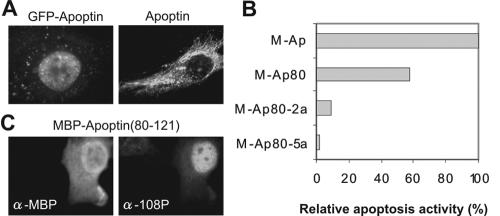
Wadia et al. (7) recently claimed that apoptin, a virally encoded protein with tumor-selective apoptosis activity, contains a concentration-dependent nuclear localization signal (NLS) that is not tumor selective as previously reported (1, 2, 4). Their Fig. 1B shows, however, that under all the conditions tested, nuclear import of green fluorescent protein (GFP)-apoptin70-121 remains 200 to 300% higher in tumor cells than in primary cells. The apparent concentration dependence of apoptin's NLS is intriguing. We agree with their interpretation that presentation of multiple apoptin NLS domains by a molecular aggregate could generate more efficient nuclear trafficking than NLS exposure at a single site. This result is, however, somewhat academic, as full-length apoptin can only be harvested as a multimer from live cells, with a dissociation rate constant (koff) that is so slow that it can hardly be measured, whereas the C-terminal fragment forms only monomers (5, 9). This could explain why Wadia et al. could measure a cooperative, concentration-dependent effect of apoptin's NLS, as they used a GFP fusion of a C-terminal fragment of apoptin lacking the multimerization domain (5). Had they used full-length apoptin, all the NLS sequences would likely have been clustered, resulting in effective nuclear import over all concentration ranges. Nevertheless, we suggest caution in using GFP-apoptin fusions in functional studies. For example, fusion of GFP to full-length apoptin results in increased levels of nuclear GFP-apoptin versus wild-type apoptin in primary cells (Fig. 1A).
FIG. 1.
(A) Apoptin but not GFP-apoptin is mainly localized in the cytoplasm of human primary cells. Human mesenchymal stem cells were microinjected with plasmids encoding GFP-apoptin (left) or wild-type apoptin (right). GFP-apoptin was analyzed directly by fluorescence microscopy, whereas apoptin was stained by indirect immunofluorescence (2). (B) Mutation of the phosphorylation sites at locus positions 106 to 108 eliminates the apoptotic activity of the apoptin C-terminal region. (Apoptin also has an autonomous, phosphorylation-independent N-terminal death domain [3] that appears to act in an additive fashion with the C-terminal death domain; both must be disrupted for full inactivity.) Human HeLa cells were transfected (Fugene; Roche) with DNA plasmids encoding maltose binding protein (MBP)-fusion proteins containing full-length apoptin (M-Ap), a C-terminal (80 to 121) fragment (M-Ap80), or two mutated C-terminal (80 to 121) fragments: M-Ap80-2a with alanines at position 107 and 108 instead of threonines and M-Ap80-5ala with alanines from 106 to 110 inclusive. The cells were examined for relative apoptosis activity at various days posttransfection: the endpoint at 4 days is presented. The apoptosis activities were corrected for the nonapoptotic desmin control (2). (C) The C-terminal apoptin (positions 80 to 121) fragment used in panel B is phosphorylated on Thr108 in human tumor cells—mainly the nuclear pool, as previously reported for the wild type (6). Human tumorigenic HeLa cells were transfected with a plasmid encoding MBP-apoptin (positions 80 to 121). Cells were stained with antibodies (α-) against MBP (left panel) or with 108-P, a purified phosphospecific polyclonal rabbit antibody (6) against phosphorylated Thr108 of apoptin (right panel).
The current hypothesis is that nuclear trafficking and tumor-specific phosphorylation of apoptin at Thr108 are essential for induction of apoptosis (3, 6, 8). Wadia et al. reported a failure to detect phosphorylation with radioactive labeling of GFP-apoptin70-121; we do not know why this is so, as phosphorylation of full-length apoptin has been thoroughly documented by the use of mass spectrometry and a phospho-specific antibody (6). Our preliminary experiments suggest that abolishing the phosphorylation site of apoptin does not significantly disrupt its nuclear import in tumor cells. In attempting to address this observation with a GFP-apoptin70-121 T108A mutant, Wadia et al. used a construct that is not phospho-null in vivo; in our hands, as Thr108 is the last in a run of three threonine residues, the adjacent Thr107 becomes opportunistically phosphorylated instead, which yields the same phenotype. Mutation at both positions 107 and 108 is required to eliminate phosphorylation and function. As Fig. 1B indicates, phosphorylation of apoptin is required for apoptosis induced by its C-terminal death domain (3); a control experiment confirms that the apoptosis-competent fragment is phosphorylated on Thr108 in vivo (Fig. 1C).
