Abstract
Accumulating evidence suggests that natural bioactive compounds, alone or in combination with traditional chemotherapeutic agents, could be used as potential therapies to fight cancer. In this study, we employed four natural bioactive compounds (curcumin, resveratrol, melatonin, and silibinin) and studied their role in redox control and ability to promote apoptosis in androgen sensitive and insensitive prostate cancer cells. Here is shown that curcumin and resveratrol promote ROS production and induce apoptosis in LNCaP and PC-3. An increase in reactive species is a trigger event in curcumin-induced apoptosis and a consequence of resveratrol effects on other pathways within these cells. Moreover, here we demonstrated that these four compounds affect differently one of the main intracellular redox regulator, the thioredoxin system. Exposure to curcumin and resveratrol promoted TRX1 oxidation and altered its subcellular location. Furthermore, resveratrol diminished TRX1 levels in PC-3 cells and increased the expression of its inhibitor TXNIP. Conversly, melatonin and silibinin only worked as cytostatic agents, reducing ROS levels and showing preventive effects against TRX oxidation. All together, this work explores the effect of compounds currently tested as chemo-preventive agents in prostate cancer therapy, on the TRX1 redox state and function. Our work shows the importance that the TRX system might have within the differences found in their mechanisms of action. These bioactive compounds trigger different responses and affect ROS production and redox systems in prostate cancer cells, suggesting the key role that redox-related pathways might play in processes like differentiation or survival in prostate cancer.
Abbreviations: AP-1, activator protein 1; ASK1, apoptosis signal-regulating kinase 1; DHE, Dehydroepiandrosterone; DMEN-F12, Dulbecco's modified eagle medium; DCFH2-DA, 2′-7′- dichlorodihydrofluorescein diacetate; GSH, glutathione; HDA2, Histone deacetylase 2; IAA, iodoacetic acid; IAM, iodoacetamide; MitoSOX, red mitochondrial superoxide indicator; NAC, N-acetyl-cysteine; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; PCa, prostate cancer; REF-1, redox factor – 1; TXNIP, thioredoxin-interacting protein; TRX, thioredoxin; TRXR, thioredoxin reductase
Keywords: Thioredoxin, Thioredoxin reductase, TXNIP, Prostate cancer, Redox signaling, Apoptosis
Graphical abstract
Highlights
-
•
Resveratrol decreases TRX1 by increasing TXNIP while curcumin induces TRX1 oxidation.
-
•
Antioxidants decrease TRX1 oxidation and nuclear translocation to prevent cell death.
-
•
TRX1 oxidation and nuclear translocation play a key role in apoptosis.
-
•
Differences in the apoptosis induction of bioactive compounds relay on TRX1 oxidation.
1. Introduction
Reactive oxygen species (ROS) are generated in response to endogenous and exogenous stimuli. Under physiological conditions, basal low levels of ROS are crucial mediators of multiple cell processes including growth, migration or differentiation. However, an excess of ROS production induces the so called oxidative stress, causing oxidative damage to macromolecules and thus leading to cell death, apoptosis and/or senescence. As a consequence, oxidative stress has been related to the pathogenesis of various diseases including cancer, diabetes and neurodegenerative diseases [1], [2]. Many studies suggest that cancer cells show increased oxidative stress associated with alterations in metabolic activity and oncogenic transformation. Higher ROS production plays an important role in tumor initiation and progression [3]. However, oxidative stress also renders cancer cells vulnerable to be damaged by further ROS production induced by exogenous agents. Therefore, manipulating ROS levels by redox modulation become an effective therapeutic approach to selectively kill cancer cells without causing significant toxicity to normal cells [4], [5].
Physiologically generated ROS are controlled by non-enzymatic and enzymatic anti-oxidants, such as superoxide dismutases, catalase, peroxiredoxins and glutathione peroxidases. The thioredoxin (TRX) system composed of TRX, thioredoxin reductase (TRXR), and NADPH, is one of the main thiol-dependent electron donors systems in the cell. It plays a critical role in the regulation of cellular redox environment and in a wide range of signaling pathways [6], [7], [8]. TRX acts as an intermediate that senses cellular redox state and transmits information to signaling molecules such as apoptosis signal-regulating kinase 1 (ASK1) [9]. ASK1 is a member of the mitogen-activated protein kinase (MAP3K) family that is involved in apoptosis and differentiation [10]. Reduced TRX negatively regulates ASK1 preventing apoptosis by binding to its N-terminal domain [9], [11].
Prostate cancer (PCa) is one of the most common malignancies and the second leading cause of cancer-related male mortality in western countries [12]. PCa growth is initially androgen-dependent and thus androgen ablation is the standard therapeutic option. Nevertheless, malignant prostate tumors eventually relapse after treatment, becoming hormone-independent and resistant to conventional therapies.
Several bioactive compounds derived from natural sources have been at the forefront research of new therapies against PCa [13]. First, epidemiologic data suggested that some diet habits are related to a lower incidence of certain tumors types. Then, preclinical studies using cells or animal models supported the role of some bioactive compounds as chemo-preventive agents. Their relatively low toxicity, low cost, easy availability and their possible role as adjuvants that potentiate the effect of different chemotherapeutic drugs has been investigated for the last years [14], [15], [16], [17]. In the current study, the effect of four natural compounds, i.e.: curcumin, a natural dye extracted from Curcuma longa L.; resveratrol, a polyphenolic stilbene found in grapes; silibinin, a naturally-occurring flavonoid produced by milk thistle and melatonin, an indole that is mainly produced by the pineal gland in vertebrates but also found in edible plants [18], was investigated. All of them have shown effects against tumor growth in cells or in rodent models of prostate cancer [19], [20], [21], though, the molecular pathways involved in their mechanisms of action, in particular the redox signaling pathways altered, still remain unclear. In this study, we aim to investigate ROS-mediated apoptosis signaling pathways induced by these bioactive compounds that have displayed antioxidant or pro-oxidant activities in vitro [22], [23], [24], [25]. Since TRX1 has been proposed as a subcellular biomarker of redox imbalance in human prostate cancer progression [26], its role in androgen-dependent and independent prostate cancer cells apoptosis caused by these bioactive compounds was evaluated.
2. Material and methods
2.1. Cell culture and drugs treatments
Human androgen-dependent epithelial prostate cancer cells (LNCaP) were purchased from European Collection of Cell Cultures (Catalog number 89110211, Salisbury, UK) and were cultured in RPMI 1640 medium supplemented with 10% FBS, 2 mM L-glutamine, 15 mM HEPES, 100 µg/ml ampicillin and 100 µg/ml kanamycin. PC-3 cells, a human prostate androgen-independent adenocarcinoma cell line, purchased from American Type Culture Collection (Catalog number CRL-1435, Manassas, VA, USA) were grown in DMEM/F12 medium supplemented with 10% FBS, 2% L-glutamine and 1% antibiotic-antimycotic cocktail (100 U/ml penicillin, 10 µg/ml streptomycin and 0.25 µg/ml amphotericin B). Both cell lines were grown at 37 °C in a humidified 5% CO2 environment. The medium was changed every 2 days and cultures were split at least once a week, when they reached 80% confluence. IC50 concentration of each compound based on cell viability and proliferation assays was employed. Thus, in the following studies 1 mM melatonin, 50 µM silibinin, 25 µM curcumin and 50 µM resveratrol were chosen. Natural compounds were added directly in complete culture medium from a stock solution using dimethyl sulfoxide (DMSO, Sigma-Aldrich) as a vehicle. DMSO was added to each control and experimental group.
2.2. Cell viability
Cell viability was evaluated by MTT reduction assay (BQCell™ MTT, Bioquochem, Oviedo, Spain). Cells were seeded in 96 well plates and then treated with several concentrations of each compound for 48 h. Base on previous reports, concentrations range for each compound were chosen as follow, curcumin (1.6–25 µM), resveratrol (6.12–100 µM), melatonin (0.062–1 mM) and silibinin (6.12–100 µM). After treatment, MTT was added to each well. Four hours later, cells were lysed by adding one volume of lysis buffer and left overnight at 37 °C. Absorbance at 570 nm was measured using a microplate reader (Cary 50-MPR; Agilent Technologies, Santa Clara, CA, USA). Experiments were repeated at least three times. Results are shown as the media of six samples ±SEM.
2.3. Apoptosis. Annexin V staining
Apoptosis was evaluated by annexin V/propidium iodide staining by using Annexin V-FITC Apoptosis Detection Kit (BD Biosciences, 2350 Qume Drive, San Jose, California, USA) following manufacturer´s instructions. Fluorescence was analyzed by using a Becton-Dickinson Immunocytometry Systems FACStar Plus flow cytometer, equipped with an argon-ion laser. At least 104 cells were analyzed in each experimental group.
2.4. Cell cycle analysis
Cell cycle distribution was determined by flow cytometry (Becton–Dickinson and Beckman– Coulter). After 48 h incubation with compounds, cells were harvested and fixed in 70% ethanol at 4 °C for 24 h. Then, cells were incubated with RNaseA (100 mg/l) at 37 °C for 30 min and stained with propidium iodide (10 mg/l) (Sigma-Aldrich). Samples were analyzed for DNA content using a Coulter EPICS-XL Cytometer.
2.5. Detection of intracellular and mitochondrial ROS levels
MitoSOX red (Invitrogen, Carlsbad, CA, USA), Dihydroethidium (DHE, Sigma-Aldrich), and 2′-7′-dichlorodihydrofluorescein diacetate (DCFH2-DA, Sigma-Aldrich) staining were employed to detect intra-mitochondrial or intracellular O2•- levels and general ROS production, respectively. For MitoSOX and DHE staining, cells were treated with each bioactive compound for 30 min and for H2-DCFDA, cells were treated for 3, 6 or 24 h. Cells were collected by trypsinization and resuspended in 1 ml of PBS plus glucose (1 g/l glucose) and 0.1% FBS containing 1 μM MitoSOX, 2 µM DHE or 1 μM DCFH2-DA. Cells were then incubated with MitoSOX for 10 min and with DHE and DCFH2-DA for 30 min at 37 °C. Stained cells were analyzed by flow cytometry (Beckman-Coulter EPICS-XL Cytometer). Data analysis was performed using Kaluza Analysis Software (Beckman-Coulter).
2.6. Hydrogen peroxide determination
Hydrogen peroxide (H2O2) was electrochemically assayed as previously reported [27] using screen-printed electrodes (Bioquochem SL, Oviedo, Spain). Briefly, cells were cultured in complete medium at a density of 75×105 cells/ml and incubated with compounds for 24 h. Samples of the medium were collected and a small aliquot of 30 µl of cell-free culture medium was placed on the electrode surface. Intensity (µA) was registered for 200 s employing +0.4 V as working potential. To determine H2O2, each sample was measured before and 5 min after adding catalase to blank sample. Measurements were done using an e-BQC portable device.
