14789-14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckenrode, V. K., J. Arnold, and R. B. Meagher. 1984. Comparison of the nucleotide sequence of soybean 18S rRNA with the sequences of other small-subunit rRNAs. J. Mol. Evol. 21:259-269. [DOI] [PubMed] [Google Scholar]
- 22.Erickson, F. L., S. Holzberg, A. Calderon-Urrea, V. Handley, M. Axtell, C. Corr, and B. Baker. 1999. The helicase domain of the TMV replicase proteins induces the N-mediated defence response in tobacco. Plant J. 18:67-75. [DOI] [PubMed] [Google Scholar]
- 23.Fellers, J. P., D. Tremblay, M. F. Handest, and S. A. Lommel. 2002. The Potato virus Y MSNR NIb-replicase is the elicitor of a veinal necrosis-hypersensitive response in root knot nematode resistant tobacco. Mol. Plant Pathol. 3:145-152. [DOI] [PubMed] [Google Scholar]
- 24.Feys, B. J., L. J. Moisan, M.-A. Newman, and J. E. Parker. 2001. Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. EMBO J. 20:5400-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flor, H. H. 1971. Current status of the gene-for-gene concept. Annu. Rev. Phytopathol. 9:275-296. [Google Scholar]
- 26.Goulden, M. G., B. A. Kohm, S. Santa Cruz, T. A. Kavanagh, and D. C. Baulcombe. 1993. A feature of the coat protein of potato virus X affects both induced virus resistance in potato and viral fitness. Virology 197:293-302. [DOI] [PubMed] [Google Scholar]
- 27.Guo, D., M.-L. Rajamaki, M. Saarma, and J. P. T. Valkonen. 2001. Towards a protein interaction map of potyviruses: protein interaction matrixes of two potyviruses based on the yeast two-hybrid system. J. Gen. Virol. 82:935-939. [DOI] [PubMed] [Google Scholar]
- 28.Hajimorad, M. R., and J. H. Hill. 2001. Rsv1-mediated resistance against Soybean mosaic virus-N is hypersensitive response-independent at inoculation site, but has the potential to initiate a hypersensitive response-like mechanism. Mol. Plant-Microbe Interact. 14:587-598. [DOI] [PubMed] [Google Scholar]
- 29.Hajimorad, M. R., A. L. Eggenberger, and J. H. Hill. 2003. Evolution of Soybean mosaic virus-G7 molecularly cloned genome in Rsv1-genotype soybean results in emergence of a mutant capable of evading Rsv1-mediated recognition. Virology 314:497-509. [DOI] [PubMed] [Google Scholar]
- 30.Hall, T. J. 1980. Resistance at the Tm-2 locus in the tomato to tomato mosaic virus. Euphytica 29:189-197. [Google Scholar]
- 31.Hammond-Kosack, K. E., B. J. Staskawicz, J. D. G. Jones, and D. C. Baulcombe. 1995. Functional expression of a fungal avirulence gene from a modified potato virus X genome. Mol. Plant-Microbe Interact. 8:181-185. [Google Scholar]
- 32.Hammond-Kosack, K. E., and J. D. G. Jones. 1996. Resistance gene-dependent plant defense responses. Plant Cell 8:1773-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hull, R. 2002. Matthew's plant virology. Academic Press, New York, N.Y.