Apoptin's tumor-specific activity does not result from a single characteristic. Its multimerization behavior, its potential to be phosphorylated, its cooperativity in nuclear trafficking, and many of its other physical, chemical, and functional activities all shape the mechanism by which it induces tumor-specific apoptosis. Extrapolations of results obtained from studies that disrupt apoptin's integrity should therefore be approached with caution.
REFERENCES
- 1.Cheng, C.-M., S.-P. Huang, Y. F. Chang, W.-Y. Chung, and C.-Y. You. 2003. The viral death protein Apoptin interacts with Hippi, the protein interactor of Huntingtin-interacting protein 1. Biochem. Biophys. Res. Commun. 305:359-364. [DOI] [PubMed] [Google Scholar]
- 2.Danen-van Oorschot, A. A. A. M., D. Fischer, J. M. Grimbergen, B. Klein, S.-M. Zhuang, J. H. F. Falkenburg, C. Backendorf, P. H. A. Quax, A. J. Van der Eb, and M. H. M. Noteborn. 1997. Apoptin induces apoptosis in human transformed and malignant cells but not in normal cells. Proc. Natl. Acad. Sci. USA 94:5843-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danen-van Oorschot, A. A. A. M., Y.-H. Zhang, S. R. Leliveld, J. L. Rohn, M. C. M. J. Seelen, M. W. Bolk, A. Van Zon, S. J. Erkeland, J.-P. Abrahams, D. Mumberg, and M. H. M. Noteborn. 2003. Importance of nuclear localization of apoptin for tumor-specific induction of apoptosis. J. Biol. Chem. 278:27729-27736. [DOI] [PubMed] [Google Scholar]
- 4.Guelen, L., H. Paterson, J. Gaeken, M. Meyers, F. Farzaneh, and M. Tavassoli. 2004. TAT-apoptin is efficiently delivered and induces apoptosis in cancer cells. Oncogene 23:1153-1165. [DOI] [PubMed] [Google Scholar]
- 5.Leliveld, S. R., Y.-H. Zhang, L. R. Rohn, M. H. M. Noteborn, and J. P. Abrahams. 2003. Apoptin induces tumor-specific apoptosis as globular multimer. J. Biol. Chem. 278:9042-9051. [DOI] [PubMed] [Google Scholar]
- 6.Rohn, J. L., Y.-H. Zhang, R. I. J. M. Aalbers, N. Otto, J. Den Hertog, N. Henriquez, C. J. H. Van de Velde, P. J. K. Kuppen, D. Mumberg, P. Donner, and M. H. M. Noteborn. 2002. A tumor-specific kinase activity regulates the viral death protein Apoptin. J. Biol. Chem. 277:50820-50827. [DOI] [PubMed] [Google Scholar]
- 7.Wadia, J. S., M. V. Wagner, S. A. Ezhevsky, and S. F. Dowdy. 2004. Apoptin/VP3 contains a concentration-dependent nuclear localization signal (NLS), not a tumorigenic selective NLS. J. Virol. 78:6077-6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang, Y.-H., K. Kooistra, A. M. Pietersen, J. L. Rohn, and M. H. M. Noteborn. 2004. Activation of the tumor-specific death effector apoptin and its kinase by an N-terminal determinant of simian virus 40 large T antigen. J. Virol. 78:9965-9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang, Y.-H., S. R. Leliveld, K. Kooistra, C. Molenaar, J. L. Rohn, H. J. Tanke, J. P. Abrahams, and M. H. M. Noteborn. 2003. Recombinant Apoptin multimers kill tumor cells but are not-toxic and epitope-shielded in a normal-cell-specific fashion. Exp. Cell Res. 289:36-46. [DOI] [PubMed] [Google Scholar]