2.7. Transient transfections
Two of the natural compounds employed in this study seemed to induce differentiation in prostate cancer cells while the others induced apoptosis. Thus, the possible role of the MAP kinase ASK1, which has been linked to both processes, within these differences was investigated. Cells were transiently transfected with hASK1-HA-pcDNA3 or the catalytically inactive form hASK1-KM-HApcDNA3. Plasmids were a gift from Dr. Hidenori Ichijo (University of Tokyo). LNCaP and PC-3 cells were plated at a density of 5×104 cells/ml in 24-well cell culture dishes. After 48 h cells were transfected with 1.0 μg of plasmid DNA using Lipofectamine (Invitrogen) following the manufacturer's instructions.
2.8. SDS-PAGE and immunoblotting
Cells were seeded in 100 mm dishes and allowed to attach overnight. After treatment for 48 h, cells were washed with ice cold phosphate buffered saline (PBS) and lysed in RIPA lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% Igepal C, 0.5% sodium deoxycholate, 1 mM Dithiothreitol (DTT), and protease inhibitors (10 μg/ml leupeptin, 2 μg/ml aprotinin, 1 μg/ml pepstatin, 200 μM sodium orthovanadate and 1 mM phenylmethylsulfonyl fluoride (PMSF)) (Sigma-Aldrich). Fifty micrograms of protein were loaded in 12–15% SDS polyacrylamide gels and electrotransferred to PVDF membranes. The following antibodies were employed: anti-CuZnSOD (Calbiochem, MerckChemicals Ltd, Nottingham, UK), anti-catalase, anti-Bcl-2 (Calbiochem), anti-Bax, anti-Trx1, anti-GAPDH and anti-β actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Immunoreactivity was detected by using enhanced Immobilon Western Substrates ECL (Millipore).
2.9. Nuclear and cytosolic fractionation
Cells were seeded in 100 mm dishes at a density of 5×104 cells/ml and allowed to attach overnight. After 48 h treatment, cells were washed with ice-cold PBS and nuclear and cytosolic proteins were extracted after harvesting cells by scrapping. Cells were lysed in ice-cold Buffer A (10 mM HEPES, pH 7.9, 15 mM KCl, 2 mM MgCl2, 1 mM DTT, Sigma-Aldrich) and 0.1 mM EDTA) supplemented with protease inhibitors (1 mM PMSF, 1g/ml aprotinin, 0.3g/ml leupeptin) and 0.2% Igepal C (Sigma-Aldrich). Nuclear fractions were collected by centrifugation at 14,000×g 5 min at 4 °C and supernatants (cytosolic fraction) were transferred to clean tubes. Nuclei were resuspended in 20 µl of Buffer B (250 mM Tris–HCl, pH 7.8, 60 mM KCl, 1 mM DTT, 1 mM PMSF, 1g/ml aprotinin, 0.3g/ml leupeptin and 10% glycerol) and 30 µl of Buffer C (50 mM KCl, 20 mM HEPES, pH 7.8, 0.2 mM EDTA and 20% glycerol). After incubation on ice for 15 min, nuclear extracts were clarified by centrifugation at 13,000×g for 30 min at 4 °C. Protein concentration was estimated using Bradford protein assay (Bio-Rad Laboratories Inc., Madrid, Spain).
2.10. Urea-PAGE for detection of TRX1 redox state
The method used for the detection of TRX redox state was developed by Bersani et al. [28] and Takahashi and Hirose [29] and modified in Du et al. [30]. Briefly, to prepare mobility standards, cell lysates were denatured and unfolded with urea and fully reduced with DTT. Solutions with different molar ratios of iodoacetic acid (IAA, Sigma-Aldrich) and iodoacetamide (IAM, Sigma-Aldrich) were incubated with the reduced proteins containing “n” cysteines, leading to “n+1” possible labelled protein isoforms with introduced number of acidic carboxymethyl thiol adducts (-SA-) and neutral amidomethyl thiol adducts (-SM). During Urea-PAGE, the ionized -SA- adducts resulted in faster protein migration toward the anode. Therefore, the “n+1” isoforms were separated and used as a mobility standard for representing the number of -SA-. To determine the redox state of TRX1 in vivo, cells were harvested by trypsinization and washed in ice-cold PBS to remove secreted oxidized Trx1. Pelleted cells were immediately dissolved in TEU buffer (50 mM Tris– HCl, pH 8.2, 1 mM EDTA, 8 M Urea) containing 30 mM IAA. Samples were incubated at 37 °C for 30 min, centrifuged at 13,000×g for 10 min and transferred to fresh tubes. To wash away excess IAA, proteins were precipitated with ice-cold acetone-HCl and centrifuged at 13,000×g for 10 min, supernatants were removed. Washing procedure was repeated two more times. The final pellet was dissolved in 100 µl TEU buffer with 3.5 mM DTT, incubated for 30 min at 37 °C and subsequently alkylated with 10 mM IAM for 15 min at 37 °C and centrifuged. Then protein concentration was determined by Bradford protein assay and equal amounts of protein were loaded into Urea-PAGE and electrotransfered to PVDF membranes. Membranes were probed with Trx1 primary antibody (IMCO Ltd. Stockholm, Sweden) and visualized by binding of horseradish peroxidase conjugated anti-rabbit (Santa Cruz Biotechnology). Immunoreactivity was detected by using enhanced Immobilon Western Substrates ECL (Millipore).
2.11. Quantitative real-time PCR
Total RNA was isolated by using Tri Reagent (Sigma-Aldrich), according to manufacturer's instructions. Two micrograms of mRNA were used to synthesize cDNA with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Each sample was analyzed in triplicate. The primers used in this assay were: Trx1 (5′-GATCAAGCCTTTCTTTCATTCCC-CCCACCTTTTGTCCCTTCTTAA-3′), TrxR1 (5′-GGTCCAACCTTGAAGGCTTA-CATATTGGGCTGCCTCCTTA-3′) TXNIP (5′- CTTACTGATCTATGTTAGGCGTTC-GGATGTTCAGATCTACCCAACT-3′) and β-Actin (5′-ATCAAGATCATTGCTCCTCCT-CATAGTCCGCCTAGAAGCA-3′). β-Actin was employed as internal control. Relative quantification values are expressed as 2 (–delta CT).
2.12. Enzymatic activity
For the DTNB (5,5′-dithio-bis(2-nitrobenzoic acid)) endpoint assay the thioredoxin reactions were coupled with insulin as protein substrate according to method previously described [31]. Briefly, 20 µg of proteins were incubated with 85 mM HEPES pH 7.6, 3 mM EDTA, 0.3 mM insulin and 660 µM NADPH and with or without 50 nM TRXR. After 30 min at 37 °C, a solution of 1 mM DTNB in 6 M guanidine-HCl was added to stop the reaction and label and determine thiols. Absorbance of samples was measured at 412 nm and the thiols concentration was calculated using the extinction coefficient of TNB (13,600 M−1 cm−1).
2.13. Statistical analysis
Results shown represent the mean±standard error of the mean (SEM) of at least three samples per group. Normality was assessed using Kolmogorov-Smirnov test and then differences among means were calculated using one-way ANOVA, followed by a Student-Newman-Keuls (SNK) t-test. Values were considered statistically significant when p<0.05.
3. Results
3.1. Melatonin and silibinin inhibit cell growth while curcumin and resveratrol induce apoptosis in prostate cancer cells
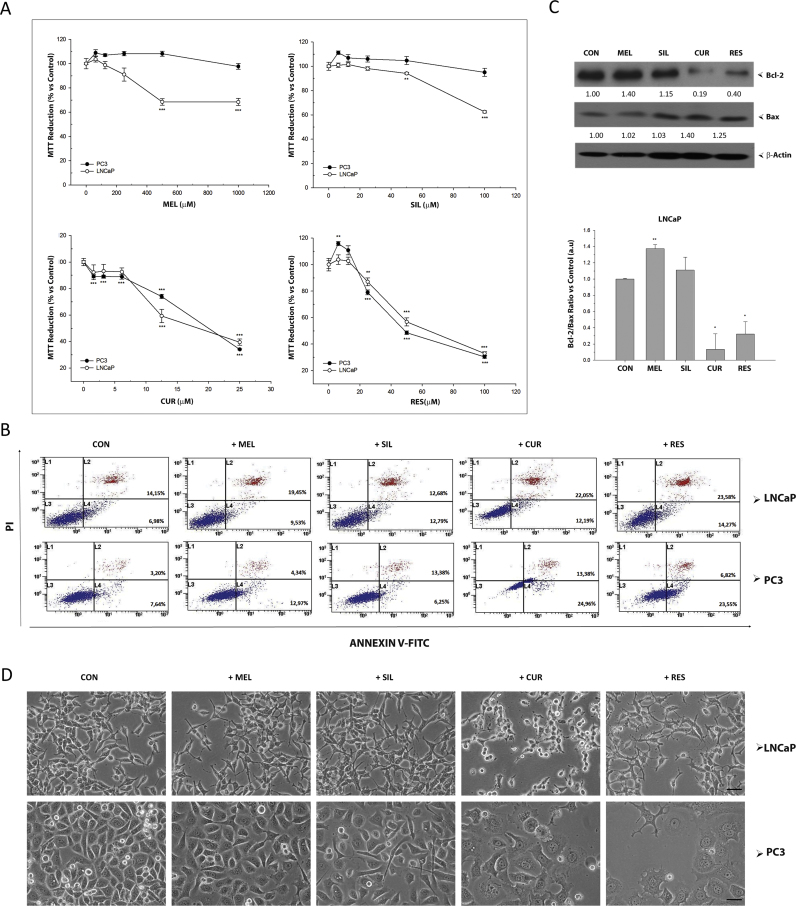
The effects of four bioactive compounds (melatonin, silibinin, curcumin and resveratrol) on the viability and proliferation of prostate cancer cells were evaluated using MTT assay (Fig. 1A). After 48 h treatment, the growth of androgen-sensitive LNCaP cells was significantly inhibited by melatonin 500 µM and 1 mM and silibinin 50 µM, while no significant effect was found in androgen-insensitive PC-3 cells (Fig. 1A). Curcumin and resveratrol inhibited the viability of both cell lines at concentrations over 10 and 20 µM, respectively. MTT assay cannot discriminate cell growth inhibition from cell death. Thus, annexinV staining in combination with flow cytometry quantification of stained cells was performed after culturing LNCaP and PC-3 cells with melatonin (1 mM), silibinin (50 µM), curcumin (25 µM) or resveratrol (50 µM) (Fig. 1B). Only incubation with curcumin and resveratrol induced a significant increase in apoptotic cells in both cell types. To confirm this, the levels of anti-apoptotic protein BCL2 and pro-apoptotic protein BAX were assessed by western blot. In LNCaP cells, a clear decrease in BCL2 and an increase in BAX protein levels were found after incubation with curcumin or resveratrol. On the contrary, an increased in BCL2 was observed after melatonin incubation while no significant differences were observed in cells treated with silibinin in BCL2/BAX ratio (Fig. 1C). Fig. 1D shows the morphology of LNCaP and PC-3 cells after incubation for 48 h with all compounds. The characteristic morphological features of apoptotic cells such as cell shrinkage, rounded shape and partial detachment were all observed after curcumin incubation in both cell types. Similarly, PC-3 cells undergoing resveratrol incubation showed morphological signs of apoptosis but those were preceded by cytoplasmic vacuolization. Significant changes in the morphology, including long processes (neurites) and rounded nuclei, typical features of neuroendocrine differentiation, were observed in cells cultured with melatonin and silibinin as previously reported [32].