- 34.Jackson, R. W., E. Athanassopoulos, G. Tsiamis, J. W. Mansfield, A. Sesma, D. L. Arnold, M. J. Gibbon, J. Murillo, J. D. Taylor, and A. Vivian. 1999. Identification of a pathogenicity island, which contains genes for virulence and avirulence, on a large native plasmid in the bean pathogen Pseudomonas syringae pathovar phaseolicola. Proc. Natl. Acad. Sci. USA 96:10875-10880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jayaram, C. H., J. H. Hill, and W. A. Miller. 1992. Complete nucleotide sequences of two Soybean mosaic virus strains differentiated by response of soybean containing the Rsv resistance gene. J. Gen. Virol. 73:2067-2077. [DOI] [PubMed] [Google Scholar]
- 36.Jenner, C. E., K. Tomimura, K. Ohshima, S. L. Hughes, and J. A. Walsh. 2002. Mutations in Turnip mosaic virus P3 and cylindrical inclusion proteins are separately required to overcome two Brassica napus resistance genes. Virology 300:50-59. [DOI] [PubMed] [Google Scholar]
- 37.Jenner, C. E., X. Wang, K. Tomimura, K. Ohshima, F. Ponz, and J. A. Walsh. 2003. The dual role of the potyvirus P3 protein of Turnip mosaic virus as a symptom and avirulence determinant in brassicas. Mol. Plant-Microbe Interact. 16:777-784. [DOI] [PubMed] [Google Scholar]
- 38.Kamoun, S., E. Huitema, and V. G. A. A. Vleeshouwers. 1999. Resistance to oomycetes: a general role for the hypersensitive response? Trends Plant Sci. 4:196-200. [DOI] [PubMed] [Google Scholar]
- 39.Kamoun, S., G. Honee, R. Weide, R. Lauge, M. Kooman-Gersmann, K. de Groot, F. Govers, and P. J. G. M. De Wit. 1999. The fungal gene Avr9 and the oomycete gene inf1 confer avirulence to potato virus X on tobacco. Mol. Plant-Microbe Interact. 12:459-462. [Google Scholar]
- 40.Karasawa, A., I. Okada, K. Akashi, Y. Chida, S. Hase, Y. Nakazawa-Nasu, A. Ito, and Y. Ehara. 1999. One amino acid change in cucumber mosaic virus RNA polymerase determines virulent/avirulent phenotypes on cowpea. Phytopathology 89:1186-1192. [DOI] [PubMed] [Google Scholar]
- 41.Kim, C.-H., and P. Palukaitis. 1997. The plant defense response to cucumber mosaic virus in cowpea is elicited by the viral polymerase gene and affects virus accumulation in single cells. EMBO J. 16:4060-4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiraly, Z., B. Barna, A. Kecskes, and J. Fodor. 2002. Down-regulation of antioxidative capacity in a transgenic tobacco which fails to develop acquired resistance to necrotization caused by TMV. Free Radical Res. 36:981-991. [DOI] [PubMed] [Google Scholar]
- 43.Klein, P. G., R. R. Klein, E. Rodriguez-Cerezo, A. G. Hunt, and J. G. Shaw. 1994. Mutational analysis of the tobacco vein mottling virus genome. Virology 204:759-769. [DOI] [PubMed] [Google Scholar]
- 44.Kohm, B. A., M. G. Goulden, J. E. Gilbert, T. A. Kavanagh, and D. C. Baulcombe. 1993. A potato virus X resistance gene mediates an induced, nonspecific resistance in protoplasts. Plant Cell 5:913-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanfermeijer, F. C., J. Dijkhuis, M. J. G. Sturre, P. de Hann, and J. Hille. 2003. Cloning and characterization of the durable tomato mosaic virus resistance gene Tm-22 from Lycopersicon esculentum. Plant Mol. Biol. 52:1037-1049. [DOI] [PubMed] [Google Scholar]
- 46.Lauge, R., M. H. A. J. Joosten, J. P. W. Haanstra, P. H. Goodwin, P. Lindhout, and P. J. G. De Wit. 1998. Successful search for a resistance gene in tomato targeted against a virulence factor of a fungal pathogen. Proc. Natl. Acad. Sci. USA 95:9014-9018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leach, J. E., C. M. Vera Cruz, J. Bai, and H. Leung. 2001. Pathogen fitness penalty as a predictor of durability of disease resistance genes. Annu. Rev. Phytopathol. 39:187-224. [DOI] [PubMed] [Google Scholar]
- 48.Lim, S. M. 1985. Resistance to soybean mosaic virus in soybeans. Phytopathology 75:199-201. [Google Scholar]
- 49.Merits, A., D. Guo, L. Jarvekulg, and M. Saarma. 1999. Biochemical and genetic evidence for interactions between potato A potyvirus-encoded proteins P1 and P3 and proteins of the putative replication complex. Virology 263:15-22. [DOI] [PubMed] [Google Scholar]
- 50.Meshi, T., F. Motoyoshi, A. Adachi, Y. Watanabe, N. Takamatsu, and Y. Okada. 1988. Two concomitant base substitutions in the putative replicase genes of tobacco mosaic virus confer the ability to overcome the effects of a tomato resistance gene, Tm-1. EMBO J. 7:1575-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mestre, P., G. Brigneti, and D. C. Baulcombe. 2000. An Ry-mediated resistance response in potato requires the intact active site of the NIa proteinase from potato virus Y. Plant J. 23:653-661. [DOI] [PubMed] [Google Scholar]
- 52.Moury, B., K. G. Selassie, G. Marchoux, A.-M. Daubeze, and A. Palloix. 1998. High temperature effects on hypersensitive resistance to tomato spotted wilt Tospovirus (TSWV) in pepper (Capsicum chinense Jacq.). Eur. J. Plant Pathol. 104:489-498. [Google Scholar]
- 53.Padgett, H. S., and R. N. Beachy. 1993. Analysis of a tobacco mosaic virus strain capable of overcoming N gene-mediated resistance. Plant Cell 5:577-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palanichelvam, K., A. B. Cole, M. Shababi, and J. E. Schoelz. 2000. Agroinfiltration of Cauliflower mosaic virus gene VI elicits hypersensitive response in Nicotiana species. Mol. Plant-Microbe Interact. 13:1275-1279. [DOI] [PubMed] [Google Scholar]
- 55.Pawlowski, K., R. Kunze, S. De Vries, and T. Bisseling. 1994. Isolation of total poly(A) and polysomal RNA from plant tissues, p. D5/1-D5/4. In S. B. Gelvin and R. A. Schilperoort (ed.), Plant molecular biology manual. Kluwer Academic, Dordrecht, The Netherlands.