Fig. 1.
Effect of bioactive compounds on viability in prostate cancer cells. (A) Androgen-sensitive LNCaP and androgen-insensitive PC-3 were exposed to melatonin, silibinin, curcumin or resveratrol and MTT assay was performed after 48 incubation. Data are shown as mean±SEM. of six independent samples. Experiments were repeated at least three times. * p<0.05; ** p<0.01; *** p<0.001 versus CON. (B) Apoptosis was evaluated in LNCaP and PC-3 after 48 h incubation with IC50 of the compounds (1 mM melatonin, 50 µM silibinin, 25 µM curcumin and 50 µM resveratrol), using Annexin V-FITC assay by flow cytometry. (C) LNCaP cells were cultured with or without 1 mM melatonin, 50 µM silibinin, 25 µM curcumin or 50 µM resveratrol for 48 h. BCL-2 and BAX protein levels were assessed by western blot. GAPDH and β-Actin were employed as loading control. (D) Changes in cell morphology were examined under a phase contrast microscope with a 200× magnification and photographed (scale bar, 25 µm).
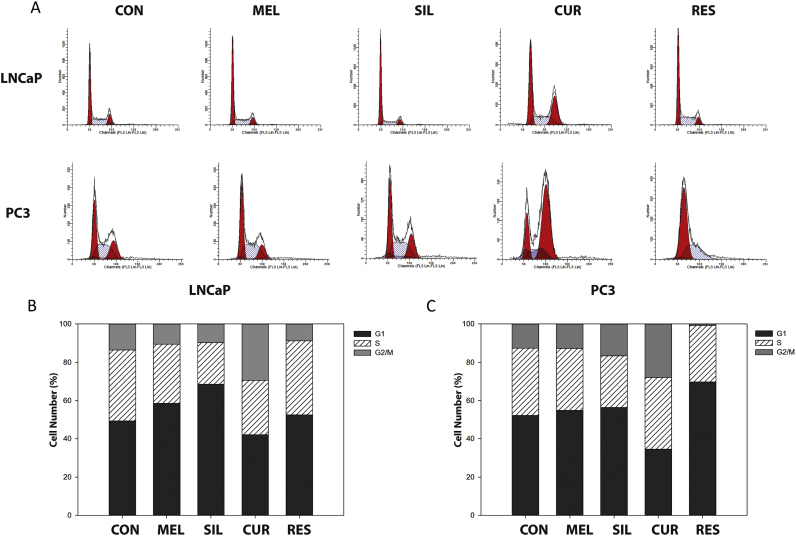
To determine whether the decrease in cell viability was due to an arrest of the cell cycle, LNCaP and PC-3 cells were treated with all compounds for 48 h and cell cycle distribution was analyzed by flow cytometry (Fig. 2A). Melatonin and silibinin caused a significant arrest in G0/G1 in LNCaP cells (Fig. 2B), whereas no significant differences were found in androgen-insensitive PC-3 after incubation with these agents. On the other hand, curcumin caused a G2 arrest in both cell types besides increasing apoptosis (Fig. 2C). Interestingly, resveratrol only promoted a significant arrest in G1/S phase in androgen-insensitive PC-3 cells. All together these results show that melatonin and silibinin act as cytostatic agents but only in androgen-dependent cells while curcumin and resveratrol display potent pro-apoptotic effects in both cell lines.
Fig. 2.
Impact of bioactive compounds on cell cycle distribution in prostate cancer cells. (A) LNCaP and PC-3 cells were incubated with 1 mM melatonin, 50 µM silibinin, 25 µM curcumin or 50 µM resveratrol for 24 h and cell cycle was analyzed by flow cytometry. Data shown in the graphs is representative of three independent experiments. Bars represents the percentage of cells in each phase of cell cycle for LNCaP (B) and PC-3 (C) after 24 h incubation with compounds. G0/G1 phase; S phase; G2/M phase.
3.2. Curcumin increases oxidative stress in prostate cancer cells
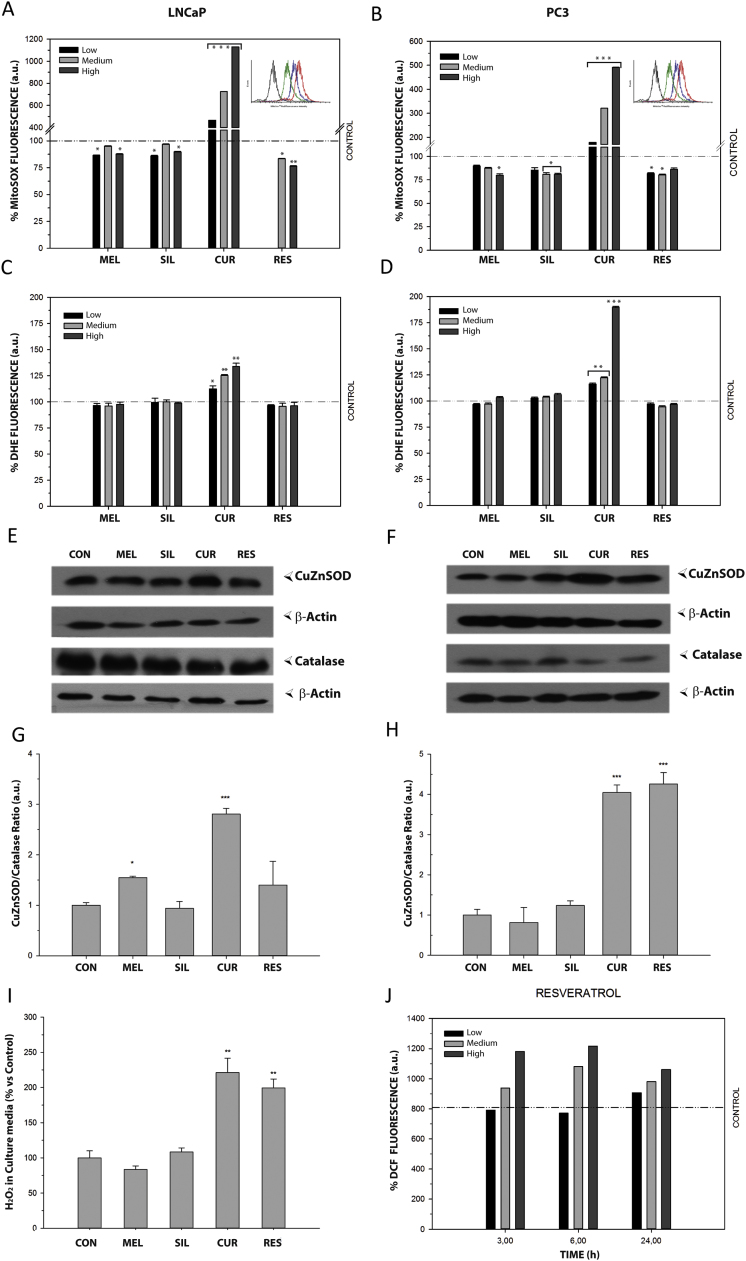
The four bioactive compounds employed in this study have been found to exert antioxidant properties in vitro [22], [23], [25], [33], but their effects on growth inhibition in some tumor cell lines have been sometimes related to ROS production [34], [35]. We aim to investigate whether an increase or a decrease in ROS levels occurred in PCa cells after treatment with these compounds and if that effect was an early triggering event or a consequence of the apoptosis pathway. For this purpose, cells were incubated for 30 min with the four compounds. After incubation, cells were then stained with DHE and MitoSOX red probes to evaluate the production of cytosolic or mitochondrial O2•-, respectively (Fig. 3). As shown, only curcumin induced a significant increase in MitoSOX staining, being the fluorescent intensity more than ten times higher than in control cells. Not only did not melatonin, silibinin and resveratrol increased but they rather decreased MitoSOX fluorescence, when compared to controls (Figs. 3A, B). Again, after DHE staining, only curcumin-incubated cells showed a clear increase in fluorescence.
Fig. 3.
Effect of bioactive compounds on ROS production and on antioxidant protein levels in prostate cancer cells. LNCaP and PC-3 cells were incubated with melatonin (250, 500, 1000 µM), silibinin (25, 50, 100 µM), curcumin (10, 25, 50 µM) or resveratrol (25, 50, 100 µM) for 30 min and stained with MitoSOX red (A, B) or DHE (C, D) for 30 min. Representative histogram of MitoSOX fluorescence shift after curcumin incubation in shown in the top corner of figures A and B. ROS levels were measured by flow cytometry and are presented as the percentage fold change relative to control cells. LNCaP (E) and PC-3 (F) were cultured with 1 mM melatonin, 50 µM silibinin, 25 µM curcumin or 50 µM resveratrol for 48 h and CuZnSOD and catalase protein levels were assessed by western blot. β-Actin was employed as loading control. Experiment was repeated at least 3 times and a representative experiment is shown. CuZnSOD/catalase protein ratio was calculated in LNCaP (G) and PC-3 (H). (I) Effect of bioactive compounds on H2O2 release in prostate cancer cells. Cells were cultured in complete medium at a density of 75×105 cells/ml and incubated with 1 mM melatonin, 50 µM silibinin, 25 µM curcumin or 50 µM resveratrol for 24 h. An electro-oxidation method was used for H2O2 determination. (J) PC-3 cells were exposed to resveratrol (25, 50, 100 µM) and ROS production was measured using DCFH2-DA after 3,6 or 24 h incubation. Data are shown as mean ±S.E.M of three independent samples. *p<0.05; **p<0.01; ***p<0.001 versus CON. Experiment was repeated 3 times and a representative experiment is shown.
Since MitoSOX and DHE fluorescence are indicatives of intracellular O2•-, protein levels of SOD2/MnSOD, SOD1/CuZnSOD and catalase were analyzed by Western blotting. No significant differences were found in SOD2 levels after any of the treatments (data not shown). However, CuZnSOD protein levels were increased after melatonin treatment in androgen-dependent LNCaP cells. Likewise, curcumin and resveratrol increased CuZnSOD protein levels in both cell types (Figs. 3E, F). Using CuZnSOD/Catalase ratio as a measurement of redox balance, it was significantly increased after curcumin treatment in androgen-sensitive and insensitive cells, while resveratrol only showed an effect in PC-3 cells (Fig. 3G-H).
Given the imbalance in CuZnSOD/catalase observed in PC-3 cells after incubation with both, curcumin and resveratrol, the levels of H2O2 were further analyzed to assay whether these compounds could also alter intracellular ROS in a long-time fashion (Fig. 3I). Consequent with that, a significant increase in H2O2 released into cell culture media was found after curcumin and resveratrol incubation. Altered CuZnSOD/Catalase ratio and H2O2 levels prompted us to next measure ROS production with DCFH2-DA (a general oxidative stress indicator) after a longer exposure time to the stilbene. As shown in Fig. 3J, resveratrol, at concentrations ≥ IC50 significantly increased ROS levels in PC-3 cells at all times of incubation. These results suggest that both polyphenols increase O2•- at shorter and H2O2 at longer exposure times thus promoting oxidative stress in PC-3 cells.
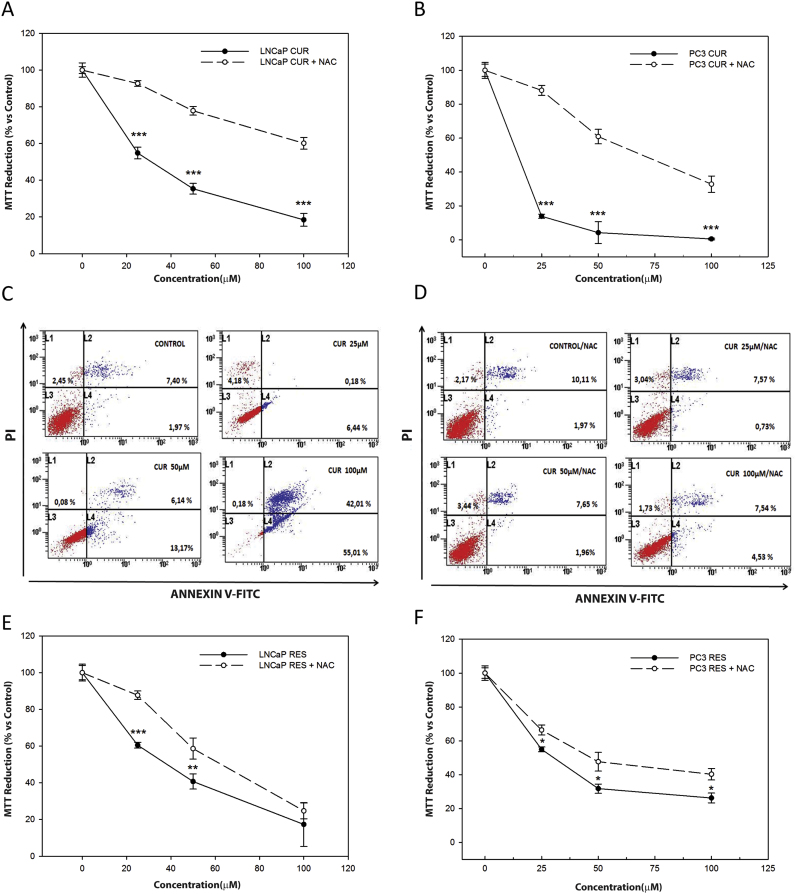
3.3. NAC prevents curcumin-induced apoptosis
Since an early increase in ROS levels combined with a decrease in catalase likely explains the mechanism of action of curcumin in LNCaP and PC-3 cells, we tried to evaluate whether curcumin effect on cell viability could be prevented by incubation with N-acetyl-cysteine (NAC), a precursor of glutathione (GSH). LNCaP (Fig. 4A) and PC-3 cells (Fig. 4B) were pre-treated with 20 mM NAC for 3 h and then exposed to increasing concentrations of curcumin (25, 50, and 100 µM). The effect of curcumin on cell viability was greatly inhibited by pre-incubation with NAC in both cell lines (Fig. 4A, B). In addition, pre-incubation with NAC decreased nearly 90% the number of apoptotic cells in the group treated with 100 µM curcumin (Fig. 4C), supporting that the toxicity mediated by this compound might involve changes in redox state in prostate cancer cells.
Fig. 4.
Influence of NAC on curcumin and resveratrol-induced toxicity. LNCaP (A) and PC-3 cells (B) were incubated with curcumin (25, 50 and 100 μM) alone or prior 3 h incubation with 20 mM NAC. Cell viability was measured by MTT assay after 24 h treatment. PC-3 cells were treated with curcumin alone (C) or after prior incubation with NAC (D) for 36 h. Apoptosis was measured by flow cytometry after staining with Annexin V/PI. LNCaP (E) and PC-3 cells (F) were treated with resveratrol (25, 50 and 100 µM). Cell viability was measured with MTT assay after 24 h. *p<0.05; **p<0.01; ***p<0.001 versus CON.
Comparably, resveratrol changed antioxidant enzymes levels and induced an increase in H2O2 in PC-3 cells; therefore, the effect of co-incubation of NAC with the stilbene was also studied. A much less significant reduction on cell toxicity was observed when cells were pre-incubated with NAC (Fig. 4D, E) suggesting that curcumin and resveratrol induced apoptosis in prostate cancer cells following different mechanisms.
3.4. Inhibition of ASK1 activity prevents curcumin induced apoptosis
Melatonin and silibinin induce differentiation in prostate cells while curcumin and resveratrol are able to induced apoptosis. For this reason, the role of ASK1 in the biological activity of the four bioactive compounds was evaluated. The phosphorylation of ASK1 at Ser-967 has been related to differentiation in other cell types [36] whereas phosphorylation of ASK1 at Thr-845 has been associated with apoptosis induction [37]. The level of endogenous ASK1 and phosphorylated forms, P-ASK1Thr845 or P-ASK1Ser 967 were studied in prostate cancer cells. Endogenous protein levels were too low to be detected by western blot in LNCaP and PC-3 cells (data not shown) so both cell lines were transfected with hASK1-HA-pcDNA3 and then immunoprecipitation was carried out. No significant signal for P-ASK1Thr-845 was detected and only a slight increase in P-ASK1Ser 967 was observed in LNCaP cells (data not shown).
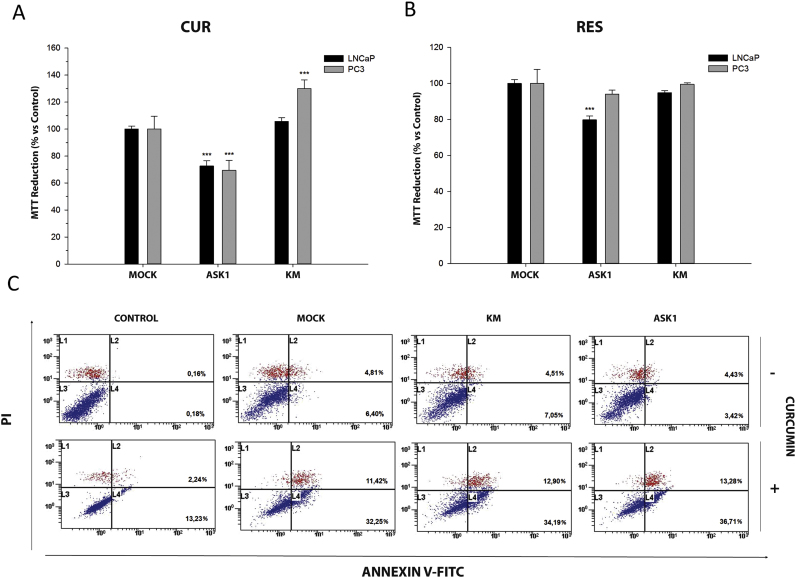
In order to evaluate indirectly the participation of ASK1 in prostate cancer survival, LNCaP and PC-3 cells were transfected with hASK1-HA-pcDNA3 or the kinase mutant hASK1-KM-HApcDNA3 and cell viability was assessed after treatment with curcumin and resveratrol by MTT assay (Fig. 5A, B).
Fig. 5.
Effect of curcumin and resveratrol on ASK1-overexpressed cells viability. LNCaP and PC-3 cells were transiently transfected with vector pcDNA3.1 (MOCK), wild-type ASK1 (ASK1) or kinase inactive mutant (KM-ASK1). Cells were set overnight and then treated with 25 µM curcumin (A) or 50 µM resveratrol (B). Cell viability was studied using MTT assay after 24 treatment. Data are shown as mean±S.E.M. of 6 independent samples. *p<0.05; **p<0.01; ***p<0.001 versus CON. PC-3 cells (C) transiently transfected with vector, ASK1 or KM-ASK1 were incubated with or without curcumin (25 µM) for 24 h. Apoptosis was evaluated after staining with Annexin V/PI by flow cytometry.
As expected (see above), curcumin and resveratrol reduced the viability of both androgen-dependent and independent cells. Overexpression of native ASK1 enhanced toxicity induced by both polyphenols in LNCaP cells. In the case of the androgen independent PC-3 cells, ASK1 overexpression only enhanced the toxicity caused by curcumin (Fig. 5A, B). Overexpression of a mutant KM-ASK1 isoform partially protected PC-3 cells from curcumin-caused toxicity (Fig. 5A) while it had no effect when cells were incubated with resveratrol. These results suggested that ASK1 could play a role in curcumin but not in resveratrol-induced toxicity. Thus, to further investigate the role of this kinase in curcumin-mediated apoptosis, Annexin V-PI assay was performed in PC-3 cells. However, in this case no significant differences were found between cells transfected with either, native or KM-ASK1 mutant (Fig. 5C).