- 56.Pilowsky, M., R. Frankel, and S. Cohen. 1981. Studies of the variable reaction at high temperature of F1 hybrid tomato plants resistant to tobacco mosaic virus. Phytopathology 71:319-323. [Google Scholar]
- 57.Riechmann, J. L., S. Lain, and J. A. Garcia. 1992. Highlights and prospects of potyvirus molecular biology. J. Gen. Virol. 73:1-16. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez-Cerezo, E., E. D. Ammar, T. P. Pirone, and J. G. Shaw. 1993. Association of the non-structural P3 viral protein with cylindrical inclusions in potyvirus-infected cells. J. Gen. Virol. 74:1945-1949. [DOI] [PubMed] [Google Scholar]
- 59.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 60.Stahl, E. A., and J. B. Bishop. 2000. Plant-pathogen arms races at the molecular level. Curr. Opin. Plant Biol. 3:299-304. [DOI] [PubMed] [Google Scholar]
- 61.Takken, F. L. W., and M. H. A. J. Joosten. 2000. Plant resistance genes: their structure, function and evolution. Eur. J. Plant Pathol. 106:699-713. [Google Scholar]
- 62.Taraporewala, Z. F., and J. N. Culver. 1996. Identification of an elicitor active site within the three-dimensional structure of the tobacco mosaic tobamovirus coat protein. Plant Cell 8:169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taraporewala, Z. F., and J. N. Culver. 1997. Structural and functional conservation of the tobamovirus coat protein elicitor active site. Mol. Plant-Microbe Interact. 10:597-604. [Google Scholar]
- 64.Tobias, C. M., G. E. D. Oldroyd, J. H. Chang, and B. J. Staskawicz. 1999. Plants expressing the Pto disease resistance gene confer resistance to recombinant PVX containing the avirulence gene AvrPto. Plant J. 17:41-50. [DOI] [PubMed] [Google Scholar]
- 65.Tsiamis, G., J. W. Mansfield, R. Hockenhull, R. W. Jackson, A. Sesma, E. Athanassopoulos, M. A. Bennett, C. Stevens, A. Vivian, J. D. Taylor, and J. Murillo. 2000. Cultivar-specific avirulence and virulence functions assigned to avrPphF in Pseudomonas syringae pv. phaseolicola, the cause of bean halo-blight disease. EMBO J. 19:3204-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsuda, S., M. Kirita, and Y. Watanabe. 1998. Characterization of a Papper mild mottle tobamovirus strain capable of overcoming the L3 gene-mediated resistance, distinct from the resistance-breaking Italian isolate. Mol. Plant-Microbe Interact. 11:327-331. [DOI] [PubMed] [Google Scholar]
- 67.Urcuqui-Inchima, S., A.-L. Haenni, and F. Bernardi. 2001. Potyvirus proteins: a wealth of functions. Virus Res. 74:157-175. [DOI] [PubMed] [Google Scholar]
- 68.Vance, V. B., and R. N. Beachy. 1984. Translation of soybean mosaic virus RNA in vitro: evidence of protein processing. Virology 132:271-281. [DOI] [PubMed] [Google Scholar]
- 69.Weintraub, M., W. G. Kemp, and H. W. J. Ragetli. 1961. Some observations on hypersensitivity to plant viruses. Phytopathology 51:290-293. [Google Scholar]
- 70.Weststeijn, E. A. 1981. Lesion growth and virus localization in leaves of Nicotiana tabacum cv. Xanthi nc. after inoculation with tobacco mosaic virus and incubation alternately at 22°C and 32°C. Physiol. Plant Pathol. 18:357-368. [Google Scholar]
- 71.Yu, Y. G., M. A. Saghai Maroof, G. R. Buss, P. J. Maughan, and S. A. Tolin. 1994. RFLP and microsatellite mapping of a gene for soybean mosaic virus resistance. Phytopathology 84:60-64. [Google Scholar]
- 72.Zaitlin, M. 1962. Graft transmissibility of a systemic virus infection to a hypersensitive host—an interpretation. Phytopathology 52:1222-1223. [Google Scholar]