3.5. TRX1 oxidation mediates curcumin and resveratrol-induced apoptosis
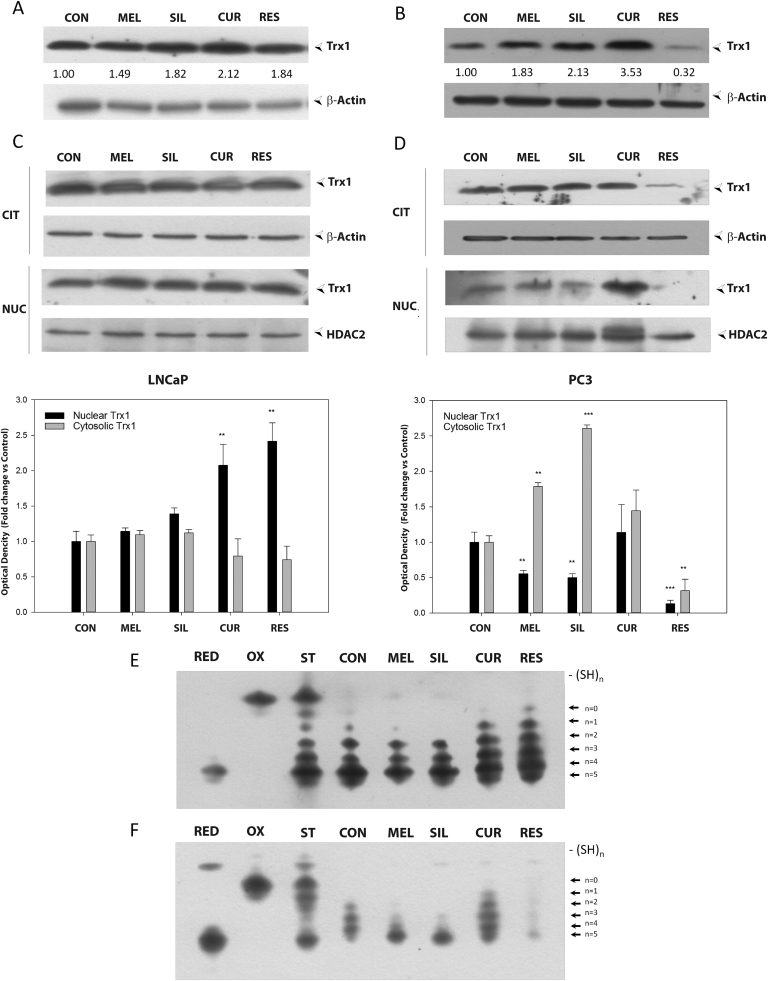
Since TRX1 is the major redox regulator of ASK1, we evaluated the effect of melatonin, silibinin, curcumin and resveratrol on TRX1 protein levels, subcellular location and redox state. Total protein levels of TRX1 in LNCaP and PC-3 are shown in Fig. 6A and 6B, respectively. Melatonin, silibinin and curcumin induced an increase in TRX1 in both cell lines, while resveratrol displayed different effects in androgen-sensitive and insensitive cells. Therefore, incubation with the stilbene caused an increase in TRX1 protein levels in LNCaP but a significant decrease in PC-3 cells (Fig. 6A and B). Because TRX1 translocates into the nucleus under oxidative stress, we analyzed its subcellular location after 48 h incubation with the four compounds. Following incubation with melatonin and silibinin, no significant differences were found in TRX1 location in LNCaP (Fig. 6C), although both clearly decreased nuclear TRX1 levels in the androgen-independent cells (Fig. 6D). Curcumin significantly induced nuclear TRX1 translocation in both cell types while resveratrol promoted an increase in its nuclear localization in LNCaP, but dramatically decreased protein levels in both compartments in PC-3 cells, consistent with the results mentioned above (Fig. 6B).
Fig. 6.
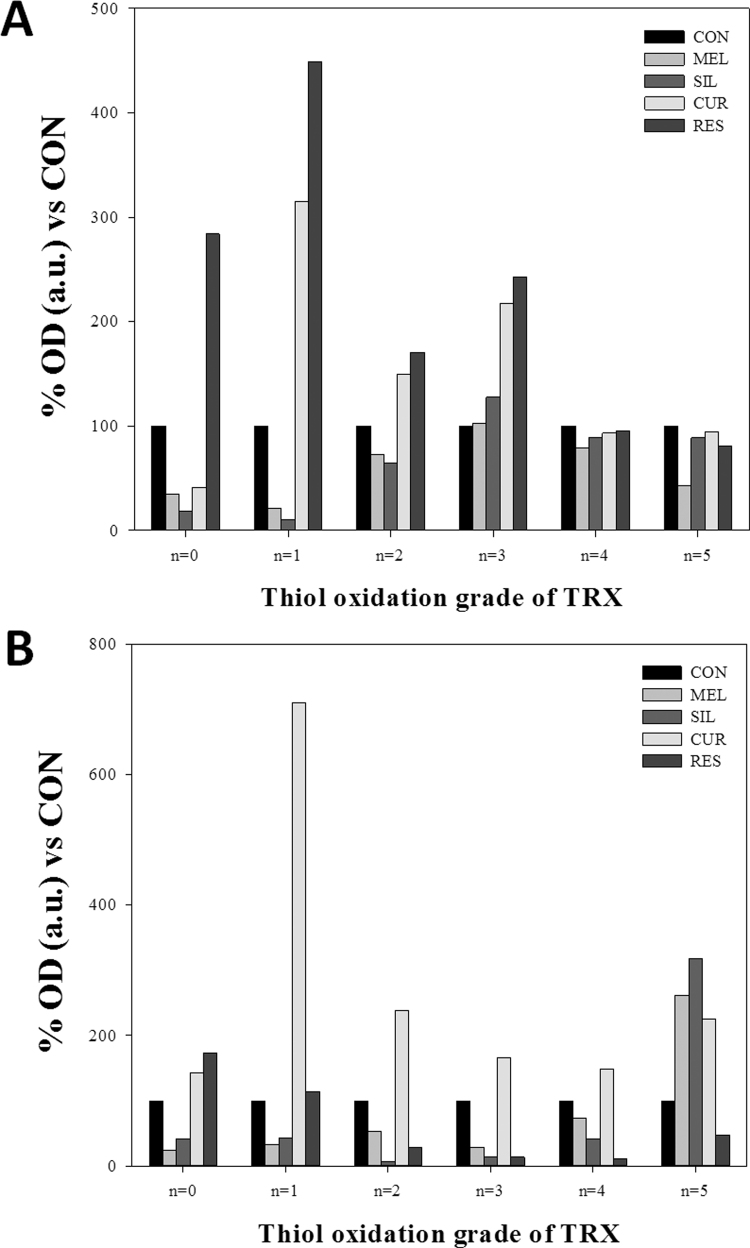
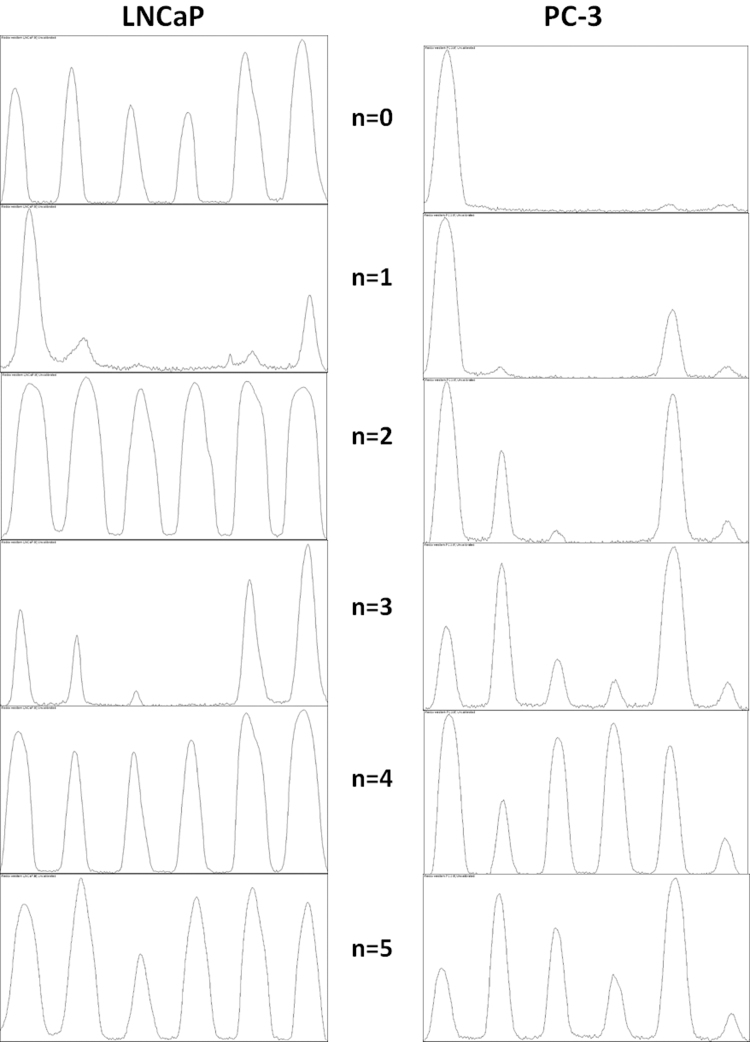
Influence of bioactive compounds on TRX1 levels, subcellular localization and redox state. LNCaP (A, C) and PC-3 (B, D) cells were treated with 1 mM melatonin, 50 µM silibinin, 25 µM curcumin or 50 µM resveratrol for 48 h and TRX1 protein levels were evaluated by western blot. HDAC2 and β-Actin were employed as loading controls. Redox state of TRX1 in LNCaP (E) and PC-3 (F) was analyzed after 48 h of treatment. Experiments were repeated at least 3 times and data of a representative experiment is shown.
TRX1 activity is regulated by its redox state. Accordingly, the oxidative state of TRX1 was evaluated by using a modified redox western blot technique as previously described [30], [38]. As shown in Fig. 6E and F, TRX1 is not fully reduced in untreated LNCaP and PC-3 cells. Melatonin and silibinin did not change the basal redox state in LNCaP and these compounds even caused a further TRX1 reduction in PC-3 cells. On the other hand, treatment with curcumin increased TRX1 oxidation in both cells lines. Resveratrol showed a different effect and led to a clear oxidation of TRX1 in LNCaP cells while in androgen-independent PC-3 cells, although there is an increment of oxidation, we observed a strong decrease in total TRX1 protein levels once more.
3.6. Resveratrol decreases TRX1 by increasing TXNIP mRNA levels in PC-3 cells
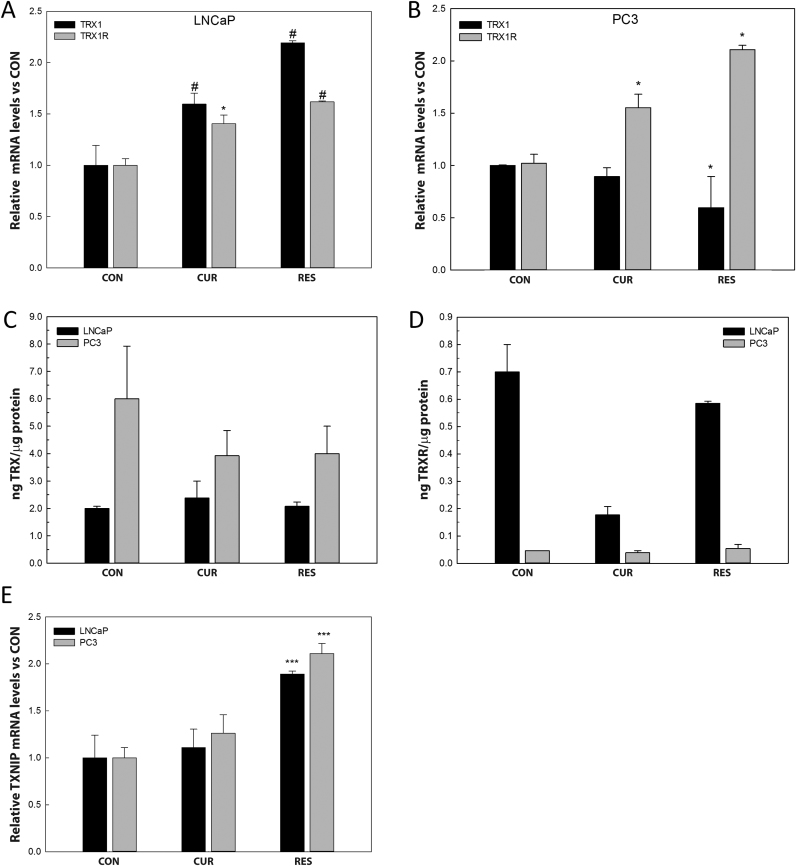
In order to confirm whether curcumin and resveratrol treatment modified TRX1 and TRXR1 activities or their mRNA levels, they were analyzed after 48 h of exposure to the compounds in both cell lines. Curcumin and resveratrol incubation increased TRX1 and TRXR1 transcription in LNCaP cells (Fig. 7A). On the other hand, curcumin increased TRXR1 mRNA levels but no significant differences were found in TRX1. Resveratrol dramatically decreased TRX1 transcription while significantly increased TRXR1 mRNA levels in PC-3 cells (Fig. 7B). No differences were found in total TRX1 activity, at the concentrations employed, neither in LNCaP nor in PC-3 cells treated with curcumin or resveratrol (Fig. 8C). However, TRXR1 activity was significantly decreased after curcumin treatment in LNCaP cells (Fig. 7D).
Fig. 7.
Effect of curcumin and resveratrol on TRX1, TRXR1 and TXNIP mRNA levels and enzymatic activities in prostate cancer cells. LNCaP and PC-3 cells were incubated with 25 µM curcumin or 50 µM resveratrol for 48 h. Trx1 and TrxR1mRNA levels in LNCaP (A) and PC-3 (B) were analyzed by RT-qPCR. TRX (C) and TRXR (D) enzymatic activities were analyzed in LNCaP and PC-3 cells after 25 µM curcumin or 50 µM resveratrol for 48 h. Txnip mRNA levels (E) were analyzed by RT-qPCR. Graphs represent the mean ±SEM obtained from three independent experiments performed in triplicates. *p<0.05; #p<0.01; ***p<0.001 versus CON.
Fig. 8.
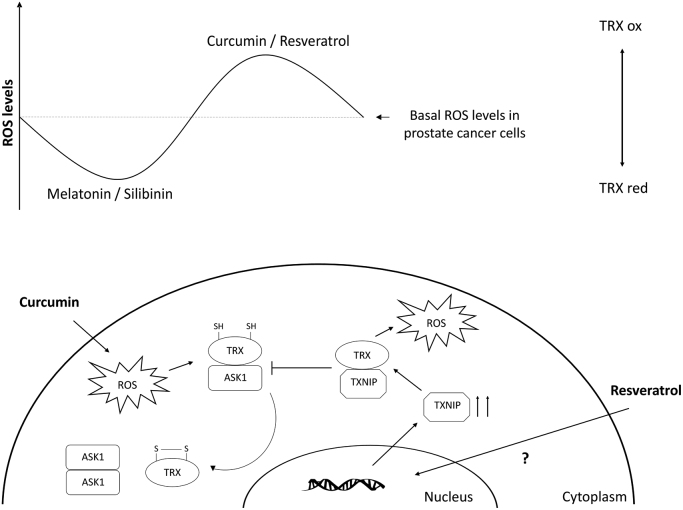
Schematic illustration of curcumin and resveratrol regulation of thioredoxin signaling in prostate cancer cells.
In PC-3 cells TRX1 mRNA levels decreased after incubation with resveratrol but this reduction did not seem to be significant enough to explain the substantial decrease in TRX1 protein levels found. Although, this fact could be explained by a slow translational rate or a rapid proteasomal degradation. To analyze whether TRX1 could be released after resveratrol treatment in PC-3 cells, we evaluated the protein levels in the cultured media. However, no significant differences were found (data not shown). Then, the potential role of TRX1 inhibitor, TXNIP, in the TRX1 reduction induced by resveratrol was considered. A remarkable increase in TXNIP mRNA levels was found after resveratrol incubation in both, LNCaP and PC-3 cells, while curcumin showed no effect on TXNIP transcription. (Fig. 7E). Interestingly, melatonin and silibinin decreased the mRNA levels for the inhibitor in both cell lines (data not shown).
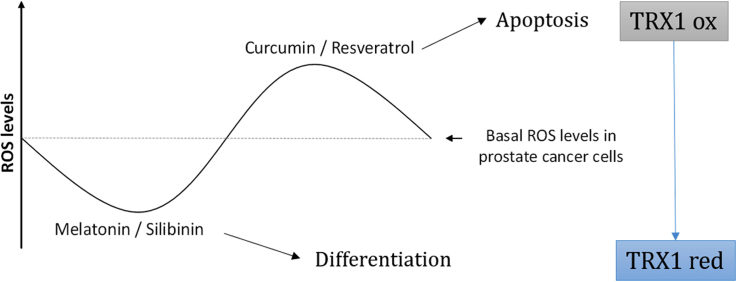
All together, these data suggest that curcumin and resveratrol affect differently TRX1 but that in both, an increase in TRX1 oxidation is associated with an increase in cell death (Fig. 8).
4. Discussion
Chemo-preventive properties of bioactive compounds and their potential use in cancer treatment have been tested for years. However, their specific targets, the limited knowledge of their mechanisms of action as well as their mechanistic differences have restricted their utility. Here it is confirmed that these four different bioactive compounds display cell type-dependent effects resulting in diverse outcomes in terms of cell redox modulation, pointing out the complexity of their biological properties. While melatonin and silibinin show antioxidant activity, curcumin and resveratrol induce cell death, with different effects on the TRX system.
Previous studies had found that silibinin decreases both, intracellular and secreted levels of PSA in LNCaP as well as promotes cell cycle arrest without inducing apoptosis but rather causing neuroendocrine differentiation of PCa cells [39]. Interestingly, similar results were found with melatonin, where it was shown that the indole increases neuroendocrine markers and that this neuroendocrine-like phenotype increases cells sensitivity to apoptosis induced by cytokines [32], [40]. On the other hand, several reports demonstrate that curcumin and resveratrol have pro-apoptotic effects in prostate cancer both in vitro and in vivo [19], [41], [42], [43]. The pro-apoptotic and anti-proliferative properties of these compounds have been linked to their effects on protein kinases, transcription factors, cell cycle proteins, cell adhesion molecules, pro and anti-apoptotic proteins, inflammatory pathways and growth signaling pathways [44] but its role as antioxidants or pro-oxidants was also postulated.
Here it is confirmed that melatonin and silibinin exhibited an inhibitory growth effect on androgen-dependent LNCaP cells, without inducing cell death. LNCaP cells are by far the most commonly used androgen-dependent PCa cell model. As a result, the specificity of the indole and the flavonoid on LNCaP cells might indicate an androgenic pathway-related target. This is not the case for curcumin and resveratrol, which exhibit pro-apoptotic effects in both cell lines, being the apoptosis promotion more remarkable in the more aggressive androgen-independent PC-3 cells.
Curcumin inhibits cell proliferation at multiple levels within the transcriptional network. This compound is well known for its antioxidant properties acting as a free radical scavenger by inhibiting lipid peroxidation and oxidative DNA damage [45], [46]. Additionally, it has been proposed that curcumin-induced apoptosis might be triggered by ROS production and/or oxidative stress in some tumor cells [47]. Here we demonstrated that curcumin increases O2•- production after short-term incubation and this pro-apoptotic effect is remarkably prevented by pre-incubation with NAC, implying that ROS are key players in this cell death induction. Resveratrol also increases ROS levels but requires a much longer incubation, suggesting that the oxidative stress caused by this stilbene is rather a collateral consequence of the apoptotic process. On the contrary, melatonin and silibinin did not trigger any sign of oxidative stress, consistent with their lack of proapoptotic effects. Furthermore, both compounds reduced mitochondrial superoxide levels after short-time incubation, whereas no differences in cytoplasmic ROS or in H2O2 production were found, compared to control cells, suggesting that both compounds have a local mitochondrial action. The antioxidant potential of silibinin [48] and melatonin [49] has been described in several cellular and animal models. The role of the indole as a direct antioxidant and as a major regulator of the key antioxidant enzymes have being already reported [24], [48] although concentrations required for direct ROS scavenging are relatively high, in accordance with the results shown here.
Here, for the first time, the effect of these compounds on TRX1/TRX1 R in PCa cells was evaluated. This antioxidant system does not simply act as a ROS scavenger but instead as an oxidative stress regulator through protein-protein interaction. Recently, TRX1 has been proposed as a subcellular biomarker of redox imbalance in prostate cancer [26] and high TRX1 expression has been associated with drug resistance in tumor cells [50], [51]. TRX1 quickly translocates into the nucleus in response to oxidative stress, NF-κB activation, UVB irradiation or tumor necrosis factor α treatment [52]. In this regard, the four compounds employed affected differently the subcellular localization and redox state of TRX1. Again, a dichotomy was clearly observed, while melatonin and silibinin decreased nuclear TRX1, curcumin and resveratrol increased its nuclear translocation in PC-3 cells. Despite the fact that nuclear translocation might initiate pro-survival signals, this signaling is likely to be useless under a situation of persistent redox imbalance, which would ultimately affect TRX1 oxidation and protein activity.
Furthermore, curcumin promotes TRX1 nuclear localization and oxidation in both cell lines. Fang et al. reported that curcumin is able to irreversibly modify and inactivate TRXR1 [53], resulting in TRX1 oxidation and inactivation of NADPH oxidase activity, leading to an increase in ROS production. In agreement with this, our results show oxidation of TRX1 induced by both, curcumin and resveratrol. Nonetheless, these compounds increased not only TRX1 but also TRXR1 mRNA levels in LNCaP, which appears to be a clear compensating effect. Reduced, but not oxidized TRX1 is able to bind and inhibit ASK1 activity, whereas ASK1 activation by TRX1 oxidation results in apoptosis [9]. As shown in this work, overexpression of dominant-negative ASK1-KM inhibits curcumin-induced cell death in PC-3 cells. Therefore, ROS production, subsequent TRX1 oxidation and ASK1 activation appear as the most plausible pathway to explain curcumin-induced apoptosis in these cell lines. To our knowledge, this is the first report evaluating the effects of resveratrol on TRX1 subcellular location and redox state. Interestingly, this stilbene displays different effects on androgen-sensitive and insensitive PCa cells, i.e. promoting TRX1 nuclear translocation and oxidation in LNCaP and decreasing dramatically protein levels in PC-3 cells. The same findings have been reported with flavonoids such as quercetin or myricetin, which inhibit TRXR promoting TRX1 oxidation [54]. The decrease in total protein content found in resveratrol-treated PC-3 cells could be explained in terms of the reduced transcription but other mechanisms such as proteasomal degradation cannot be excluded.
TXNIP is the only known endogenous TRX1 inhibitor, which binds to reduced TRX1 and forms disulfide bonds with cysteine residues at its catalytic center, thereby suppressing its activity [55], [56]. Consequent with the effects on TRX1, resveratrol incubation led to an increase in mRNA levels of TXNIP in both cell types, which might be important for its antitumor activity. TXNIP expression is dramatically altered in several tumors [57], [58], [59] and has emerged as an important element in the pathogenesis of many cancers and metabolic diseases. TRX1 inhibition by TXNIP may affect cell signaling by reducing its ability to interact with other key partners. As a result, TXNIP overexpression increases ROS levels in other cell types [60]. Moreover, Yamaguchi et al. have demonstrated that TXNIP overexpression can induce G1 cell cycle arrest [61]. In agreement with those studies, resveratrol-induced cell cycle arrest and TRX1 oxidation shown here could be a consequence of its effects on TXNIP transcription.
Natural bioactive compounds such as the ones employed in this study, have gained considerable attention not only as chemopreventive agents but also as sensitizers of anticancer drugs [16], [62]. However, they usually exert poor solubility, weak bioavailability and a high metabolization, which have been a barrier for the development of therapeutic applications [63], [64]. In this context, an increasing number of studies aim to design novel formulations to overcome these problems. Current attempts have focused in the development of new delivery systems and more bioavailable analogues in order to increase bioavailability while preserving their biological activity [65], [66]. All these new approaches allow natural compounds to play a significant role in the development of new therapies against several malignancies once more.
5. Conclusion
To summarize, the present study provides new findings to explain why redox-altering bioactive compounds exert different properties in tumor cells and consequently to understand the role of redox homeostasis on tumor progression. This will help to develop new anti-tumor drugs based on redox modulation, which is a promising approach to fight the disease. Here we demonstrated that the redox protein TRX1 might play a key role in PCa survival after treatment with pro/anti-apoptotic compounds. Curcumin causes an increase in ROS and an oxidation of TRX1 while resveratrol dramatically diminishes its levels while increase the transcription of TRX1 inhibitor, TXNIP and promote a further increase in ROS levels (Fig. 8). Both substances lead to apoptosis in androgen sensitive and insensitive prostate cells by promoting oxidative stress rather than through their antioxidant properties frequently exhibited in vitro. On the contrary, cytostatic agents as melatonin and silibinin favor differentiation instead of apoptosis in prostate cancer cells by increasing their antioxidant capacity via the reduction of TRX1 oxidation and its nuclear translocation.
Funding
This work was supported by ‘Ministerio de Economía y Competitividad’ (Grant# MINECO-17-BFU2016-79139-R). A.R-G acknowledges the financial support from “Fundacion para el Fomento en Asturias de la Investigacion Cientifica aplicada y la Tecnologia” (FICYT), “Severo Ochoa” Program. Pedro González-Menéndez acknowledges sponsorship from Ministerio de Educación, Cultura y Deporte, (AP2012-4924).
Acknowledgements
We want to thank Ana Salas (Flow Cytometry Core Unit from Universidad de Oviedo), Ivan González-Pola and Maria Navarro-Rego for their helpful technical assistance.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.plefa.2016.05.006.
Appendix A. Supplementary material
Fig. S1.
Bands shown in redox western from Fig. 7 were quantified by densitometry in LNCaP (A) and PC-3 (B) cells, using Image J software. Bands from control LNCaP or PC3 cells (lane 4) were used as reference. Plots including the standard and the 5 experimental groups can be checked at supplementary material.
Fig. S2.
Scanning plots obtained from bands shown in redox western from Fig. 7 and plotted in supplementary Fig. 1. Scans were made using Image J with the Gel Analyzer add-in complement.
References
- 1.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 2.Sosa V., Moline T., Somoza R., Paciucci R., Kondoh H., ME L.L. Oxidative stress and cancer: an overview. Ageing Res. Rev. 2013;12:376–390. doi: 10.1016/j.arr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Wu W.S. The signaling mechanism of ROS in tumor progression. Cancer Metastas. Rev. 2006;25:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 4.Benhar M., Engelberg D., Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–425. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelicano H., Carney D., Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Update. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Arnér E.S., Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi T., Ueno Y., Okamoto T. Oxidoreductive regulation of nuclear factor kappa B. Involvement of a cellular reducing catalyst thioredoxin. J. Biol. Chem. 1993;268:11380–11388. [PubMed] [Google Scholar]
- 8.Hirota K., Matsui M., Iwata S., Nishiyama A., Mori K., Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Natl. Acad. Sci. USA. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saitoh M., Nishitoh H., Fujii M., Takeda K., Tobiume K., Sawada Y., Kawabata M., Miyazono K., Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iriyama T., Takeda K., Nakamura H., Morimoto Y., Kuroiwa T., Mizukami J., Umeda T., Noguchi T., Naguro I., Nishitoh H., Saegusa K., Tobiume K., Homma T., Shimada Y., Tsuda H., Aiko S., Imoto I., Inazawa J., Chida K., Kamei Y., Kozuma S., Taketani Y., Matsuzawa A., Ichijo H. ASK1 and ASK2 differentially regulate the counteracting roles of apoptosis and inflammation in tumorigenesis. EMBO J. 2009;28:843–853. doi: 10.1038/emboj.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang R., Al-Lamki R., Bai L., Streb J.W., Miano J.M., Bradley J., Min W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ. Res. 2004;94:1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- 12.Ferlay J., Parkin D.M., Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur. J. Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Shukla S., Gupta S. Dietary agents in the chemoprevention of prostate cancer. Nutr. Cancer. 2005;53:18–32. doi: 10.1207/s15327914nc5301_3. [DOI] [PubMed] [Google Scholar]
- 14.Deeb D., Xu Y.X., Jiang H., Gao X., Janakiraman N., Chapman R.A., Gautam S.C. Curcumin (diferuloyl-methane) enhances tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in LNCaP prostate cancer cells. Mol. Cancer Ther. 2003;2:95–103. [PubMed] [Google Scholar]
- 15.Gill C., Walsh S.E., Morrissey C., Fitzpatrick J.M., Watson R.W. Resveratrol sensitizes androgen independent prostate cancer cells to death-receptor mediated apoptosis through multiple mechanisms. Prostate. 2007;67:1641–1653. doi: 10.1002/pros.20653. [DOI] [PubMed] [Google Scholar]
- 16.Dhanalakshmi S., Agarwal P., Glode L.M., Agarwal R. Silibinin sensitizes human prostate carcinoma DU145 cells to cisplatin- and carboplatin-induced growth inhibition and apoptotic death. Int. J. Cancer J. Int. Cancer. 2003;106:699–705. doi: 10.1002/ijc.11299. [DOI] [PubMed] [Google Scholar]
- 17.Sainz R.M., Reiter R.J., Tan D.X., Roldan F., Natarajan M., Quiros I., Hevia D., Rodriguez C., Mayo J.C. Critical role of glutathione in melatonin enhancement of tumor necrosis factor and ionizing radiation-induced apoptosis in prostate cancer cells in vitro. J. Pineal Res. 2008;45:258–270. doi: 10.1111/j.1600-079X.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- 18.Reiter R.J., Tan D.X. Melatonin: an antioxidant in edible plants. Ann. N.Y. Acad. Sci. 2002;957:341–344. doi: 10.1111/j.1749-6632.2002.tb02938.x. [DOI] [PubMed] [Google Scholar]
- 19.Harper C.E., Patel B.B., Wang J., Arabshahi A., Eltoum I.A., Lamartiniere C.A. Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis. 2007;28:1946–1953. doi: 10.1093/carcin/bgm144. [DOI] [PubMed] [Google Scholar]
- 20.Zhou D.Y., Ding N., Van Doren J., Wei X.C., Du Z.Y., Conney A.H., Zhang K., Zheng X. Effects of curcumin analogues for inhibiting human prostate cancer cells and the growth of human PC-3 prostate xenografts in immunodeficient mice. Biol. Pharm. Bull. 2014;37:1029–1034. doi: 10.1248/bpb.b14-00044. [DOI] [PubMed] [Google Scholar]
- 21.Tyagi A., Bhatia N., Condon M.S., Bosland M.C., Agarwal C., Agarwal R. Antiproliferative and apoptotic effects of silibinin in rat prostate cancer cells. Prostate. 2002;53:211–217. doi: 10.1002/pros.10146. [DOI] [PubMed] [Google Scholar]
- 22.Ak T., Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Khanduja K.L., Bhardwaj A. Stable free radical scavenging and antiperoxidative properties of resveratrol compared in vitro with some other bioflavonoids. Indian J. Biochem. Biophys. 2003;40:416–422. [PubMed] [Google Scholar]
- 24.Tan D.X., Reiter R.J., Manchester L.C., Yan M.T., El-Sawi M., Sainz R.M., Mayo J.C., Kohen R., Allegra M., Hardeland R. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002;2:181–197. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 25.Katiyar S.K. Silymarin and skin cancer prevention: anti-inflammatory, antioxidant and immunomodulatory effects (Review) Int. J. Oncol. 2005;26:169–176. [PubMed] [Google Scholar]
- 26.Shan W., Zhong W., Zhao R., Oberley T.D. Thioredoxin 1 as a subcellular biomarker of redox imbalance in human prostate cancer progression. Free Radic. Biol. Med. 2010;49:2078–2087. doi: 10.1016/j.freeradbiomed.2010.10.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fanjul-Bolado P., Queipo P., Lamas-Ardisana P.J., Costa-Garcia A. Manufacture and evaluation of carbon nanotube modified screen-printed electrodes as electrochemical tools. Talanta. 2007;74:427–433. doi: 10.1016/j.talanta.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Bersani N.A., Merwin J.R., Lopez N.I., Pearson G.D., Merrill G.F. Protein electrophoretic mobility shift assay to monitor redox state of thioredoxin in cells. Methods Enzymol. 2002;347:317–326. doi: 10.1016/s0076-6879(02)47031-0. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi N., Hirose M. Determination of sulfhydryl groups and disulfide bonds in a protein by polyacrylamide gel electrophoresis. Anal. Biochem. 1990;188:359–365. doi: 10.1016/0003-2697(90)90621-f. [DOI] [PubMed] [Google Scholar]
- 30.Du Y., Zhang H., Lu J., Holmgren A. Glutathione and glutaredoxin act as a backup of human thioredoxin reductase 1 to reduce thioredoxin 1 preventing cell death by aurothioglucose. J. Biol. Chem. 2012;287:38210–38219. doi: 10.1074/jbc.M112.392225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.E.S. Arner, A. Holmgren, Measurement of thioredoxin and thioredoxin reductase. Current protocols in toxicology/editorial board, Mahin D. Maines Chapter 7:Unit 7 4, 2001. [DOI] [PubMed]
- 32.Rodriguez-Garcia A., Mayo J.C., Hevia D., Quiros-Gonzalez I., Navarro M., Sainz R.M. Phenotypic changes caused by melatonin increased sensitivity of prostate cancer cells to cytokine-induced apoptosis. J. Pineal Res. 2013;54:33–45. doi: 10.1111/j.1600-079X.2012.01017.x. [DOI] [PubMed] [Google Scholar]
- 33.Hevia D., Mayo J.C., Tan D.X., Rodriguez-Garcia A., Sainz R.M. Melatonin enhances photo-oxidation of 2′,7′-dichlorodihydrofluorescein by an antioxidant reaction that renders N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) PLoS One. 2014;9:e109257. doi: 10.1371/journal.pone.0109257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo H., Yang A., Schulte B.A., Wargovich M.J., Wang G.Y. Resveratrol induces premature senescence in lung cancer cells via ROS-mediated DNA damage. PLoS One. 2013;8:e60065. doi: 10.1371/journal.pone.0060065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thayyullathil F., Chathoth S., Hago A., Patel M., Galadari S. Rapid reactive oxygen species (ROS) generation induced by curcumin leads to caspase-dependent and -independent apoptosis in L929 cells. Free Radic. Biol. Med. 2008;45:1403–1412. doi: 10.1016/j.freeradbiomed.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Takeda K., Hatai T., Hamazaki T.S., Nishitoh H., Saitoh M., Ichijo H. Apoptosis signal-regulating kinase 1 (ASK1) induces neuronal differentiation and survival of PC12 cells. J. Biol. Chem. 2000;275:9805–9813. doi: 10.1074/jbc.275.13.9805. [DOI] [PubMed] [Google Scholar]
- 37.Madan E., Gogna R., Kuppusamy P., Bhatt M., Mahdi A.A., Pati U. SCO2 induces p53-mediated apoptosis by Thr845 phosphorylation of ASK-1 and dissociation of the ASK-1-Trx complex. Mol. Cell. Biol. 2013;33:1285–1302. doi: 10.1128/MCB.06798-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X., Zheng Y., Fried L.E., Du Y., Montano S.J., Sohn A., Lefkove B., Holmgren L., Arbiser J.L., Holmgren A., Lu J. Disruption of the mitochondrial thioredoxin system as a cell death mechanism of cationic triphenylmethanes. Free Radic. Biol. Med. 2011;50:811–820. doi: 10.1016/j.freeradbiomed.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zi X., Agarwal R. Silibinin decreases prostate-specific antigen with cell growth inhibition via G1 arrest, leading to differentiation of prostate carcinoma cells: implications for prostate cancer intervention. Proc. Natl. Acad. Sci. USA. 1999;96:7490–7495. doi: 10.1073/pnas.96.13.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sainz R.M., Mayo J.C., Tan D.X., Leon J., Manchester L., Reiter R.J. Melatonin reduces prostate cancer cell growth leading to neuroendocrine differentiation via a receptor and PKA independent mechanism. Prostate. 2005;63:29–43. doi: 10.1002/pros.20155. [DOI] [PubMed] [Google Scholar]
- 41.Dorai T., Cao Y.C., Dorai B., Buttyan R., Katz A.E. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47:293–303. doi: 10.1002/pros.1074. [DOI] [PubMed] [Google Scholar]
- 42.Sheth S., Jajoo S., Kaur T., Mukherjea D., Sheehan K., Rybak L.P., Ramkumar V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS One. 2012;7:e51655. doi: 10.1371/journal.pone.0051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harper C.E., Cook L.M., Patel B.B., Wang J., Eltoum I.A., Arabshahi A., Shirai T., Lamartiniere C.A. Genistein and resveratrol, alone and in combination, suppress prostate cancer in SV-40 tag rats. Prostate. 2009;69:1668–1682. doi: 10.1002/pros.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aggarwal B.B., Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Sharma O.P. Antioxidant activity of curcumin and related compounds. Biochem. Pharmacol. 1976;25:1811–1812. doi: 10.1016/0006-2952(76)90421-4. [DOI] [PubMed] [Google Scholar]
- 46.Pinlaor S., Yongvanit P., Prakobwong S., Kaewsamut B., Khoontawad J., Pinlaor P., Hiraku Y. Curcumin reduces oxidative and nitrative DNA damage through balancing of oxidant-antioxidant status in hamsters infected with Opisthorchis viverrini. Mol. Nutr. Food Res. 2009;53:1316–1328. doi: 10.1002/mnfr.200800567. [DOI] [PubMed] [Google Scholar]
- 47.Chanvorachote P., Pongrakhananon V., Wannachaiyasit S., Luanpitpong S., Rojanasakul Y., Nimmannit U. Curcumin sensitizes lung cancer cells to cisplatin-induced apoptosis through superoxide anion-mediated Bcl-2 degradation. Cancer Investig. 2009;27:624–635. doi: 10.1080/07357900802653472. [DOI] [PubMed] [Google Scholar]
- 48.Vue B., Chen Q.H. The potential of flavonolignans in prostate cancer management. Curr. Med. Chem. 2016;23:3925–3950. doi: 10.2174/0929867323666160823151833. [DOI] [PubMed] [Google Scholar]
- 49.Reiter R.J., Mayo J.C., Tan D.X., Sainz R.M., Alatorre-Jimenez M., Qin L. Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 50.Kim S.J., Miyoshi Y., Taguchi T., Tamaki Y., Nakamura H., Yodoi J., Kato K., Noguchi S. High thioredoxin expression is associated with resistance to docetaxel in primary breast cancer. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 2005;11:8425–8430. doi: 10.1158/1078-0432.CCR-05-0449. [DOI] [PubMed] [Google Scholar]
- 51.Yokomizo A., Ono M., Nanri H., Makino Y., Ohga T., Wada M., Okamoto T., Yodoi J., Kuwano M., Kohno K. Cellular levels of thioredoxin associated with drug sensitivity to cisplatin, mitomycin C, doxorubicin, and etoposide. Cancer Res. 1995;55:4293–4296. [PubMed] [Google Scholar]
- 52.Hirota K., Murata M., Sachi Y., Nakamura H., Takeuchi J., Mori K., Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. J. Biol. Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 53.Fang J., Lu J., Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J. Biol. Chem. 2005;280:25284–25290. doi: 10.1074/jbc.M414645200. [DOI] [PubMed] [Google Scholar]
- 54.Lu J., Papp L.V., Fang J., Rodriguez-Nieto S., Zhivotovsky B., Holmgren A. Inhibition of Mammalian thioredoxin reductase by some flavonoids: implications for myricetin and quercetin anticancer activity. Cancer Res. 2006;66:4410–4418. doi: 10.1158/0008-5472.CAN-05-3310. [DOI] [PubMed] [Google Scholar]
- 55.Nishiyama A., Matsui M., Iwata S., Hirota K., Masutani H., Nakamura H., Takagi Y., Sono H., Gon Y., Yodoi J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- 56.Patwari P., Higgins L.J., Chutkow W.A., Yoshioka J., Lee R.T. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J. Biol. Chem. 2006;281:21884–21891. doi: 10.1074/jbc.M600427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cadenas C., Franckenstein D., Schmidt M., Gehrmann M., Hermes M., Geppert B., Schormann W., Maccoux L.J., Schug M., Schumann A., Wilhelm C., Freis E., Ickstadt K., Rahnenfuhrer J., Baumbach J.I., Sickmann A., Hengstler J.G. Role of thioredoxin reductase 1 and thioredoxin interacting protein in prognosis of breast cancer. Breast Cancer Res.: BCR. 2010;12:R44. doi: 10.1186/bcr2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dutta K.K., Nishinaka Y., Masutani H., Akatsuka S., Aung T.T., Shirase T., Lee W.H., Yamada Y., Hiai H., Yodoi J., Toyokuni S. Two distinct mechanisms for loss of thioredoxin-binding protein-2 in oxidative stress-induced renal carcinogenesis. Lab. Investig.; J. Tech. Methods Pathol. 2005;85:798–807. doi: 10.1038/labinvest.3700280. [DOI] [PubMed] [Google Scholar]
- 59.Kopantzev E.P., Monastyrskaya G.S., Vinogradova T.V., Zinovyeva M.V., Kostina M.B., Filyukova O.B., Tonevitsky A.G., Sukhikh G.T., Sverdlov E.D. Differences in gene expression levels between early and later stages of human lung development are opposite to those between normal lung tissue and non-small lung cell carcinoma. Lung Cancer. 2008;62:23–34. doi: 10.1016/j.lungcan.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 60.Yu Y., Xing K., Badamas R., Kuszynski C.A., Wu H., Lou M.F. Overexpression of thioredoxin-binding protein 2 increases oxidation sensitivity and apoptosis in human lens epithelial cells. Free Radic. Biol. Med. 2013;57:92–104. doi: 10.1016/j.freeradbiomed.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamaguchi F., Takata M., Kamitori K., Nonaka M., Dong Y., Sui L., Tokuda M. Rare sugar D-allose induces specific up-regulation of TXNIP and subsequent G1 cell cycle arrest in hepatocellular carcinoma cells by stabilization of p27kip1. Int. J. Oncol. 2008;32:377–385. [PubMed] [Google Scholar]
- 62.Fulda S., Debatin K.M. Sensitization for anticancer drug-induced apoptosis by the chemopreventive agent resveratrol. Oncogene. 2004;23:6702–6711. doi: 10.1038/sj.onc.1207630. [DOI] [PubMed] [Google Scholar]
- 63.C.R. Ireson, D.J. Jones, S. Orr, M.W. Coughtrie, D.J. Boocock, M.L. Williams, P.B. Farmer, W.P. Steward, A.J. Gescher, Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiology, Biomarkers and Prevention : a Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, vol. 11, 2002, pp. 105–111. [PubMed]
- 64.Wenzel E., Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005;49:472–481. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- 65.Yallapu M.M., Khan S., Maher D.M., Ebeling M.C., Sundram V., Chauhan N., Ganju A., Balakrishna S., Gupta B.K., Zafar N., Jaggi M., Chauhan S.C. Anti-cancer activity of curcumin loaded nanoparticles in prostate cancer. Biomaterials. 2014;35:8635–8648. doi: 10.1016/j.biomaterials.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Augustin M.A., Sanguansri L., Lockett T. Nano- and micro-encapsulated systems for enhancing the delivery of resveratrol. Ann. N.Y. Acad. Sci. 2013;1290:107–112. doi: 10.1111/nyas.12130. [DOI] [PubMed] [Google Scholar]